LETTER
Nephrotoxicity associated with the polymyxins has been a significant barrier to their use. The conventional approach of monitoring renal function by measuring serum creatinine is relatively insensitive in acute kidney injury (AKI), and more sensitive biomarkers are needed to detect early AKI (1). Previous studies have looked at urinary biomarkers for predicting nephrotoxicity in animals treated with polymyxins. KIM-1 and α-glutathione S-transferase were reported to predict renal injury in rats, while others found that neutrophil gelatinase-associated lipocalin and KIM-1 levels were correlated with kidney injury in several animal models (1, 2). However, these biomarkers have not been investigated for clinical use in patients.
We report here our preliminary findings on the utility of KIM-1 for the prediction of AKI in adult (≥18 years old) patients treated intravenously with polymyxin B. An open-label, prospective, observational study was conducted at Catholic Health Initiatives (CHI) Baylor St. Luke's Medical Center (Houston, TX). It was approved by the CHI Institutional Review Board. Written informed consent was obtained from each patient or legal guardian. The following were excluded: patients with allergy to polymyxin B, pediatric and pregnant patients, patients with fluctuating renal function (>50% change in serum creatinine in the 3 days prior to initiation of polymyxin therapy), and patients on any form of renal replacement therapy. Polymyxin B dosing was based on institution recommendations (i.e., a 25,000-U/kg loading dose and then 12,500 to 15,000 U/kg every 12 h). Renal function was monitored by measuring serum creatinine in accordance with routine care procedures. Urine samples were collected within 24 h of polymyxin B initiation and daily thereafter. Samples were aliquoted and frozen at −80°C for later analysis.
Urinary creatinine (UCr) was quantified via a colorimetric assay from Abcam (Cambridge, MA), and urine was assayed for KIM-1 with an enzyme-linked immunosorbent assay kit from Enzo (Farmingdale, NY). Urine KIM-1 levels were normalized against the appropriate UCr values to account for variation in urine concentration due to hydration status. A daily normalized ratio (fold change from the baseline) was calculated for each patient.
Three patients met the inclusion criteria. One patient experienced AKI with an elevation in serum creatinine to >1.5 times the baseline on day 9 of treatment. KIM-1 peaked in a median time of 6 days. The peak normalized KIM-1/UCr ratio was 16.9 in the patient who experienced AKI, while it was 8.6 and 5.6 in the other two patients. While KIM-1 peaked on day 10 in the patient with AKI, a normalized ratio of >10 was seen by day 3 of treatment, 6 days prior to the clinically significant increase in serum creatinine (Fig. 1). These findings are consistent with previous observations that renal injury associated with polymyxin B is primarily confined to the proximal tubules in an animal model (3).
FIG 1.
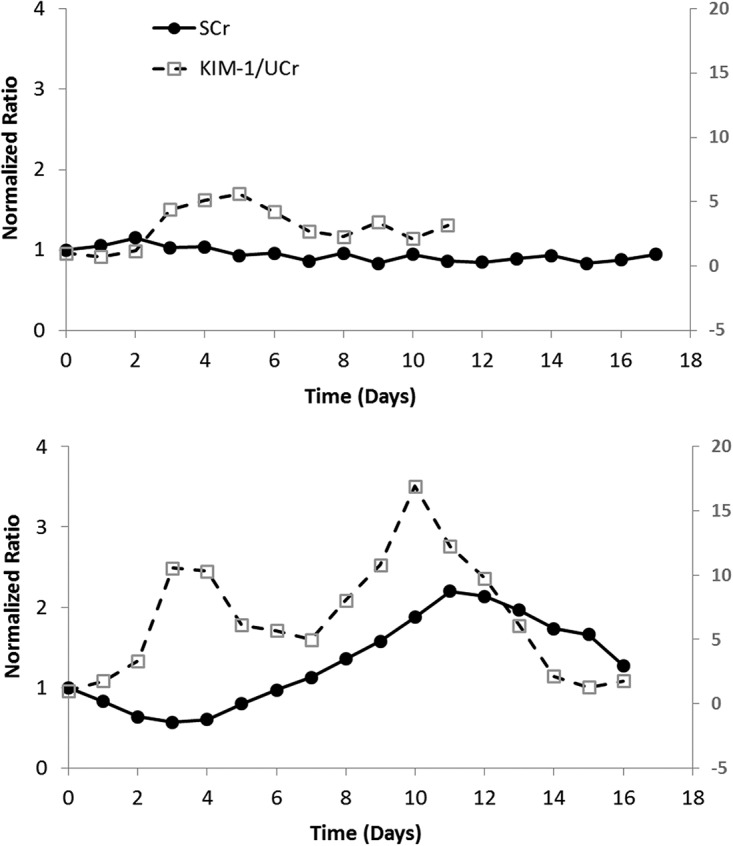
Changes in biomarker concentrations over time. Each panel represents observations from a single patient. The patient whose results are depicted in the top panel did not experience AKI, while an elevated serum creatinine (SCr) level was observed in the patient whose results are shown in the bottom panel.
To our knowledge, this is the first study to examine the clinical utility of urinary biomarkers for the prediction of polymyxin B-induced nephrotoxicity. We hypothesize that a 10-fold elevation in KIM-1 from the baseline could be a target threshold to predict AKI in patients treated with polymyxin B. Further clinical validation in a larger number of subjects is warranted.
REFERENCES
- 1.Keirstead ND, Wagoner MP, Bentley P, Blais M, Brown C, Cheatham L, Ciaccio P, Dragan Y, Ferguson D, Fikes J, Galvin M, Gupta A, Hale M, Johnson N, Luo W, McGrath F, Pietras M, Price S, Sathe AG, Sasaki JC, Snow D, Walsky RL, Kern G. 2014. Early prediction of polymyxin-induced nephrotoxicity with next-generation urinary kidney injury biomarkers. Toxicol Sci 137:278–291. doi: 10.1093/toxsci/kft247. [DOI] [PubMed] [Google Scholar]
- 2.Burt D, Crowell SJ, Ackley DC, Magee TV, Aubrecht J. 2014. Application of emerging biomarkers of acute kidney injury in development of kidney-sparing polypeptide-based antibiotics. Drug Chem Toxicol 37:204–212. doi: 10.3109/01480545.2013.834360. [DOI] [PubMed] [Google Scholar]
- 3.Abdelraouf K, Braggs KH, Yin T, Truong LD, Hu M, Tam VH. 2012. Characterization of polymyxin B-induced nephrotoxicity: implications for dosing regimen design. Antimicrob Agents Chemother 56:4625–4629. doi: 10.1128/AAC.00280-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


