ABSTRACT
In the screening of natural plant extracts for antifungal activity, assessment of their effects on the growth of cells in suspension or in the wells of microtiter plates is expedient. However, microorganisms, including Candida albicans, grow in nature as biofilms, which are organized cellular communities with a complex architecture capable of conditioning their microenvironment, communicating, and excluding low- and high-molecular-weight molecules and white blood cells. Here, a confocal laser scanning microscopy (CLSM) protocol for testing the effects of large numbers of agents on biofilm development is described. The protocol assessed nine parameters from a single z-stack series of CLSM scans for each individual biofilm analyzed. The parameters included adhesion, thickness, formation of a basal yeast cell polylayer, hypha formation, the vertical orientation of hyphae, the hyphal bend point, pseudohypha formation, calcofluor white staining of the extracellular matrix (ECM), and human white blood cell impenetrability. The protocol was applied first to five plant extracts and derivative compounds and then to a collection of 88 previously untested plant extracts. They were found to cause a variety of phenotypic profiles, as was the case for 64 of the 88 extracts (73%). Half of the 46 extracts that did not affect biofilm thickness affected other biofilm parameters. Correlations between specific effects were revealed. The protocol will be useful not only in the screening of chemical libraries but also in the analysis of compounds with known effects and mutations.
KEYWORDS: Candida albicans, natural agents, antibiofilm agents
INTRODUCTION
Plants and other organisms have developed a variety of mechanisms to protect themselves from fungal infections (1–5). They therefore provide a potentially rich source of natural antifungal agents, which in some cases may translate into potential drugs for treating human fungal infections (6–8). The screening of large numbers of extracts, fractions of extracts, or known compounds for new antimicrobial agents usually involves expedient methods for assessing the effects of a large number of candidate compounds on cell multiplication in suspension or standing cultures (9, 10). However, in natural settings pathogenic microorganisms grow predominantly as biofilms on the surfaces of tissues, catheters, and prosthetics (11–16). Biofilms are complex multicellular communities. Therefore, screening for new antimicrobial agents using a single growth parameter, while expedient, may benefit from the addition of procedures that assess their effects on biofilm formation (17–22). The number of studies in which the effects of antimicrobial agents on biofilm preparations have been screened has therefore increased in recent years (19, 23–29). However, the majority of these studies, as is the case for the screening of the effects of antimicrobial agents on planktonic cultures, is limited to the evaluation of one or more growth or metabolic parameters, such as dry weight (23, 25, 29), 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt (XTT) reduction (19, 24, 26, 27, 29–32), crystal violet staining (33–35), and the number of CFU (32). In a limited number of cases, such as a study by Chandra et al. (36), parameters evaluated by combinations of methods, including fluorescence microscopy, scanning electron microscopy, confocal laser scanning microscopy, and determination of XTT reduction and dry weight, have been assessed, but such evaluations require separate biofilm preparations. The protocol by Chandra et al. (36) highlighted the fact that biofilms are not simply carpets of equivalent cells growing on a two-dimensional substrate. Rather, as Costerton and colleagues first described for bacteria (37, 38) and Douglas and colleagues described for Candida species (39, 40), biofilms are highly organized, developmental communities of cells capable of exhibiting a complex three-dimensional architecture, differentiating into multiple phenotypes with very different growth patterns and gene expression patterns, and forming a complex extracellular matrix (ECM). Biofilms facilitate cell-cell communication; conditioning of their microenvironment; exclusion of harmful molecules, including antibiotics; and exclusion of phagocytic polymorphonuclear leukocytes (PMNs) (13, 41–43). Moreover, the biofilms of a single strain can be phenotypically diverse, depending upon the conditions under which biofilms form (40, 44–46). In the case of Candida albicans and related Candida species, pathogenic or sexual biofilms form depending upon the configuration of the mating type locus (47–49). These alternative biofilms exhibit major differences in permeability to high- and low-molecular-weight molecules, impenetrability by human white blood cells, and drug resistance and are regulated by alternative signal transduction pathways (40, 49–53).
Recognizing the complexity of C. albicans biofilms and realizing that the use of multiple preparations is too time-consuming for screens of large chemical, natural extract, or mutant libraries, we have developed a protocol for screening the effects of chemical agents on biofilm development that is based on high-resolution confocal laser scanning microscopy (CLSM) and that quantitatively assesses nine parameters from the same stack of z-series scans of a single CLSM preparation. The parameters included adherence to a substrate, architectural parameters, cell phenotypes, ECM staining, and PMN impenetrability (Fig. 1B). The power of the protocol is that all nine parameters are obtained from a single z-stack of CLSM scans of an individual biofilm preparation rather than from separate preparations in separate experiments. We first assessed the effectiveness of this protocol on five crude extracts and extract-derived compounds previously demonstrated to inhibit the growth of C. albicans in suspension or on a plastic surface (54–62). All five extracts and all five extract-derived antifungal agents caused aberrant biofilm formation, on the basis of a comparison with the phenotypic profiles afforded by the nine parameters of the untreated control preparations, but the profiles differed between extracts in some cases and between the extract and the extract-derived agent in two cases. Having confirmed the efficacy of the protocol with a single preparation for the five extracts and derivative compounds with known antifungal activities, we then used it to analyze 88 previously untested plant extracts. Seven of the extracts affected adherence and therefore could not be assessed for the eight remaining parameters by CLSM. The remaining 81 extracts that were assessable by CLSM were then analyzed for the remaining eight parameters. Six of the 10 extracts (60%) causing biofilms to be significantly thicker than the biofilms of the controls affected one or more of the remaining parameters. Twenty-one of the 25 extracts (84%) that caused the biofilms to be significantly thinner and 24 of the 46 extracts (52%) that had no significant effect on thickness produced changes in one or more of the eight remaining biofilm characteristics. The procedure therefore revealed that 72% of the extracts affected one or more aspects of biofilm development. Our results support the effectiveness of the protocol described here for identifying new antifungal agents that affect biofilm development. The results also revealed correlations between particular parameters that suggest cause-effect relationships. The protocol, which can also be used to characterize the effects of compounds with known functions and mutations on biofilm development, provides a large data set that can be expanded with new data if the protocol is rigidly followed in future experiments.
FIG 1.
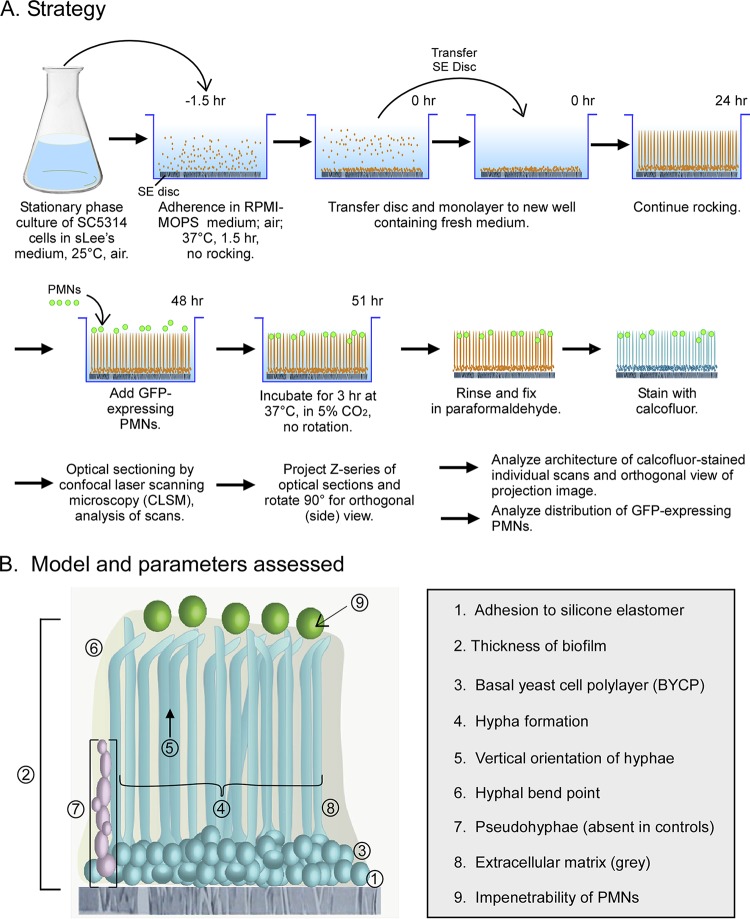
Procedure for generating biofilm preparations for analysis and the parameters that were assessed in comparing the effects of extracts and compounds on individual biofilm development. (A) Flowchart of the procedure. See Materials and Methods and Results for details. (B) Model of a control biofilm and the assessed parameters. Note that pseudohyphae (parameter 7) are not formed in untreated control biofilms. SE disc, silicone elastomer disc; sLee's medium, supplemented Lee's medium; BYCP, basal yeast cell polylayer; CLSM, confocal laser scanning microscopy, GFP-expressing PMNs, green fluorescent protein-expressing polymorphonuclear leukocytes.
RESULTS
Strategy for testing.
In the procedure outlined in Fig. 1A (40), untreated cells of strain SC5314 form a basal yeast cell polylayer (BYCP) approximately eight cells thick after 6 to 8 h of incubation (Fig. 1B and 2A). In the next 35 to 40 h of incubation, an upper layer of hypha forms, accounting for approximately 80% of the biofilm height. The hyphae are separated by an extracellular matrix (ECM) and vertically oriented through 60 to 70% of their length, which is evidenced by the punctate profiles in a scan through the midpoint of a 48-h-old control biofilm in Fig. 2C. At 60 to 70% of their length, the hyphae then bend, forming a mat in the most distal (top) region of the 48-h-old control biofilm (Fig. 1B and 2E). ECM can be visualized (Fig. 2F) by calcofluor white staining (63) using confocal laser scanning microscopy (CLSM), as described in Materials and Methods. The growth of the biofilm under these conditions slows with time and is completed by 48 h, as described previously by Hawser and Douglas (64). When green fluorescent protein (GFP)-expressing PMNs of the cell line HL-60 were distributed on the surface of a fresh 48-h-old untreated biofilm, they remained at the top after 3 h of incubation, reflecting PMN impenetrability (65). The parameters that were used to assess the effects of the extracts on biofilm formation (Fig. 1B) included (i) adherence to the silicone elastomer, (ii) absolute thickness (in micrometers) and the percent difference in thickness from the thickness of the control, (iii) the thickness of the BYCP, (iv) hypha formation, (v) vertical orientation of the hyphae, (vi) the percentage of the length of the hyphae at which there was a bend, (vii) induction of pseudohypha formation, which is absent in untreated control preparations, (viii) the intensity of calcofluor white staining of the ECM, and (ix) the impenetrability of PMNs (Fig. 1B). These parameters are defined in detail in Materials and Methods.
FIG 2.
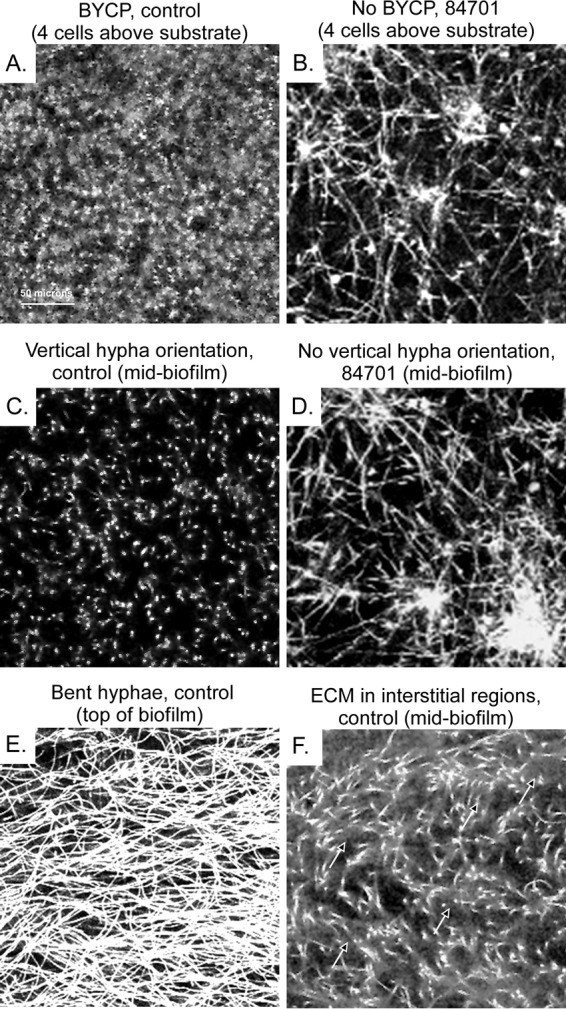
Individual CLSM scans at different heights of a control biofilm (untreated) and an extract 84701-treated biofilm, which exhibits a loss of vertical hypha orientation and defects in other biofilm parameters. (A) Scan at the 4-cell level through the basal yeast cell polylayer (BYCP) of a 48-h-old untreated (control) biofilm. (B) Scan at the same height as the scan of the control biofilm in panel A of a preparation treated with extract 84701 after 48 h. Note that the biofilm treated with extract 84701 lacked a BYCP and vertical hypha orientation. (C) Scan at the midpoint (80 μm) through the hyphal region of a 48-h-old untreated control biofilm. Note the punctate pattern of the vertically oriented hyphae. (D) Scan at the same height as the scan of the control in panel C of a preparation treated with extract 84701. Note the randomly oriented hyphae, as was the case at the lower level in panel B. (E) The matted top of a 48-h-old untreated (control) biofilm after the most distal ends had bent. (F) Visualization of the ECM at the midpoint of a 48-h-old control biofilm in the interstitial regions between cells by increasing midgrayscale intensity.
DMSO control.
Since all extracts and compounds tested in this study contained 0.4% (0.05 M) dimethyl sulfoxide (DMSO), control biofilms were assessed both in the absence (Fig. 3A and B; see Table 2) and in the presence (Fig. 3C and D; see Table 2) of DMSO. Biofilms treated with DMSO exhibited adhesiveness, architectural parameter, and PMN impenetrability characteristics highly similar to those of untreated biofilms (see Table 2).
FIG 3.
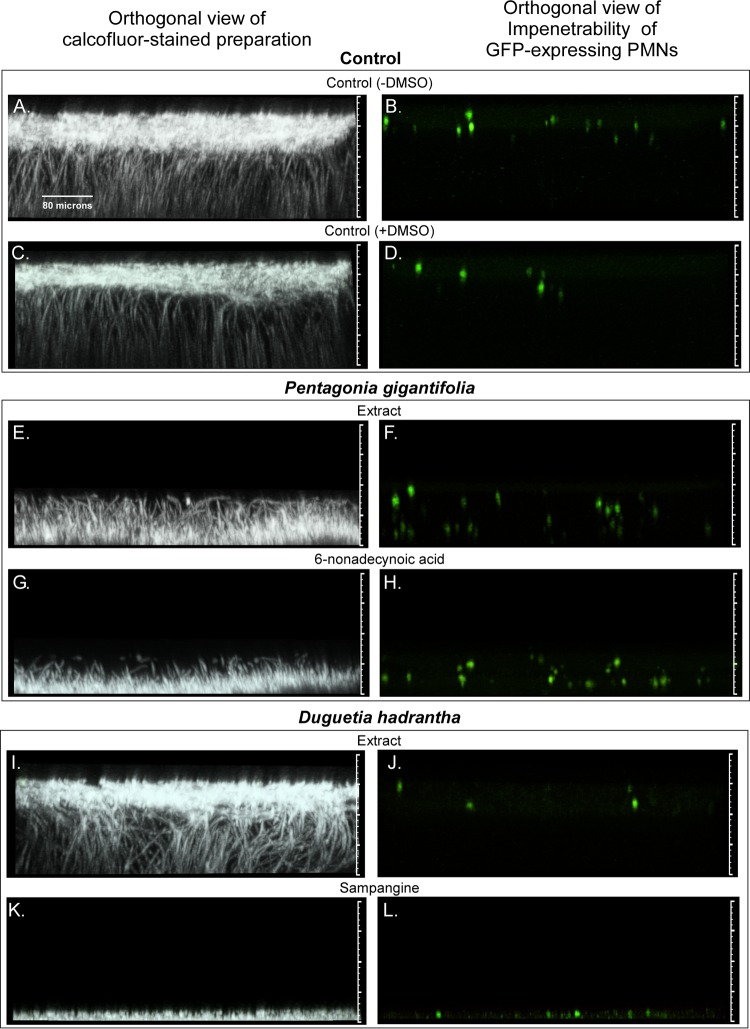
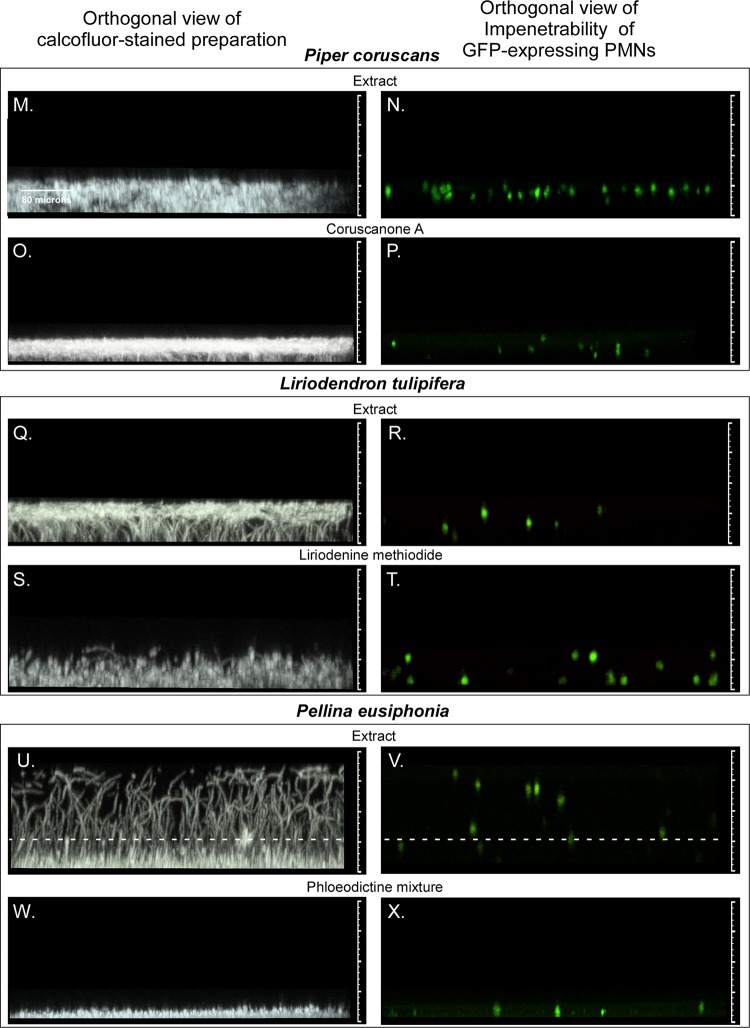
Orthogonal (side) views of biofilm staining with calcofluor white or imaging of green fluorescent protein (GFP)-expressing PMNs after treatment with five pairs of extracts and derivative compounds previously shown to inhibit the growth of C. albicans. The five pairs of extracts and derivative agents are described in Table 1, and biofilm characteristics are described in Table 2. (A to D) Untreated biofilm control in the absence of DMSO (A, B) and untreated biofilm control in the presence of 0.4% DMSO (C, D). Note that all test biofilms treated with extracts or derivative agents contained 0.4% DMSO. (E to H) Representative biofilms treated with the extract of Pentagonia gigantifolia (E, F) or the derivative 6-nonadecynoic acid (G, H). (I to L) Representative biofilms treated with the extract of Duguetia hadrantha (I and J) or the derivative sampangine (K and L). (M to P) Representative biofilms treated with the extract of Piper coruscans (M, N) or the derivative coruscanone A (O, P). (Q to T) Representative biofilms treated with the extract of Liriodendron tulipifera (Q, R) or the derivative liriodenine methiodide (S, T). (U to X) Representative biofilms treated with the extract of Pellina eusiphonia (U, V) or the derivative phloeodictine mixture (W, X). The matted hyphae in the Pellina eusiphonia extract-treated preparation unwound when inverted, causing the biofilm, which was devoid of ECM, to appear thicker than it was. The dashed lines in panels U and V reflect the actual upright thickness in this case. The orthogonal views represent vertical slices (sections) through the stack of CLSM scans (projected image) that had been rotated 90°. The view is of an orthogonal slice taken 40 pixels into the projected image.
TABLE 2.
Effects of five natural extracts and molecular derivatives on Candida albicans biofilm formationd
| Organism | Extract, deriv. compd., or presence of DMSO | Biofilm thickness (μm) | % change in biofilm thickness | BYCP formation score | Hypha formation score | Vertical hypha orientation score | Hypha bend pointc | Pseudohypa formation score | ECM staining score | PMN impenetrability score |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Without DMSO | 161 ± 12 | ++++ | ++++ | ++++ | 70 | − | ++++ | ++++ | |
| With DMSO | 159 ± 11 | ++++ | ++++ | ++++ | 74 | − | ++++ | ++++ | ||
| Pentagonia gigantifolia | Extract | 64 ± 4 | 60 | ++++ | ++ | − | 0 | +++ | − | − |
| 6-Nonadecynoic acid | 40 ± 9 | 75 | + | + | − | NA | ++++ | − | − | |
| Duguetia hadrantha | Extract | 143 ± 5 | 15 | + | ++++ | − | 78 | − | ++ | +++ |
| Sampanginea | 9 ± 1 | 94 | + | − | NA | NA | − | − | NA | |
| Piper coruscans | Extract | 71 ± 4 | 55 | − | +++++(+)b | − | 0 | − | ++ | + |
| Coruscanone A | 45 ± 6 | 72 | − | +++++(+) | − | 0 | − | − | − | |
| Liriodendron tulipifera | Extract | 63 ± 15 | 61 | − | ++++ | − | 30 | − | − | − |
| Liriodenine methiodide | 28 ± 3 | 83 | +++ | + | − | 0 | ++++ | − | − | |
| Pellina eusiphonia | Extract | 61 ± 5 | 62 | ++++ | + | − | 77 | ++++ | − | − |
| Phloeodictine mixturea | 10 ± 2 | 94 | − | − | NA | NA | − | − | NA |
Because only a monolayer was formed, preparations treated with sampangine or the phloeodictine mixture could not be assessed for some characteristics.
++++(+), hypherhypha formation, assessed as a highly dense hyphal upper layer.
The hypha bend point is the percentage of the hyphal length in the distal direction at which hyphae bend.
Deriv. compd., derivative compound; BYCP, basal yeast cell polylayer; ECM staining, staining of extracellular matrix with calcofluor white; PMN impenet., polymorphonuclear leukocyte impenetrability; NA, not assessable.
Testing of five extracts with identified antifungal components.
To assess the efficacy of the strategy for discriminating the selective effects of untested natural products on biofilm architecture and PMN impenetrability, we analyzed the biofilms formed in five natural extracts and extract-derived compounds previously shown to have antifungal activity by growth assays (54–62) (Table 1). Four of the five pairs of extracts and derivative antifungal compounds were from plants, and the one remaining pair was from a sponge (Table 1).
TABLE 1.
Five extracts and derivatives previously demonstrated to inhibit Candida albicans growth
| Genus and species (organism type) of extract source | Derivative antifungal | Mechanism of action of derivative | Derivative structure | Reference(s) |
|---|---|---|---|---|
| Pentagonia gigantifolia (plant) | 6-Nonadecynoic acid | Fatty acid metabolism | 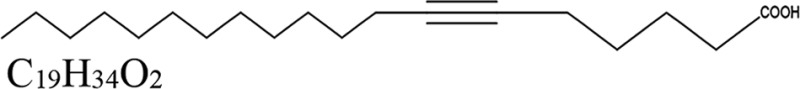 |
59, 60, 62 |
| Duguetia hadrantha (plant) | Sampangine | Heme biosynthesis | 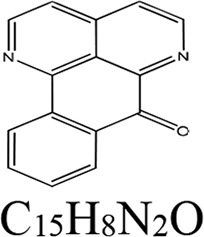 |
54, 61 |
| Piper coruscans (plant) | Coruscanone A | Mitochondrial function | 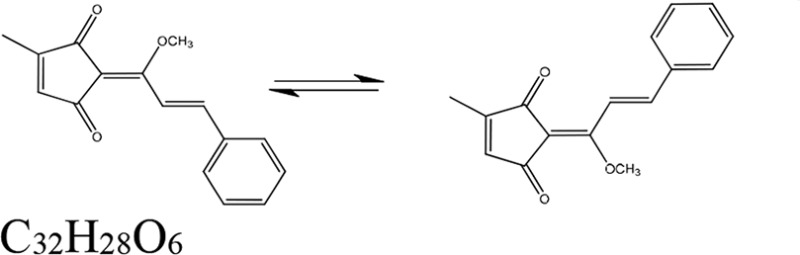 |
55, 58 |
| Liriodendron tulipifera (plant) | Liriodenine methiodide | Iron homeostasis | 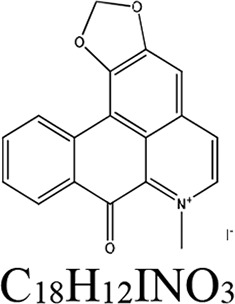 |
56 |
| Pellina eusiphonia (animal) | Phloeodictine mixture | Unknown | 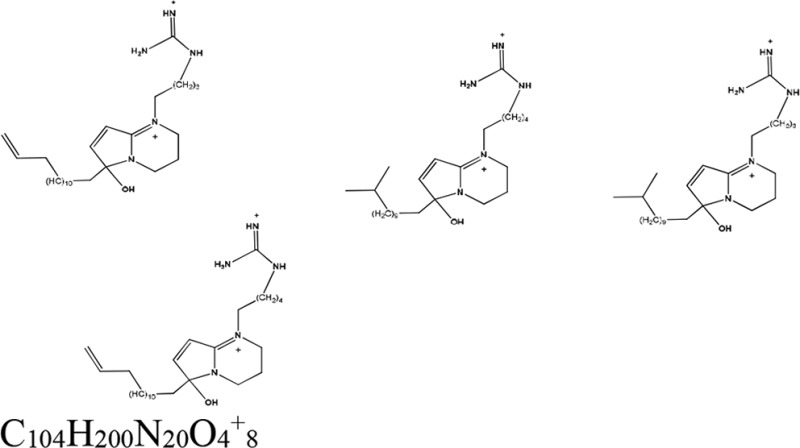 |
57 |
(i) 6-Nonadecynoic acid.
The extract from the plant Pentagonia gigantifolia and the identified antifungal, 6-nonadecynoic acid, caused similar, major reductions in biofilm thickness of 60% and 75%, respectively (Table 2). These reductions in thickness compared to the thickness of the controls (Fig. 3A and C) were apparent in projected orthogonal (side) CLSM images of treated preparations (Fig. 3E and G, respectively). However, analyses of the individual CLSM scans revealed that the extract and derivative compound differed in their effects on the formation of the BYCP. The extract had no effect on the formation of the BYCP, whereas 6-nonadecynoic acid dramatically reduced the thickness of the BYCP (Table 2). Both caused major reductions in hypha formation and the vertical hypha orientation (Table 2; Fig. 3E and G, respectively), and both induced the formation of pseudohyphae (Table 2), which, as evident in Fig. 3A and C, did not occur in the untreated controls under the conditions employed. The dramatic decrease in hypha formation in both extract- and 6-nonadecynoic acid-treated preparations made it difficult to assess the point of hyphal bending. Both the extract and 6-nonadecynoic acid caused a major reduction in the intensity of calcofluor white staining of the ECM (Table 2) and the complete loss of PMN impenetrability (Table 2; Fig. 3F and H, respectively).
(ii) Sampangine.
The extract from the plant Duguetia hadrantha and the derivative sampangine (Table 1) had dramatically different effects on thickness, producing 15% and 94% decreases, respectively (Table 2). Both caused a dramatic decrease in BYCP formation (Table 2). The extract did not affect hypha formation, but the vertical hypha orientation was lost (Table 2; Fig. 3I). The extract caused a modest decrease in calcofluor white staining of the ECM but had no significant effect on PMN impenetrability (Table 2; Fig. 3J). Because sampangine almost completely inhibited biofilm development (Fig. 3K and L; Table 2), most of the parameters were scored negative and could not be compared to the parameters for the extract. It should be noted that neither the extract nor sampangine induced pseudohypha formation (Table 2).
(iii) Coruscanone A.
The extract from the plant Piper coruscans and the derivative coruscanone A (Table 1) caused 55% and 72% reductions in biofilm thickness, respectively, and similar architectural defects (Table 2; Fig. 3M and O, respectively). Both the extract and coruscanone A inhibited the formation (a score of +) of a BYCP (Table 2). Both caused an increase in hyphal density over that in control biofilms (Table 2). Neither induced pseudohypha formation (Table 2). Both caused a loss of vertical hypha orientation (Table 2). The hyphae meshed to form a mat in both cases. Bending was not assessable in either case due to the absence of vertical hypha orientation. The extract and coruscanone A caused a dramatic decrease or the complete loss of ECM staining, respectively (Table 2), and both caused PMN impenetrability (Table 2; Fig. 3N and P, respectively).
(iv) Liriodenine methiodide.
The extract from the plant Liriodendron tulipifera and the derivative liriodenine methiodide caused major reductions in thickness of 61% and 83%, respectively (Table 2; Fig. 3Q and S, respectively). The two agents affected several select parameters differently. The extract inhibited the formation of a BYCP but did not block hypha formation (Table 2; Fig. 3Q). Liriodenine methiodide did not inhibit BYCP formation but did cause a decrease in hypha formation (Table 2; Fig. 3S). Bending of the hyphae occurred early in the development of the extract-treated biofilms (Table 2) but was not assessable in liriodenine methiodide-treated biofilms because of the scarcity of hyphae (Table 2; Fig. 3S). Both the extract and liriodenine methiodide inhibited ECM formation and caused a loss of PMN impenetrability (Table 2; Fig. 3R and T, respectively). Whereas liriodenine methiodide induced pseudohypha formation, the extract did not (Table 2). The differences between the effects of the extract and liriodenine methiodide were the most dramatic among the differences in the three pairs of extracts and the derivative compounds that could be fully compared.
(v) Phloeodictine mixture.
The extract from the sponge Pellina eusiphonia and the derivative compounds, consisting of a phloeodictine mixture (Table 1), caused 62% and 94% decreases in thickness, respectively (Table 2; Fig. 3U and W, respectively). Extract-treated preparations formed a BYCP and formed pseudohyphae in the upper layer, which was devoid of ECM (Table 2; Fig. 3U and W, respectively). The extract caused a loss of PMN impenetrability (Table 2; Fig. 3V). When treated with the phloeodictine mixture, only a yeast cell monolayer formed on the silicone elastomer disc (Table 2; Fig. 3W) and only the sporadic formation of isolated hyphae was detected. Therefore, hypha-related parameters, ECM staining, and PMN impenetrability could not be assessed in the phloeodictine mixture-treated preparation (Table 2).
(vi) Summary of extract compound data.
All five extracts caused a decrease in thickness of between 15 and 62%. All derivative compounds had greater effects on biofilm formation than the extract, decreasing the biofilm thickness between 72% and 94% (Table 2). For BYCP formation, three of the five extracts and four of the five identified derivative compounds caused nearly complete or complete inhibition (Table 2). Only one of the extracts, that of L. tulipifera, caused a greater decrease in a parameter, BYCP formation, than the derivative compound, liriodenine methiodide (Table 2). For hyphal density, two of the five extracts and four of the five derivative compounds caused a dramatic decrease. Treatment with sampangine and phloeodictine mixtures inhibited not only BYCP formation but hypha and ECM formation as well, resulting in only a cell monolayer or bilayer after 48 h. The vertical hypha orientation was totally lost in all extract-treated preparations and in the three compound-treated preparations that formed hyphae and that could therefore be assessed for that parameter (Table 2). Bending was not assessable in two of these extract-treated preparations due to the collapse of the hyphae and was not assessable in all five derivative compound-treated preparations because of the absence of hyphae, a decrease in hyphal length, or the predominance of pseudohyphae (Table 2). All extracts and derivative compounds caused dramatic reductions in calcofluor white staining of the ECM, and all but the one extract, that of Duguetia hadrantha, caused a reduction in PMN impenetrability (Table 2). Interestingly, two of the five extracts induced pseudohypha formation and two of the five derivative compounds did so as well (Table 2). In only one case, Pentagonia gigantifolia, both the extract and the derivative compound 6-nonadecynoic acid induced pseudohyphae (Table 2). Most importantly, these results indicate that, at least for the five extracts with known antifungal activity, the protocol distinguishes four different phenotypic profiles on the basis of the nine parameters assessed, providing us with justification for its use in the extensive analysis of 88 untested plant extracts.
Screening of 88 previously untested extracts.
Using the same protocol (Fig. 1) applied to the five pair of extracts and derivative compounds (Table 1), we screened 88 untested plant extracts (Table 3) randomly selected from the extract collection of the National Center for Natural Products Research (https://pharmacy.olemiss.edu/ncnpr/). In the case of seven extracts (extracts 84687, 84709, 84710, 84712, 84729, 84733, and 84744), the treated preparations formed biofilms that did not remain attached to the silicone elastomer disc prior to fixation and, therefore, could not be assessed for the eight remaining biofilm parameters by CLSM. The eight remaining parameters (Fig. 1B) were assessed in two independent biofilms formed in the presence of each of the 81 assessable extracts. All were analyzed for adhesion prior to CLSM. Three regions of each of the two biofilms were analyzed for the remaining eight CLSM-based parameters. The CLSM-based data for the 81 extracts were separated into three categories on the basis of whether the extract caused the biofilms to be significantly thicker (P ≤ 0.05), caused them to be significantly thinner (P < 0.05), or had no effect on thickness (P > 0.05) (Table 4). In this case, the Student t test was employed to assess significance. The numbers of extracts in the three categories were 10, 25, and 46, respectively. The categorized data for biofilms formed in each of the 81 individual extracts are presented in Table 4, and averaged data for the three categories are presented in Table 5. Projected orthogonal (side) images of the CLSM scans of select biofilms are provided for each category in Fig. 4.
TABLE 3.
Characteristics of the 88 extracts used in the screen for those affecting biofilm architecture and white blood cell impenetrability
| Natural product identifier | Species | Family | Source sample subtype | Common name |
|---|---|---|---|---|
| 84657 | Phalaris canariensis | Poaceae | Grass family | Canary grass |
| 84658 | Bromus carinatus | Poaceae | Grass family | Californian brome |
| 84659 | Eruca vesicaria | Brassicaceae | Mustard family | Rocketsalad |
| 84660 | Caulanthus lasiophyllus | Brassicaceae | Mustard family | California mustard |
| 84661 | Coronopus didymus | Brassicaceae | Mustard family | Lesser swinecress |
| 84662 | Delphinium andersonii | Ranunculaceae | Buttercup family | Anderson's larkspur |
| 84663 | Rumex hymenosepalus | Polygonaceae | Smartweed family | Canaigre |
| 84664 | Rumex hymenosepalus | Polygonaceae | Smartweed family | Canaigre |
| 84665 | Centaurea melitensis | Asteraceae | Daisy family | Maltese star thistle |
| 84666 | cf. Ericameria brachylepis | Asteraceae | Daisy family | Chaparral goldenbush |
| 84667 | Mirabilis bigelovii | Nyctaginaceae | Four o'clock family | Bigelow's desert four o'clock, desert wishbone bush |
| 84668 | Chamaecrista fasciculata | Fabaceae | Legume family | Golden cassia, partridge pea |
| 84669 | Cliftonia monophylla | Cyrillaceae | Flowering dicot | Buckwheat tree |
| 84670 | Viburnum nudum | Adoxaceae | Moschatel family | Smooth withe rod, possumhaw |
| 84671 | Viburnum nudum | Adoxaceae | Moschatel family | Smooth withe rod, possumhaw |
| 84672 | Viburnum sp. | Adoxaceae | Moschatel family | Honeysuckle |
| 84673 | Elephantopus elatus | Asteraceae | Daisy family | Tall elephantsfoot |
| 84674 | Pinus palustris | Pinaceae | Conifer | Pitch pine |
| 84675 | Heuchera bracteata | Saxifragaceae | Saxifrage family | Bracted alumroot |
| 84676 | Potentilla fissa | Rosaceae | Rose family | Bigflower cinquefoil |
| 84677 | Cornus stolonifera | Cornaceae | Dogwood family | Redosier dogwood |
| 84678 | Vaccinium erythrocarpum | Ericaceae | Heath family | Southern mountain cranberry |
| 84679 | Ceratozamia mexicana | Zamiaceae | Cycad | Mexican horncone |
| 84680 | Philodendron radiatum | Araceae | Arum family | Philodendron |
| 84681 | Samanea saman | Fabaceae | Legume family | Raintree |
| 84682 | Pinus palustris | Pinaceae | Conifer | Pitch pine |
| 84683 | Quercus pumila | Fagaceae | Beech family | Running oak |
| 84684 | Sabal palmetto | Arecaceae | Palm family | Cabbage palmetto |
| 84685 | Pinus serotina | Pinaceae | Conifer | Pond pine |
| 84686 | Lyonia fruticosa | Ericaceae | Heath family | Coastal plain staggerbush |
| 84687 | Livistona chinensis | Arecaceae | Palm family | Chinese Fan Palm |
| 84688 | Bactris militaris | Arecaceae | Palm family | Bactris palm |
| 84689 | Coix lacryma-jobi | Poaceae | Grass family | Job's tears |
| 84690 | Rhapis excelsa | Arecaceae | Palm family | Broadleaf lady palm, bamboo palm |
| 84691 | Quercus laevis | Fagaceae | Beech family | American turkey oak |
| 84692 | Aspidotis densa | Pteridaceae | Fern | Indian's dream |
| 84693 | Podocarpus macrophyllus | Podocarpaceae | Conifer | Kusamaki |
| 84694 | Aglaonema commutatum | Araceae | Arum family | Chinese or Philippine evergreen |
| 84695 | Oplismenus hirtellus | Poaceae | Grass family | Basketgrass |
| 84696 | cf. Ceiba pentandra | Bombacaceae | Mallow family | Mallow |
| 84697 | Zamia furfuracea | Zamiaceae | Cycad | Cardboard Sago, Jamaican Sago, Mexican cycad |
| 84698 | Arenga undulatifolia | Arecaceae | Palm family | Aren gelora |
| 84699 | Desmodium sandwicense | Fabaceae | Legume family | Hawai'i ticktrefoil |
| 84700 | Dissotis rotundifolia | Melastomataceae | Flowering dicot | Pinklady |
| 84701 | Yucca harrimaniae | Agavaceae | Agave family | Spanish bayonet |
| 84702 | Nicotiana attenuata | Solanaceae | Nightshade family | Coyote tobacco |
| 84703 | Vitis arizonica | Vitaceae | Grape family | Canyon grape |
| 84704 | Caesalpinia pulcherrima | Fabaceae | Legume family | Poinciana, peacock flower, red bird of paradise, pride of Barbados |
| 84705 | Arundo donax | Poaceae | Grass family | Giant reed |
| 84706 | Atriplex fruticulosa | Chenopodiaceae | Goosefoot family | Ball saltbush |
| 84707 | Veronica anagallis-aquatica | Plantaginaceae | Plantain family | Water speedwell |
| 84708 | Agrostis stolonifera | Poaceae | Grass family | Creeping bentgrass |
| 84709 | Ribes montigenum | Grossulariaceae | Gooseberry family | Gooseberry currant |
| 84710 | Atriplex hymenelytra | Chenopodiaceae | Goosefoot family | Desert holly |
| 84711 | Atriplex lentiformis | Chenopodiaceae | Goosefoot family | Quail bush |
| 84712 | Quercus alba | Fagaceae | Beech family | White oak |
| 84713 | Encelia farinosa | Asteraceae | Daisy family | Brittle bush |
| 84714 | Hemerocallis fulva | Liliaceae | Lily family | Orange daylily, tawny daylily, tiger lily, ditch lily |
| 84715 | Leea rubra | Vitaceae | Grape family | West Indian holly, red leea |
| 84716 | Philodendron ferrugineum | Araceae | Arum family | Philodendron |
| 84717 | Urera baccifera | Urticaceae | Nettle family | Scratchbush |
| 84718 | Ribes missouriense | Grossulariaceae | Gooseberry family | Missouri gooseberry |
| 84719 | Mahonia aquifolium | Berberidaceae | Barberry family | Oregon grape |
| 84720 | Coccoloba pubescens | Polygonaceae | Smartweed family | Grandleaf seagrape |
| 84721 | Cercocarpus minutiflorus | Rosaceae | Rose family | Smooth mountain mahogany |
| 84722 | Rhus integrifolia | Anacardiaceae | Cashew family | Lemonade berry |
| 84723 | Quercus agrifolia | Fagaceae | Beech family | Encina, coast live oak |
| 84724 | Artemisia californica | Asteraceae | Daisy family | Wormwood |
| 84725 | Genista monspessulana | Fabaceae | Legume family | French broom |
| 84726 | Quercus turbinella | Fagaceae | Beech family | Turbinella oak |
| 84727 | Adiantum capillus-veneris | Pteridaceae | Fern | Maidenhair fern, avenca |
| 84728 | Foeniculum vulgare | Apiaceae | Umbellifer | Fennel |
| 84729 | Pinus elliottii | Pinaceae | Conifer | Slash pine |
| 84730 | Quercus incana | Fagaceae | Beech family | Bluejack oak |
| 84731 | Hypericum hypericoides | Hypericaceae | St. John's wort family | St. Andrews cross |
| 84732 | Myrica cerifera | Myricaceae | Sweet gale family | Wax myrtle |
| 84733 | Asimina obovata | Annonaceae | Custard apple family | Bigflower pawpaw |
| 84734 | Cinnamomum camphora | Lauraceae | Laurel family | Camphor |
| 84735 | Cinnamomum camphora | Lauraceae | Laurel family | Camphor |
| 84736 | Rumex verticillatus | Polygonaceae | Smartweed family | Swamp dock |
| 84737 | Nuphar lutea | Nymphaeaceae | Water lily family | Yellow water lily |
| 84738 | Nuphar lutea | Nymphaeaceae | Water lily family | Yellow water lily |
| 84739 | Lachnocaulon minus | Eriocaulaceae | Pipewort family | Small's bogbutton |
| 84740 | Lyonia fruticosa | Ericaceae | Heath family | Coastal plain staggerbush |
| 84741 | Rhus copallinum | Anacardiaceae | Cashew family | Shining, winged, or dwarf sumac |
| 84742 | Dombeya tiliacea | Malvaceae | Mallow family | NA |
| 84743 | Musa ornata | Musaceae | Banana family | Flowering or ornamental banana |
| 84744 | Spathiphyllum wallisii | Araceae | Arum family | Peace lily |
TABLE 4.
Effects of 81 natural extracts on biofilm formationa
| Category | Natural product identifier | Biofilm thickness (μm) | % change in biofilm thickness | BYCP formation score | Hypha formation score | Vertical hypha orientation score | Hyphal bend pointb | Pseudohyphal formation score | ECM staining score | PMN impenet.c score |
|---|---|---|---|---|---|---|---|---|---|---|
| Control (without DMSO) | 160 ± 16 | ++++ | ++++ | ++++ | 70 | − | ++++ | ++++ | ||
| Significantly thicker (n = 10) | 84666 | 186 ± 9 | +16 | ++++ | ++++ | ++++ | 88 | − | ++++ | ++++ |
| 84705 | 188 ± 10 | +18 | +++ | ++++ | ++++ | 83 | − | ++ | ++ | |
| 84706 | 192 ± 9 | +20 | +++ | ++++ | +++ | 85 | − | ++ | ++ | |
| 84708 | 210 ± 8 | +31 | ++++ | ++++ | +++ | 76 | − | +++ | ++ | |
| 84711 | 218 ± 11 | +36 | ++++ | ++++ | +++ | 62 | − | ++ | ++ | |
| 84714 | 210 ± 6 | +31 | ++++ | ++ | ++ | 39 | ++ | ++ | ++++ | |
| 84716 | 208 ± 11 | +30 | ++++ | ++++ | +++ | 84 | + | ++ | ++ | |
| 84718 | 238 ± 11 | +49 | ++++ | ++++ | +++ | 60 | − | ++++ | +++ | |
| 84724 | 218 ± 11 | +36 | ++++ | ++++ | ++++ | 70 | − | ++++ | ++++ | |
| 84741 | 220 ± 14 | +38 | ++++ | ++++ | +++ | 62 | − | +++ | ++++ | |
| Significantly thinner (n = 25) | 84660 | 118 ± 6 | −26 | ++++ | ++++ | ++++ | 64 | − | ++++ | ++++ |
| 84663 | 132 ± 5 | −18 | +++ | ++++ | ++++ | 67 | − | ++++ | ++++ | |
| 84664 | 82 ± 0 | −49 | − | ++++ | ++ | 49 | − | ++ | ++ | |
| 84672 | 78 ± 0 | −51 | ++++ | ++++ | +++ | 85 | − | ++ | ++++ | |
| 84675 | 84 ± 2 | −48 | ++++ | ++ | +++ | 93 | ++ | ++ | − | |
| 84676 | 98 ± 2 | −39 | ++++ | ++ | +++ | 94 | ++ | + | − | |
| 84677 | 96 ± 3 | −40 | ++++ | ++ | +++ | 75 | + | ++ | − | |
| 84678 | 116 ± 7 | −28 | ++++ | ++++ | +++ | 81 | − | +++ | ++++ | |
| 84679 | 118 ± 0 | −26 | − | ++++ | +++ | 68 | − | +++ | ++++ | |
| 84682 | 122 ± 9 | −24 | ++++ | ++++ | +++ | 66 | − | ++++ | ++++ | |
| 84690 | 126 ± 10 | −15 | − | ++++ | ++ | 32 | − | ++ | + | |
| 84694 | 98 ± 4 | −39 | − | ++++ | ++ | 78 | − | ++ | ++ | |
| 84695 | 68 ± 2 | −58 | ++ | ++ | ++ | 44 | − | ++ | − | |
| 84696 | 112 ± 9 | −30 | − | ++++ | ++ | 45 | − | +++ | + | |
| 84697 | 126 ± 6 | −21 | ++++ | ++++ | ++ | 59 | − | +++ | ++++ | |
| 84699 | 135 ± 7 | −20 | − | ++++ | ++++ | 91 | − | ++++ | ++++ | |
| 84701 | 100 ± 7 | −38 | − | ++ | + | 0 | ++ | +++ | − | |
| 84702 | 120 ± 6 | −25 | +++ | ++++ | ++ | 74 | − | +++ | ++ | |
| 84707 | 120 ± 14 | −25 | ++++ | ++++ | ++++ | 80 | − | +++ | ++ | |
| 84713 | 116 ± 6 | −28 | ++ | ++++ | ++ | 59 | ++ | ++ | + | |
| 84719 | 130 ± 8 | −19 | ++++ | ++++ | ++ | 58 | − | ++ | + | |
| 84720 | 120 ± 9 | −25 | ++++ | ++++ | +++ | 60 | − | ++ | + | |
| 84727 | 60 ± 8 | −63 | − | +++ | + | 20 | − | ++ | − | |
| 84737 | 120 ± 6 | −25 | + | +++ | ++ | 65 | − | +++ | ++ | |
| 84740 | 120 ± 6 | −25 | ++++ | ++++ | +++ | 70 | − | ++ | ++ | |
| Similar thickness (n = 46) | 84657 | 140 ± 9 | −13 | ++++ | ++++ | ++++ | 77 | − | ++++ | ++++ |
| 84658 | 172 ± 8 | +8 | ++++ | ++++ | ++++ | 79 | − | ++++ | ++++ | |
| 84659 | 162 ± 4 | 0 | +++ | ++++ | ++++ | 77 | − | +++ | +++ | |
| 84661 | 150 ± 4 | −6 | ++++ | ++++ | ++++ | 80 | − | ++++ | ++++ | |
| 84662 | 156 ± 5 | −3 | ++++ | +++ | +++ | 55 | − | ++++ | ++++ | |
| 84665 | 144 ± 6 | −10 | ++++ | ++++ | +++ | 83 | − | +++ | ++++ | |
| 84667 | 140 ± 80 | −12 | +++ | ++++ | ++ | 67 | − | ++++ | ++++ | |
| 84668 | 146 ± 3 | −8 | ++++ | ++++ | ++++ | 82 | − | ++++ | ++++ | |
| 84669 | 180 ± 8 | +13 | ++++ | ++++ | ++++ | 81 | − | ++++ | ++++ | |
| 84670 | 156 ± 13 | −1 | ++++ | ++++ | +++ | 60 | − | ++++ | ++++ | |
| 84671 | 160 ± 6 | 0 | ++++ | ++++ | ++++ | 61 | − | ++++ | ++ | |
| 84673 | 156 ± 2 | −4 | ++++ | ++++ | ++++ | 83 | − | ++ | ++ | |
| 84674 | 142 ± 10 | −11 | ++++ | ++++ | ++++ | 73 | − | ++++ | ++++ | |
| 84680 | 154 ± 7 | −4 | ++++ | ++++ | +++ | 68 | − | ++++ | ++++ | |
| 84681 | 142 ± 15 | −11 | ++++ | ++++ | ++++ | 85 | − | ++++ | ++++ | |
| 84683 | 150 ± 2 | −6 | − | ++++ | +++ | 69 | − | ++ | ++ | |
| 84684 | 144 ± 5 | −10 | +++ | ++++ | +++ | 74 | − | +++ | +++ | |
| 84685 | 156 ± 10 | −3 | +++ | ++++ | ++ | 74 | − | ++++ | ++++ | |
| 84686 | 156 ± 8 | −2 | +++ | ++++ | ++ | 77 | − | +++ | ++++ | |
| 84688 | 146 ± 3 | −9 | − | ++++ | +++ | 75 | − | ++++ | ++++ | |
| 84689 | 168 ± 12 | +5 | − | ++++ | +++ | 87 | − | ++ | ++ | |
| 84691 | 142 ± 7 | −11 | ++++ | ++++ | +++ | 82 | − | ++ | − | |
| 84692 | 164 ± 6 | +3 | ++ | ++++ | +++ | 85 | − | ++++ | ++++ | |
| 84693 | 176 ± 6 | +10 | ++++ | +++ | ++ | 92 | − | ++ | ++ | |
| 84698 | 166 ± 0 | +4 | − | ++++ | ++ | 48 | − | ++++ | ++++ | |
| 84700 | 148 ± 8 | −8 | ++++ | ++++ | +++ | 82 | − | ++++ | ++++ | |
| 84703 | 180 ± 5 | +13 | ++++ | ++++ | +++ | 58 | + | ++ | ++ | |
| 84704 | 138 ± 3 | −14 | ++++ | +++ | +++ | 70 | + | ++ | ++ | |
| 84715 | 160 ± 6 | 0 | ++++ | ++ | ++ | 64 | ++ | ++ | + | |
| 84717 | 130 ± 11 | −19 | ++++ | ++++ | +++ | 62 | − | ++++ | ++++ | |
| 84721 | 174 ± 12 | +9 | ++++ | ++++ | ++++ | 80 | − | ++++ | ++++ | |
| 84722 | 176 ± 12 | +10 | ++++ | ++++ | +++ | 68 | − | ++ | ++ | |
| 84723 | 170 ± 8 | +6 | ++++ | ++++ | ++++ | 88 | − | +++ | ++ | |
| 84725 | 160 ± 14 | 0 | ++++ | ++++ | +++ | 75 | − | ++++ | ++ | |
| 84726 | 186 ± 10 | +16 | ++++ | ++++ | ++++ | 91 | − | ++ | ++ | |
| 84728 | 174 ± 6 | +9 | ++++ | +++ | ++++ | 71 | − | +++ | +++ | |
| 84730 | 150 ± 11 | −6 | ++++ | ++ | ++ | 81 | − | ++ | ++ | |
| 84731 | 170 ± 11 | +6 | ++++ | ++++ | ++ | 60 | − | ++ | ++ | |
| 84732 | 154 ± 6 | −3 | ++++ | ++++ | +++ | 70 | − | +++ | ++++ | |
| 84734 | 151 ± 10 | −6 | ++++ | ++++ | +++ | 79 | − | ++++ | ++++ | |
| 84735 | 140 ± 7 | −13 | ++++ | ++++ | ++ | 61 | − | ++ | − | |
| 84736 | 140 ± 8 | −13 | ++++ | ++++ | ++++ | 84 | − | ++ | − | |
| 84738 | 164 ± 9 | +3 | ++++ | ++++ | ++++ | 87 | − | ++++ | ++++ | |
| 84739 | 166 ± 10 | +4 | ++++ | ++++ | +++ | 82 | − | ++++ | ++++ | |
| 84742 | 180 ± 11 | +13 | ++++ | ++++ | +++ | 78 | − | ++++ | ++++ | |
| 84743 | 180 ± 11 | +13 | ++++ | ++++ | ++++ | 22 | − | ++ | ++++ |
The origins of the extracts are provided in Table 3.
The hypha bend point is the percentage of the hyphal length in the distal direction at which hyphae bend.
PMN impenet., polymorphonuclear leukocyte impenetrability.
TABLE 5.
Summary of defects caused by 81 extracts on biofilm architecture and PMN impenetrability in the three thickness categoriesa
| Category | No. | % of extracts in each category in which the following had a score of −, +, or ++: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Presence of BYCP | Hypha formation | Vertical hypha orientation | ECM staining | PMN impenet. | Hyphal bend point | Pseudohypha formation | One or more affected parameters | ||
| Thicker | 10 | 0 | 10 | 10 | 50 | 50 | 10 | 20 | 60 |
| Thinner | 25 | 44 | 20 | 44 | 52 | 68 | 36 | 16 | 84 |
| Similar | 46 | 10 | 4 | 20 | 33 | 37 | 9 | 7 | 52 |
Comparisons to the untreated control were made. No., number of extracts resulting in biofilms that were thicker than the control biofilm, thinner than the control biofilm, or similar in thickness to the control biofilm. BYCP, basal yeast cell polylayer; ECM staining, staining if extracellular matrix with calcofluor white; PMN impenet., polymorphonuclear leukocyte impenetrability; hyphal bend point, the proportion of extract-treated biofilms in a category in which the bend point occurred at less than 60% of the hyphal length.
FIG 4.
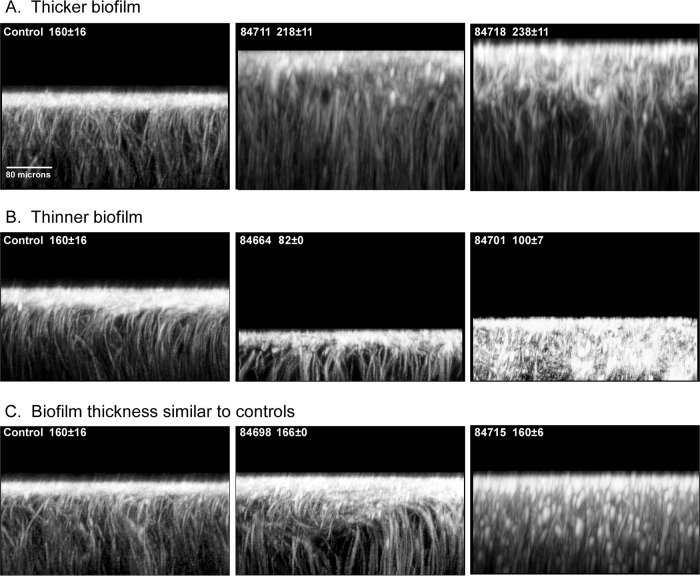
Orthogonal (side) views of calcofluor white-stained biofilms treated with extracts causing them to be significantly thicker than control biofilms (A), thinner than control biofilms (B), or similar in thickness to control biofilms (C). The extract name and thickness (mean ± standard deviation thickness [in micrometers]) of each preparation are presented in the upper left corner of each image. The orthogonal views are of slices of a stack of LSCM scans rotated 90° taken 40 pixels into the projected image.
Significantly thicker biofilms.
Of the 10 extracts that caused a significant increase in thickness of between 16 and 49% (P = 0.03 to 0.0007), none had a major effect on the formation of the BYCP (Table 4 and 5). Only one extract, extract 84714, affected hypha formation, causing a decrease in the density of hyphae, a decrease in vertical hypha orientation, and early hyphal bending (Table 4). Both extract 84714 (+31%) and extract 84716 (+30%) caused low levels of pseudohypha formation (Table 4). Five of the 10 extracts caused a decrease in ECM staining, and five caused decreases in PMN impenetrability (Table 4). Four of the 10 extracts (extracts 84666, 84718, 84724, and 84741) did not affect any of the assessed parameters except thickness (Table 4). Four of the five extracts caused a decrease in ECM staining and PMN impenetrability without affecting BYCP formation or hyphal parameters (Table 4). One (extract 84714) affected ECM staining and hyphal parameters but had no effect on PMN impenetrability (Table 4). It caused pseudohypha formation (Table 4). Figure 4A presents two orthogonal views of thicker biofilms treated with extracts 84711 (in which the biofilm was 36% thicker than the control biofilm) and 84718 (in which the biofilm was 49% thicker than the control biofilm). The extract 84711-treated biofilm exhibited normal BCYP formation and normal hypha parameters but reduced ECM staining and PMN impenetrability (Table 4). The extract 84718-treated biofilms were normal for all parameters but underwent almost a 50% increase in thickness (+49%) (Table 4). In both biofilms, there was noticeably more disorganization in the uppermost regions (Fig. 4A). Two of the 10 extracts (20%), extracts 84714 and 84716, induced pseudohypha formation, and both caused a decrease in ECM staining.
Significantly thinner biofilms.
Twenty-five extracts caused a significant decrease in biofilm thickness of between 18% and 63% (Table 4). All decreases were found to be significant (P = 0.05 to 0.00002). A greater proportion of extracts in this group than in the group causing thicker biofilms caused decreases in BYCP formation, hyphal density, vertical hypha orientation, and bending point (Tables 4 and 5). The proportions of extract-induced thicker and thinner biofilms that exhibited a dramatic decrease in BYCP formation were 0% for thicker biofilms versus 44% for thinner biofilms, the proportions with decreased vertical hypha orientation were 10% for thicker biofilms versus 44% for thinner biofilms, and the proportions with a hyphal bend point at <60% of the biofilm thickness were 10% for thicker biofilms versus 36% for thinner biofilms (Table 5). The proportion of thinner biofilms with decreased ECM staining was similar to that of thicker biofilms (52% and 50%, respectively), and the proportion of thinner biofilms with decreased PMN impenetrability was similar to that of thicker biofilms (68% and 50%, respectively) (Table 5). Figure 4B presents CLSM orthogonal views of two extract-treated biofilms that were thinner due to treatment with extracts 84664 and 84701. Extract 84664-treated biofilms exhibited a reduction in thickness of 49%, no basal yeast cell polylayer, a moderate reduction in vertical hypha orientation, reduced ECM staining, and reduced PMN impenetrability but still exhibited the general calcofluor white-stained hyphal architecture observed in the upper region of control biofilms (Table 4; Fig. 4B). Extract 84701-treated biofilms were reduced in thickness by 38%, formed no BYCP, and exhibited reduced hyphal density and associated hyphal parameters (Table 4; Fig. 2B and D). Extract 84701-treated biofilms produced a moderate level of pseudohyphae (Table 4) which were stained by calcofluor white throughout the CLSM orthogonal view (Fig. 4B). Of the 18 extract-treated biofilms that were thinner and exhibited decreases in ECM staining and/or PMN impenetrability, 12 (67%) exhibited decreases in both (Table 4). Five (28%) exhibited a decrease in PMN impenetrability in the absence of a decrease in ECM staining, but none exhibited a decrease in ECM staining in the absence of a decrease in PMN impenetrability (Table 4), suggesting that the latter depends upon a normal ECM, as evidenced by normal calcofluor white staining.
Biofilms with a thickness similar to that of control biofilms.
A comparison of the data for biofilms treated with the 46 extracts that did not have a significant effect on thickness (P > 0.05) revealed that only one extract-treated biofilm had reduced BYCP formation and only four (9%) lacked a BYCP (a score of −) (Tables 4 and 5). Only one extract-treated biofilm exhibited a reduction in hypha formation, nine (20%) had decreases in vertical hypha orientation, and two (4%) had decreases in vertical hypha orientation and early bending (Tables 4 and 5). Only three (7%) formed pseudohyphae (Tables 4 and 5). Even so, the proportions of extracts that caused decreases in calcofluor white staining of the ECM and PMN impenetrability were surprisingly high, 33% and 37%, respectively, although they were not as high as the proportions of extracts that caused thicker and thinner biofilms (Table 5). Figure 4C presents a CLSM orthogonal view of a biofilm treated with extract 84698, which did not affect thickness but which blocked the formation of the basal yeast cell polylayer (Table 4). The extract had a moderate effect on the vertical hypha orientation and bending but had no effect on ECM staining or PMN impenetrability (Table 4). The uppermost zone that sequesters calcofluor white (40) was noticeably expanded in orthogonal views (Fig. 4C). Figure 4C presents an example of a biofilm treated with extract 84715 which was notable for distal bullet-shaped pseudohyphae in the upper region in calcofluor white-stained orthogonal images. The majority of the 46 extract-treated biofilms with thicknesses similar to those of the controls exhibited in CLSM orthogonal views staining patterns similar to those of the untreated controls. Of the 18 extract-treated biofilms with thicknesses similar to those of the controls that exhibited a defect in ECM staining and/or PMN impenetrability, 14 (78%) exhibited both defects, while 4 (22%) exhibited one or the other defect (Table 4). Of the 15 extract-treated biofilms with a decrease in ECM staining, 14 (93%) also exhibited a decrease in PMN impenetrability (Table 4). Of the 17 extract-treated biofilms with a decrease in PMN impenetrability, 14 (82%) also exhibited a decrease in ECM staining, but 3 (18%) did not (Table 4). These results again suggest that a decrease in PMN impenetrability occurs in a minority of extract-treated biofilms in the absence of a change in ECM staining.
Correspondence of thickness and other parameters.
To assess further the correlations between the changes in biofilm parameters caused by extracts, we focused on the thinner category of biofilms because these biofilms exhibited, on average, the largest number of parameter changes (Tables 4 and 5). BYCP formation, vertical hypha orientation, and PMN impenetrability were plotted as functions of the reduction in thickness in the scatter plots in Fig. 5A to C, respectively. Each scatter plot was separated into quadrants (quadrant 1 [Q1], Q2, Q3, and Q4) by two dashed lines, with the horizontal dashed line demarcating a ≤30% decrease in thickness versus a >30% decrease in thickness and the vertical dashed line demarcating a dramatic decrease in the parameter value (a score of −, +, or ++) versus a normal parameter value (a score of +++ or ++++). The quadrants were then compared pairwise, as indicated by double-headed arrows (Fig. 5). Percentages were computed as the number of biofilms in a quadrant divided by the total number in the two quadrants compared multiplied by 100.
FIG 5.
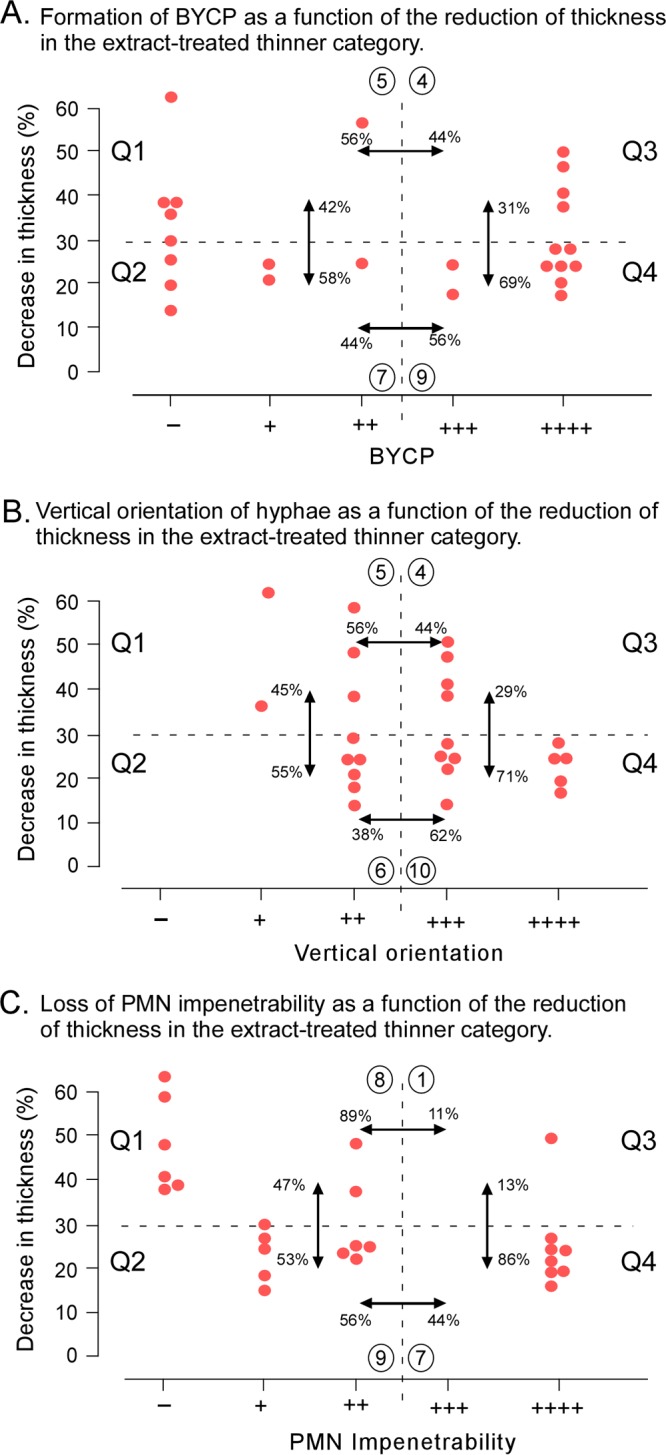
Correlations between biofilm thickness and BYCP formation (A), vertical hypha orientation (B), and PMN impenetrability (C) among the 25 extract-treated biofilms that were significantly thinner than control biofilms. The three parameters are plotted as functions of the decrease in thickness. The plot is separated into four quadrants (Q1, Q2, Q3, and Q4) by dashed lines. The horizontal dashed line in each panel separates decreases of >30% from decreases of ≤30%. The vertical dashed line in each panel separates defective (a score of −, ++, or +++) from normal (a score of +++ or ++++) biofilms. Comparisons are noted by double-headed arrows between quadrants. The compared percentages were computed as the number of extract-treated biofilms in a quadrant (circled) divided by the total number in the two quadrants being compared multiplied by 100.
(i) Biofilm thickness and BYCP.
If the thickness of the BYCP were a function of the thickness of the biofilm, then the proportion of defective BYCPs (a score of −, +, or ++) would be much higher for extract-treated biofilms with a >30% decrease in biofilm thickness than for those with a ≤30% decrease. The proportion of defective BYCPs in extract-treated biofilms with a >30% decrease in thickness was similar to that in biofilms with a ≤30% decrease at 42% versus 58% (Fig. 5A, Q1 versus Q2). Reversing the dependency, we asked if biofilm thickness was a function of a reduction of BYCP formation. If that were the case, then the proportion of biofilms with normal BYCPs would be lower when the decrease in thickness was ≤30% (Q4) than when the decrease was >30% (Q3) (Fig. 5A). The proportions for normally thick biofilms were 69% and 31%, respectively (Fig. 5A), suggesting a weak correlation. Using the chi-square test and Fisher's exact test, the differences between quadrants was not found to be significant (P > 0.05). Using the Pearson correlation test, we found no significant correlation between the two parameters. These results indicate that even when a biofilm is reduced in thickness by >30% by treatment with extracts, 44% of biofilms can still form normal BYCPs (Fig. 5A, Q4).
(ii) Biofilm thickness and vertical orientation.
Comparisons of quadrants demarcated by the percent decrease in biofilm thickness and the vertical orientation of hyphae revealed proportions between quadrants that were highly similar to those for the quadrants demarcated by biofilm thickness and BYCP thickness (Fig. 5A). A weak effect was again revealed in Q3, demarcated by a decrease in biofilm thickness of >30% and a normal (a score of +++ or ++++) vertical hypha orientation (Fig. 5B). In a comparison of Q3 to Q4, demarcated by a decrease in biofilm thickness of ≤30% and a normal hyphal orientation, there appeared to be a major difference of 29% versus 71%, respectively (Fig. 5B). However, the chi-square test and Fisher's exact test failed to show significant correlations between quadrants, and Pearson's correlation test found no correlation between the two parameters. These results suggest that extract-induced decreases in biofilm thickness of >30% resulted in no significant decrease in the proportion exhibiting a normal vertical hypha orientation. Four extracts still caused a decrease in thickness of >30% but not a decrease in vertical orientation.
(iii) Biofilm thickness and PMN impenetrability.
A much stronger correlation between biofilm thickness and PMN impenetrability was observed, however. The proportion of extract-treated biofilms that retained normal PMN impenetrability and underwent a ≤30% decrease in thickness was 86%, whereas the proportion that retained normal PMN impenetrability but underwent a >30% decrease in thickness was 13% (Fig. 5C). The chi-square test produced significant P values between Q1 and Q3 and between Q3 and Q4 of 0.020 and 0.034, respectively. Pearson's correlation coefficient with a confidence interval of 95% resulted in a P value of 0.514 and a coefficient of correlation range of from −0.755 (maximum) to −0.150 (minimum), suggesting a correlation between the two parameters. These results suggest that extract-induced decreases in biofilm thickness are usually accompanied by a loss of PMN impenetrability.
Correspondence of ECM and PMN impenetrability.
The data in both Tables 2 and 4 suggest that when an extract caused a defect in the level of calcofluor white staining of the ECM, it was in most cases accompanied by a defect in PMN impenetrability. To explore this correlation further, we plotted in Fig. 6 calcofluor white staining of the ECM as a function of PMN impenetrability for the collection of 25 extract-treated preparations that were thinner than the control preparations. Four quadrants were demarcated by a horizontal dashed line separating decreased (a score of −, +, or ++) and normal (a score of +++ or ++++) calcofluor white staining of ECM and a vertical dashed line separating decreased (a score of −, +, or ++) and normal (a score of +++ or ++++) PMN impenetrability. Of the 13 extract-treated biofilms exhibiting a decrease in calcofluor white staining of the ECM (Fig. 6, Q2 and Q4), 12 (92%) were defective and only 1 (8%) was normal for PMN impenetrability (Fig. 6). Of the eight extract-treated biofilms with normal PMN impenetrability, seven (88%) were normal and one (12%) showed a decrease in calcofluor white staining of the ECM (Fig. 6, Q3 and Q4). The chi-square test produced P values between Q3 and Q4 and between Q2 and Q4 of 0.034 and 0.002, respectively. Pearson's correlation coefficient with a confidence interval of 95% resulted in a P value of 0.0692 and a coefficient of correlation range of from 0.853 (maximum) to 0.409 (minimum), suggesting a correlation between the two parameters. These results demonstrate a close correlation between a defective ECM and a loss of PMN impenetrability.
FIG 6.
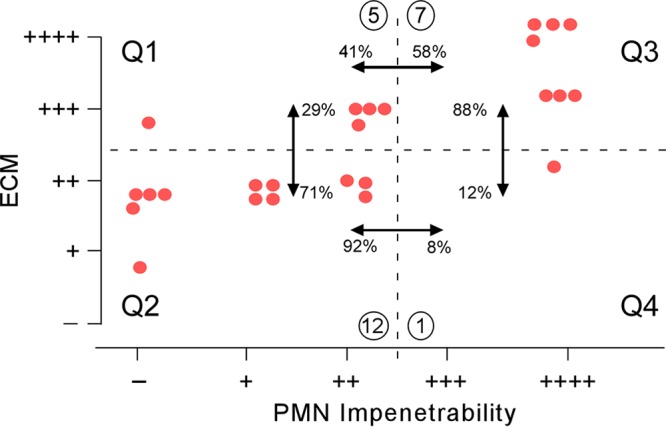
Correlation between ECM staining and PMN impenetrability among the 25 extract-treated biofilms that were significantly thinner than control biofilms. Both the horizontal and vertical dashed lines demarcating the quadrants were based on separation of decreased (a score of −, +, or ++) versus normal (a score of +++ or ++++) assessments. Comparisons of quadrants are noted by double-headed arrows. The compared percentages were computed as the number of extract-treated biofilms in a quadrant (circled) divided by the total number in the two quadrants being compared multiplied by 100.
DISCUSSION
Many antifungal agents may affect specific characteristics of biofilm development, disrupting its integrity, and, in so doing, decrease its resistance to the host immune system and antifungal drug therapy. This may occur in some cases without significantly affecting one or more metabolic and general growth parameters. Based upon this premise, we set out to develop a protocol for assessing in each individual biofilm preparation the effects of extracts on growth, architecture, cellular phenotype, ECM staining, and PMN impenetrability. The approach employs a rigid set of experimental conditions that resulted in the reproducible formation of an architecturally complex, PMN-impenetrable biofilm preparation and employed a CLSM protocol to assess all nine parameters for each individual biofilm preparation. To assess initially the efficiency of the protocol, we applied it to a set of five pairs of extracts and derivative antifungal agents previously demonstrated to inhibit the growth of C. albicans, using the inhibition of growth in a suspension or on a plastic surface (54–62). The protocol revealed selective defects in the nine assayed parameters, resulting in a set of diverse and reproducible phenotypic profiles for both the extracts and the derivative compounds. These results justified an expanded analysis of 88 plant extracts not previously tested for antifungal activity. The expanded study revealed that 57 of 88 (66%) extracts affected one or more of the nine parameters. Moreover, the correlative results provided insights into the possible dependencies and independencies of several of the parameters in the process of biofilm development.
Five known antifungal extracts and derivative compounds.
The results revealed that all five extracts and their derivative antifungal agents affected thickness, vertical hypha formation, the hyphal bend point, ECM staining, and PMN penetrability and selectively affected BYCP formation, hypha formation, and pseudohypha formation. The protocol identified four different phenotypic profiles for the five extracts. Four of the five derivative compounds had an effect on biofilm development more extreme than that of the extracts from which they were isolated. For one pair of extracts and derivative compounds, that of Liriodendron tulipifera, the phenotypic profiles differed markedly. The diversity of phenotypes between the five extracts and derivative compounds was not unexpected, given the diversity of the interpreted mechanisms of action of the derivative compounds (Table 1). These ranged from iron homeostasis (56) to mitochondrial function (55, 58) and fatty acid metabolism (59, 62). Interestingly, the observation that all extracts and compounds caused both a decrease in calcofluor white staining of the ECM and a decrease in PMN impenetrability, when assessable, suggested a possible cause-effect relationship between the two parameters that was also observed in many of the profiles for biofilms treated with the 88 random extracts. Previous work had, in fact, indicated that the ECM indeed plays a major role in the reduction of human white cell penetrability of normal Candida species biofilms (49, 66, 67).
Previously untested extracts.
Of the 88 previously untested extracts, 7 (extracts 84687, 84709, 84710, 84712, 84729, 84733, and 84744) could not be analyzed by CLSM because the extracts affected biofilm adhesion to the silicone elastomer substrate, causing them to peel off or fragment due to weak adherence prior to CLSM analysis. Adherence to catheters and other abiotic surfaces presents a major target for therapeutics (16). The protocol that we have developed may, therefore, identify agents that inhibit the firm attachment of biofilms to catheters.
Ten (12%) of the 81 extracts caused significant increases in biofilm thickness. One extract, extract 84718, caused a 49% increase in the thickness of an otherwise architecturally normal biofilm. All but one of the thicker extract-treated biofilms were normal for hypha formation, and only two abnormally formed pseudohyphae. However, half of the 10 extracts caused a decrease in calcofluor white staining of the ECM, and half diminished PMN impenetrability. Four of the five thicker biofilms with decreased ECM staining also exhibited decreased PMN impenetrability, indicating, as did the results for the five previously studied extracts and derivative compounds, that the defects in the two parameters correlate. However, the biofilm treated with one of the extracts (extract 84714) exhibited normal PMN impenetrability, even though ECM staining was reduced, as were hypha formation and vertical hypha orientation.
Approximately half (n = 46) of the 81 extract-treated biofilms exhibited no significant change in biofilm thickness, but approximately half of this group (52%) still exhibited one or more defects in the eight remaining parameters. Five (11%) made no or a diminished BYCP, 9 (20%) exhibited a decrease in the vertical orientation of hyphae, and 18 (39%) exhibited diminished calcofluor white staining of the ECM and/or a decrease in PMN impenetrability. Fourteen of the 46 (30%) exhibited a decrease in both ECM staining and PMN impenetrability. Five (11%) exhibited a decrease in PMN impenetrability but not a decrease in ECM staining. Fourteen of the 15 with decreased ECM staining also exhibited decreased PMN impenetrability. However, calcofluor white staining may be an inadequate method for assessing ECM, because it is differentially sequestered in the upper regions of a biofilm (40). Therefore, one cannot rule out the possibility that in all cases of decreased PMN impenetrability the ECM is compromised. It should also be noted that extracts that cause a decrease in PMN impenetrability may also cause a decrease in the impermeability of the biofilm to high- and low-molecular-weight molecules and, thus, increased susceptibility to antifungals. MTL-homozygous cells form biofilms that are far less impenetrable by PMNs and far more permeable to both low- and high-molecular-weight molecules, including dyes and fluconazole (40, 49). For these reasons, we are now pursuing a method for incorporating a measure of impermeability to molecules with low molecular weights in the range of those of common antifungals into the single preparation protocol presented here by adding before fixation with PMNs a vital dye that has a red excitation-emission spectrum different from those for calcofluor white and green fluorescent protein.
By far the greatest effects on architecture, cell phenotype, ECM staining, and PMN impenetrability were observed in extract-treated biofilms that were significantly thinner than control biofilms. Of the 25 biofilms in this group, 44% exhibited a decrease or an absence of BYCP formation, 20% exhibited a decrease in hypha formation, 44% exhibited a defect in vertical hypha orientation, 36% exhibited early hyphal bending, 52% exhibited a decrease in calcofluor white staining of the ECM, and 68% exhibited a decrease or a loss of PMN impenetrability (Table 5). As was the case for extract-treated biofilms thicker than control biofilms or with a thickness similar to that of control biofilms, the least affected parameters were hypha related. Again, there was a strong correlation between effects on calcofluor white staining of the ECM and PMN impenetrability. Eighteen exhibited a decrease in ECM staining and/or PMN impenetrability; of these, 12 with a decrease in ECM staining exhibited a decrease in PMN impenetrability as well and 11 with a decrease in PMN impenetrability exhibited normal ECM staining.
Conclusion.
Both the analysis of five previously analyzed pairs of extracts and derivative compounds and that of 81 extracts revealed that a dense upper hyphal layer could form in the absence of a BYCP, as was previously shown to be the case when biofilms are formed in supplemented Lee's defined medium (65). Both sets of data also revealed that a BYCP could form without the formation of a dense upper hyphal region. The strongest correlations, however, were between thickness and PMN impenetrability and between calcofluor white staining of the ECM and PMN impenetrability. We also found that the thinner that an extract-treated biofilm was the more likely it was to lose PMN impenetrability as well and that the lower that the level of ECM calcofluor white staining was the more likely the biofilm was to lose PMN impenetrability. The level of calcofluor white staining may reflect differences in ECM density or composition, such as a decrease in the amount of chitin, which is stained by calcofluor white (10, 68–70). Our results clearly show that extract-treated biofilms that are as thick as or thicker than untreated controls and that have dense upper hyphal regions can still exhibit decreases in calcofluor white-stained ECM and decreases in PMN impenetrability. The results presented here suggest that the ECM may be the parameter that is the most susceptible to the variety of tested extracts and, because of its potential role in drug resistance and PMN impenetrability, deserves more attention (i.e., through the use of more methods for quantitative assessment) in screening for antifungals. The value of some of the tested extracts therefore may be to increase the vulnerability of biofilms to the immune system, both to phagocytic white blood cells and to antibodies, and to increase the permeability and, therefore, the vulnerability of cells to known antifungal drugs when the extracts are used in combination with them.
Based on two premises, that the growth or metabolism of a mature biofilm alone may not be sufficient to identify the antifungal activity of natural extracts and that biofilms may be the major growth form of C. albicans in hosts and on catheters, dentures, and other prosthetics, we developed a high-resolution CLSM-based protocol for testing extracts and chemical libraries through the use of nine parameters of biofilm development. The protocol is unique in that all nine parameters can be assessed from the stack of CLSM z-scans of a single biofilm, resulting in a very large volume of relevant data. Our results both with a previously selected group of five pairs of extracts and derivative antifungal compounds and with a random collection of 88 natural extracts previously untested suggest that the described procedure provides a new and extremely effective method for testing for new antifungal agents. It should also serve as a method for assessing the effects of mutants and inhibitors of known cellular functions on biofilm development. It is meant to complement previous and established methods for assessing the effects of agents on biofilm development, such as determination of dry weight, XTT reduction assays, crystal violet assays, and live-dead assays. The analysis of several more parameters, especially ones related to the ECM, could potentially be added to the procedure to increase the resolution of the phenotypic profiles of biofilms treated with test agents. In addition, the data provided by the protocol reveal both the independence of some biofilm parameters and an obvious correlation between others, with the latter suggesting dependent relationships, such as the dependence of PMN impenetrability and vertical hypha orientation on a normal ECM. One of the specific aims of this study was to engender an awareness that the increased number of parameters used to assess the biofilm phenotype can be incorporated into screens for new antifungals, screens of mutations, and analyses of agents with known function for selective effects on different aspects of biofilm development.
MATERIALS AND METHODS
Preparation of natural extracts and antifungal compounds.
The natural product materials were freeze-dried, ground, and subsequently extracted sequentially three times with 95% aqueous ethanol at 37°C for 10 min. The extracts were evaporated under reduced pressure, weighed, and dissolved at 20 mg/ml in 100% DMSO. Fifty microliters of the extract dissolved in DMSO was loaded into each well of a 96-well polypropylene plate, and the plate was stored at −80°C until employed experimentally. The plant-derived antifungal compounds 6-nonadecynoic acid (60), sampangine (61), coruscanone A (58), and liriodenine methiodide (56) were isolated from Pentagonia gigantifolia, Duguetia hadrantha, Piper coruscans, and Liriodendron tulipifera, respectively. The phloeodictine mixture (57) was isolated from the marine sponge Pellina eusiphonia.
Formation of biofilms.
The procedure used for biofilm formation and subsequent analysis by confocal laser scanning microscopy is diagrammed in Fig. 1A. C. albicans cells of the a/α strain SC5314 (71) were first grown to stationary phase at 25°C in supplemented Lee's (sLee's) medium (40, 72, 73). Cells of the suspension culture were collected, washed, and resuspended in RPMI 1640 medium (Gibco, Grand Island, NY) buffered with 3-(N-morpholino)propanesulfonic acid (MOPS; 0.165 M) to stabilize the pH at 7.0. This medium is referred to as “RPMI-MOPS medium.” The cell concentration was determined with a hemocytometer, and the cells were diluted to 2 × 107 cells per ml in RPMI-MOPS medium. Over 90% of the cells were unbudded singlets. Two milliliters was dispersed on a hydrated silicon elastomer disc (Cardiovascular Instrument Corp., Wakefield, MA) in the well of a Costar 24-well cluster plate. The disc was punched out of a 0.1-cm-thick sheet of silicone elastomer with a 10-mm biopsy punch (Acu-Punch; Acuderm, Ft. Lauderdale, FL). The elastomer-supported culture was preincubated for 1.5 h at 37°C, and then the disc with the adhering culture was removed, gently rinsed with phosphate-buffered saline (PBS), and transferred into a fresh well of a Costar 12-well cluster plate. Each well was filled with 2.5 ml of RPMI-MOPS medium alone, RPMI-MOPS medium containing an extract (50 μg per ml), or, in five cases, RPMI-MOPS medium containing an extract-derived antifungal agent (20 μg per ml). The extract concentration was chosen by testing the five previously studied extracts for growth inhibition at 25, 50, 100, and 200 μg per ml in liquid cultures in RPMI 1640 medium. An extract concentration of 50 μg per ml caused 25 to 50% inhibition, on average, and affected population and/or hypha formation. This was also the approximate concentration used in previous studies of the extracts. A concentration of 20 μg per ml of the five identified compounds was used in previous studies of growth inhibition (56–58, 60, 61). The plates were rocked gently as previously described for aeration and mixing (40). Since all treated test cultures contained 0.4% DMSO, a set of control biofilms was also analyzed in untreated RPMI-MOPS medium containing 0.4% DMSO. After 48 h of incubation at 37°C in air, 3 × 105 differentiated HL-60 cells expressing GFP (HL-60-GFP cells) in 50 μl of RPMI-MOPS medium were gently dispersed on the top of the biofilms, and the biofilms were incubated for an additional 3 h at 37°C in 5% CO2 (65). HL-60-GFP cells were derived from the human neutrophilic promyelocytic cell line HL-60 (74, 75), and when these cells are in the differentiated state, they exhibit the same incapacity to penetrate a wild-type biofilm (impenetrability) as freshly isolated human polymorphonuclear leukocytes (65). The incapacity of HL-60-GFP cells to penetrate a wild-type biofilm is referred to as “PMN impenetrability.” At least two biofilms were generated and analyzed for each of the agents tested. Three fields of each of the two biofilms were scanned by laser scanning confocal microscopy (LSCM), resulting in a total of six or more assessments. Prior to microscopic analysis, biofilms were fixed in 2% paraformaldehyde (PFA) and stained with calcofluor white (Fluorescent Brightener 28; Sigma).
CLSM analysis and parameters assessment.
To assess the effects of the extracts or compounds on the biofilm architecture and PMN impenetrability, biofilms were fixed with paraformaldehyde, stained with calcofluor white, and scanned using a Bio-Rad Radiance 2100 MP multiphoton confocal laser scanning microscope (CLSM) equipped with a 20× Plan Fluor water immersion objective and a Nikon TE 2000 inverted microscope. Calcofluor white (800-nm excitation, 420-nm emission) and GFP (488-nm excitation, 515-nm emission) fluorescent images were acquired as a z-series at 2.0-μm intervals through each individual biofilm, resulting in at least 100 scans per biofilm, depending on the thickness, with Laser Sharp software (Bio-Rad). Individual scans were analyzed using Imaris software (Bitplane, Concord, MA) and ImageJ software (76). Orthogonal (side) images of the stack of z-scans of each biofilm were obtained by rotating the projected stack image 90° and capturing the orthogonal image 40 pixels into the stack. Processing of the orthogonal image was performed with Imaris software using a Gaussian smoothing function at 1.22 μm. The phenotypic characteristics analyzed by CLSM are diagrammed and listed in Fig. 1B and are described in the following sections.
(i) Adhesion of biofilm to silicone elastomer.
If a biofilm remained attached to the substrate through fixation (i.e., after 48 h), it was considered adhesive. If it released from the substrate, as is the case for the a/a Δtec1/Δtec1 mutant biofilms under increased rotation (45), the biofilms were considered nonadhesive.
(ii) Thickness.
The thickness of a biofilm was computed by counting the number of scans from the substrate to the interpreted top of the biofilm and multiplying this value by 2.0 μm, the distance between scans. The Student t test was used to discriminate the categories of biofilms thicker than the control biofilms, thinner than the control biofilms, and similar in thickness to the control biofilms.
(iii) BYCP.
The untreated biofilms exhibited a yeast cell polylayer 8 to 10 cells deep (a score of ++++). Biofilms that formed hyphae directly from the cells adhering to the substrate, as was observed previously in Spider medium (65), and had no more than the original number of yeast cells on the substrate were assessed to be basal yeast cell polylayer (BYCP) negative. Biofilms with a polylayer of 2 yeast-phase cells were scored +, those with a polylayer of 3 to 4 yeast-phase cells were scored ++, and those with a polylayer of 5 to 6 yeast-phase cells were scored +++.
(iv) Hypha formation.
The assessment of hypha formation was based on the average number of hyphal profiles, presented previously as dots for vertical hyphae and as oblong or tangential images for bent hyphae (45, 77), at the halfway point through the z-axis stack of scans. For untreated control biofilms, the average number of dots for five biofilms was 502 ± 28 per 624- by 624-μm field. In two extract-treated preparations in which the hyphae collapsed, hyphal density was determined by analyzing the orthogonal images. This allowed us to compare the cross sections of hyphae with a horizontal orientation. For all comparisons, a density similar to that for the controls was scored ++++. No hyphae was scored negative, a decrease of 75% was scored +, a decrease of 50% was scored ++, and a decrease of 25% was scored +++.
(v) Vertical hypha orientation.
In the middle scans of the z-axis stack of untreated control biofilms, the hypha profiles appeared as equidistance dots separated by ECM, indicating a high degree of vertical orientation (40, 45), which was scored ++++. Images with up to 10% tangential profiles and 90% dots, indicating a loss of vertical orientation, were scored +++, those with approximately 25% tangential profiles and 75% dots were scored ++, those with approximately 50% to 75% tangential profiles and 50% to 25% dots were scored +, and those with no dots or tangential profiles was scored negative.
(vi) Hyphal bend.
The hyphae of untreated controls bend apically, losing their vertical orientation. The controls bent at approximately 70% of the biofilm thickness. To determine the point at which hyphae bend, each scan through the biofilm in the CLSM z-series, beginning at the origin of hyphal growth (the distal edge of the BYCP), was examined. The bend point was determined to be the scan in which at least 25% of the profiles became tangential and is represented as a percentage of the total biofilm thickness.
(vii) Pseudohypha formation.
Pseudohyphae were identified as end-to-end strings of elongate cells that did not detach and that had constricted septae (78–80). Pseudohyphae were assessed by analyzing the orthogonal views of stacked scans through multiple depths and by examining individually each stack of scans. Scoring was as follows: ++++ when over 90% of the filamentous morphologies were pseudohyphae, +++ when 75% were pseudohyphae, ++ when 50% were pseudohyphae, + when 10% to 25% were pseudohyphae, and − when no pseudohyphae were present.
(viii) ECM staining.
Calcofluor white stains the chitin in the cell walls of yeast and hyphae and, to a lesser extent, the chitin shed into the ECM biofilms (63, 81). The level of calcofluor white staining was assessed as the fluorescence intensity in the interstitial regions of biofilms, between cells in the midregions of the biofilm. Using Imaris software, the gamma correction was increased by 0.4 unit in the interstitial regions in a midbiofilm z-series stack. The ECM intensity was measured as the grayscale pixel intensity, which ranged from 50 to 250. This linear intensity range was divided into quartiles, and each quartile was given a corresponding qualitative rank as follows: below 50, −; 50 to 100, +; 101 to 150, ++; 151 to 200, +++; and 201 to 250, ++++.
(ix) PMN impenetrability.
DMSO-induced differentiated HL-60 cells constitutively expressing GFP (74, 75) were used to assess impenetrability by PMNs (49, 65, 82). An aliquot of 50 μl of a suspension of differentiated HL-60 cells (3 × 105) was gently pipetted onto the surface of a 48-h-old biofilm and incubated in the absence of rotation at 37°C in 5% CO2 (Fig. 1A) for 3 h. Orthogonal views and individual z-stacks of the CLSM images of biofilms were then analyzed for the depth of PMN penetration (65). In control biofilms, PMNs were retained at the surface or migrated into the control biofilms to a depth of 5% of the total depth. This was assigned a score of ++++. PMNs that migrated without restriction to the elastomer substrate were given an impenetrability-negative score. PMNs that penetrated through 10% to 30% of a biofilm were assigned a score of +++, those that penetrated through 40 to 60% were assigned a score of +++, those that penetrated through 70% to 90% were assigned a score of ++, and those that penetrated through 100% were assigned a score of +.
For all nine parameters, each assessment was performed in three separate areas in each of the two biofilms and quantified, and the average parameter values are reported. In all cases, reproducibility was high, as is evident in the low standard deviations for thickness in Tables 2 and 4.
ACKNOWLEDGMENTS
This work was supported in part by the Developmental Studies Hybridoma Bank, a National Resource created by NIH and housed at the University of Iowa, and a USDA-ARS cooperative agreement (no. 58-6408-2-0009) in association with the National Center for Natural Products Research at the University of Mississippi.
We are indebted to Claude Pujol for his help with statistical analyses.
REFERENCES
- 1.Blanco JL, Garcia ME. 2008. Immune response to fungal infections. Vet Immunol Immunopathol 125:47–70. doi: 10.1016/j.vetimm.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Dangl JL, Jones JD. 2001. Plant pathogens and integrated defence responses to infection. Nature 411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 3.Dickman MB, Figueiredo P. 2011. Comparative pathobiology of fungal pathogens of plants and animals. PLoS Pathog 7:e1002324. doi: 10.1371/journal.ppat.1002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoham S, Levitz SM. 2005. The immune response to fungal infections. Br J Haematol 129:569–582. doi: 10.1111/j.1365-2141.2005.05397.x. [DOI] [PubMed] [Google Scholar]
- 5.Vera-Estrella R, Higgins VJ, Blumwald E. 1994. Plant defense response to fungal pathogens (II. G-protein-mediated changes in host plasma membrane redox reactions). Plant Physiol 106:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arif T, Bhosale JD, Kumar N, Mandal TK, Bendre RS, Lavekar GS, Dabur R. 2009. Natural products—antifungal agents derived from plants. J Asian Nat Prod Res 11:621–638. doi: 10.1080/10286020902942350. [DOI] [PubMed] [Google Scholar]
- 7.Lacerda AF, Vasconcelos EA, Pelegrini PB, Grossi de Sa MF. 2014. Antifungal defensins and their role in plant defense. Front Microbiol 5:116. doi: 10.3389/fmicb.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidyasagar GM. 2016. Plant-derived antifungal agents: past and recent developments. Recent Trends Antifungal Agents Antifungal Ther 2016:123–147. [Google Scholar]
- 9.Cos P, Vlietinck AJ, Berghe DV, Maes L. 2006. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol 106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Monteiro MC, de la Cruz M, Cantizani J, Moreno C, Tormo JR, Mellado E, De Lucas JR, Asensio F, Valiante V, Brakhage AA, Latge JP, Genilloud O, Vicente F. 2012. A new approach to drug discovery: high-throughput screening of microbial natural extracts against Aspergillus fumigatus using resazurin. J Biomol Screen 17:542–549. doi: 10.1177/1087057111433459. [DOI] [PubMed] [Google Scholar]
- 11.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 12.Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. 2009. Characterization of mucosal Candida albicans biofilms. PLoS One 4:e7967. doi: 10.1371/journal.pone.0007967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumamoto CA, Vinces MD. 2005. Alternative Candida albicans lifestyles: growth on surfaces. Annu Rev Microbiol 59:113–133. doi: 10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]
- 15.Nett JE, Andes DR. 2015. Fungal biofilms: in vivo models for discovery of anti-biofilm drugs. Microbiol Spectr 3(3):MB-0008-2014. doi: 10.1128/microbiolspec.MB-0008-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramage G, Martinez JP, Lopez-Ribot JL. 2006. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res 6:979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 17.Delattin N, Cammue BP, Thevissen K. 2014. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med Chem 6:77–90. doi: 10.4155/fmc.13.189. [DOI] [PubMed] [Google Scholar]
- 18.Pierce CG, Srinivasan A, Ramasubramanian AK, Lopez-Ribot JL. 2015. From biology to drug development: new approaches to combat the threat of fungal biofilms. Microbiol Spectr 3(3):MB-0007-2014. doi: 10.1128/microbiolspec.MB-0007-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan A, Leung KP, Lopez-Ribot JL, Ramasubramanian AK. 2013. High-throughput nano-biofilm microarray for antifungal drug discovery. mBio 4:e00331-13. doi: 10.1128/mBio.00331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, You J, King JB, Powell DR, Cichewicz RH. 2012. Waikialoid A suppresses hyphal morphogenesis and inhibits biofilm development in pathogenic Candida albicans. J Nat Prod 75:707–715. doi: 10.1021/np2009994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watamoto T, Egusa H, Sawase T, Yatani H. 2015. Screening of pharmacologically active small molecule compounds identifies antifungal agents against Candida biofilms. Front Microbiol 6:1453. doi: 10.3389/fmicb.2015.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong SS, Samaranayake LP, Seneviratne CJ. 2014. In pursuit of the ideal antifungal agent for Candida infections: high-throughput screening of small molecules. Drug Discov Today 19:1721–1730. doi: 10.1016/j.drudis.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Coleman JJ, Okoli I, Tegos GP, Holson EB, Wagner FF, Hamblin MR, Mylonakis E. 2010. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem Biol 5:321–332. doi: 10.1021/cb900243b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furletti VF, Teixeira IP, Obando-Pereda G, Mardegan RC, Sartoratto A, Figueira GM, Duarte RM, Rehder VL, Duarte MC, Hofling JF. 2011. Action of Coriandrum sativum L. essential oil upon oral Candida albicans biofilm formation. Evid Based Complement Alternat Med 2011:985832. doi: 10.1155/2011/985832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 46:1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pires RH, Montanari LB, Martins CH, Zaia JE, Almeida AM, Matsumoto MT, Mendes-Giannini MJ. 2011. Anticandidal efficacy of cinnamon oil against planktonic and biofilm cultures of Candida parapsilosis and Candida orthopsilosis. Mycopathologia 172:453–464. doi: 10.1007/s11046-011-9448-0. [DOI] [PubMed] [Google Scholar]
- 27.Siles SA, Srinivasan A, Pierce CG, Lopez-Ribot JL, Ramasubramanian AK. 2013. High-throughput screening of a collection of known pharmacologically active small compounds for identification of Candida albicans biofilm inhibitors. Antimicrob Agents Chemother 57:3681–3687. doi: 10.1128/AAC.00680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taff HT, Nett JE, Andes DR. 2012. Comparative analysis of Candida biofilm quantitation assays. Med Mycol 50:214–218. doi: 10.3109/13693786.2011.580016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tournu H, Van Dijck P. 2012. Candida biofilms and the host: models and new concepts for eradication. Int J Microbiol 2012:845352. doi: 10.1155/2012/845352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gobor T, Corol G, Ferreira LE, Rymovicz AU, Rosa RT, Campelo PM, Rosa EA. 2011. Proposal of protocols using d-glutamine to optimize the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay for indirect estimation of microbial loads in biofilms of medical importance. J Microbiol Methods 84:299–306. doi: 10.1016/j.mimet.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn DM, Balkis M, Chandra J, Mukherjee PK, Ghannoum MA. 2003. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J Clin Microbiol 41:506–508. doi: 10.1128/JCM.41.1.506-508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother 45:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peeters E, Nelis HJ, Coenye T. 2008. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods 72:157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Pitts B, Hamilton MA, Zelver N, Stewart PS. 2003. A microtiter-plate screening method for biofilm disinfection and removal. J Microbiol Methods 54:269–276. doi: 10.1016/S0167-7012(03)00034-4. [DOI] [PubMed] [Google Scholar]
- 35.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179. doi: 10.1016/S0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 36.Chandra J, Mukherjee PK, Ghannoum MA. 2008. In vitro growth and analysis of Candida biofilms. Nat Protoc 3:1909–1924. doi: 10.1038/nprot.2008.192. [DOI] [PubMed] [Google Scholar]
- 37.Costerton JW, Geesey GG, Cheng KJ. 1978. How bacteria stick. Sci Am 238:86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 38.Ehrlich GD, Arciola CR. 2012. From Koch's postulates to biofilm theory. The lesson of Bill Costerton. Int J Artif Organs 35:695–699. [DOI] [PubMed] [Google Scholar]
- 39.Hawser SP, Baillie GS, Douglas LJ. 1998. Production of extracellular matrix by Candida albicans biofilms. J Med Microbiol 47:253–256. doi: 10.1099/00222615-47-3-253. [DOI] [PubMed] [Google Scholar]
- 40.Soll DR, Daniels KJ. 2016. Plasticity of Candida albicans biofilms. Microbiol Mol Biol Rev 80:565–595. doi: 10.1128/MMBR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Ramage G, Rajendran R, Sherry L, Williams C. 2012. Fungal biofilm resistance. Int J Microbiol 2012:528521. doi: 10.1155/2012/528521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie Z, Thompson A, Sobue T, Kashleva H, Xu H, Vasilakos J, Dongari-Bagtzoglou A. 2012. Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing. J Infect Dis 206:1936–1945. doi: 10.1093/infdis/jis607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F. 2011. Resistance of bacterial biofilms to disinfectants: a review. Biofouling 27:1017–1032. doi: 10.1080/08927014.2011.626899. [DOI] [PubMed] [Google Scholar]
- 45.Daniels KJ, Srikantha T, Pujol C, Park YN, Soll DR. 2015. Role of Tec1 in the development, architecture, and integrity of sexual biofilms of Candida albicans. Eukaryot Cell 14:228–240. doi: 10.1128/EC.00224-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 47.Daniels KJ, Srikantha T, Lockhart SR, Pujol C, Soll DR. 2006. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J 25:2240–2252. doi: 10.1038/sj.emboj.7601099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park YN, Daniels KJ, Pujol C, Srikantha T, Soll DR. 2013. Candida albicans forms a specialized “sexual” as well as “pathogenic” biofilm. Eukaryot Cell 12:1120–1131. doi: 10.1128/EC.00112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi S, Sahni N, Daniels KJ, Lu KL, Srikantha T, Huang G, Garnaas AM, Soll DR. 2011. Alternative mating type configurations (a/alpha versus a/a or alpha/alpha) of Candida albicans result in alternative biofilms regulated by different pathways. PLoS Biol 9:e1001117. doi: 10.1371/journal.pbio.1001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soll DR. 2011. Evolution of a new signal transduction pathway in Candida albicans. Trends Microbiol 19:8–13. doi: 10.1016/j.tim.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Soll DR. 2014. The evolution of alternative biofilms in an opportunistic fungal pathogen: an explanation for how new signal transduction pathways may evolve. Infect Genet Evol 22:235–243. doi: 10.1016/j.meegid.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Yi S, Sahni N, Daniels KJ, Lu KL, Huang G, Garnaas AM, Pujol C, Srikantha T, Soll DR. 2011. Utilization of the mating scaffold protein in the evolution of a new signal transduction pathway for biofilm development. mBio 2:e00237-10. doi: 10.1128/mBio.00237-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sahni N, Yi S, Daniels KJ, Huang G, Srikantha T, Soll DR. 2010. Tec1 mediates the pheromone response of the white phenotype of Candida albicans: insights into the evolution of new signal transduction pathways. PLoS Biol 8:e1000363. doi: 10.1371/journal.pbio.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agarwal AK, Xu T, Jacob MR, Feng Q, Lorenz MC, Walker LA, Clark AM. 2008. Role of heme in the antifungal activity of the azaoxoaporphine alkaloid sampangine. Eukaryot Cell 7:387–400. doi: 10.1128/EC.00323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Babu KS, Li XC, Jacob MR, Zhang Q, Khan SI, Ferreira D, Clark AM. 2006. Synthesis, antifungal activity, and structure-activity relationships of coruscanone A analogues. J Med Chem 49:7877–7886. doi: 10.1021/jm061123i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark AM, Watson ES, Ashfaq MK, Hufford CD. 1987. In vivo efficacy of antifungal oxoaporphine alkaloids in experimental disseminated candidiasis. Pharm Res 4:495–498. doi: 10.1023/A:1016479622383. [DOI] [PubMed] [Google Scholar]
- 57.Li XC, Babu KS, Jacob MR, Khan SI, Agarwal AK, Clark AM. 2011. Natural product based 6-hydroxy-2,3,4,6-tetrahydropyrrolo[1,2-a]pyrimidinium scaffold as a new antifungal template. ACS Med Chem Lett 2:391–395. doi: 10.1021/ml200020h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li XC, Ferreira D, Jacob MR, Zhang Q, Khan SI, ElSohly HN, Nagle DG, Smillie TJ, Khan IA, Walker LA, Clark AM. 2004. Antifungal cyclopentenediones from Piper coruscans. J Am Chem Soc 126:6872–6873. doi: 10.1021/ja048081c. [DOI] [PubMed] [Google Scholar]
- 59.Li XC, Jacob MR, ElSohly HN, Nagle DG, Smillie TJ, Walker LA, Clark AM. 2003. Acetylenic acids inhibiting azole-resistant Candida albicans from Pentagonia gigantifolia. J Nat Prod 66:1132–1135. doi: 10.1021/np030196r. [DOI] [PubMed] [Google Scholar]
- 60.Li XC, Jacob MR, Khan SI, Ashfaq MK, Babu KS, Agarwal AK, Elsohly HN, Manly SP, Clark AM. 2008. Potent in vitro antifungal activities of naturally occurring acetylenic acids. Antimicrob Agents Chemother 52:2442–2448. doi: 10.1128/AAC.01297-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muhammad I, Dunbar DC, Takamatsu S, Walker LA, Clark AM. 2001. Antimalarial, cytotoxic, and antifungal alkaloids from Duguetia hadrantha. J Nat Prod 64:559–562. doi: 10.1021/np000436s. [DOI] [PubMed] [Google Scholar]
- 62.Xu T, Tripathi SK, Feng Q, Lorenz MC, Wright MA, Jacob MR, Mask MM, Baerson SR, Li XC, Clark AM, Agarwal AK. 2012. A potent plant-derived antifungal acetylenic acid mediates its activity by interfering with fatty acid homeostasis. Antimicrob Agents Chemother 56:2894–2907. doi: 10.1128/AAC.05663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol 183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hawser SP, Douglas LJ. 1994. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect Immun 62:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daniels KJ, Park YN, Srikantha T, Pujol C, Soll DR. 2013. Impact of environmental conditions on the form and function of Candida albicans biofilms. Eukaryot Cell 12:1389–1402. doi: 10.1128/EC.00127-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alonso MF, Gow NAR, Erwig LP, Bain JM. 2017. Macrophage migration is impaired within Candida albicans biofilms. J Fungi 3:31. doi: 10.3390/jof3030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson CJ, Cabezas-Olcoz J, Kernien JF, Wang SX, Beebe DJ, Huttenlocher A, Ansari H, Nett JE. 2016. The extracellular matrix of Candida albicans biofilms impairs formation of neutrophil extracellular traps. PLoS Pathog 12:e1005884. doi: 10.1371/journal.ppat.1005884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez-Ramirez AI, Ramirez-Granillo A, Medina-Canales MG, Rodriguez-Tovar AV, Martinez-Rivera MA. 2016. Analysis and description of the stages of Aspergillus fumigatus biofilm formation using scanning electron microscopy. BMC Microbiol 16:243. doi: 10.1186/s12866-016-0859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rajendran R, Williams C, Lappin DF, Millington O, Martins M, Ramage G. 2013. Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot Cell 12:420–429. doi: 10.1128/EC.00287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, Bernhardt J, Lounes-Hadj Sahraoui A, Fontaine J, Sanchez H, Hatfield RD, Ntambi JM, Nett JE, Mitchell AP, Andes DR. 2014. Novel entries in a fungal biofilm matrix encyclopedia. mBio 5:e01333-14. doi: 10.1128/mBio.01333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 72.Bedell GW, Soll DR. 1979. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun 26:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee KL, Buckley HR, Campbell CC. 1975. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- 74.Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, Altschuler SJ, Wu LF, Weiner OD. 2012. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell 148:175–188. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Servant G, Weiner OD, Neptune ER, Sedat JW, Bourne HR. 1999. Dynamics of a chemoattractant receptor in living neutrophils during chemotaxis. Mol Biol Cell 10:1163–1178. doi: 10.1091/mbc.10.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao X, Daniels KJ, Oh SH, Green CB, Yeater KM, Soll DR, Hoyer LL. 2006. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology 152:2287–2299. doi: 10.1099/mic.0.28959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Odds FC. 1988. Candida and candidosis: a review and bibliography, 2nd ed Baillière Tindall, London, United Kingdom. [Google Scholar]
- 79.Sudbery P, Gow N, Berman J. 2004. The distinct morphogenic states of Candida albicans. Trends Microbiol 12:317–324. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 80.Veses V, Gow NA. 2009. Pseudohypha budding patterns of Candida albicans. Med Mycol 47:268–275. doi: 10.1080/13693780802245474. [DOI] [PubMed] [Google Scholar]
- 81.Al-Fattani MA, Douglas LJ. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol 55:999–1008. doi: 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 82.Srikantha T, Daniels KJ, Pujol C, Kim E, Soll DR. 2013. Identification of genes upregulated by the transcription factor Bcr1 that are involved in impermeability, impenetrability, and drug resistance of Candida albicans a/alpha biofilms. Eukaryot Cell 12:875–888. doi: 10.1128/EC.00071-13. [DOI] [PMC free article] [PubMed] [Google Scholar]