ABSTRACT
Norovirus is a major cause of acute gastroenteritis worldwide and has emerged as an important issue of chronic infection in transplantation patients. Since no approved antiviral is available, we evaluated the effects of different immunosuppressants and ribavirin on norovirus and explored their mechanisms of action by using a human norovirus (HuNV) replicon-harboring model and a surrogate murine norovirus (MNV) infectious model. The roles of the corresponding drug targets were investigated by gain- or loss-of-function approaches. We found that the calcineurin inhibitors cyclosporine (CsA) and tacrolimus (FK506) moderately inhibited HuNV replication. Gene silencing of their cellular targets, cyclophilin A, FKBP12, and calcineurin, significantly inhibited HuNV replication. A low concentration, therapeutically speaking, of mycophenolic acid (MPA), an uncompetitive IMP dehydrogenase (IMPDH) inhibitor, potently and rapidly inhibited norovirus replication and ultimately cleared HuNV replicons without inducible resistance following long-term drug exposure. Knockdown of the MPA cellular targets IMPDH1 and IMPDH2 suppressed HuNV replication. Consistent with the nucleotide-synthesizing function of IMPDH, exogenous guanosine counteracted the antinorovirus effects of MPA. Furthermore, the competitive IMPDH inhibitor ribavirin efficiently inhibited norovirus and resulted in an additive effect when combined with immunosuppressants. The results from this study demonstrate that calcineurin phosphatase activity and IMPDH guanine synthase activity are crucial in sustaining norovirus infection; thus, they can be therapeutically targeted. Our results suggest that MPA shall be preferentially considered immunosuppressive medication for transplantation patients at risk of norovirus infection, whereas ribavirin represents as a potential antiviral for both immunocompromised and immunocompetent patients with norovirus gastroenteritis.
KEYWORDS: norovirus, mycophenolic acid, calcineurin inhibitors, ribavirin, cell culture model, cell culture, noroviruses
INTRODUCTION
Following widespread implementation of rotavirus vaccination, norovirus infection is now becoming the major cause of acute gastroenteritis worldwide (1). As a single-strand positive-RNA virus belonging to the family Caliciviridae, norovirus is classified into 6 distinct genogroups (GI to GVI). Genogroup 2, genotype 4 (GII.4) is the most prevalent, accounting for 96% of all sporadic infections (2). Although norovirus infection is often self-limiting in adults, every year, it is estimated that noroviruses resulted in up to 200,000 deaths in children below 5 years of age, mainly in developing countries (3). It is recently estimated that norovirus causes more than 200,000 deaths across all ages per year worldwide, of which more than 90,000 are in children under five (4).
Accumulating evidence indicates that transplant recipients are highly susceptible to norovirus infection, irrespective of their status as a pediatric or adult patient (5, 6). Norovirus infection has been described in many types of transplant recipients, including those receiving orthotopic transplantation of lung (7), kidney (8–10), liver (11), heart (12, 13), renal (14–16), intestinal (17–19), small bowel (20), or hematopoietic stem cells (21–24). Norovirus infection in such patients often resulted in severe clinical pathology with prolonged illness (21, 25). Although the exact mechanism of norovirus susceptibility in orthotopic organ transplantation recipients remains unclear, the use of immunosuppressants for preventing organ rejection is conceivably an important risk factor (5, 26). It is plausible that an immunosuppressed status weakens host immunity defending virus invasion. Therefore, a cautious reduction and withdrawal of immunosuppressants has been taken into consideration for managing chronic norovirus gastroenteritis in transplant recipients (27). Intriguingly, immunosuppressants have been reported to directly modulate viral infection (28), but the direct effects on norovirus infection remain to be investigated.
Despite its significant impact on public health, no vaccination or specific antiviral treatment is available. Ribavirin has broad antiviral activity against many viruses in vitro and has been approved for treating chronic hepatitis C virus (HCV) patients for decades. A recent study reported that chronic norovirus infection in two patients with common variable immunodeficiency (CVID) was successfully treated by oral ribavirin, indicating the use of ribavirin as a potential therapy against norovirus infection (29), although the definitive effect requires further evaluation.
In this study, we profiled the effects of different immunosuppressants on norovirus in cell culture models. We reveal that cellular calcineurin and IMPDH enzymes are essential factors supporting norovirus replication, which could be targeted by the corresponding immunosuppressants, calcineurin inhibitors and mycophenolic acid, to exert antinorovirus effects. Furthermore, we demonstrate that ribavirin, a general antiviral drug of a nonimmunosuppressive IMPDH inhibitor, has potent antinorovirus effects and exerts an additive effect when combined with calcineurin inhibitors or MPA. These results provide an important reference for managing immunosuppression and antiviral treatment for transplant patients infected by norovirus or at risk of norovirus infection.
RESULTS
Not all immunosuppressants directly affect norovirus replication.
A major challenge in antinorovirus drug development is the lack of an infectious human isolate that recapitulates the entire life cycle of the virus in a cell culture model. Therefore, we used an HuNV subgenomic replicon model that closely mimicked viral replication in the absence of the production of infectious particles (30). In our study, we observed that glucocorticoids, including prednisone (see Fig. S2A in the supplemental material) and dexamethasone (Fig. S2B), rapamycin (Fig. S2C), brequinar (Fig. S2D), and leflunomide (Fig. S2E) showed no effect on HuNV replication, demonstrating that modulation of norovirus infection is not a general property of immunosuppressants.
CsA and its immunosuppressive analogue, voclosporin, inhibited HuNV replication through a CyPA-dependent mechanism.
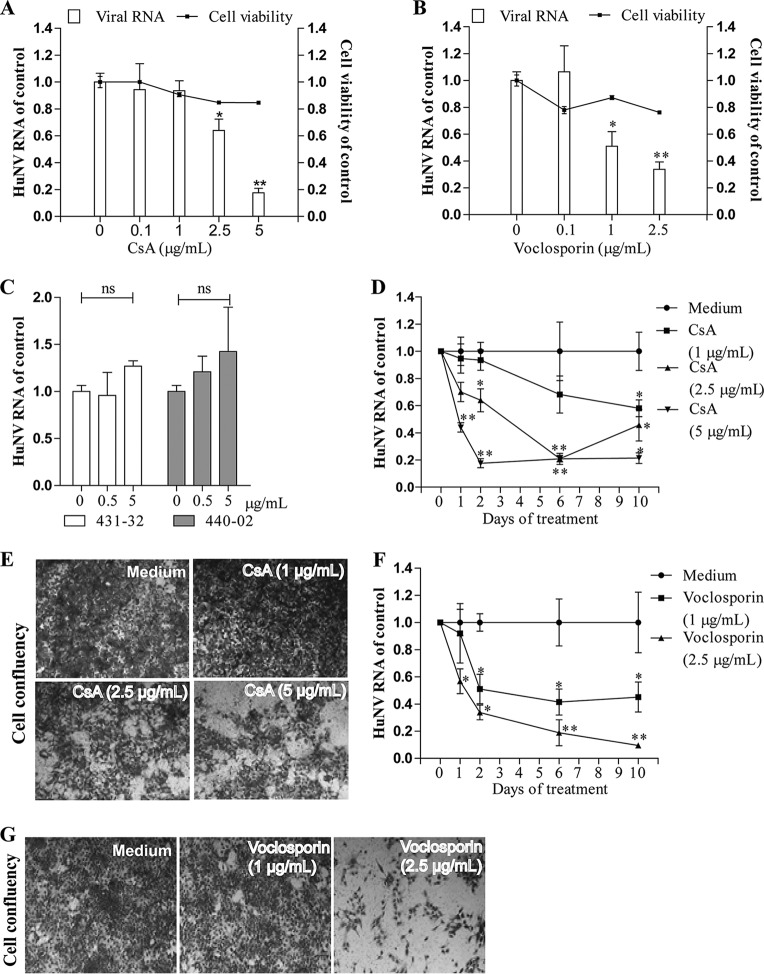
CsA is a calcineurin inhibitor targeting cellular cyclophilins. In the HuNV model, treatment with CsA (5 μg/ml) for 48 h inhibited HuNV replication by 82% ± 3.3% (P < 0.05, n = 6; Fig. 1A). To further confirm that this inhibitory effect relates to the compound targeting cyclophilins, a series of CsA derivatives were tested. Voclosporin, a novel immunosuppressive cyclophilin inhibitor, diminished cellular HuNV RNA levels by 49% ± 10% (P < 0.05, n = 6; Fig. 1B) after 48 h of treatment, even at the low concentration of 1 μg/ml, whereas the parental compound CsA at this concentration had no effect on HuNV. This observation relates well with the notion that voclosporin is a more potent calcineurin inhibitor than CsA (31). Two nonimmunosuppressive CsA derivatives, 431-32 and 440-02, which are chemically similar to CsA, if functionally distinct, had no effect on HuNV replication (P > 0.05, n = 4; Fig. 1C), suggesting that the effects of CsA analogues on norovirus replication are related to calcineurin inhibition.
FIG 1.
Antiviral activities of CsA and its derivatives against HuNV replication. CsA (A) and its immunosuppressive derivative voclosporin (B) dose-dependently inhibited HuNV replication without potent toxicity to host cells after 48 h of treatment. The level of HuNV RNA was quantified by qRT-PCR and was compared to that in cells treated with 0.5% DMSO (control) (n = 3 independent experiments with 2 replicates each). (C) Its nonimmunosuppressive derivatives 431-32 and 440-02 failed to inhibit HuNV replication after 48 h of treatment, even at the highest concentration tested (n = 2 independent experiments with 2 replicates each). (D and F) Clearance assay with CsA and voclosporin. HG23 cells were treated with CsA or voclosporin for 1, 2, 6, or 10 days. At the end of each treatment period, the level of HuNV RNA was determined by qRT-PCR and normalized to that of the control cells from the same treatment time (n = 3 independent experiments with 2 replicates each). (E and G) Rebound assay with CsA and voclosporin. After 10 days of treatment, drugs were omitted, and HG23 cells (48-well tissue culture plate with 2.5 × 104 cells per well) were cultured under the selective pressure of G418 (1.5 mg/ml). With another 5 days of culture, the cell layers were stained with hematoxylin and visualized by an inverted light microscope. Images are representative of three independent experiments with 2 replicates each. Data are presented as the means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant).
It is necessary to determine whether long-term exposure to these immunosuppressants completely eliminated HuNV replicons from host cells. For this purpose, a clearance and rebound assay was employed. If the replicons are completely cleared, the cells could not survive and proliferate in the presence of G418. If the cells still carry replicons, they will proliferate. CsA at 5 μg/ml caused a reduction in HuNV RNA levels by 79% ± 3.8% (P < 0.05, n = 6; Fig. 1D) after 10 days of treatment. Consistently, CsA-treated cells survived and proliferated in the presence of the selection marker (Fig. 1E), demonstrating that although CsA inhibited HuNV replication, it did not completely cause elimination of all replicons from cells. Voclosporin at 2.5 μg/ml provoked potent inhibition of HuNV replication, as evidenced by a reduction of 91% ± 1.5% (P < 0.05, n = 6; Fig. 1F) in viral RNA. Following subsequent challenge with the selection marker, only a small portion of cells stayed alive (Fig. 1G), showing that voclosporin treatment cleared most HuNV replicons from cultures, but a replicon-containing compartment remained.
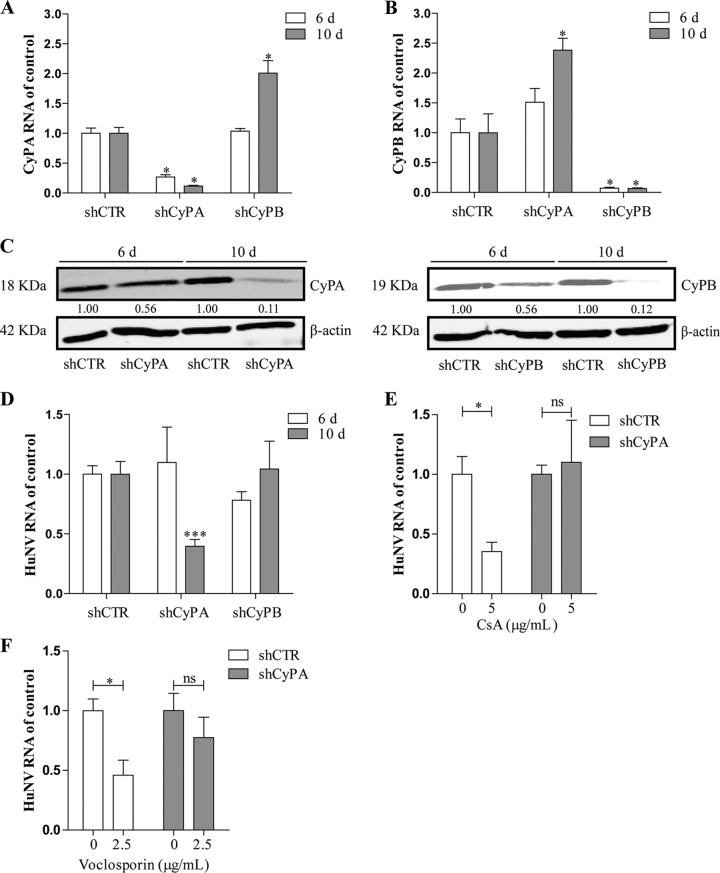
Cellular cyclophilins, in particular the cyclophilin A (CyPA) and cyclophilin B (CyPB) isoforms, are the canonical drug targets of CsA. To study the potential involvement of CyPA and CyPB in CsA-caused inhibition of HuNV replication, an RNA interference (RNAi)-mediated loss-of-function assay was performed. Upon transduction of lentiviral vectors targeting CyPA (shCyPA) or CyPB (shCyPB), specific downregulation of the target genes at the mRNA level was observed at both day 6 and day 10 postransduction, compared to a scrambled control (shCTR) (Fig. 2A and B). Downregulation at the protein level was observed only at day 10 (Fig. 2C). Consistently, following knockdown, no effect on HuNV was observed at day 6, but a significant reduction in viral replication by 60% ± 5.7% (P < 0.05, n = 8; Fig. 2D) was observed by knockdown of CyPA, but not that of CyPB, at day 10 postransduction. Notably, knockdown of CyPA also resulted in an elevation of CyPB mRNA expression at day 10 postransduction and vice versa, suggesting that a compensatory mechanism may exist between CyPA and CyPB (Fig. 2A and B). Concomitant with CyPA knockdown, CsA (P < 0.05, n = 5; Fig. 2E) and voclosporin (P < 0.05, n = 5; Fig. 2F) lost their capacity to inhibit HuNV after 48 h of treatment. Collectively, these results show that CsA and voclosporin inhibited HuNV replication through targeting CyPA, not CyPB.
FIG 2.
CyPA, but not CyPB, is involved in CsA-caused inhibition of HuNV replication. (A and B) Knockdown of CyPA and CyPB through lentiviral shRNA vectors. HG23 cells were transduced with shRNA vectors against CyPA (shCyPA), CyPB (shCyPB), or control (shCTR). At the indicated time points, the level of cyclophilin RNA was measured by qRT-PCR and compared to the control (n = 2 independent experiments with 2 replicates each). (C) Western blot analysis of CyPA and CyPB in HG23 cells transduced with shRNAs. Images are representative of three independent experiments. Transduction of CyPA and CyPB shRNAs resulted in a dramatic downregulation of CyPA and CyPB expression at day 10 (10 d) but not at day 6 (6 d) postransduction. (D) qRT-PCR analysis of HuNV RNA in HG23 cells transduced with shRNAs. Knockdown of CyPA but not CyPB resulted in a significant decrease in viral replication at day 10 but not at day 6 postransduction (n = 3 independent experiments with 2 to 3 replicates each). (E and F) Knockdown of CyPA abrogated the inhibition of HuNV replication by CsA and voclosporin. After successful knockdown of mRNA and protein of CyPA, the responsiveness of HuNV to CsA and voclosporin treatment was detected after 48 h of treatment. CsA and voclosporin possessed the antinorovirus activity against shCTR-treated HG23 cells but failed to suppress HuNV replication in shCyPA HG23 cells. Data presented as the means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
FK506 moderately inhibited HuNV replication via targeting FKBP12.
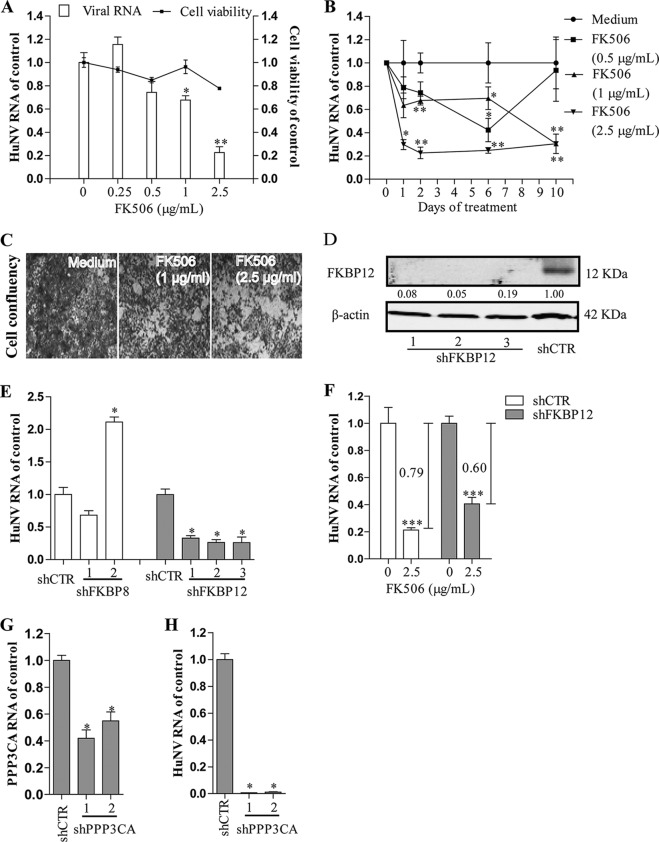
FK506 (tacrolimus) is another calcineurin inhibitor. FK506 at 2.5 μg/ml caused a reduction in HuNV replication by 77% ± 5.0% (P < 0.05, n = 6) and 70% ± 8.3% (P < 0.05, n = 6) after short-term (2 days, Fig. 3A) and long-term (10 days, Fig. 3B) treatment, respectively. Of note, FK506 at high concentration of 2.5 μg/ml quickly inhibited HuNV replication being evident from effects even after 1 day of treatment. Similar to CsA analogues, FK506 did not completely clear HuNV replicons from the cultures (Fig. 3C).
FIG 3.
FK506 moderately inhibited HuNV replication through FKBP12 and calcineurin. (A) Concentration-dependent antiviral effects of FK506 on HuNV replication after 48 h of treatment (n = 3 independent experiments with 2 replicates each). (B) Clearance assay with FK506. Long-term exposure to FK506 resulted in a moderate reduction of HuNV replication (n = 3 independent experiments with 2 replicates each). (C) Rebound assay with FK506. After long-term clearance phase, HG23 cells were cultured in the presence of G418 for another 5 days. Reduced cell confluence was observed, indicating that FK506 partially cleared the HuNV replicons from host cells. (D) Western blot analysis of FKBP12 knockdown by lentiviral shRNA vectors. Images are representative of three independent experiments. (E) qRT-PCR analysis of HuNV RNA level in shCTR, shFKBP8, and shFKBP12 cells after transduction of lentiviral shRNA vectors for 10 days. FKBP12 but not FKBP8 knockdown inhibited HuNV replication (n = 2 independent experiments with 2 replicates each). (F) FKBP12 knockdown partially blocked FK506-induced inhibition of HuNV after 48 h of treatment (n = 3 independent experiments with 2 to 3 replicates each). (G) qRT-PCR analysis of calcineurin knockdown at the RNA level. The level of calcineurin subunit PPP3CA RNA was presented as the relative value to the shRNA control (n = 2 independent experiments with 2 replicates each). (H) PPP3CA knockdown inhibited HuNV replication, as determined by qRT-PCR (n = 2 independent experiments with 2 replicates each). Data are presented as the means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
FK506 suppresses the immune system by binding to FKBP12, resulting in inhibition of the phosphatase activity of calcineurin. To understand the role of FKBP12 in FK506-mediated inhibition of HuNV, we knocked down the expression of FKBP12. It is demonstrated that knockdown of FKBP12 (Western blot analysis in Fig. 3D and reverse transcription-quantitative PCR [qRT-PCR] analysis in Fig. S3), but not FKBP8 (a control; qRT-PCR analysis in Fig. S3) resulted in reduced HuNV replication (Fig. 3E). At the same time, FKBP12 knockdown partially abrogated FK506-mediated inhibition of HuNV (P < 0.05, n = 8; Fig. 3F), suggesting that FK506 inhibited HuNV replication through a pathway involving FKBP12. Thus, the calcineurin pathway appears to be essential for norovirus propagation, and experiments were initiated to confirm this notion.
Calcineurin activation is indispensable for efficient replication of norovirus.
Calcineurin inhibitors are effective against norovirus replication, whereas nonimmunosuppressive CsA derivatives are ineffective, suggesting that calcineurin activity is required for CsA and FK506-mediated inhibition of norovirus. To confirm this hypothesis, we used RNAi to knock down protein phosphatase 3, catalytic subunit, alpha isozyme (PPP3CA) to inhibit calcineurin phosphatase activity. After successful knockdown of PPP3CA (Fig. 3G), it is shown that HuNV replication was inhibited (Fig. 3H). Thus, calcineurin activation is vital for norovirus replication, supporting the idea that CsA and FK506 exerted antinorovirus activity through the inhibition of calcineurin.
MPA potently inhibited norovirus replication and completely cleared HuNV replicons from host cells.
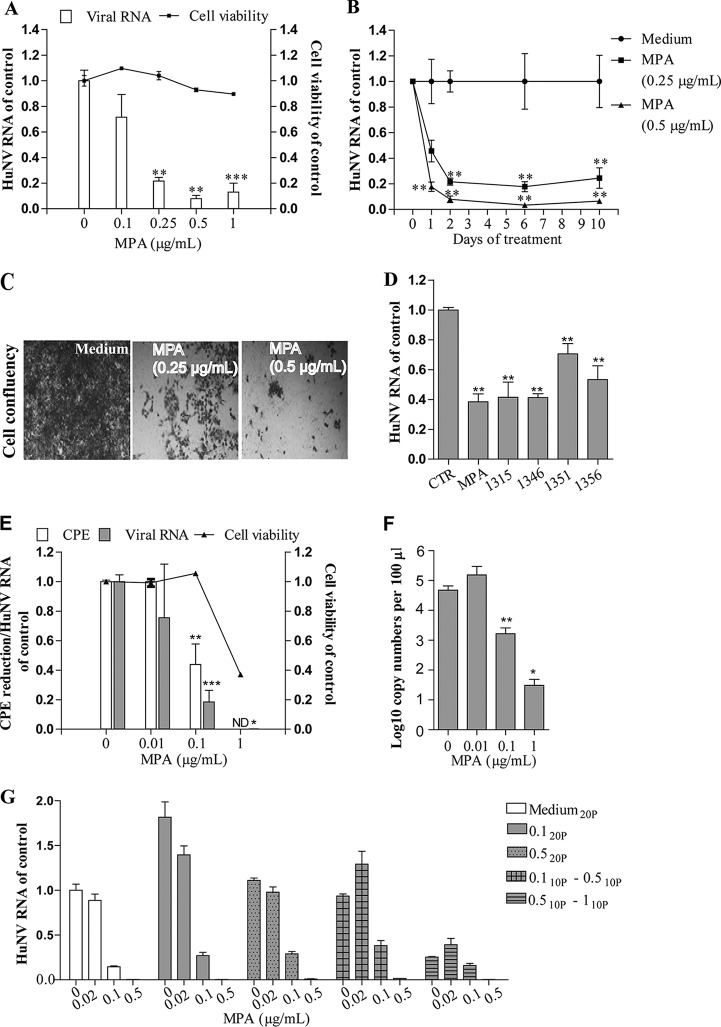
Examining the effects of MPA on HuNV replication, we observed that treatment with only 0.25 μg/ml MPA, which was even lower than the blood concentration (approximately 1 to 10 μg/ml) in transplantation patients (32), potently inhibited HuNV replication by 79% ± 2.7% (P < 0.05, n = 6; Fig. 4A) after 48 h of treatment. Likewise, following long-term treatment with 0.5 μg/ml MPA (10 days; Fig. 4B), HuNV replication was reduced by 94% ± 1.7% (P < 0.05, n = 6). Correspondingly, in the rebound experiment (Fig. 4C), MPA-treated cells completely lost the ability to proliferate and succumbed to the selection marker, suggesting that MPA treatment totally cleared replicons from the host cells. In apparent support, when we tested other 4 IMPDH inhibitors with differential inhibitory efficacies on IMPDH enzymatic activity (Ki values toward IMPDH1 and IMPDH2 are shown in the Table S4), all of them exerted significant antinorovirus activities at a 1 μM concentration (P < 0.05, n = 6; Fig. 4D).
FIG 4.
MPA potently inhibited norovirus replication and completely eliminated HuNV replicons from host cells without concomitant drug resistance. (A) Treatment with MPA for 48 h potently inhibited HuNV replication, as determined by qRT-PCR (n = 3 independent experiments with 2 replicates). (B) Clearance assay with MPA (n = 3 independent experiments with 2 replicates). (C) Rebound assay with MPA. Long-term treatment with 0.5 μg/ml MPA completely cleared HuNV replicons from host cells, and no colony formation was observed. (D) Comparison of the antinorovirus effects of MPA and 4 IMPDH inhibitors on HuNV. In the HG23 cells, treatment with 4 IMPDH inhibitors (the same concentration of 1 μM as MPA) resulted in a reduction of HuNV replication (n = 3 independent experiments with 2 replicates). (E) The antinorovirus effects of MPA were validated on MNV-1 as quantified by means of an MTT-based CPE reduction assay and qRT-PCR (n = 2 independent experiments with 2 to 3 replicates). ND, not detected. (F) Same as panel E for detecting the cellular MNV-1 RNA level; the viral RNA copy numbers in the supernatant (secreted viruses) were also detected after 24 h of treatment with MPA. MPA potently inhibited MNV-1 virus particle production (n = 2 independent experiments with 2 or 3 replicates). (G) The inhibitory efficacy of MPA against MNV-1 following 20 passages (20P) exposed to MPA or vehicle control. In the selection process, MNV-1 was either directly cultured in the presence of a fixed MPA concentration (0.1 or 0.5 μg/ml) or in a lengthy stepwise selection concentration from 0.1 to 0.5 μg/ml or from 0.5 to 1 μg/ml. After selection, the antinorovirus effects of MPA on the 20th passage of MNV-1 were determined by qRT-PCR. Data are presented as the means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001). 10P, 10 passages.
In the face of a lack of a cell culture system for HuNV, we considered MNV a suitable surrogate for studying HuNV biology and pathogenesis. Consistent with the results in the replicon model, MPA at 0.1 μg/ml reduced MNV-1 cellular viral RNA by 83% ± 8% (P < 0.05, n = 6 to 8; Fig. 4E) and virus production in the supernatant (P < 0.05, n = 6; Fig. 4F) after 24 h of treatment. The inhibitory effect was also confirmed in a cytopathic effect (CPE) inhibition assay, demonstrating that MPA at 0.1 μg/ml protected RAW cells from MNV-1 induced CPE formation by 57% ± 14% (P < 0.05, n = 8; Fig. 4E). Collectively, our results demonstrated that MPA potently inhibited norovirus replication and could even completely clear the virus.
High barrier to resistance development.
In the HuNV replicon model, MPA completely cleared the viral replicons, which obviously prevented the emergence of resistance and thus leaves the question unanswered about whether norovirus develops resistance to MPA treatment. To address this issue, MNV-infected RAW cells were exposed to MPA in different setups for 20 passages. As shown in Fig. 4G, MNV-1 retained its sensitivity to MPA treatment, even at passage 20, with inhibitory efficacy varying from 98.5% ± 0.07% to 99.6% ± 0.07% following treatment with 0.5 μg/ml MPA, which was comparable to that in the unchallenged control (99.6% ± 0.05% inhibition). Thus, norovirus is not prone to developing resistance to MPA.
MPA inhibited norovirus replication by simultaneously targeting IMPDH1 and IMPDH2.
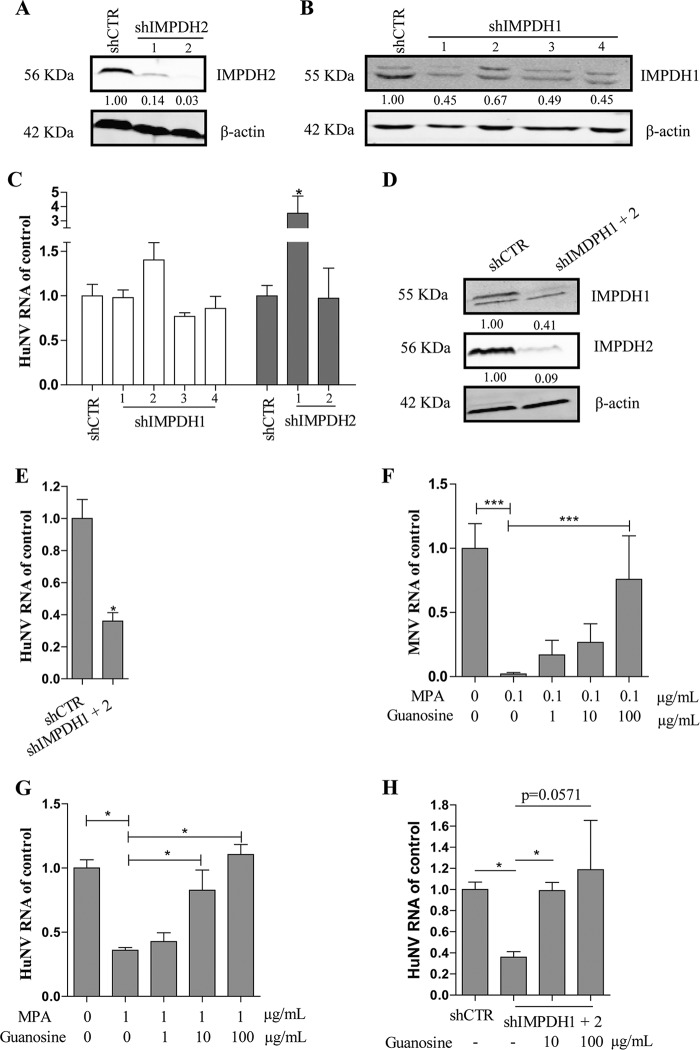
MPA acts through inhibition of IMPDH. There are two isoforms of IMPDH, IMPDH1 and IMPDH2, in mammals. MPA has a 5-fold more potent inhibitory effect on IMPDH2 than on IMPDH1 (33). In view of the low concentrations of MPA required to counteract norovirus, IMPDH2 appears to be the more likely drug target with respect to its effects on the norovirus life cycle. However, knockdown of IMPDH2 had no significant effect on HuNV replication (Fig. 5C), even though such knockdown profoundly decreased IMPDH2 mRNA and protein levels, as assessed by qRT-PCR (Fig. S4) and Western blotting (Fig. 5A). Similarly, knockdown of IMPDH1 decreased IMPDH1 expression at the RNA level (Fig. S4) and protein level (Fig. 5B) but caused no significant change in HuNV replication (Fig. 5C). Surprisingly, simultaneous knockdown of IMPDH1 and IMPDH2 (qRT-PCR analysis in Fig. S5 and Western blot analysis in Fig. 5D) suppressed HuNV replication by 64% ± 5.3% (P < 0.05, n = 4; Fig. 5E), showing the interchangeable roles of IMPDH1 and IMPDH2 in the norovirus life cycle.
FIG 5.
Simultaneous knockdown of IMPDH1/2 reduced HuNV replication, which was reversed by exogenous guanosine. (A and B) Validation of IMPDH downregulation in shCTR cells and shIMPDH cells by Western blotting. Images are representative of three independent experiments. (C) qRT-PCR analysis of HuNV RNA level in IMPDH knockdown cells. Compared to control cells, knockdown of IMPDH1 or IMPDH2 alone had no significant effect on HuNV replication (n = 2 independent experiments with 2 replicates each). (D) Validation of IMPDH downregulation after simultaneous knockdown of IMPDH1/2 by Western blotting. Images are representative of three independent experiments. (E) Simultaneous silence of IMPDH1/2 decreased HuNV replication (n = 2 independent experiments with 2 replicates). (F and G) Guanosine restored norovirus replication in MPA-treated cells. MNV-1 and the HuNV replicon were treated with MPA alone or combined with guanosine (1, 10, or 100 μg/ml). After 24 h of incubation, norovirus replication was quantified by qRT-PCR. (H) IMPDH1/2 knockdown cells were cultured with medium or increasing concentrations of guanosine. After 24 h of incubation, the HuNV RNA level was analyzed by qRT-PCR and was compared to the shCTR cells (n = 2 independent experiments with 2 replicates). Data presented as means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Guanosine restored norovirus replication in MPA-treated and IMPDH1/2 knockdown cells.
MPA inhibits de novo guanosine nucleotide biosynthesis by inhibiting IMPDH. To examine whether MPA exerts an inhibitory effect on norovirus via guanosine depletion, we challenged norovirus cultures either with only MPA or with a combination of MPA and guanosine (with guanosine at 1, 10, or 100 μg/ml concentrations). After 24 h of incubation, norovirus replication was determined by qRT-PCR analysis. We observed that the inhibitory effect of MPA on norovirus replication was sensitive to supplementation with guanosine. A 100 μg/ml guanosine concentration completely negated MPA effects in both the MNV-1 model (P < 0.05, n = 6 to 8; Fig. 5F) and HuNV replicon model (P < 0.05, n = 5; Fig. 5G). In apparent agreement, exogenous supplementation of guanosine reversed the inhibitory effects of IMPDH1/2 knockdown on HuNV replication (P < 0.05, n = 5; Fig. 5H). Thus, norovirus biology requires substantial intracellular guanosine levels, and the inhibitory effects of MPA and IMPDH knockdown are mediated by the depletion of this guanosine pool.
Ribavirin efficiently inhibited norovirus partially through nucleotide depletion.
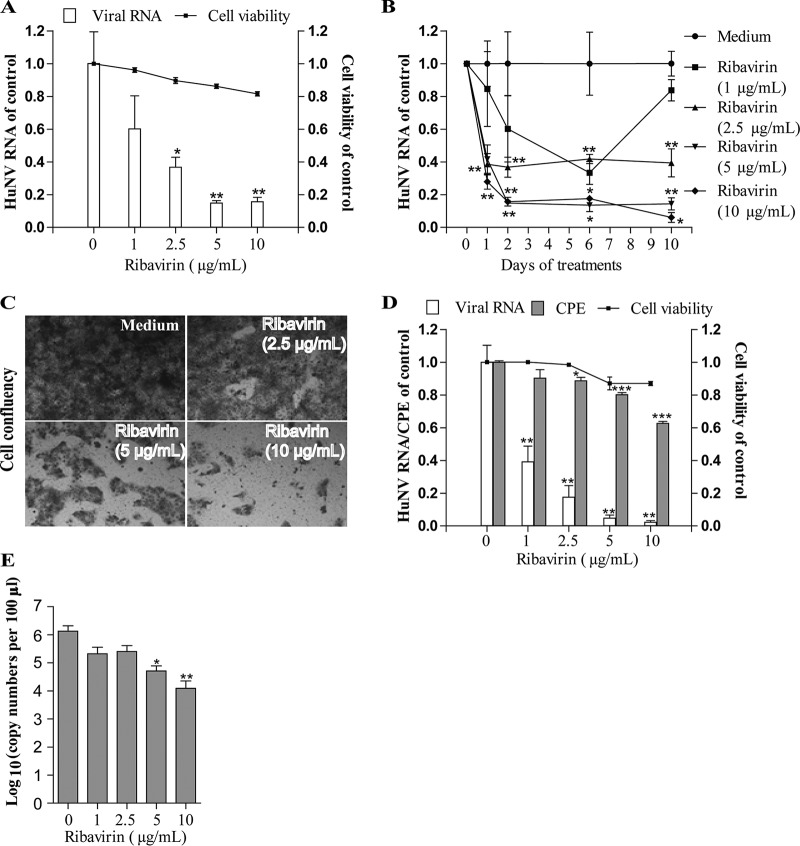
Ribavirin at a 2.5 μg/ml concentration resulted in a reduction of HuNV replication by 64% ± 6.1% (P < 0.05, n = 6; Fig. 6A) and 61% ± 8.5% (P < 0.05, n = 6; Fig. 6B) after short-term and long-term treatment, respectively. Additionally, ribavirin at higher concentrations (5 and 10 μg/ml) was capable of clearing cells of most replicons following 10 days of consecutive culture (Fig. 6C). The antiviral activity of ribavirin was confirmed in the MNV-1 model. Ribavirin at concentration of 5 μg/ml effectively decreased MNV-1 cellular RNA (P < 0.05, n = 6; Fig. 6D) and extracellular viral particles in the supernatant (P < 0.05, n = 6; Fig. 6E). In the CPE inhibition experiment, treatment with ribavirin for 3 days dose-dependently increased cell viability and decreased CPE formation by inhibiting MNV-1 replication (P < 0.05, n = 8; Fig. 6D), further validating the antiviral activity of ribavirin on MNV-1. To further explore the mechanism of ribavirin, we investigated whether the addition of exogenous guanosine would revert the antiviral effect of ribavirin. As shown in Fig. S6, a high concentration of guanosine partially reverted the antiviral activity of ribavirin for both HuNV and MNV, indicating that guanosine depletion contributed to the antiviral activity of ribavirin.
FIG 6.
Ribavirin effectively inhibited norovirus replication. (A) Treatment with ribavirin for 48 h dose-dependently decreased HuNV replication, as determined by qRT-PCR (n = 3 independent experiments with 2 replicates each). (B) Clearance assay with ribavirin (n = 3 independent experiments with 2 replicates each). (C) Rebound assay with ribavirin. (D) The antinorovirus activity of ribavirin was also confirmed on MNV-1. Ribavirin treatment decreased MNV-1-induced CPE and viral RNA replication in RAW 264.7 cells (n = 3 independent experiments with 2 to 3 replicates). (E) Ribavirin treatment also decreased MNV-1 virus particle production in supernatant as quantified by viral RNA copy numbers (n = 3 independent experiments with 2 replicates). Data are presented as the means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Combinatory effects of ribavirin and immunosuppressants on norovirus replication.
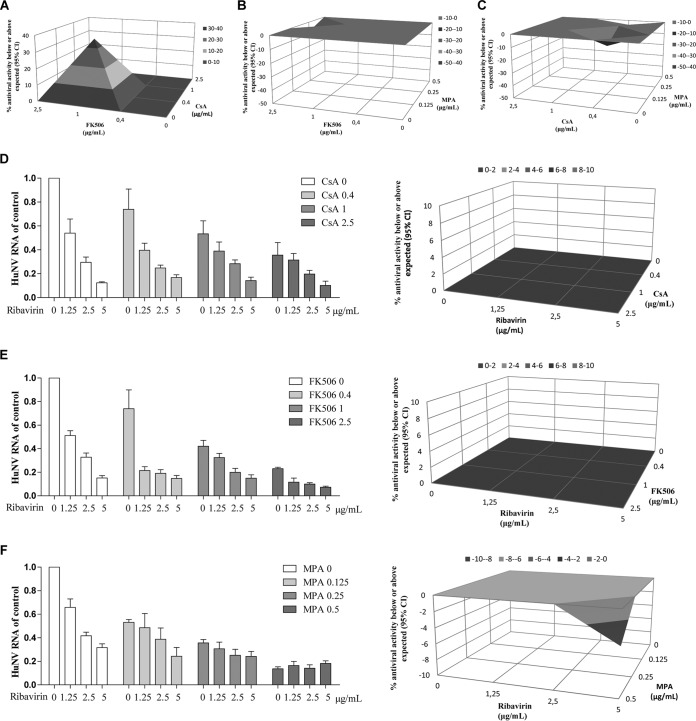
Immunosuppressants are often used in combination in the clinic. First, we evaluated the interaction of immunosuppressants on HuNV using MacSynergy 2 combination analysis. As shown in Fig. 7A, the combined calcineurin inhibitors, FK506 and CsA, exerted a moderately synergistic antiviral effect, with a log volume of 5.55. The maximal degree of combined inhibition between FK506 and CsA was achieved at 1 μg/ml FK506 and 0.4 μg/ml CsA, with a synergy volume of 35.06 μM2% above the expected value. The combination of MPA with FK506 (Fig. 7B) or CsA (Fig. 7C) achieved a synergy volume of −7.79 μM2% (log volume of −0.93) or −14.58 μM2% (log volume of −1.32), respectively, indicating an additive effect. These results highlight the dependency of norovirus biology on both calcineurin phosphatase activity and the presence of an adequate intracellular guanosine pool.
FIG 7.
The combinatory effects of ribavirin and immunosuppressants on norovirus replication. The combinatory effects of two drugs in the 48-h antiviral assay with HuNV were analyzed using the mathematical model MacSynergy. The three-dimensional surface plot represents the differences (within 95% confidence interval [95% CI]) between actual experimental effects and theoretical additive effects of the combination at various concentrations of the two compounds (n = 5). The antiviral effects of FK506 in combination with CsA (A) or MPA (B) as well as CsA in combination with MPA (C) was analyzed by MacSynergy model. Combinations of ribavirin with CsA (D), FK506 (E) or MPA (F) were analyzed by the relative HuNV RNA level compared to the control and MacSynergy model.
Next, we assessed the combinatory antiviral effects of ribavirin and immunosuppressants. The combined effects of ribavirin and immunosuppressants, including CsA (Fig. 7D), FK506 (Fig. 7E), and MPA (Fig. 7F) were additive, with synergy volumes of 0 μM2% (log volume of 0), 0 μM2% (log volume of 0), and −4.04 μM2% (log volume of −0.37), respectively.
DISCUSSION
Immunosuppressants are universally used to deliberately induce immunosuppression after organ transplantation to prevent allograft rejection. However, this results in increased susceptibility to opportunistic infections, and especially norovirus infection has emerged as a major concern in this respect. A recent study reported that among 116 immunocompromised pediatric hematopoietic stem cell and solid organ transplant recipients, norovirus infection was associated with prolonged diarrhea and frequent intensive care unit (ICU) admission (5). Although new classes of immunosuppressive medications have been introduced to the transplant community during the last decades, T-lymphocytes remain the key targets of these agents, as this cell type is a major effector in graft rejection. T-lymphocytes, however, also play an important role in the adaptive immune response following viral infection; thus, reduced resistance to viral infection is an expected side effect of graft tolerance-inducing medications. Intriguingly, immunosuppressive medications may also directly interfere with the viral life cycle. For various viruses, it is now clear that the choice of immunosuppressive medications relates to patient sensitivity to infection (34). Norovirus biology, however, is relatively poorly understood, and its interaction with different immunosuppressive regimens remains obscure at best. Here, we have profiled the commonly used immunosuppressants on norovirus infection and related the effects to their cellular effectors. Thus, our data provide guidance as to the host biochemical mechanisms essential for norovirus replication and may guide clinical management of transplantation patients at risk for norovirus infection. With respect to the latter, the remarkable sensitivity of norovirus to MPA is especially important, particularly in view of the absence of norovirus-directed vaccines or antiviral medications.
Interestingly, our study reveals a hitherto unexpected role for calcineurin phosphatase activity in the norovirus life cycle. Calcineurin inhibitors include FK506 and CsA. FK506 is currently a widely used immunosuppressive agent for managing transplantation patients. CsA binds to cyclophilins, and FK506 binds to FK binding proteins (FKBPs). Both events result in a profound inhibition of the phosphatase activity of calcineurin, which in turn suppresses T cell proliferation. In general, FK506 is thought to have no effect on viral infections (28). In contrast, CsA has been demonstrated to interact with the biology of a broad range of viruses, mainly through inhibiting cyclophilins. Of note, CsA inhibited HCV replication both in vitro and in vivo in an apparently specific manner. HCV RNA replication requires the nonstructural HCV NS5A protein that serves as a direct ligand for CyPA. CsA could disrupt the CyPA-NS5A interaction, further reducing HCV RNA replication (35). In our study, FK506 and CsA exerted a moderate inhibitory effect on norovirus replication through FKBP12 and CyPA, respectively. Furthermore, nonimmunosuppressive CsA analogues, which do not inhibit calcineurin, failed to suppress norovirus replication, supporting the hypothesis that calcineurin activation is required for the norovirus life cycle.
Calcineurin is a heterodimeric Ca2+- and calmodulin-dependent serine/threonine protein phosphatase composed of a 60-kDa catalytic subunit (PPP3CA) and a 19-kDa calcium binding regulatory subunit (PPP3R1) (36). Our results showed that knockdown of PPP3CA resulted in a dramatic reduction of HuNV replication, demonstrating that calcineurin is required for HuNV replication. Although the exact mechanism as to how calcineurin facilitates norovirus replication is unknown, it may resemble its role in the life cycle of the polyomavirus BK (37). The observation that CsA, but not NIM811 (a nonimmunosuppressive CsA derivative), inhibited BK virus (BKV) replication by inhibiting CyPA and calcineurin activation is highly reminiscent of the results obtained in the present study, but concluding that the effects are mechanistically identical requires further validation, especially with respect to the role of the nuclear factor of activated T cells (NFAT).
Mycophenolate mofetil (MMF) has been widely used in kidney, pancreas, liver, and heart transplantation (38). MPA, the active form of MMF in vivo, is a potent inhibitor of IMPDH, a rate-limiting enzyme in the de novo synthesis of guanosine nucleotides. By inhibiting IMPDH, MPA results in the depletion of the intracellular GTP and dGTP pools and exerts potent immunosuppressive effects. MPA has been demonstrated to inhibit a variety of viruses, ranging from DNA virus, including hepatitis B virus (HBV), to RNA viruses, including HCV, dengue virus, yellow fever virus, and Chikungunya virus (28). Here, we demonstrate that MPA potently inhibited both MNV and HuNV replication through simultaneously targeting IMPDH1 and IMPDH2. Furthermore, MPA at levels below the serum concentrations in patients completely eliminated HuNV replicons from cells after long-term treatment. In apparent agreement, 4 IMPDH inhibitors also inhibited HuNV replication in our experiments. Apparently, MPA has a high barrier against developing drug resistance with respect to its action on norovirus biology. Of note, characterization of the potential mutagenesis of the viral genome in response to MPA exposure is interesting for future research. In conjunction, our data provide compelling evidence that MPA should be the immunosuppressant of choice in transplantation patients at risk for norovirus infection.
The optimization of immunosuppression might help the clearance of norovirus infection in a proportion of infected organ recipients. However, for the transplant or other types of patients who fail to clear the virus, effective antiviral therapy is urgently needed, whereas no proven antiviral medications are available. Ribavirin, a general antiviral, has been used in the clinic for decades to treat various types of viral infections. A recent study reported that ribavirin cleared norovirus and resulted in complete symptomatic and histological recovery in two CVID patients, which raises the possibility of ribavirin as a potential antiviral therapy for norovirus infection. Several studies have attempted to understand the antiviral activity and the mechanism of ribavirin on norovirus. It has been reported that the addition of guanosine to the ribavirin treatment could moderately reverse ribavirin inhibition on HuNV (39), which is consistent with our results. Another two studies demonstrated that ribavirin inhibited MNV through increased mutagenesis and quasispecies diversity (40, 41). In our study, ribavirin at concentrations comparable to serum concentrations in patients (42) inhibited norovirus replication. Ribavirin, combined with MPA or calcineurin inhibitors, additively inhibited norovirus replication, which supports the potential of ribavirin as a therapy for norovirus infection in transplant patients. However, it remains to be further investigated whether the optimal combination with ribavirin is MPA, CsA or FK506.
In summary, we profiled the effects of clinically relevant immunosuppressants on norovirus replication and observed that norovirus was sensitively dependent on IMPDH guanine-synthesizing activity but also required calcineurin phosphatase activity. MPA represents a potent inhibitor of norovirus replication, with a high barrier toward the development of drug resistance. Ribavirin is a promising candidate in treating norovirus infection, although further evaluation, particularly in patients, is needed.
MATERIALS AND METHODS
Reagents.
The immunosuppressants, including dexamethasone (DEX; Chemical Abstracts Service [CAS] no. 50-02-2), prednisolone (PRED; CAS no. 50-24-8), rapamycin (RAP; CAS no. 53123-88-9), leflunomide (LEF; CAS no. 75706-12-6), brequinar (BQR) sodium salt hydrate (Method Detection Limit [MDL] no. MFCD21363375), and mycophenolic acid (MPA; CAS no. 24280-93-1) were purchased from Sigma-Aldrich (St. Louis, MO). The calcineurin inhibitors (CNIs) cyclosporine (CsA; CAS no. 59865-13-3) and tacrolimus (FK506; CAS no. 104987-11-3) were obtained from Bio-Connect (Huizen, The Netherlands) and Abcam (Cambridge, MA), respectively. Two nonimmunosuppressive CsA derivatives, 431-32 and 440-02, together with a novel calcineurin inhibitor, voclosporin, were provided by the Isotechnika Pharma Incorporation (Edmonton, Alberta, Canada). All the compounds were dissolved in dimethyl sulfoxide (DMSO) and stored in aliquots at −20°C before use.
Primary antibodies targeting cyclophilin A (CyPA; polyclonal rabbit, 1:1,000 dilution; Abcam), cyclophilin B (CyPB; polyclonal rabbit, 1:1,000 dilution; Abcam), IMPDH1 (polyclonal rabbit, ab33039, 1:1,000 dilution; Abcam), IMPDH2 (monoclonal rabbit, ab131158, 1:1,000 dilution; Abcam), FKBP12 (polyclonal rabbit, sc-28814, 1:500 dilution; Santa Cruz), and β-actin (monoclonal mouse, 1:1,000 dilution, Santa Cruz) were used. Secondary antibodies IRDye 800CW-conjugated goat anti-rabbit and goat anti-mouse IgGs (1:10,000 dilution; Li-Cor Bioscience, Lincoln, NE, USA) were used, as appropriate.
MTT assay.
The cytotoxicities of the compounds on host cells were determined by a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. In brief, cells were seeded into 96-well plates containing 0.5% DMSO (control) or increasing concentrations of drugs. After the time indicated, 10 mM MTT (Sigma, Zwijndrecht, The Netherlands) was added. With another 3 h of incubation, the medium was removed and 100 μl of DMSO was added to each well. The plate was incubated at 37°C for 50 min. The absorbance at 490 nm was recorded on the microplate absorbance reader (Bio-Rad, CA, USA).
Cell cultures and virus propagation.
HG23 (Huh7 cells containing a stable subgenomic HuNV replicon), RAW 264.7, and human embryonic kidney 293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Lonza Verviers, Belgium) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS; HyClone, Logan, UT, USA). A marker cassette containing the neomycin phosphotransferase gene was inserted between nucleotides (nt) 5456 and 6753 of open reading frame 2 (ORF2) of HuNV, conferring HG23 resistance to neomycin. Gentamicin (G418; Gibco) was added to HG23 culture medium at 1.5 mg/ml for selection before experimentation.
Murine norovirus 1 (MNV-1) (43) was produced by consecutively inoculating MNV-1 (kindly provided by Herbert Virgin, IV, Department of Pathology and Immunology, Washington University School of Medicine) into RAW 264.7 cells. After 4 consecutive passages, the MNV-1 cultures were purified, aliquoted, and stored at −80°C for all subsequent experiments. The MNV-1 stock was quantified three independent times by the 50% tissue culture infective dose (TCID50).
TCID50.
MNV-1 was quantified by TCID50 assay. Briefly, 10-fold dilutions of MNV-1 were inoculated into RAW cells grown in 96-well tissue culture plate at 1,000 cells/well. The plate was incubated at 37°C for another 5 days, followed by observing the cytopathic effect (CPE) of each well under a light scope. The TCID50 was calculated by using the Reed-Muench method.
Quantitative real-time PCR.
Total RNA was isolated with a Macherey NucleoSpin RNA II kit (Bioke, Leiden, The Netherlands) and measured with a NanoDrop ND-1000 (Wilmington, DE, USA). cDNA was reverse transcribed from 500 ng of RNA using a cDNA synthesis kit (TaKaRa Bio, Inc.). The cDNA of a targeted gene transcript was amplified for 50 cycles and quantified with a SYBR Green-based real-time PCR (Applied Biosystems), according to the manufacturer's instructions. All the PCRs were performed in duplicate, and amplification specificity was confirmed by melting-curve analysis. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and murine GAPDH genes were used as reference genes. The relative expression of targeted gene was calculated as 2−ΔΔCT, where ΔΔCT = ΔCTsample − ΔCTcontrol (ΔCT = CT[targeted gene] − CT[GAPDH]). All primer combinations are listed in Table S1.
Antiviral assay with HuNV replicon model.
Before the experiments, HG23 cells were cultured overnight without G418. The next day, HG23 cells were seeded into 48-well tissue culture plates at 5 × 104 cells per well and treated with 0.5% DMSO (control) or drugs. After 48 h of treatment, total RNA was extracted from cells, and HuNV replication was determined by qRT-PCR analysis.
Antiviral assay with MNV-1.
The antiviral assay with MNV-1 was initiated by inoculating MNV-1 into RAW cells at a multiplicity of infection (MOI) of 0.1. After 1 h of infection, cells were washed with phosphate-buffered saline (PBS) for 4 times to remove free virus and were subsequently treated with 0.5% DMSO (control) or drugs. After 24 h of treatment, extracellular RNA and intracellular RNA were extracted from the cell culture supernatant (100 μl) and cell layer, respectively. The relative intracellular MNV-1 RNA level was normalized to murine GAPDH and calculated with the 2−ΔΔCT method. For absolute quantification of MNV-1 RNA in the supernatant, the virus genome copy number was detected using qRT-PCR. In brief, a cDNA-containing target sequence positioned between nt 4972 and 5064 in MNV-1 was amplified, purified, and 10 times serially diluted. The dilutions were quantified by qRT-PCR to generate a standard curve, which was expressed by plotting the log copy numbers against the cycle threshold (CT) value (Fig. S1). The viral genome copy numbers in the MNV-1 culture were calculated by comparing the CT with that of the standard curve.
MNV-1 could induce cytopathic effect (CPE) in RAW cells (43). The antiviral activity of drugs on MNV-1 was further verified by using an MTT-based CPE inhibition assay. Briefly, MNV-infected RAW cells were seeded into 96-well tissue culture plates at 10,000 cells/well. After 72 h of treatment with 0.5% DMSO or drugs, more than 95% CPE was observed in the control well. Then, an MTT assay was initiated, and the absorbance (optical density [OD]) at 490 nm was recorded. CPE inhibition was calculated as [(ODtreated)MNV − ODVC]/[ODCC − ODVC], where (ODtreated)MNV represented the OD of virus-infected cells treated with drugs, while ODCC and ODVC represented the OD of the cell control and virus control, respectively.
Clearance and rebound assay.
A clearance and rebound assay was performed according to the procedure described by Joana et al., with modifications (44). In brief, HG23 cells were seeded into 48-well tissue culture plates at 2.5 × 104 cells per well and treated with increasing concentration of drugs but no G418. After 2 days of treatment, the cells were trypsinized, 2.5 × 104 cells were subcultured into 48-well plates containing the same concentration of drugs for another 4 days of treatment and 2 × 104 cells were collected for qRT-PCR analysis. After 6 days of treatment, the same treatment process was performed for the second time. After 10 days of treatment, 2.5 × 104 cells were subcultured into a 48-well plate with fresh medium containing G418 (1.5 mg/ml). With another 5 days of culture, the cell layers were stained with hematoxylin and were visualized with an inverted light microscope. For HuNV RNA level quantification, each passage of drug-treated cells was compared to 0.5% DMSO-treated control cells with the same passage number.
In vitro selection of MPA-resistant MNV.
MPA-resistant MNV-1 was selected by culturing MNV-1 for 20 passages under antiviral pressure. Briefly, MNV-infected RAW (MOI, 0.1) cells were treated with 0.5% DMSO or MPA (0.1 or 0.5 μg/ml). After 24 h of incubation, the MNV-1 cultures were collected by freezing-thawing process and titrated by qRT-PCR for use in the next passage. After 10 passages, MNV-1 was continuously exposed to the same MPA concentrations for another 10 passages, and MNV-1 was exposed to increased MPA concentrations for another 10 passages. When MNV-1 serial passaging with MPA was done, the inhibitory effect of MPA on 20th-passage MNV-1 was quantified by qRT-PCR.
Short-hairpin RNA delivery by lentiviral transduction.
Gene knockdown was performed as described previously (45). In brief, pLKO.1-based vectors containing the RNA interference sequences were obtained from the Biomics Center at the Erasmus Medical Center. The lentiviral vectors were generated in HEK293T cells and condensed by high-speed centrifugation (if indicated). Transduction was initiated by inoculating short-hairpin RNA (shRNA) vectors into HG23 cells. As the vectors also express a puromycin resistance gene, following 3 days of transduction, cells were selected with 3 μg/ml puromycin (Sigma-Aldrich). A validated nonsilencing scrambled vector (shCTR) was used as a control. All the shRNA vector sequences are listed in Table S2. For simultaneous knockdown, HG23 cells were transduced with a first lentiviral vector. After selection with puromycin, HG23 cells were subsequently transduced with a second condensed lentiviral vector. The knockdown efficacy and specificity were confirmed by qRT-PCR and Western blot analysis.
Western blotting.
Cell samples were lysed and loaded onto a 10 to 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel. After electrophoresis at 120 V for 100 min, the proteins were electrotransferred to a polyvinylidene difluoride (PVDF) membrane (pore size, 0.45 μM; Invitrogen) for 1.5 h, with an electric current of 250 mA. The membrane was probed with primary antibody plus secondary antibody and detected with Odyssey 3.0 infrared imaging system (Li-Cor Biosciences). Beta-actin served as a standardization for sample loading.
Synergy analysis.
To evaluate the interaction of ribavirin and immunosuppressants on norovirus replication, MacSynergy 2 (kindly provided by Mark Prichard) (46), a mathematical model based on the Bliss Independence theory, was employed to analyze the data from 48 h of combined treatment of ribavirin and immunosuppressants on an HuNV model. In the MacSynergy model, the theoretical additive effect with two compounds could be calculated using the equation Z = X + Y (1 − X), where X and Y represent the inhibition produced by the individual drugs, and Z represents the theoretical effect produced by the combination of two drugs. The theoretical additive surface is subtracted from the actual experimental surface. When a combination is additive, the data points of the calculated surface lie in the zero plane. A surface that lies >20% above the zero plane indicates a synergistic effect of the combination, and a surface >20% below the zero plane indicates antagonism. The 95% confidence interval was considered to be statistically significant.
Statistics.
Data are presented as mean ± standard error of the mean (SEM). Comparisons between groups were performed with Mann-Whitney test using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). Differences were considered significant at a P value of <0.05. The 50% cytotoxic concentration (CC50) and 50% inhibitory concentration (IC50) were determined by fitting the data with a variable-slope dose response-inhibition nonlinear regression equation using GraphPad Prism (Table S3).
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Herbert W. Virgin (Washington University, St. Louis, MO, USA) for providing us the MNV-1 and Kyeong-Ok Chang (Kansas State University, USA) for providing the HuNV replicon. We also thank the general support of the Center for Drug Design (University of Minnesota, USA) for developing the IMPDH inhibitors.
We declare no conflicts of interest.
This work was supported by the Dutch Digestive Foundation (MLDS) for a career development grant (no. CDG 1304) and the China Scholarship Council for funding Ph.D. fellowships to W. Dang (grant 201406180072) and Y. Yin (grant 201307720045).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01095-17.
REFERENCES
- 1.McAtee CL, Webman R, Gilman RH, Mejia C, Bern C, Apaza S, Espetia S, Pajuelo M, Saito M, Challappa R, Soria R, Ribera JP, Lozano D, Torrico F. 2016. Burden of norovirus and rotavirus in children after rotavirus vaccine introduction, Cochabamba, Bolivia. Am J Trop Med Hyg 94:212–217. doi: 10.4269/ajtmh.15-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoa Tran TN, Trainor E, Nakagomi T, Cunliffe NA, Nakagomi O. 2013. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J Clin Virol 56:185–193. doi: 10.1016/j.jcv.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. 2008. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis 14:1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartsch SM, Lopman BA, Ozawa S, Hall AJ, Lee BY. 2016. Global economic burden of norovirus gastroenteritis. PLoS One 11:e0151219. doi: 10.1371/journal.pone.0151219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye X, Van JN, Munoz FM, Revell PA, Kozinetz CA, Krance RA, Atmar RL, Estes MK, Koo HL. 2015. Noroviruses as a cause of diarrhea in immunocompromised pediatric hematopoietic stem cell and solid organ transplant recipients. Am J Transplant 15:1874–1881. doi: 10.1111/ajt.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Beek J, van der Eijk AA, Fraaij PL, Caliskan K, Cransberg K, Dalinghaus M, Hoek RA, Metselaar HJ, Roodnat J, Vennema H, Koopmans MP. 2017. Chronic norovirus infection among solid organ recipients in a tertiary care hospital, the Netherlands, 2006–2014. Clin Microbiol Infect 23:265.e9–265.e13. doi: 10.1016/j.cmi.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Boillat Blanco N, Kuonen R, Bellini C, Manuel O, Estrade C, Mazza-Stalder J, Aubert JD, Sahli R, Meylan P. 2011. Chronic norovirus gastroenteritis in a double hematopoietic stem cell and lung transplant recipient. Transpl Infect Dis 13:213–215. doi: 10.1111/j.1399-3062.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 8.Coste JF, Vuiblet V, Moustapha B, Bouin A, Lavaud S, Toupance O, de Rougemont A, Benejat L, Megraud F, Wolak-Thierry A, Villena I, Chemla C, Le Magrex E, de Champs C, Andreoletti L, Rieu P, Leveque N. 2013. Microbiological diagnosis of severe diarrhea in kidney transplant recipients by use of multiplex PCR assays. J Clin Microbiol 51:1841–1849. doi: 10.1128/JCM.03366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florescu DF, Hermsen ED, Kwon JY, Gumeel D, Grant WJ, Mercer DF, Kalil AC. 2011. Is there a role for oral human immunoglobulin in the treatment for norovirus enteritis in immunocompromised patients? Pediatr Transplant 15:718–721. doi: 10.1111/j.1399-3046.2011.01556.x. [DOI] [PubMed] [Google Scholar]
- 10.Schorn R, Hohne M, Meerbach A, Bossart W, Wuthrich RP, Schreier E, Muller NJ, Fehr T. 2010. Chronic norovirus infection after kidney transplantation: molecular evidence for immune-driven viral evolution. Clin Infect Dis 51:307–314. doi: 10.1086/653939. [DOI] [PubMed] [Google Scholar]
- 11.Lee BE, Pang XL, Robinson JL, Bigam D, Monroe SS, Preiksaitis JK. 2008. Chronic norovirus and adenovirus infection in a solid organ transplant recipient. Pediatr Infect Dis J 27:360–362. doi: 10.1097/INF.0b013e31815f5b5a. [DOI] [PubMed] [Google Scholar]
- 12.Engelen MA, Gunia S, Stypmann J. 2011. Elimination of norovirus in a chronic carrier under immunosuppression after heart transplantation–effect of everolimus. Transpl Int 24:e102–103. doi: 10.1111/j.1432-2277.2011.01330.x. [DOI] [PubMed] [Google Scholar]
- 13.Ebdrup L, Bottiger B, Molgaard H, Laursen AL. 2011. Devastating diarrhoea in a heart-transplanted patient. J Clin Virol 50:263–265. doi: 10.1016/j.jcv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Chagla Z, Quirt J, Woodward K, Neary J, Rutherford C. 2013. Chronic norovirus infection in a transplant patient successfully treated with enterally administered immune globulin. J Clin Virol 58:306–308. doi: 10.1016/j.jcv.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Westhoff TH, Vergoulidou M, Loddenkemper C, Schwartz S, Hofmann J, Schneider T, Zidek W, van der Giet M. 2009. Chronic norovirus infection in renal transplant recipients. Nephrol Dial Transplant 24:1051–1053. doi: 10.1093/ndt/gfn693. [DOI] [PubMed] [Google Scholar]
- 16.Roos-Weil D, Ambert-Balay K, Lanternier F, Mamzer-Bruneel MF, Nochy D, Pothier P, Avettand-Fenoel V, Anglicheau D, Snanoudj R, Bererhi L, Thervet E, Lecuit M, Legendre C, Lortholary O, Zuber J. 2011. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation 92:61–69. doi: 10.1097/TP.0b013e31821c9392. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman SS, Chatterjee NK, Fuschino ME, Magid MS, Gordon RE, Morse DL, Herold BC, LeLeiko NS, Tschernia A, Florman SS, Gondolesi GE, Fishbein TM. 2003. Calicivirus enteritis in an intestinal transplant recipient. Am J Transplant 3:764–768. doi: 10.1034/j.1600-6143.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman SS, Chatterjee NK, Fuschino ME, Morse DL, Morotti RA, Magid MS, Gondolesi GE, Florman SS, Fishbein TM. 2005. Characteristics of human calicivirus enteritis in intestinal transplant recipients. J Pediatr Gastroenterol Nutr 40:328–333. doi: 10.1097/01.MPG.0000155182.54001.48. [DOI] [PubMed] [Google Scholar]
- 19.Morotti RA, Kaufman SS, Fishbein TM, Chatterjee NK, Fuschino ME, Morse DL, Magid MS. 2004. Calicivirus infection in pediatric small intestine transplant recipients: pathological considerations. Hum Pathol 35:1236–1240. doi: 10.1016/j.humpath.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Florescu DF, Hill LA, McCartan MA, Grant W. 2008. Two cases of Norwalk virus enteritis following small bowel transplantation treated with oral human serum immunoglobulin. Pediatr Transplant 12:372–375. doi: 10.1111/j.1399-3046.2007.00875.x. [DOI] [PubMed] [Google Scholar]
- 21.Robles JD, Cheuk DK, Ha SY, Chiang AK, Chan GC. 2012. Norovirus infection in pediatric hematopoietic stem cell transplantation recipients: incidence, risk factors, and outcome. Biol Blood Marrow Transplant 18:1883–1889. doi: 10.1016/j.bbmt.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Saif MA, Bonney DK, Bigger B, Forsythe L, Williams N, Page J, Babiker ZO, Guiver M, Turner AJ, Hughes S, Wynn RF. 2011. Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: a cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr Transplant 15:505–509. doi: 10.1111/j.1399-3046.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- 23.Lemes LG, Correa TS, Fiaccadori FS, Cardoso D, Arantes Ade M, Souza KM, Souza M. 2014. Prospective study on norovirus infection among allogeneic stem cell transplant recipients: prolonged viral excretion and viral RNA in the blood. J Clin Virol 61:329–333. doi: 10.1016/j.jcv.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Roddie C, Paul JP, Benjamin R, Gallimore CI, Xerry J, Gray JJ, Peggs KS, Morris EC, Thomson KJ, Ward KN. 2009. Allogeneic hematopoietic stem cell transplantation and norovirus gastroenteritis: a previously unrecognized cause of morbidity. Clin Infect Dis 49:1061–1068. doi: 10.1086/605557. [DOI] [PubMed] [Google Scholar]
- 25.Echenique IA, Stosor V, Gallon L, Kaufman D, Qi C, Zembower TR. 2016. Prolonged norovirus infection after pancreas transplantation: a case report and review of chronic norovirus. Transpl Infect Dis 18:98–104. doi: 10.1111/tid.12472. [DOI] [PubMed] [Google Scholar]
- 26.Karst SM, Baric RS. 2015. What is the reservoir of emergent human norovirus strains? J Virol 89:5756–5759. doi: 10.1128/JVI.03063-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atmar J, Mullen E. 2013. Norovirus in immunocompromised patients. Oncol Nurs Forum 40:434–436. doi: 10.1188/13.ONF.434-436. [DOI] [PubMed] [Google Scholar]
- 28.Pan Q, Tilanus HW, Metselaar HJ, Janssen HL, van der Laan LJ. 2012. Virus-drug interactions–molecular insight into immunosuppression and HCV. Nat Rev Gastroenterol Hepatol 9:355–362. doi: 10.1038/nrgastro.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodward JM, Gkrania-Klotsas E, Cordero-Ng AY, Aravinthan A, Bandoh BN, Liu H, Davies S, Zhang H, Stevenson P, Curran MD, Kumararatne D. 2015. The role of chronic norovirus infection in the enteropathy associated with common variable immunodeficiency. Am J Gastroenterol 110:320–327. doi: 10.1038/ajg.2014.432. [DOI] [PubMed] [Google Scholar]
- 30.Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. 2006. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology 353:463–473. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Stalder M, Birsan T, Hubble RW, Paniagua RT, Morris RE. 2003. In vivo evaluation of the novel calcineurin inhibitor ISATX247 in non-human primates. J Heart Lung Transplant 22:1343–1352. doi: 10.1016/S1053-2498(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 32.Patel CG, Akhlaghi F. 2006. High-performance liquid chromatography method for the determination of mycophenolic acid and its acyl and phenol glucuronide metabolites in human plasma. Ther Drug Monit 28:116–122. doi: 10.1097/01.ftd.0000177664.96726.56. [DOI] [PubMed] [Google Scholar]
- 33.Carr SF, Papp E, Wu JC, Natsumeda Y. 1993. Characterization of human type I and type II IMP dehydrogenases. J Biol Chem 268:27286–27290. [PubMed] [Google Scholar]
- 34.Brennan DC, Aguado JM, Potena L, Jardine AG, Legendre C, Saemann MD, Mueller NJ, Merville P, Emery V, Nashan B. 2013. Effect of maintenance immunosuppressive drugs on virus pathobiology: evidence and potential mechanisms. Rev Med Virol 23:97–125. doi: 10.1002/rmv.1733. [DOI] [PubMed] [Google Scholar]
- 35.Gallay PA. 2012. Cyclophilin inhibitors: a novel class of promising host-targeting anti-HCV agents. Immunol Res 52:200–210. doi: 10.1007/s12026-011-8263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusnak F, Mertz P. 2000. Calcineurin: form and function. Physiol Rev 80:1483–1521. [DOI] [PubMed] [Google Scholar]
- 37.Li YJ, Wu HH, Weng CH, Chen YC, Hung CC, Yang CW, Wang RY, Sakamoto N, Tian YC. 2012. Cyclophilin A and nuclear factor of activated T cells are essential in cyclosporine-mediated suppression of polyomavirus BK replication. Am J Transplant 12:2348–2362. doi: 10.1111/j.1600-6143.2012.04116.x. [DOI] [PubMed] [Google Scholar]
- 38.Shipkova M, Armstrong VW, Oellerich M, Wieland E. 2005. Mycophenolate mofetil in organ transplantation: focus on metabolism, safety and tolerability. Expert Opin Drug Metab Toxicol 1:505–526. doi: 10.1517/17425255.1.3.505. [DOI] [PubMed] [Google Scholar]
- 39.Chang KO, George DW. 2007. Interferons and ribavirin effectively inhibit Norwalk virus replication in replicon-bearing cells. J Virol 81:12111–12118. doi: 10.1128/JVI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arias A, Thorne L, Goodfellow I. 2014. Favipiravir elicits antiviral mutagenesis during virus replication in vivo. Elife 3:e03679. doi: 10.7554/eLife.03679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Julian TR, Baugher JD, Rippinger CM, Pinekenstein R, Kolawole AO, Mehoke TS, Wobus CE, Feldman AB, Pineda FJ, Schwab KJ. 2016. Murine norovirus (MNV-1) exposure in vitro to the purine nucleoside analog ribavirin increases quasispecies diversity. Virus Res 211:165–173. doi: 10.1016/j.virusres.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Nicot F, Legrand-Abravanel F, Lafont T, Dubois M, Saune K, Pasquier C, Chatelut E, Izopet J. 2008. Serum concentrations of ribavirin and pegylated interferon and viral responses in patients infected with HIV and HCV. J Med Virol 80:1523–1529. doi: 10.1002/jmv.21227. [DOI] [PubMed] [Google Scholar]
- 43.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW IV. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol 2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rocha-Pereira J, Jochmans D, Debing Y, Verbeken E, Nascimento MS, Neyts J. 2013. The viral polymerase inhibitor 2′-C-methylcytidine inhibits Norwalk virus replication and protects against norovirus-induced diarrhea and mortality in a mouse model. J Virol 87:11798–11805. doi: 10.1128/JVI.02064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan Q, de Ruiter PE, von Eije KJ, Smits R, Kwekkeboom J, Tilanus HW, Berkhout B, Janssen HL, van der Laan LJ. 2011. Disturbance of the microRNA pathway by commonly used lentiviral shRNA libraries limits the application for screening host factors involved in hepatitis C virus infection. FEBS Lett 585:1025–1030. doi: 10.1016/j.febslet.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 46.Prichard MN, Shipman C Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antiviral Res 14:181–205. doi: 10.1016/0166-3542(90)90001-N. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.