Abstract
Background
The impact of ruptured hepatocellular carcinoma (HCC) on a patients outcome after hepatic resection remains insufficient. We aimed to identify the independent predictive factors of spontaneous tumor rupture (STR) for curative resection of HCC and to investigate the impact of STR of HCC on long-term survival after resection.
Patients and methods
The clinicopathological parameters of 106 patients with ruptured HCC and 201 patients with non-ruptured HCC who underwent hepatic resection from 2007 to 2011 were investigated. Clinical features and factors associated with the clinical outcomes were compared between both groups.
Results
Of 774 HCC patients who underwent surgical resection, 106 (13.7%) had tumor rupture. Multivariate stepwise logistic regression analysis revealed hypertension, liver cirrhosis, total bilirubin (TB), tumor size and ascites to be independent prognostic factors for patients with ruptured HCC. The overall survival (OS) of patients in the ruptured HCC group was significantly poorer compared with those in the non-ruptured HCC group. The 1-, 3- and 5-year OS rates were 77.7%, 56.9% and 41.6%, respectively, in the non-ruptured HCC group and 37.7%, 19.7%, 14.%, respectively, in the ruptured HCC group (P<0.001). Similar OS rates were found in patients with non-ruptured and ruptured HCC; patients in the non-ruptured HCC group had a significantly better recurrence-free survival (RFS) rate compared with those in the ruptured group (P=0.016).
Conclusion
The presence of hypertension, liver cirrhosis, higher TB levels, tumor size >5 cm and ascites are the independent indicators of poorer prognosis for patients undergoing hepatic resection after ruptured HCC. The present study confirmed that tumor rupture itself had a negative impact on patient survival, but hepatic resection, when technically feasible, is safe and appropriate in selected patients and can result in OS and RFS rates comparable to that of patients with non-ruptured HCC.
Keywords: hepatocellular carcinoma, spontaneous rupture, hepatectomy, overall survival, recurrence-free survival
Background
Hepatocellular carcinoma (HCC) is one of the leading etiologies of global cancer-related mortalities, which is especially prevalent in China.1–4 Spontaneous tumor rupture (STR), which is an uncommon but potentially fatal complication of HCC, accompanied by intraperitoneal hemorrhage is a potentially fatal condition, with reported incidence ranging between 10% and 15%.5–8
When confronted with this condition, hemostasis should be urgently attempted as the initial treatment, followed by hepatic resection as secondary. Partial hepatectomy is a well-validated treatment strategy for HCC, which has become more feasible with satisfactory safety and efficacy profiles due to more advanced surgical technique and perioperative care. Partial hepatectomy is an effective treatment for spontaneously ruptured HCC, through which long-term survival can be achieved in selected patients.9,10 It should be performed when technically feasible as either an emergency or a staged operation (following embolization or other hemostatic procedures). However, the short- and long-term outcomes of patients undergoing partial hepatectomy due to spontaneous rupture of HCC warrant further validations.3 The present study aimed to investigate the clinicopathological characteristics of ruptured HCC and to demonstrate the impact of tumor rupture and other prognostic factors on the prognosis of this condition in a large patient cohort.
Patients and methods
This is a retrospective study of consecutive patients who underwent partial hepatectomy for HCC between April 2007 and November 2011 at the Department of Hepatic Surgery, Eastern Hepatobiliary Surgery Hospital, Shanghai, China. Data were prospectively collected in a computer database. Data analysis was done retrospectively. Additional data were obtained by reviewing medical records. The exclusion criteria were 1) incomplete clinical data; 2) presence of severe comorbidities that could affect life expectancy, such as with a history of severe cardiac disorders; 3) preoperative portal vein embolization; 4) surgical portosystemic shunts before or at the same time as hepatic resection, were treated as an emergency and 5) a palliative resection or had presented at pathologic examination. According to whether the HCC had ruptured or not, all participants were divided into two groups. Informed consent was obtained from all participants. The research protocol of this study was discussed and approved by the Clinical Research Ethics Committee of Eastern Hepatobiliary Surgery Hospital.
Preoperative care, surgical procedures and follow-up
Preoperative checkups, including electrocardiography, chest X-ray, complete blood counts, liver and renal function tests, serum α-fetoprotein (AFP) level, serological markers for hepatitis B and coagulation profile, were done for every patient. Tumor location and extent were assessed by computed tomography (CT) and/or magnetic resonance imaging (MRI), and resectability was determined accordingly. A previously described criterion was used for resection and remained identical over the study period.11 Child–Pugh grade C liver function was considered as an absolute contraindication to partial hepatectomy.12
All operations were performed by experienced surgeons. Pringle manoeuver was routinely used with cycles of clamp/unclamp for 15/5 minutes. Transection of the hepatic parenchyma was done using the clamp-crushing technique, and hemostasis was done with suture ligations and argon beam coagulators. Anatomical resection was preferred when possible, while nonanatomical resection was reserved for tumors that were peripherally located or situated at the junction of several liver segments, as well as for patients with serious cirrhosis. Major hepatectomy was defined as resection of three or more Couinaud liver segments, while minor hepatectomy was defined as resection of fewer than three segments. The presence of cirrhosis was confirmed by histopathological examination.
When all microscopic and macroscopic tumors were resected, it was defined as R0 resection. A complication was defined as the occurrence of postoperative pulmonary, renal, cardiac or liver failure; biliary complications; sepsis of any etiology and wound complications. Ascites and pleural effusion that required diuretics or paracentesis were defined as morbidities. Postoperative liver failure was defined by a postoperative serum total bilirubin (TB) level of >60 µmol/L, prothrombin time >18 s and/or the development of postoperative hepatic encephalopathy. Postoperative conditions and complications were assessed daily from the day of surgery until discharge.
Patients were followed-up every 2–3 months during the first year after surgery and 3–6 months thereafter until November 30, 2016. All patients were followed up with AFP measurement, CT or MRI and chest X-ray at 4 weeks after operation for 6 months at a 2-month interval and every 3 months thereafter. Patients underwent positron emission tomography, digital subtraction angiography or bone scan when necessary. All follow-up examinations were done by two physicians blinded of patient information and study data. Diagnostic criteria for recurrences were the same as for preoperative diagnosis of HCC. Once recurrence occurred, treatment plan was made based on the pattern of recurrence, hepatic functional reserve and the patient’s general condition. A multidisciplinary approach, including re-resection, local ablative therapy, transcatheter arterial chemoembolization (TACE), external irradiation, systemic chemotherapy/immunotherapy or sorafenib (since 2008), was adopted for patients with recurrence.
Special procedures for ruptured HCC
The diagnosis of HCC rupture was based on symptoms and signs upon admission, as well as bedside ultrasonography. For hemodynamically unstable patients, active resuscitation with intravascular fluid was initiated with correction of coagulopathy and patients were monitored closely in the ward or in the intensive care unit. In the majority of patients, bleeding would stop spontaneously with conservative treatment and watchful waiting. When the bleeding exacerbated, emergency transcatheter arterial embolization (TAE) was done. Some of the patients in this study had already undergone TAE before transferring to the authors’ hospital for further treatment.
When bleeding could not be controlled by nonsurgical approaches, emergency laparotomy was indicated. For patients with resectable tumor, adequate liver functional reserves and satisfactory general condition, partial hepatectomy was conducted. Surgical hemostatic procedures, such as packing or hepatic artery ligation, suturing, plication and alcohol injection, were used to stop active bleeding at the site of rupture.
Pringle manoeuver and occlusion of the hepatic artery proper were used in emergency partial hepatectomy.13 Tumor was reassessed for resectability after clot removal in the peritoneal cavity. Ideally, a resection margin of >1 cm was planned. After hepatectomy, peritoneal lavage with distilled water (DWPL; 5000–10000 mL) was done, and 5-fluorouracil (5-FU; 500 mg) was left in the abdominal cavity before closure.14
Staged hepatectomy would be planned 2–6 weeks after the episode of spontaneous rupture for patients with resectable HCC whose bleeding had stopped either spontaneously or with laparotomy.
Statistical analysis
All data are presented as mean values with standard deviation (SD) or percentages. Nominal data were compared with the Pearson c2 test; multiple forward stepwise logistic regressions were used when appropriate. The distribution of continuous data was tested using the Kolmogorov–Smirnov test; values are shown as mean (SD) if distributed normally and as median (range) otherwise. These data were analyzed by mean values of the independent samples t-test and Wilcoxon rank-sum test (Mann–Whitney U-test). Survival was calculated and compared with the log-rank test and presented with the Kaplan–Meier approach. Hospital mortality was defined as death during the hospital stay or within 60 days of surgery. Overall survival (OS) was defined as the length of time between surgery and death or the last follow-up examination. Recurrence-free survival (RFS) was calculated from the date of tumor resection until tumor recurrence or last observation. To identify factors predictive of survival, univariable and multivariable analyses were performed using the log-rank test and Cox proportional hazards model, respectively. All statistical analyses were conducted using SPSS software version 19.0 (IBM Corporation, Armonk, NY, USA). P<0.05 was considered as statistically significant.
Results
Characteristics of ruptured HCC
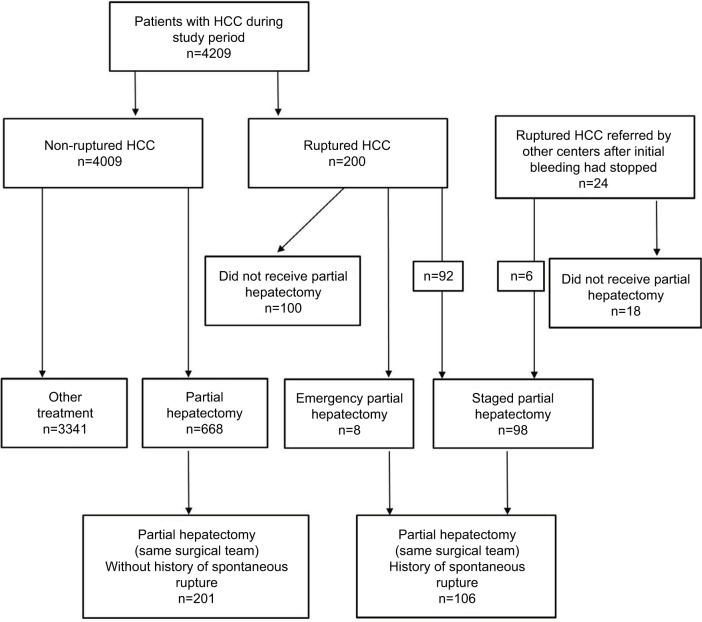
During the study period, a total of 4209 patients with HCC were admitted in the authors’ unit, of which 200 (4.8%) had tumor rupture. Of the 774 patients who underwent elective partial hepatectomy, 106 (13.7%) had STR before surgery (Figure 1). Patients included in the study were divided into ruptured (n=106) and non-ruptured (n=201) groups. Both groups underwent hepatectomy from the same team of surgeons. In all, 94 patients with ruptured HCC did not receive hepatectomy due to various reasons: preoperative decision of unresectable HCC (51, 54.3%), intraoperative decision as not suitable for hepatectomy (4, 4.3%), inadequate liver functional reserve (17, 18.1%), poor general condition (10, 10.6%), patient refusal (3, 3.2%) or for undefined reasons (9, 9.5%).
Figure 1.
Study flow chart.
Abbreviation: HCC, hepatocellular carcinoma.
A total of 99 men and seven women with a mean age of 47.9 years (range 22–75 years) were in the ruptured HCC group. No significant difference was found in age distributions and sex ratios between the ruptured and non-ruptured groups (Table 1). Sudden-onset abdominal pain (71 patients, 67%) and shock (54 patients, 51%) were more common in the ruptured HCC group. However, patients in the ruptured group presented with significantly more symptoms and higher prevalence of cirrhosis than those in the non-ruptured group. Meanwhile, patients in the ruptured group were more likely to have the presence of arterial hypertension (hypertension), ascites, higher Child–Pugh grade, a lower serum albumin level, a higher TB level, a higher serum AFP level, seropositivity for hepatitis B and worse tumor characteristics (including size,).
Table 1.
Demographic and laboratory data for patients with ruptured and non-ruptured HCC undergoing hepatectomy
| Variables | Ruptured HCC (n=106) | Non-ruptured HCC (n=201) | P-value |
|---|---|---|---|
| Age (years) | 47.9 (22–75) | 50.5 (21–78) | 0.066 |
| Sex (M:F) | 99/7 | 179/22 | 0.216 |
| Diabetes | 3/103 | 5/196 | 0.858 |
| Hypertension | 10/96 | 6/195 | 0.016* |
| Liver cirrhosis | 89/17 | 113/88 | <0.001* |
| Child–Pugh (A:B) | 96/10 | 199/2 | <0.001* |
| PT (s) | 13.0 (10.4–17.8) | 13.3 (9.8–23.5) | 0.165 |
| Hemoglobin (g/L) | 126 (60–141) | 140 (90–155) | <0.001* |
| TB (µmol/L) | 21.8 (6.3–130) | 16.1 (5.2–48.3) | <0.001* |
| Albumin (g/L) | 39.7 (23.2–49.4) | 40.8 (24.7–55.5) | 0.029* |
| ALT (IU/L) | 60.3 (9.1–559.8) | 59.4 (9.6–411.4) | 0.892 |
| AST (IU/L) | 67.9 (12.4–785.1) | 59.9 (17.3–353.5) | 0.280 |
| Creatinine (µmol/L) | 71.9 (39–103) | 74.4 (35–242) | 0.223 |
| AFP (µg/L) | 643.3 (1.5–1000) | 771.7 (2–63159) | 0.768 |
| AFP >100 µg/L | 75/31 | 113/88 | 0.013* |
| Positive HBsAg | 98/8 | 164/37 | 0.013* |
| Tumor size (cm) | 8.6 (1.8–17.5) | 7.1 (1–22) | 0.002* |
| Tumor size >5 cm | 89/17 | 116/85 | <0.001* |
| Tumor location (R/L/both) | 72/24/10 | 145/38/18 | 0.715 |
| Protrusion | 55/51 | 90/111 | 0.235 |
| Vascular thrombus | 61/45 | 121/80 | 0.653 |
| Capsule formation | 64/42 | 130/71 | 0.459 |
| Satellite lesions | 23/83 | 35/166 | 0.362 |
| Ascites | 51/55 | 16/185 | <0.001* |
Notes: Data presented as ratio or mean value (range minimum to maximum).
P<0.05.
Abbreviations: AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; F, female; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; L, left; M, male; PT, prothrombin time; R, right; TB, total bilirubin.
Perioperative outcomes
Perioperative parameters, including macroscopic and microscopic findings, are summarized in Table 2. Tumor size in the ruptured HCC group was significantly greater than that in the non-ruptured group (P=0.002). Characteristics of the tumors, such as capsule formation, vascular invasion, satellite lesions, recurrence rate and surgical margins, were similar between the two groups. Patients with ruptured HCC were more likely to present with more intraoperative blood loss (P=0.006) and intraoperative blood transfusion (P<0.001) but without a longer duration of operation (P=0.885) compared to those in the non-ruptured group. In addition, there was a lower R0 resection rate in the ruptured group (83.9% vs 87.6%). Patients with ruptured HCC were more likely to have a nonanatomical partial hepatectomy. No significant difference in morbidity and mortality was found between both groups (Table 2). One hospital death (due to hepatic failure) occurred in the ruptured group.
Table 2.
Operative detail of patients with ruptured and non-ruptured HCC undergoing hepatectomy
| Detail | Ruptured HCC (n=106) | Non-ruptured HCC (n=201) | P-value |
|---|---|---|---|
| Extent of hepatectomy | 0.169 | ||
| Minor | 44 | 100 | |
| Major | 62 | 101 | |
| Type of hepatectomy | 0.022* | ||
| Anatomical | 56 | 133 | |
| Nonanatomical | 50 | 68 | |
| Operating time (min) | 166.8±70.2 | 165.6±69.0 | 0.885 |
| Duration of clamping (min) | 17.1±8.5 | 16.0±7.3 | 0.233 |
| Blood loss (mL) | 819.8±987.0 | 521.3±779.5 | 0.006* |
| Blood transfusion (mL) | 876.1±1237.8 | 371.4±1048.5 | <0.001* |
| Margin >1 cm | 7/99 | 26/175 | 0.089 |
| Surgical margins | 0.655 | ||
| R0 resection | 89 | 176 | |
| R1 resection | 16 | 24 | |
| R2 resection | 1 | 1 | |
| Hospital death | 1 | 1 | 0.644 |
| Major complications | |||
| Postoperative bleeding | 0 | 1 | 0.467 |
| Liver failure | 1 | 2 | 0.239 |
| Bile leak | 1 | 3 | 0.687 |
| Pleural effusion | 51 | 106 | 0.441 |
| Postoperative hospital stay | 20.2±7.3 | 18.6±7.4 | 0.083 |
| Recurrence | 57/49 | 124/77 | 0.183 |
Notes: Data presented as number or mean ± SD.
P<0.05.
Factors associated with spontaneous rupture of HCC
Univariate analysis indicated that sudden-abdominal pain, underlying diseases of hypertension, liver cirrhosis, Child–Pugh grade, hemoglobin, TB, serum albumin level, AFP level, HBsAg, tumor size and ascites were significantly associated with STR (Table 1). That is, STR was more frequently observed in patients with sudden-abdominal pain, hypertension and cirrhosis, positive HBsAg, larger tumor size and a poorer liver functional reserve. However, STR was also more frequently observed in patients with the presence of ascites, a lower serum albumin level, a higher TB and AFP level.
Parameters associated with spontaneous rupture of HCC (multivariate analyses)
Multivariate analysis with logistic regression revealed the following parameters as independent prognostic factors for STR: maximum tumor diameter (hazard ratio [HR]: 0.187 [>5 cm vs ≤5 cm]), the presence of hypertension (HR: 0.036), liver cirrhosis (HR: 0.195), TB (HR: 0.373) and the presence of ascites (HR: 0.127; Table 3). Cox proportional hazards analyses of OS and RFS in 307 patients with HCC are shown in Tables 4 and 5.
Table 3.
Multiple forward stepwise logistic regression analysis of clinicopathological features
| Variables | SE | Wald | HR | 95% CI for HR | P-value |
|---|---|---|---|---|---|
| Hypertension (presence vs absence) | 0.817 | 16.451 | 0.036 | 0.01–0.18 | <0.001* |
| Liver cirrhosis (presence vs absence) | 0.384 | 18.142 | 0.195 | 0.09–0.41 | <0.001* |
| Tumor size (≤5 vs >5 cm) | 0.380 | 19.516 | 0.187 | 0.09–0.39 | <0.001* |
| TB (≤18.8 vs >18.8 µmol/L) | 0.314 | 9.866 | 0.373 | 0.20–0.69 | 0.002* |
| Ascites (presence vs absence) | 0.393 | 27.499 | 0.127 | 0.06–0.28 | <0.001* |
Note:
P<0.05.
Abbreviations: CI, confidence interval; HR, hazard ratio; SE, standard error; TB, total bilirubin.
Table 4.
Cox proportional hazards analyses of OS in 307 patients with HCC
| Variables | SE | Wald | Exp(B) | 95% CI | P-value |
|---|---|---|---|---|---|
| Maximum tumor size (≥5 vs <5 cm) | 0.183 | 9.047 | 1.736 | 1.212–2.486 | 0.003 |
| AFP (≥400 vs <400 µg/L) | 0.153 | 9.251 | 1.591 | 1.179–2.145 | 0.002 |
| Tumor type (unifocal vs multiple/diffuse) | 0.169 | 9.704 | 1.692 | 1.215–2.355 | 0.002 |
| Microscopic vascular invasion (yes vs no) | 0.208 | 16.134 | 2.302 | 1.533–3.459 | <0.001 |
| Child–Pugh grade (A vs B) | 0.417 | 4.068 | 0.431 | 0.190–0.977 | 0.044 |
| STR (yes vs no) | 0.181 | 12.328 | 1.889 | 1.324–2.694 | <0.001 |
Abbreviations: AFP, α-fetoprotein; CI, confidence interval; HCC, hepatocellular carcinoma; OS, overall survival; SE, standard error; STR, spontaneous tumor rupture.
Table 5.
Cox proportional hazards analyses of RFS in 307 patients with HCC
| Variables | SE | Wald | Exp(B) | 95% CI | P-value |
|---|---|---|---|---|---|
| Maximum tumor size (≥5 vs <5 cm) | 0.198 | 12.022 | 1.985 | 1.347–2.926 | 0.001 |
| AFP (≥400 vs <400 µg/L) | 0.167 | 4.927 | 1.448 | 1.044–2.009 | 0.026 |
| Microscopic vascular invasion (yes vs no) | 0.225 | 9.622 | 2.009 | 1.293–3.121 | 0.002 |
| STR (yes vs no) | 0.203 | 0.077 | 0.945 | 0.635–1.407 | 0.782 |
Abbreviations: AFP, α-fetoprotein; CI, confidence interval; HCC, hepatocellular carcinoma; RFS, recurrence-free survival; SE, standard error; STR, spontaneous tumor rupture.
Long-term survival outcomes
The duration of follow-up ranged from 1 to 104 months (median 35.9 months). Among the 307 surviving patients, 106 patients were in the ruptured group and 201 patients were in the non-ruptured group. The 1-, 3- and 5-year OS rates for all 307 patients were 54.0%, 37.3% and 33.8%, respectively, with a median OS of 17 months (95% confidence interval [CI]: 12.0–25.0). The 1-, 3- and 5-year OS rates for the 307 survivors who had an R0 resection were 88.8%, 64.6% and 53.7%, respectively, with a median OS of 41 months. The Kaplan–Meier survival curves of RFS and overall actuarial survival of both groups of patients are shown in Figure 2. The median disease-free survival was 10 months (95% CI: 5.0–16.0) in the ruptured HCC group and 23 months (95% CI: 14.0–32.0) in the non-ruptured HCC group.
Figure 2.
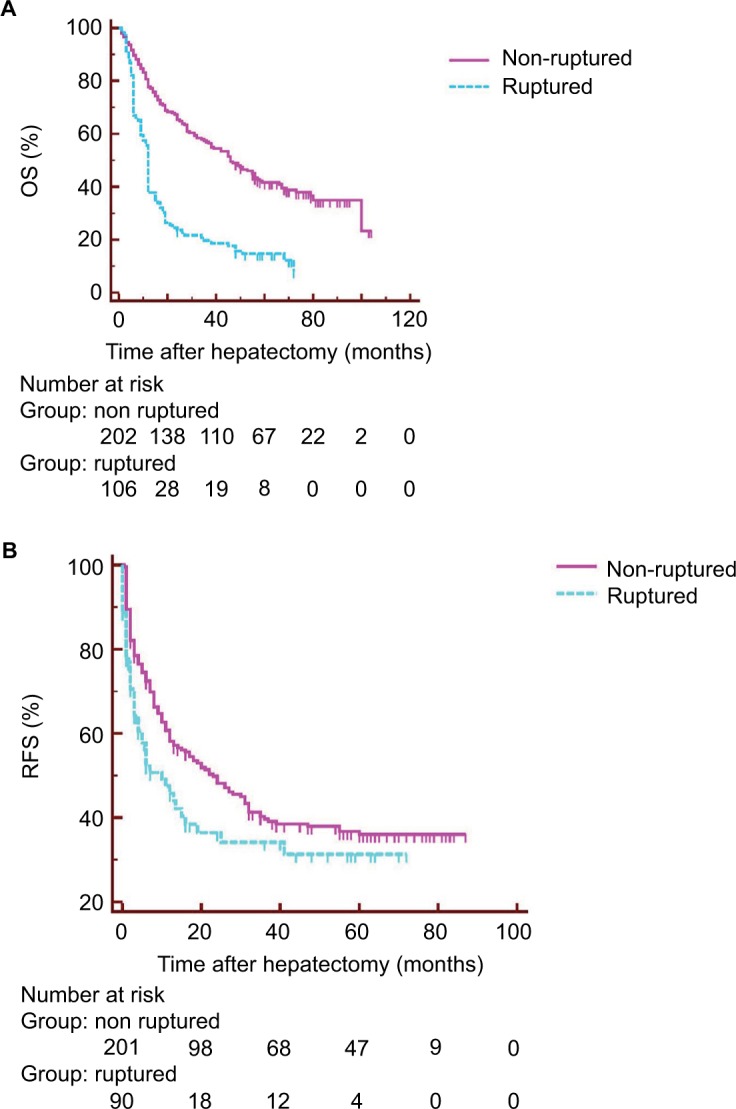
(A) OS and (B) RFS after hepatectomy for ruptured and non-ruptured HCC.
Note: (A and B) P<0.001 (log-rank test).
Abbreviations: HCC, hepatocellular carcinoma; OS, overall survival; RFS, recurrence-free survival.
After partial hepatectomy, 92 (86.8%) of 106 patients in the ruptured group and 124 (61.7%) of 201 patients in the non-ruptured group died during follow-up. Peritoneal dissemination developed in 20 (7.5%) of 265 patients after R0 resection and occurred more often in the ruptured group: 29 (32.6%) of 89 patients versus nine (5.1%) of 176 patients (P<0.001).
Impact of STR on survival
The ruptured HCC group had significantly lower OS rates (37.7%, 19.7% and 14.7% at 1, 3 and 5 years, respectively) than the non-ruptured HCC group (77.7%, 56.9% and 41.6%; P<0.001; Figure 2A). There was also a significant difference in RFS between the ruptured (45.8%, 34.2% and 31.3% at 1, 3 and 5 years, respectively) and non-ruptured (58.2%, 39.7% and 36.0%) groups (P=0.016; Figure 2B).
Both univariable and multivariable analyses showed that STR was independently associated with poor OS (HR 1.89, 95% CI: 1.32–2.69). Furthermore, although univariable analyses showed that RFS was lower in the ruptured group than in the non-ruptured group (P=0.016), STR could not independently predict poor RFS after partial hepatectomy for HCC in multivariable analyses (HR 0.95, 95% CI: 0.64–1.41, P=0.782; Tables S1 and S2).
Discussion
The incidence of spontaneous rupture of HCC shows an obvious geographical difference in previously reported studies, and ~10%–15% of patients with HCC develop this complication.7 In the present study, 106 (13.7%) of the 774 patients underwent hepatic resection due to HCC rupture.
In this study, we found a similar baseline characteristic between groups with and without rupture in physical findings, biochemical data, hepatitis status and extent of cirrhosis. Patients with ruptured HCC were more likely to have clinical manifestations such as sudden attack of severe abdominal pain, signs of bleeding during physical examination, a lower hemoglobin level, a larger tumor size, higher TB levels and greater intraoperative blood loss during hepatic resection.
Sudden abdominal pain in hemodynamic unstable HCC patients should indicate the diagnosis of ruptured HCC. Nevertheless, multiple logistic regression analysis did not confirm sudden-onset abdominal pain to be a prognostic factor in patients with ruptured HCC, which was not consistent with the study by Yeh et al.10 A possible explanation may be that the patients enrolled in the present series were all treated in a single surgical team. Moreover, we found that STR was more frequent among patients with a larger tumor size and a poorer liver functional reserve (a higher serum TB level and presence of ascites), which is consistent with previous reports.6,15
Interestingly, we also found the presence of hypertension and liver cirrhosis to be predictive factors for spontaneous rupture of HCC. It may be attributed to the fact that hypertension can directly result in an increase in pressure within the tumor; the rupture of a vascular tumor like HCC may thus lead to the tearing of vessels with uncontrollable blood loss. The condition may be further exacerbated in cirrhotic patients with underlying coagulopathy. Both of the two factors may promote the process of rupture described earlier.16
Treatments for ruptured HCC include control of hemorrhage and liver resection when possible. The feasibility of hepatectomy depends largely on the extent of the tumor (e.g., caval involvement). Hepatic resection is technically challenging in cirrhotic livers. Lai et al17 reported that 60.7% of patients with ruptured HCC had macroscopic cirrhosis, and only 12.5% of patients were managed by hepatectomy. Dewar et al18 reported that 36 of 37 patients with ruptured HCC had underlying cirrhosis of the liver, 11 of whom underwent hepatectomy. In our study, 89 of the 106 patients with ruptured HCC had underlying cirrhosis, and over half of the patients (60.4%, 64/106) underwent resection. With recent improvements in hepatic surgery, hepatectomy for ruptured HCC is feasible and may be potentially curative.
Previous Japanese19 and Italian20 studies have confirmed that emergency hepatic resection for ruptured HCC may achieve a long-term outcome comparable with that of elective surgery in selected patients. We also demonstrated that patients with ruptured HCC who underwent hepatectomy had worse OS compared with those in the non-ruptured HCC group.
Although it is generally presumed that STR is a risk factor impacting the prognosis of HCC patients undergoing hepatic resection, controversies remain on this issue.9,10,21 For instance, Aoki et al6 and Liu et al9 reported that the survival of patients with ruptured HCC was significantly worse than that of patients with non-ruptured HCC. In contrast, Yeh et al10 reported that patients with ruptured HCC had a similar OS compared to those with non-ruptured HCC; however, disease-free survival rate was significantly lower in those with ruptured HCC. As observed elsewhere,19 a trend toward lower disease-free survival rate was observed in patients with tumor rupture who underwent hepatic resection. The present study has identified in a large patient cohort that tumor rupture itself had a negative impact on patient survival; furthermore, of note, STR, which was not independently associated with poorer RFS (HR 0.95, 0.64–1.41) after hepatectomy, predicted poor OS and RFS after hepatectomy for HCC.
It is worth pointing out that all surgeons in our unit had abundant operating experience of hepatectomies. As a result, the rate of hepatectomy in the present cohort was much higher than previous counterparts.9,20 The 1-, 3- and 5-year OS and RFS rates after hepatectomy for ruptured HCC were comparable with those reported elsewhere.9,10,22
Perioperative morbidity and mortality rates were comparable between both groups, suggesting that hepatectomy did not pose additional perioperative risk for ruptured HCC in selected patients whose operations were performed by experienced surgeons. Peritoneal dissemination is not uncommon after curative resection of ruptured HCC;22 DWPL and intraperitoneal 5-FU were thus administered to prevent peritoneal dissemination. DWPL would remove tumor cells to a large extent, and thus, tumor recurrence would be delayed and better survival would be achieved in patients with spontaneously ruptured HCCs.14,23 Currently, the role of 5-FU in HCC treatment regarding adjuvant therapy after surgery, based on a randomized, controlled trial, placebo controlled,24 that showed lower recurrence rate and higher time-to-tumor progression as well as 5-year OS was better in the 5-FU group. In addition to the tumors being more advanced in the ruptured group, the R0 resection rate was significantly lower than that in the non-ruptured group (83.9% vs 87.6 %). In some patients with ruptured HCC, the intraoperative extent of tumor was more advanced than on preoperative assessment, with intraperitoneal seeding being detected at operation. Finally, peritoneal secondaries were more often found on follow-up in the ruptured group after R0 resection.
Yamagata et al19 claimed that increased intratumor pressure with venous invasion was the main culprit for recurrence in the ruptured HCC. However, in the present series, a similar percentage of vascular invasion was observed between ruptured and non-ruptured groups. Greater blood loss and more blood transfusion needs might contribute to the poorer disease-free survival in patients with ruptured HCC. Perioperative blood transfusion has been related to shorter disease-free survival because of the immunosuppressive effect of transfusion.25
Battula et al26 reported that the multifocality of the tumor and large tumor size can help predict the risk of decompensation and poor outcomes. Kirikoshi et al27 demonstrated that tumor size was the only independent factor for long-term survival among patients who underwent successful initial TAE. In the present series, tumor size was an important factor influencing survival in patients undergoing successful hepatectomy for ruptured HCC; moreover, we identified that tumor size was an independent factor influencing OS (HR: 1.74, 95% CI: 1.21–2.49, P=0.003) and RFS (HR: 1.99, 95% CI: 1.35–2.93, P=0.001).
In addition, the present study, together with the studies of Sun et al28 and Hung et al,29 showed that hepatitis B positivity was an independent factor for OS and disease-free survival and was associated with a higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B virus (HBV)-related HCC (within 1 year),28–30 suggesting HBsAg positivity impact on OS and RFS after partial hepatectomy for HCC. Although there was not a significant difference in OS or RFS between the ruptured and non-ruptured groups, the HBsAg-negative group had lower OS rates (63.2%, 45.6% and 42.1% at 1, 3 and 5 years respectively) than the HBsAg-positive group (52.1%, 35.4%, 32%); meanwhile, the 1-, 3-, 5-year OS rates for HBsAg-negative patients were 70.5%, 54.4% and 43.1%, respectively, in contrast to 62.7%, 42.2% and 30.2% for HBsAg-positive patients.
The present study has several flaws. First, although the data were collected prospectively, this is actually a retrospective cohort study with all its inherent shortcomings. Second, the study population in the present study was limited to Chinese HCC patients and the dominant etiology of the liver disease was HBV, which is different from that seen in Western countries.
Conclusion
The presence of hypertension, liver cirrhosis, higher serum TB level, tumor size >5cm and ascites are the independent indicators of ruptured HCC. The present study confirmed that tumor rupture itself had a negative impact on patient survival, and hepatic resection for patients with spontaneous HCC rupture is often feasible and is the treatment of choice for ruptured HCC, which can result in OS and RFS rates that are comparable to those of patients without rupture.
Supplementary materials
Table S1.
Univariable and multivariable analyses of overall survival in 307 patients with hepatocellular carcinoma
| Variable | n | Overall survival (%)
|
Univariable P# | Multivariable P* | Hazard ratio | ||
|---|---|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||||
| Sex | |||||||
| Male | 278 | 63.4 | 43.3 | 31.7 | 0.403 | ||
| Female | 29 | 69.0 | 51.7 | 37.9 | |||
| Age (years) | |||||||
| <60 | 255 | 62.4 | 42.3 | 33.8 | 0.929 | ||
| ≥60 | 52 | 71.7 | 52.8 | 25.0 | |||
| Presentation | |||||||
| Subclinical | 149 | 87.9 | 66.0 | 54.9 | 0.016 | 0.184 | |
| Symptomatic | 158 | 79.2 | 48.5 | 39.7 | |||
| HBsAg | |||||||
| Positive | 263 | 62.7 | 42.2 | 30.2 | 0.062 | ||
| Negative | 44 | 70.5 | 54.4 | 43.1 | |||
| TB (µmol/L) | |||||||
| <18.8 | 192 | 68.2 | 49.0 | 37.7 | 0.007 | 0.871 | |
| ≥18.8 | 115 | 56.9 | 36.0 | 23.0 | |||
| α-Fetoprotein (mg/L) | |||||||
| <400 | 146 | 80.8 | 58.8 | 43.9 | <0.001 | 0.002 | 1.59 (1.18, 2.15) |
| ≥400 | 161 | 48.8 | 30.9 | 22.0 | |||
| PT (s) | |||||||
| <13 | 149 | 63.1 | 40.3 | 31.8 | 0.938 | ||
| ≥13 | 158 | 64.8 | 47.7 | 32.7 | |||
| Child–Pugh grade | |||||||
| A | 295 | 64.9 | 44.5 | 32.3 | <0.001 | 0.044 | 0.43 (0.19, 0.98) |
| B | 12 | 41.7 | 33.3 | 27.5 | |||
| Cirrhosis | |||||||
| Yes | 105 | 67.6 | 48.6 | 34.9 | 0.385 | ||
| No | 202 | 62.1 | 41.8 | 31.0 | |||
| ALT (units/L) | |||||||
| <40 | 133 | 60.9 | 47.3 | 35.4 | 0.387 | ||
| ≥40 | 174 | 66.3 | 41.7 | 30.0 | |||
| AST (units/L) | |||||||
| <40 | 103 | 73.8 | 56.2 | 44.8 | 0.008 | 0.326 | |
| ≥40 | 204 | 59.0 | 38.0 | 26.0 | |||
| Spontaneous tumor rupture | |||||||
| Yes | 106 | 37.7 | 19.7 | 14.7 | <0.001 | <0.001 | 1.89 (1.32, 2.69) |
| No | 201 | 77.7 | 56.9 | 41.6 | |||
| Maximum tumor size (cm) | |||||||
| <5 | 103 | 88.3 | 67.9 | 52.9 | <0.001 | 0.003 | 1.74 (1.21, 2.49) |
| ≥5 | 204 | 51.7 | 32.2 | 21.9 | |||
| Macroscopic vascular invasion | |||||||
| Yes | 125 | 45.2 | 12.9 | 5.7 | <0.001 | 0.806 | |
| No | 182 | 64.5 | 45.8 | 35.7 | |||
| Microscopic vascular invasion | |||||||
| Yes | 272 | 27.8 | 11.1 | 8.3 | <0.001 | <0.001 | 2.30 (1.53, 3.46) |
| No | 35 | 68.7 | 48.5 | 35.5 | |||
| Extrahepatic spread | |||||||
| Yes | 67 | 41.2 | 26.5 | 20.6 | <0.001 | 0.155 | |
| No | 240 | 70.4 | 49.1 | 35.7 | |||
| Tumor differentiation | |||||||
| I–II | 45 | 92.5 | 77.0 | 66.9 | <0.001 | 0.080 | |
| III–IV | 262 | 79.7 | 53.1 | 43.3 | |||
| Tumor type | |||||||
| Unifocal | 242 | 70.2 | 50.4 | 36.6 | <0.001 | 0.002 | 1.69 (1.22, 2.36) |
| Multiple/diffuse | 65 | 40.9 | 21.2 | 16.4 | |||
| Blood loss (mL) | |||||||
| <500 | 200 | 86.5 | 65.9 | 56.2 | 0.004 | 0.084 | |
| ≥500 | 107 | 72.0 | 40.0 | 29.0 | |||
| Blood transfusion | |||||||
| Yes | 83 | 66.7 | 34.0 | 23.2 | <0.001 | 0.221 | |
| No | 224 | 87.8 | 65.6 | 55.9 | |||
| Type of hepatectomy | |||||||
| Anatomical | 189 | 82.4 | 57.5 | 47.0 | 0.600 | ||
| Non-anatomical | 118 | 81.1 | 54.9 | 46.0 | |||
| Extent of hepatectomy | |||||||
| Major | 163 | 80.0 | 54.8 | 44.2 | 0.130 | ||
| Minor | 144 | 85.1 | 60.0 | 47.3 | |||
| Surgical resection margin | |||||||
| R0 | 265 | 88.8 | 64.6 | 53.7 | <0.001 | 0.057 | |
| R1 | 40 | 43.8 | 9.8 | 5.8 | |||
| R2 | 2 | 18.9 | 4.9 | 2.0 | |||
Notes: Values in parentheses are 95% confidence intervals.
Log rank test.
Variables with univariable P<0.05 were entered in the Cox regression model.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; PT, prothrombin time; TB, total bilirubin.
Table S2.
Univariable and multivariable analyses of recurrence-free survival in 307 patients with hepatocellular carcinoma
| Variable | n | Recurrence-free survival (%)
|
Univariable P# | Multivariable P* | Hazard ratio | ||
|---|---|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||||
| Sex | |||||||
| Male | 278 | 53.5 | 36.3 | 33.1 | 0.566 | ||
| Female | 29 | 58.2 | 45.8 | 38.8 | |||
| Age (years) | |||||||
| <60 | 255 | 52.7 | 37.4 | 34.0 | 0.778 | ||
| ≥60 | 52 | 59.8 | 36.3 | 31.7 | |||
| Presentation | |||||||
| Subclinical | 159 | 80.1 | 59.5 | 43.5 | <0.001 | 0.095 | |
| Symptomatic | 148 | 65.4 | 44.3 | 29.0 | |||
| HBsAg | |||||||
| Positive | 263 | 52.1 | 35.4 | 32.0 | 0.139 | ||
| Negative | 44 | 63.2 | 45.6 | 42.1 | |||
| TB (µmol/L) | |||||||
| <18.8 | 192 | 49.1 | 30.3 | 28.6 | 0.157 | ||
| ≥18.8 | 115 | 56.8 | 41.1 | 36.7 | |||
| α-Fetoprotein (mg/L) | |||||||
| <400 | 145 | 66.6 | 45.0 | 40.2 | <0.001 | 0.026 | 1.45 (1.04, 2.01) |
| ≥400 | 162 | 41.4 | 29.8 | 27.7 | |||
| PT (s) | |||||||
| <13 | 149 | 56.4 | 39.4 | 36.3 | 0.580 | ||
| ≥13 | 158 | 51.9 | 36.2 | 31.5 | |||
| Child–Pugh grade | |||||||
| A | 295 | 54.1 | 37.0 | 33.4 | 0.738 | ||
| B | 12 | 48.6 | 30.2 | 20.2 | |||
| Cirrhosis | |||||||
| Yes | 202 | 51.4 | 35.8 | 32.2 | 0.740 | ||
| No | 105 | 58.7 | 38.4 | 35.0 | |||
| ALT (units/L) | |||||||
| <40 | 133 | 54.9 | 39.5 | 34.2 | 0.899 | ||
| ≥40 | 174 | 52.6 | 35.5 | 33.7 | |||
| AST (units/L) | |||||||
| <40 | 103 | 65.9 | 46.0 | 40.5 | 0.029 | 0.326 | |
| ≥40 | 204 | 54.3 | 34.8 | 30.5 | |||
| Spontaneous tumor rupture | |||||||
| Yes | 106 | 45.8 | 34.2 | 31.3 | 0.016 | 0.782 | 0.95 (0.64, 1.41) |
| No | 201 | 58.2 | 39.7 | 36.0 | |||
| Maximum tumor size (cm) | |||||||
| <5 | 103 | 78.1 | 52.4 | 50.1 | <0.001 | 0.001 | 1.99 (1.35, 2.93) |
| ≥5 | 204 | 40.6 | 29.8 | 24.4 | |||
| Macroscopic vascular invasion | |||||||
| Yes | 124 | 55.1 | 34.6 | 2 | 0.664 | ||
| No | 183 | 53.1 | 32.9 | 45 | |||
| Microscopic vascular invasion | |||||||
| Yes | 270 | 10.4 | 10.1 | 6.9 | <0.001 | 0.002 | 2.01 (1.29, 3.12) |
| No | 37 | 59.2 | 40.5 | 37.1 | |||
| Extrahepatic spread | |||||||
| Yes | 68 | 35.7 | 27.0 | 19.2 | <0.001 | 0.064 | |
| No | 239 | 58.9 | 40.1 | 37.6 | |||
| Tumor differentiation | |||||||
| I–II | 50 | 85.0 | 72.0 | 67.0 | <0.001 | 0.511 | |
| III–IV | 257 | 66.0 | 44.2 | 35.4 | |||
| Tumor type | |||||||
| Unifocal | 242 | 58.8 | 40.9 | 37.0 | <0.001 | 0.661 | |
| Multiple/diffuse | 65 | 35.5 | 23.7 | 21.7 | |||
| Perioperative blood loss (mL) | |||||||
| <500 | 209 | 75.2 | 56.0 | 46.0 | <0.001 | 0.165 | |
| ≥500 | 98 | 58.0 | 34.5 | 28.7 | |||
| Intraoperative blood transfusion | |||||||
| Yes | 76 | 53.0 | 30.2 | 22.5 | <0.001 | 0.057 | |
| No | 231 | 74.5 | 54.9 | 45.4 | |||
| Type of hepatectomy | |||||||
| Anatomical | 189 | 68.8 | 49.4 | 40.2 | 0.899 | ||
| Non-anatomical | 118 | 68.6 | 48.9 | 40.8 | |||
| Extent of hepatectomy | |||||||
| Major | 163 | 61.9 | 44.0 | 31.0 | 0.079 | ||
| Minor | 144 | 70.5 | 50.9 | 41.6 | |||
Notes: Values in parentheses are 95% confidence intervals.
Log rank test.
Variables with univariable P<0.05 were entered in the Cox regression model.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; PT, prothrombin time; TB, total bilirubin.
Acknowledgments
We acknowledge Jing Zhao and Ye Cai for professional statistical analysis. This work was supported by grants from the Natural Science Foundation of Hubei Province (2016CFB442 and WJ2017M236). This study was registered on November 30, 2014 under clinical trial number ChiCTR-OCS-11001300. Please contact author for data requests.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Yang T, Sun YF, Zhang J, et al. Partial hepatectomy for ruptured hepatocellular carcinoma. Br J Surg. 2013;100(8):1071–1079. doi: 10.1002/bjs.9167. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida H, Mamada Y, Taniai N, Uchida E. Spontaneous ruptured hepatocellular carcinoma. Hepatol Res. 2016;46(1):13–21. doi: 10.1111/hepr.12498. [DOI] [PubMed] [Google Scholar]
- 6.Aoki T, Kokudo N, Matsuyama Y, et al. Liver Cancer Study Group of Japan Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg. 2014;259(3):532–542. doi: 10.1097/SLA.0b013e31828846de. [DOI] [PubMed] [Google Scholar]
- 7.Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141(2):191–198. doi: 10.1001/archsurg.141.2.191. [DOI] [PubMed] [Google Scholar]
- 8.Leung KL, Lau WY, Lai PB, Yiu RY, Meng WC, Leow CK. Spontaneous rupture of hepatocellular carcinoma: conservative management and selective intervention. Arch Surg. 1999;134(10):1103–1107. doi: 10.1001/archsurg.134.10.1103. [DOI] [PubMed] [Google Scholar]
- 9.Liu CL, Fan ST, Lo CM, et al. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001;19(17):3725–3732. doi: 10.1200/JCO.2001.19.17.3725. [DOI] [PubMed] [Google Scholar]
- 10.Yeh CN, Lee WC, Jeng LB, Chen MF, Yu MC. Spontaneous tumour rupture and prognosis in patients with hepatocellular carcinoma. Br J Surg. 2002;89(9):1125–1129. doi: 10.1046/j.1365-2168.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang T, Zhang J, Lu JH, et al. A new staging system for resectable hepatocellular carcinoma: comparison with six existing staging systems in a large Chinese cohort. J Cancer Res Clin Oncol. 2011;137(5):739–750. doi: 10.1007/s00432-010-0935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 13.Xia F, Lau WY, Qian C, Ma K, Li X, Bie P. Continuous occlusion of hepatic artery proper for prevention of blood loss in partial hepatectomy for ruptured hepatocellular carcinoma: a case-matched comparative study. Ann Surg Oncol. 2011;18(6):1638–1643. doi: 10.1245/s10434-010-1484-3. [DOI] [PubMed] [Google Scholar]
- 14.Lin CH, Hsieh HF, Yu JC, Chen TW, Yu CY, Hsieh CB. Peritoneal lavage with distilled water during liver resection in patients with spontaneously ruptured hepatocellular carcinomas. J Surg Oncol. 2006;94(3):255–256. doi: 10.1002/jso.20596. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka A, Takeda R, Mukaihara S, et al. Treatment of ruptured hepatocellular carcinoma. Int J Clin Oncol. 2001;6(6):291–295. doi: 10.1007/s10147-001-8030-z. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Q, Li J, Yan JJ, Huang L, Wu MC, Yan YQ. Predictors and clinical outcomes for spontaneous rupture of hepatocellular carcinoma. World J Gastroenterol. 2012;18(48):7302–7307. doi: 10.3748/wjg.v18.i48.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai EC, Wu KM, Choi TK, Fan ST, Wong J. Spontaneous ruptured hepatocellular carcinoma. An appraisal of surgical treatment. Ann Surg. 1989;210(1):24–28. doi: 10.1097/00000658-198907000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewar GA, Griffin SM, Ku KW, Lau WY, Li AK. Management of bleeding liver tumours in Hong Kong. Br J Surg. 1991;78(4):463–466. doi: 10.1002/bjs.1800780424. [DOI] [PubMed] [Google Scholar]
- 19.Yamagata M, Maeda T, Ikeda Y, Shirabe K, Nishizaki T, Koyanagi N. Surgical results of spontaneously ruptured hepatocellular carcinoma. Hepatogastroenterology. 1995;42(5):461–464. [PubMed] [Google Scholar]
- 20.Vergara V, Muratore A, Bouzari H, et al. Spontaneous rupture of hepatocelluar carcinoma: surgical resection and long-term survival. Eur J Surg Oncol. 2000;26(8):770–772. doi: 10.1053/ejso.2000.1001. [DOI] [PubMed] [Google Scholar]
- 21.Mizuno S, Yamagiwa K, Ogawa T, et al. Are the results of surgical treatment of hepatocellular carcinoma poor if the tumor has spontaneously ruptured? Scand J Gastroenterol. 2004;39(6):567–570. doi: 10.1080/00365520410005135. [DOI] [PubMed] [Google Scholar]
- 22.Shuto T, Hirohashi K, Kubo S, et al. Delayed hepatic resection for ruptured hepatocellular carcinoma. Surgery. 1998;124(1):33–37. [PubMed] [Google Scholar]
- 23.Brundell SM, Tucker K, Chatterton B, Hewett PJ. The effect of lavage on intraabdominal cell burden. Surg Endosc. 2002;16(7):1064–1067. doi: 10.1007/s00464-001-9111-9. [DOI] [PubMed] [Google Scholar]
- 24.Xia Y, Qiu Y, Li J, et al. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: a randomized controlled trial. Ann Surg Oncol. 2010;17(12):3137–3144. doi: 10.1245/s10434-010-1148-3. [DOI] [PubMed] [Google Scholar]
- 25.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232(1):10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battula N, Madanur M, Priest O, et al. Spontaneous rupture of hepatocellular carcinoma: a Western experience. Am J Surg. 2009;197(2):164–167. doi: 10.1016/j.amjsurg.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Kirikoshi H, Saito S, Yoneda M, et al. Outcomes and factors influencing survival in cirrhotic cases with spontaneous rupture of hepatocellular carcinoma: a multicenter study. BMC Gastroenterol. 2009;9:29. doi: 10.1186/1471-230X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun HC, Zhang W, Qin LX, et al. Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol. 2007;47(5):684–690. doi: 10.1016/j.jhep.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008;103(7):1663–1673. doi: 10.1111/j.1572-0241.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 30.Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141(3):330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Univariable and multivariable analyses of overall survival in 307 patients with hepatocellular carcinoma
| Variable | n | Overall survival (%)
|
Univariable P# | Multivariable P* | Hazard ratio | ||
|---|---|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||||
| Sex | |||||||
| Male | 278 | 63.4 | 43.3 | 31.7 | 0.403 | ||
| Female | 29 | 69.0 | 51.7 | 37.9 | |||
| Age (years) | |||||||
| <60 | 255 | 62.4 | 42.3 | 33.8 | 0.929 | ||
| ≥60 | 52 | 71.7 | 52.8 | 25.0 | |||
| Presentation | |||||||
| Subclinical | 149 | 87.9 | 66.0 | 54.9 | 0.016 | 0.184 | |
| Symptomatic | 158 | 79.2 | 48.5 | 39.7 | |||
| HBsAg | |||||||
| Positive | 263 | 62.7 | 42.2 | 30.2 | 0.062 | ||
| Negative | 44 | 70.5 | 54.4 | 43.1 | |||
| TB (µmol/L) | |||||||
| <18.8 | 192 | 68.2 | 49.0 | 37.7 | 0.007 | 0.871 | |
| ≥18.8 | 115 | 56.9 | 36.0 | 23.0 | |||
| α-Fetoprotein (mg/L) | |||||||
| <400 | 146 | 80.8 | 58.8 | 43.9 | <0.001 | 0.002 | 1.59 (1.18, 2.15) |
| ≥400 | 161 | 48.8 | 30.9 | 22.0 | |||
| PT (s) | |||||||
| <13 | 149 | 63.1 | 40.3 | 31.8 | 0.938 | ||
| ≥13 | 158 | 64.8 | 47.7 | 32.7 | |||
| Child–Pugh grade | |||||||
| A | 295 | 64.9 | 44.5 | 32.3 | <0.001 | 0.044 | 0.43 (0.19, 0.98) |
| B | 12 | 41.7 | 33.3 | 27.5 | |||
| Cirrhosis | |||||||
| Yes | 105 | 67.6 | 48.6 | 34.9 | 0.385 | ||
| No | 202 | 62.1 | 41.8 | 31.0 | |||
| ALT (units/L) | |||||||
| <40 | 133 | 60.9 | 47.3 | 35.4 | 0.387 | ||
| ≥40 | 174 | 66.3 | 41.7 | 30.0 | |||
| AST (units/L) | |||||||
| <40 | 103 | 73.8 | 56.2 | 44.8 | 0.008 | 0.326 | |
| ≥40 | 204 | 59.0 | 38.0 | 26.0 | |||
| Spontaneous tumor rupture | |||||||
| Yes | 106 | 37.7 | 19.7 | 14.7 | <0.001 | <0.001 | 1.89 (1.32, 2.69) |
| No | 201 | 77.7 | 56.9 | 41.6 | |||
| Maximum tumor size (cm) | |||||||
| <5 | 103 | 88.3 | 67.9 | 52.9 | <0.001 | 0.003 | 1.74 (1.21, 2.49) |
| ≥5 | 204 | 51.7 | 32.2 | 21.9 | |||
| Macroscopic vascular invasion | |||||||
| Yes | 125 | 45.2 | 12.9 | 5.7 | <0.001 | 0.806 | |
| No | 182 | 64.5 | 45.8 | 35.7 | |||
| Microscopic vascular invasion | |||||||
| Yes | 272 | 27.8 | 11.1 | 8.3 | <0.001 | <0.001 | 2.30 (1.53, 3.46) |
| No | 35 | 68.7 | 48.5 | 35.5 | |||
| Extrahepatic spread | |||||||
| Yes | 67 | 41.2 | 26.5 | 20.6 | <0.001 | 0.155 | |
| No | 240 | 70.4 | 49.1 | 35.7 | |||
| Tumor differentiation | |||||||
| I–II | 45 | 92.5 | 77.0 | 66.9 | <0.001 | 0.080 | |
| III–IV | 262 | 79.7 | 53.1 | 43.3 | |||
| Tumor type | |||||||
| Unifocal | 242 | 70.2 | 50.4 | 36.6 | <0.001 | 0.002 | 1.69 (1.22, 2.36) |
| Multiple/diffuse | 65 | 40.9 | 21.2 | 16.4 | |||
| Blood loss (mL) | |||||||
| <500 | 200 | 86.5 | 65.9 | 56.2 | 0.004 | 0.084 | |
| ≥500 | 107 | 72.0 | 40.0 | 29.0 | |||
| Blood transfusion | |||||||
| Yes | 83 | 66.7 | 34.0 | 23.2 | <0.001 | 0.221 | |
| No | 224 | 87.8 | 65.6 | 55.9 | |||
| Type of hepatectomy | |||||||
| Anatomical | 189 | 82.4 | 57.5 | 47.0 | 0.600 | ||
| Non-anatomical | 118 | 81.1 | 54.9 | 46.0 | |||
| Extent of hepatectomy | |||||||
| Major | 163 | 80.0 | 54.8 | 44.2 | 0.130 | ||
| Minor | 144 | 85.1 | 60.0 | 47.3 | |||
| Surgical resection margin | |||||||
| R0 | 265 | 88.8 | 64.6 | 53.7 | <0.001 | 0.057 | |
| R1 | 40 | 43.8 | 9.8 | 5.8 | |||
| R2 | 2 | 18.9 | 4.9 | 2.0 | |||
Notes: Values in parentheses are 95% confidence intervals.
Log rank test.
Variables with univariable P<0.05 were entered in the Cox regression model.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; PT, prothrombin time; TB, total bilirubin.
Table S2.
Univariable and multivariable analyses of recurrence-free survival in 307 patients with hepatocellular carcinoma
| Variable | n | Recurrence-free survival (%)
|
Univariable P# | Multivariable P* | Hazard ratio | ||
|---|---|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||||
| Sex | |||||||
| Male | 278 | 53.5 | 36.3 | 33.1 | 0.566 | ||
| Female | 29 | 58.2 | 45.8 | 38.8 | |||
| Age (years) | |||||||
| <60 | 255 | 52.7 | 37.4 | 34.0 | 0.778 | ||
| ≥60 | 52 | 59.8 | 36.3 | 31.7 | |||
| Presentation | |||||||
| Subclinical | 159 | 80.1 | 59.5 | 43.5 | <0.001 | 0.095 | |
| Symptomatic | 148 | 65.4 | 44.3 | 29.0 | |||
| HBsAg | |||||||
| Positive | 263 | 52.1 | 35.4 | 32.0 | 0.139 | ||
| Negative | 44 | 63.2 | 45.6 | 42.1 | |||
| TB (µmol/L) | |||||||
| <18.8 | 192 | 49.1 | 30.3 | 28.6 | 0.157 | ||
| ≥18.8 | 115 | 56.8 | 41.1 | 36.7 | |||
| α-Fetoprotein (mg/L) | |||||||
| <400 | 145 | 66.6 | 45.0 | 40.2 | <0.001 | 0.026 | 1.45 (1.04, 2.01) |
| ≥400 | 162 | 41.4 | 29.8 | 27.7 | |||
| PT (s) | |||||||
| <13 | 149 | 56.4 | 39.4 | 36.3 | 0.580 | ||
| ≥13 | 158 | 51.9 | 36.2 | 31.5 | |||
| Child–Pugh grade | |||||||
| A | 295 | 54.1 | 37.0 | 33.4 | 0.738 | ||
| B | 12 | 48.6 | 30.2 | 20.2 | |||
| Cirrhosis | |||||||
| Yes | 202 | 51.4 | 35.8 | 32.2 | 0.740 | ||
| No | 105 | 58.7 | 38.4 | 35.0 | |||
| ALT (units/L) | |||||||
| <40 | 133 | 54.9 | 39.5 | 34.2 | 0.899 | ||
| ≥40 | 174 | 52.6 | 35.5 | 33.7 | |||
| AST (units/L) | |||||||
| <40 | 103 | 65.9 | 46.0 | 40.5 | 0.029 | 0.326 | |
| ≥40 | 204 | 54.3 | 34.8 | 30.5 | |||
| Spontaneous tumor rupture | |||||||
| Yes | 106 | 45.8 | 34.2 | 31.3 | 0.016 | 0.782 | 0.95 (0.64, 1.41) |
| No | 201 | 58.2 | 39.7 | 36.0 | |||
| Maximum tumor size (cm) | |||||||
| <5 | 103 | 78.1 | 52.4 | 50.1 | <0.001 | 0.001 | 1.99 (1.35, 2.93) |
| ≥5 | 204 | 40.6 | 29.8 | 24.4 | |||
| Macroscopic vascular invasion | |||||||
| Yes | 124 | 55.1 | 34.6 | 2 | 0.664 | ||
| No | 183 | 53.1 | 32.9 | 45 | |||
| Microscopic vascular invasion | |||||||
| Yes | 270 | 10.4 | 10.1 | 6.9 | <0.001 | 0.002 | 2.01 (1.29, 3.12) |
| No | 37 | 59.2 | 40.5 | 37.1 | |||
| Extrahepatic spread | |||||||
| Yes | 68 | 35.7 | 27.0 | 19.2 | <0.001 | 0.064 | |
| No | 239 | 58.9 | 40.1 | 37.6 | |||
| Tumor differentiation | |||||||
| I–II | 50 | 85.0 | 72.0 | 67.0 | <0.001 | 0.511 | |
| III–IV | 257 | 66.0 | 44.2 | 35.4 | |||
| Tumor type | |||||||
| Unifocal | 242 | 58.8 | 40.9 | 37.0 | <0.001 | 0.661 | |
| Multiple/diffuse | 65 | 35.5 | 23.7 | 21.7 | |||
| Perioperative blood loss (mL) | |||||||
| <500 | 209 | 75.2 | 56.0 | 46.0 | <0.001 | 0.165 | |
| ≥500 | 98 | 58.0 | 34.5 | 28.7 | |||
| Intraoperative blood transfusion | |||||||
| Yes | 76 | 53.0 | 30.2 | 22.5 | <0.001 | 0.057 | |
| No | 231 | 74.5 | 54.9 | 45.4 | |||
| Type of hepatectomy | |||||||
| Anatomical | 189 | 68.8 | 49.4 | 40.2 | 0.899 | ||
| Non-anatomical | 118 | 68.6 | 48.9 | 40.8 | |||
| Extent of hepatectomy | |||||||
| Major | 163 | 61.9 | 44.0 | 31.0 | 0.079 | ||
| Minor | 144 | 70.5 | 50.9 | 41.6 | |||
Notes: Values in parentheses are 95% confidence intervals.
Log rank test.
Variables with univariable P<0.05 were entered in the Cox regression model.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; PT, prothrombin time; TB, total bilirubin.