Abstract
Extramedullary intradural spinal tumors are rare. Less than 15% of all central nervous system (CNS) tumors are spinal. Ninety percent of these patients are older than 20 years. Most of spinal tumors are extradural (50-55%) whereas 40-45% are intradural. Furthermore, 5% are intramedullary and 40% are extramedullary. Most common are Schwannomas (29%), followed by meningiomas (25%) and gliomas (22%). These tumors produce pain syndroms, a variety of neurological symptoms-motor, sensory, sphincter or a combination of thereof. All spinal levels may be involved. The diagnostics includes magnetic resonance imaging (MRI) including contrast enhancement, computerizing tomography (CT) scanning (bone windows with reconstruction) and possibly CT myelograms. Preferred treatment is the microsurgical radical resection. Perioperative mortality is very low as is serious morbidity
We herein discuss various aspects of presenting symptomatology, diagnostics, preoperative planning and tactics, surgical treatment and complications. In addition, we include our own retrospective experience with 14 patients treated over the 5.5 years time interval.
Keywords: spine, spinal cord, tumors, intradural, extramedullary, meningiomas, Schwannomas, ependymomas
INTRODUCTION
Spinal tumors comprise 15% of all CNS tumors. Their annual incidence is 2-10 per 100.000. Ninety percent of these patients are older than 20 years. Most common spinal tumor location is extradural (55-60%), where cancer metastasis to spine leads the way. Primary vertebral bone tumors are less frequent extradural spinal tumors (1-4). Extramedullary, intradural spinal tumors (EISTs) are rare. They comprise about 40-45% of all spinal tumors. They are distinguished from intramedullary tumors by their extra-axial location. First recorded resection of EIST has been done by Sir Victor Alexander Haden Horsley (1888) in a 42 year old patient. The lesion has been originally classified as fibromyxoma, but was probably a degenerated Schwannoma (5). The mean age of patients with EISTs is 46 years and 54-57% of them are male. Their annual incidence is 0.4 per 100.000 population. An average neurosurgeon may see 1-2 EISTs patients per year, a neurologist 1 patient every 5-6 years, whereas every third general practitioner will see a case during their carrier (5, 6).
Presenting signs and symptoms
Median time to diagnosis is 12 months and cauda equina location is not presenting earlier than other spinal locations. The symptoms are lesion nonspecific and do not differ between intramedullary and extramedullary locations. Most common initial symptom is pain, which may be local and nocturnal or radiating to arm and/or leg. Sphincter dysfunction, paraparesis and erectile dysfunction occur in 20%, 12% and 2% of patients respectively (3, 6, 7).
Diagnostics
Primary diagnostic modality for IESTs is magnetic resonance imaging (MRI) without and with contrast enhancement. Diagnostics also include plain X-ray imaging in anterior-posterior, lateral and dynamic (flexion, extension) projections. Furthermore, computerized tomography (CT) scan, thin cuts with reconstructions (“bone windows”) are important to evaluate bony anatomy. In patients who could not undergo MRI scanning, CT myelography is an alternative. Most common tumors within the EISTs group are meningiomas, nerve sheath tumors, and filum terminale ependymomas, making up to 85% of this group (3). Dumbbell appearance accounts for 18% of EISTs, with cervical location being most common. Most common tumors with dumbbell appearance are Schwannomas (69%), followed by neurofibromas (12%). Least common dumbbell appearance have meningiomas (5%) (8).
Meningiomas
Meningiomas arise from arachnoid cap cells embedded in dura near the spinal nerve root sleeve. They are second most common EISTs. Their predominant spinal canal location is lateral. Other cells of origin may be fibroblasts associated with the dura or pia. In this case the tumor has a ventral dural origin. Frequently the attachment to dura is broad based. Most common patients’ age interval is between fifty and seventy years although any age group may be involved. They are more common in women (75-85%) and in the thoracic location (80%). In 75% of meningiomas, calcifications were registered. Most commonly they are solitary although 1-2 % may be multifocal, particularly in neurofibromatosis I (NF I) patients. Majority of spinal meningiomas are intradural, although 10% may involve extradural location. Spinal meningiomas are isoor hypointense on T1 weighted images and slightly hyperintense or hypointense on T2 weighted MRI. Upon contrast application they enhance vividly (except for a calcified part) and frequently display a “dural tail” sign. Only 5% of me-ningiomas may present in a dumbbell shape (1-4, 9-14).
Nerve sheath tumors
Spinal nerve sheath tumors (SNSTs) include Schwannomas (neuromas, neurinoma, neurilemmomas) and neu-rofibromas. They are most frequent EISTs. Schwannmomas are composed of Schwann cells with fibrous tissue. These tumors may show cystic degeneration and hemorrhage. They usually displace nerve roots. If they are multiple, they may be associated with NF II patients. Neurofibromas are composed of Schwann cells, fibroblasts, and nerve fibers in a matrix of mucopolysaccha-rides, fluid and fibrous material. Typically SNSTs are found on the dorsal sensory roots which they encase. There is no gender predilection. Most commonly they are seen in cervical and lumbar regions; less frequently in the thoracic spinal segment. Predominantly they have an intradural location but 25% are completely extradural and 15% are intra/extradural. Their peak incidence is fourth decade of life. Ninety % of these tumors are benign. Multiple tumors are typical for NF I patients. SNSTs are isointense on T1 weighted MRIs and have hyperintense signal on T2 weighted images. Upon contrast application enhancement is variable. SNST may present in a dumbbell shape. If they do, there is a 80% chance that a tumor is a SNST. If they reach a large size, they may remodel intravertebral foramen or even erode or cause scalloping of the posterior aspect of the vertebral body (1-3, 7-14, 17)
Filum terminale ependymomas
Fifty percent of all ependymomas are spinal. Within spinal ependymomas, 50% are intramedullary and another 50% are located within terminal filum. Despite the neuroectodermal origin of filum terminale, from anatomical and a surgical perspective it is appropriate to group them with IESTs. Filum terminale ependymomas arise from ependymal rests in filum terminale and are of myxopapillary histologic variant. They can occur at any age but most commonly between 3rd and 5th decades. These tumors are well circumscribed and seldom infiltrate the dura. After radical resection recurrence is generally rare although subarachnoid seeding is possible. On T1 weighted images they are hypo-or isointense and are hyperintense on T2 weighted MRIs. Homogenous or heterogenous enhancement is seen upon contrast application (3, 9, 11, 12).
Preoperative planning and treatment
EISTs can significantly compress and displace the spinal cord, the nerve roots or even the surrounding structures (e.g the vertebral artery). This can impact preoperative neurologic presentation and operative morbidity. Gross total tumor resection while preserving and improving neurologic function is the usual goal of surgery. This can be achieved in great majority of cases. Intraoperative monitoring-somatosensory evoked potentials (SSEP) and motor evoked potentials (MEP) may be utilized. Intraoperative ultrasound may at times be useful to evaluate intra-operative extent of lesion and radicality of surgery. After a detailed clinical, neurologic and neuroradiologic evaluation, the operative approach is planned. Approaches are based upon location of the tumor, its extension, its size and other parameters. The goal is to provide maximal intra-operative exposure of the tumor, while minimizing damage to the surrounding structures. Excessive removal of bony structures and ligaments may result in spinal instability (4, 5,14,15, 17). A normal spine remains stable as long as two out of the three columns remain undisturbed. The anterior column consists of the anterior half of the vertebral body, the anterior half of the intervertebral disk and the anterior longitudinal ligament. The middle column consists of the posterior half of the vertebral body, the posterior half of the intervertebral disk and the posterior longitudinal ligament. The posterior column includes paired facets, the transverse and spinous processes and the paired laminae (16). For most EISTs resections, the posterior approach with midline incision is sufficient. The patient in prone position and neck and spinal alignment should remain as neutral as possible. Certain cases may require awake, fiberoptic intubation to avoid hyperextension. The extent of incision and exposure is guided with topographic anatomy and intraoperative C-arm x-ray navigation in lateral and anterior-posterior projections. While performing laminectomies, care should be taken to preserve facets, its capsules and intertransverse ligaments to avoid postoperative kyphosis and instability. Otherwise, instrument stabilization may be required. Sometimes only hemilaminectomies may suffice for tumor resection. Posterolateral approaches with removal of pedicle and/or costotransversectomy may be necessary for certain ventral thoracic EISTs locations or extraforaminal tumor extensions. Anterior approaches are sometimes needed for ventral cervical locations whereas anterolateral approaches for ventral thoracic EIST locations. Subsequent instrumented fusion is then necessary. (4, 5, 14, 15, 17-20). The results of surgery of EISTs are usually excellent. Even long lasting preoperative neurologic deficit may be improved and reversed postoperatively. Most common complications include CSF leak, pseudomeningocele formation and wound infections. Less common is postoperative spinal instability and neurologic deficit (3-5, 7, 17, 21). Recurrence for radically resected SNST was reported to be 10 and 28 % after 5 and 15 years respectively (18,19). In case with residual tumor and/or recurrence, radiation treatment or radiosurgery may be utilized (22, 23). Chemotherapy may be used in malignant EISTs (3).
Our Series
Over the period of 5 and one half years (September, 03-March, 09) the senior author has operated on 14 cases of EISTs. There were 3 men and 11 women. Their age range was 24-84 years with a mean of 56 years. Two patients were septuagenerians and two octogenerians. Most common were meningiomas (7 cases-50%), 5 were Schwannomas, and 2-filum terminale myxopapillary ependymomas. Follow up range was 1-67 months with a mean of 28 months. The overview of tumor types, ages, gender and locations are presented on Table 1. Four representative cases are demonstrated in Figures 1-4.
TABLE 1.
Overview of the EISTs tumor types, the ages, the gender and locations in our series (y-years, M-male, F-female, C-cervical, T-thoracic, L-lumbar, S-sacral)

FIGURE 1.

75 y/o F with C1-C3 meningioma presenting with quadriparesis, bowel incontinence and C4 sensory level.
a) Preoperative sagittal T-2 weighted MRI showing the hypointense meningioma (asterix).
b) Preoperative axial postcontrast T-l weighted MM showing the partially enhanced meningioma (asterix) and severy compressed spinal cord (arrowhead)
c) Postoperative sagittal T-2 weighted MRI showing radical tumor resection
FIGURE 2.

24 y/o F with C5-C7 “dumbbell” shaped meningioma presenting with quadriparesis, intermittent bladder incontinence and Tl sensory leveL
a) Preoperative sagittal postcontrastT-l weighted MRI showing the enhanced meningioma (asterix).
b) Preoperative postcontrast T-l weighted MRI showing the “dumbbell” shaped enhanced meningioma (asterix) exiting through the right intervertebral foramen severely compressing the spinal cord (arrow head).
c) Postoperative sagittal T-2 weighted MRI showing radical tumor resection
d) Postoperative axial CT scan (“bone windows)” showing status post laminectomy for tumor resection and the lateral mass screw in position for postoperative fusioa Due to contralateral partial facet resection, a potential instability was created and the spinal segment needed to be fused by instrumentation
FIGURE 3.

47 y/o F with L3 filum terminale myxopapillary ependymoma presenting with paraparesis, bowel incontinence and L2 sensory level
a) Preoperative sagittal T-l weighted MRI showing the isointense ependymoma (asterix) compressing the cauda equine nerves.
b) Preoperative postoontrast axial T-l weighted MRI showing the enhanced ependymoma occupying about 90% of the spinal canal
c) Postoperative axial T-2 weighted MRI showing the radical tumor resection Postoperative sagittal T-2 weighted MRI showing the radical tumor resection
FIGURE 4.
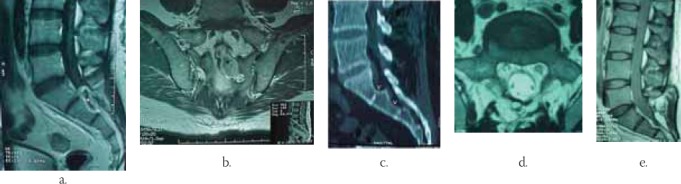
48y/o Μ presenting with perineal numbness, erection failure and severe low back pain with S1-S3 Schwannoma
a) Preoperative sagittal postcontrast T-l weighted MRI showing the rim enhancing Schwannoma (asterix).
b) Preoperative coronal T2-weighted MRI showing the “dumbbell” shaped Schwannoma (asterix).
c) Preoperative sagittal reconstruction CT scan (“bone windows)” showing the “scalloping” of sacrum (arrow heads).
d) Postoperative axial T-2 weighted MRI showing radical tumor resection
Postoperative T-l weighted MRI showing radical tumor resection
Postoperative lateral C-spine x-ray showing good spinal alignment and instrumentation in position. Except for 4 patients with Schwannomas who presented with pain and numbness at their appropriate levels and nerve distributions, all other patients presented with a quadriparesis or paraparesis with the corresponding sensory level and sphincter involvement. All patients were treated in a prone position, with microsurgical technique. For the cervical spinal EISTs locations, the head of the patient was secured in a 3 point head fixation. Radical tumor resection was confirmed on postoperative MRI scans in all patients. No tumor recurrence was noted during a mean follow up of well over 2 years (48 months). All patients completely recovered their neurologic deficit after the surgery during the follow up period. One patient with sacral Schwannoma developed pseudomeningocele 2 weeks after the surgery and was treated with surgical revision and external lumbar CSF drainage and resolved completely. There were neither perioperative nor follow up mortalities. Utilizing microscope and microsurgical technique provides the magnification, the illumination, the stereotactic vision, the communication with the remaining surgical team and the education of trainees. A cavitron ultrasonic aspirator (CUSA) may be used for debulking of the tumor. Dural opening should extend beyond the tumor limits proximally and distally and may be midline or off midline. First, proximal dural opening with the release of CSF should be done. This is to avoid cauda equina nerves herniation in dorsal direction. Section of one or more dentate ligaments frequently aids resection. A watertight dural closure is very important to prevent pseudomeningocele formation or cerebrospinal fluid (CSF) leak with resulting meningitis and infection. We have utilized harvest of 5-10 cc of abdominal fat graft via a small, 1 inch incision at the beginning of surgery. This fat tissue was used at closure to obliterate the epidural “dead space.” We postulate that resulted in the absence of CSF leaks or pseudomenin-gocele formation in our series (except the case of sacral Schwannoma where we did not utilize this maneuver). Frequently, for resection of nerve sheet tumors, sacrifice of the parent nerve may be necessary. Fortunately, this is frequently a sensory branch and ventral, motor branch may be preserved with a gentle microsurgical dissection. In meningiomas, early interruption of broad based tumor attachment to the dura provides bloodless surgery. Preserving arachnoid planes while dissecting the tumor minimizes risk of postoperative neurologic deficit. In myxopapillary ependymomas, after the tumor dissection, proximal division of filum terminale is recommended first. This is to avoid sudden tumor retraction proximally beyond the dural opening, should the division of the filum terminale is done distally first.
CONCLUSION
EISTs can be radically resected with no mortality and minimal perioperative morbidity. Thorough perioperative planning, meticulous microsurgical techniques and early mobilization and rehabilitaion are essential for good clinical outcomes.
CSF leak and pseudomeningocele formation could be prevented with meticulous dural closure, fat grafting for obliteration of the dead space and 48 hours postoperative bed rest. Patients tend to completely recover their preoperative neurologic deficits even in the case of longstanding preoperative neurological deficit. Advanced age does not seem to preclude eligibility for surgery.
REFERENCES
- 1.Van Goethem J.W.M, van den Hauwe l, Ozsarlak O, De Schepper A.M.A, Parizel P.M. Spinal tumors. Eur. J. Radiol. 2004;50:159–176. doi: 10.1016/j.ejrad.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Beall D.P, Googe D.J, Emery R.L, Thompson D.B, Campbell S.E, Ly J.Q, DeLone D, Smirniotopoulos J, Lisanti C, Currie T.J. Extramedullary intradural spinal tumors. A pictorial review. Curr. Probl. Diagn. Radiol. 2007;36:185–198. doi: 10.1067/j.cpradiol.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Zeidman S.M. Intradural intramedullary and extramedullary tumors. In: Vaccaro A.R, Betz R.R, Zeidman S.M, editors. Principles and practice of spine surgery. St.Louis: Mosby; 2003. pp. 223–239. [Google Scholar]
- 4.Parsa A.T, Lee J, Parney I.F, Weinstein P, McCormick P.C, Ames C. Spinal cord and intradural-extraparenchymal spinal tumors: current best care practices and strategies. J. Neuro. Oncology. 2004;69:291–318. doi: 10.1023/b:neon.0000041889.71136.62. [DOI] [PubMed] [Google Scholar]
- 5.El-Mehdy W, Kane P.J, Powell M.P, Crockhard H.A. Spinal intradural tumours: Part Iextramedullary. Brit. J. Neurosurg. 1999;13:550–557. doi: 10.1080/02688699943042. [DOI] [PubMed] [Google Scholar]
- 6.Jellema K, van Overbeeke J, Teepen H.L.J.M, Visser L.H. Time to diagnosis of intraspinal tumors. Eur. J. Neurol. 2005;12:621–624. doi: 10.1111/j.1468-1331.2005.01043.x. [DOI] [PubMed] [Google Scholar]
- 7.Jeon J.H, Hwang H.S, Jeong J.H, Park S.H, Moon J.G, Kim C.H. Spinal Schwannoma;analysis of 40 cases. J. Korean Neurosurg. Soc. 2008;43:135–138. doi: 10.3340/jkns.2008.43.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozawa H, Kokubun S, Aizawa T, Hoshikawa T, Kawahara C. Spinal dumbbell tumors: an analysis of a series of 118 cases. J. Neurosurg. Spine. 2007;7:587–593. doi: 10.3171/SPI-07/12/587. [DOI] [PubMed] [Google Scholar]
- 9.Abul-Kasim K, Thurnher M, McKeever P, Sundgren P.C. Intradural spinal tumors: Current classification and MRI features. Neuroradiology. 2008;50:301–314. doi: 10.1007/s00234-007-0345-7. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman R.A, Bilaniuk L.T. Imaging of tumors of the spinal canal and cord. Radiol. Clin. North. Am. 1988;26:965–1007. [PubMed] [Google Scholar]
- 11.Bloomer C.W, Ackerman A, Bhatia R.G. Imaging for spine tumors and new applications. Top. Magn. Reson. Imaging. 2006;17:69–87. doi: 10.1097/RMR.0b013e31802bb38f. [DOI] [PubMed] [Google Scholar]
- 12.Traul D.E, Shaffrey M.E, Schiff D. Part 1: Spinal-cord neoplasms-intradural neoplasms. Lancet Oncol. 2007;8:35–45. doi: 10.1016/S1470-2045(06)71009-9. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell C.I, Jaspan T, Worthington B.S, Hollan I.M. Gadolinium-enhanced magnetic resonance imaging of spinal tumours. Brit. J. Radiol. 1989;62:1067–1074. doi: 10.1259/0007-1285-62-744-1067. [DOI] [PubMed] [Google Scholar]
- 14.Albanese V, Platania N. Spinal intradural extramedullary tumors. J Neurosurg Sci. 2002;46:18–24. [PubMed] [Google Scholar]
- 15.Yasargil M.G, Tranmer B.I, Adamson T.E, Roth P. Unilateral partial hemi-laminectomy for the removal of extra-and intramedullary tumours and AVMs. Adv Tech Stand Neurosurg. 1991;18:113–132. doi: 10.1007/978-3-7091-6697-0_3. [DOI] [PubMed] [Google Scholar]
- 16.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817–831. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Safavi-Abbasi S, Senoglu N.M, Theodore N, Workman R.K, Gharabaghi A, Feiz-Erfan I, Spetzler R.F, Sonntag V.K.H. Microsurgical management of spinal schwannomas: evaluation of 128 cases. J. Neurosurg. Spine. 2008;9:40–47. doi: 10.3171/SPI/2008/9/7/040. [DOI] [PubMed] [Google Scholar]
- 18.Klekamp J, Samii M. Surgical results for spinal meningiomas. Surg. Neurol. 1999;52:552–562. doi: 10.1016/s0090-3019(99)00153-6. [DOI] [PubMed] [Google Scholar]
- 19.Klekamp J, Samii M. Surgery of spinal nerve sheath tumors with special reference to neurofibromatosis. Neurosurgery. 1998;42:279–289. doi: 10.1097/00006123-199802000-00042. [DOI] [PubMed] [Google Scholar]
- 20.Lot G, George B. Cervical neuromas with extradural components: surgical management in a series of 57 patients. Neurosurgery. 1997;41:813–820. doi: 10.1097/00006123-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Sacko O, Haegelen C, Mendes V, Brenner A, sesay M, Brauge D, Lagarrigue J, Loiseau H, Roux F.E. Spinal meningioma surgery in elderly patients with paraplegia or severe paraparesis: a multicenter study. Neurosurgery. 2009;64:503–509. doi: 10.1227/01.NEU.0000338427.44471.1D. [DOI] [PubMed] [Google Scholar]
- 22.Dodd R.L, Ryu M.R, Kamnerdsupaphon P, Gibbs I.C, Chang S.D, Adler J.R. Cyberknife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58:674–684. doi: 10.1227/01.NEU.0000204128.84742.8F. [DOI] [PubMed] [Google Scholar]
- 23.Gerszten P. C, Burton S.A, Ozhasoglu C, McCue K.J, Quinn A.E. Radiosurgery for benign intradural spinal tumors. Neurosurgery. 2008;62:887–895. doi: 10.1227/01.neu.0000318174.28461.fc. [DOI] [PubMed] [Google Scholar]


