Abstract
Acute lung injury and its more severe form acute respiratory distress syndrome (ARDS) are characterized by diffuse impairment of alveolocapillary membrane in the settings of different predisposing conditions such as sepsis, trauma and shock. Many intrahospital exposures, including aspiration, delayed resuscitation, high tidal volume mechanical ventilation and non critical use of transfusions may contribute or worsen ARDS. Therapy is targeted to treatment of predisposing condition, life supportive measures and prevention of nosocomial complications. Rigorous adherence to lung-protective mechanical ventilation is critical to prevent ventilator induced lung injury and decrease mortality. Although survival of ARDS patients has improved in the last decades ARDS mortality rates are still high and survivors encounter significant physical and psychological impairments.
Keywords: Respiratory distress syndrome, adult, mechanical ventilation, pulmonary edema
INTRODUCTION
More than 40 years ago Ashbaugh et al. first described acute respiratory distress syndrome (ARDS), a life threatening condition in patients with precipitating factors and mortality that ranged from 50 to 70%. In recent years many basic and clinical studies have improved our understanding of ARDS but the clinical impact has been limited to advances in supportive treatment. ARDS affects 200 000 people in US every year, and is associated with 75000 deaths, 3.5 millions hospital days and mortality of approximately 40% (1,2).
History
In 1967 Ashbaugh et al described 12 patients with acute respiratory failure, oxygen refractory cyanosis and diffuse alveolar infiltrates on chest X ray (3). The syndrome was first named adult respiratory distress syndrome (4), but soon after it was noted in children it was renamed to acute respiratory distress syndrome. Due to lack of clear definition in 1988 an attempt was made to quantify respiratory impairment in terms of lung injury score (LIS) based on four parameters: radiographic changes, level of hypoxia (PaO2/ FiO2), lung compliance and positive end expiratory pressure (PEEP). Factors causing direct and indirect lung injury were defined, and the role of multiorgan failure (MOF) was emphasized in terms of prognosis (5). The lack of specificity and inability to differentiate between ARDS and heart failure resulted in current definition by American-European Consensus Conference Committee in 1992 (Table 1) (6).
TABLE 1.
American-European Consensus Conference Committee Definition of Acute Respiratory Distress Syndrome; PCWP-Pulmo-nary Artery Wedge Pressure

Definition
Acute respiratory distress syndrome is a syndrome of acute respiratory failure with radiological feature of acute pulmonary edema in the absence of clinical evidence of left heart failure as a principal explanation of pulmonary edema. The condition is characterized by abrupt injury in alveolocapillary membrane resulting in alveolar flooding, inflammation and change in surfactant properties that cause severe impairment of oxygenation and respiratory failure requiring mechanical ventilation (7). The definition takes into account the degree of respiratory impairment and distinguishes acute lung injury (ALI) and a more severe form acute respiratory distress syndrome. Although early recognition of these patients facilitates enrollment into clinical trials, previous studies found no correlation between PaO2/FiO2 and survival (8, 9). The majority of patients who present with ALI progress to ARDS within first three days (10). Arterial oxygen saturation measured by pulse oximetry is useful in estimating level of respiratory impairment in both pediatric patients and adults (SaO2/ FiO2 of 315 and 263 correspond to PaO2/FiO2=300, SaO2/FiO2=235 and 201 corresponds to PaO2/ FiO2=200 in adults and children, respectively) (11, 12).
Etiology
The diagnosis of ALI is usually made in the ICU but the biological process begins much earlier. ALI/ARDS is rarely present at the time of hospital admission and usually develops in first hours to days after hospital admission in patients with predisposing conditions that can cause direct (pneumonia, aspiration, immersion, lung contusion, fat embolism) and indirect lung injury (sepsis, shock, severe trauma, transfusion, acute pancreatitis, drug intoxication) (13). (Table 2.)
TABLE 2.
Causes of ALI/ARDS
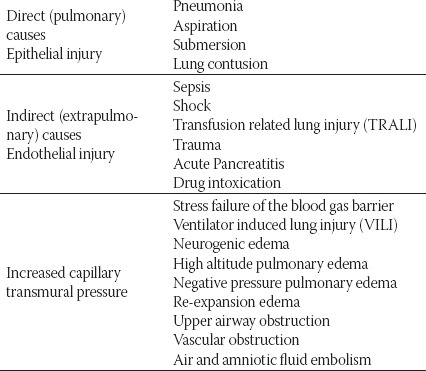
FIGURE 1.

Diffuse alveolar damage with hyaline membranes
FIGURE 2.

Radiogram of patient with ARDS
FIGURE 3.

CT findings in ARDS
Risk factors
Sepsis, and in particular pulmonary sepsis, has been recognized as the most common cause of ARDS. But only a small proportion of patients with pneumonia (10%), aspiration (16%), extrapulmonary sepsis (6%) and acute pancreatitis (1%) develop ALI/ARDS.14 Previous research identified various factors that model the development of ALI (’multiple hit hypothesis’). Primary patients’ characteristics (“first hit”) such as local and systemic inflammation, oxidative stress, epithelial cell injury caused by acid or inhalation toxins, smoking, alcoholism, chronic lung disease (interstitial diseases and COPD), acidosis and certain genetic polymorphism with additional exposures (“second hit”) to high tidal volumes, high oxygenation fraction, certain drugs (amiodarone, cytostatics) and transfusion of alloimmunized donors lead to development and progression of ARDS (15). Massive transfusions (>15units) have been previously associated with ARDS (16). It has now been widely recognized that any transfusion of fresh frozen plasma, thrombocytes and erythrocytes can cause lung injury inside 6 hours after transfusion (17). This, transfusion related lung injury-TRALI is caused by immune reaction of donor antibodies (sensibilization in previous pregnancies or during previous transfusions) and HLA antigens of recipient. Products rich with plasma are more potent in causing TRALI. Prolonged storage and accumulation of bioactive lipids may cause additional injury to the lungs (18). Risk factors associated with the development of ALI are summarized in Table 3.
TABLE 3.
Risk factors for the developement of ALI/ARDS
*unadjusted

Pathophysiology
Acute lung injury is characterized by damage and increased vascular permeability of alveolocapillary membrane that result in protein rich pulmonary edema (increased permeability edema as opposed to hydrostatic edema in cardiogenic shock) (7). Injury to alveolocapillary membrane can be physical (increased pulmonary pressure), chemical or due to an activation of immune response. These affect both alveolar epithelium and capillary endothelium. Alveolar epithelium consists mainly (90%) of alveolar epithelial cells type I that are responsible for gas exchange and prevent extravasation of fluid into alveoli. Cubic, alveolar epithelial cells type II are less prone to injury and have a role in reabsorption of the fluid and surfactant production. Proliferation of alveolar cells type II and their differentiation to alveolar epithelial cells type I allows healing of alveolocapillary membrane (30). Injury of alveolar epithelial cells type II causes dysregulation in surfactant production and results in severe derangements of respiratory mechanics and decrease in pulmonary compliance. Endothelial cells edema and widening of intercellular gaps causes increase in vascular permeability (’capillary leak syndrome’) (31). Both inadequate and hyperimmune response can cause injury of the alveolocapillary membrane. Dysregulation of inflammation in immune response is the common cause of acute lung injury (32). After initial stimuli activate macrophages to secrete tumor necrosis factor TNF α and IL-1 neutrophils migrate into intraalveolar space. Activated neutrophils, endothelial and epithelial cells induce a cascade of cytokines that amplify immune response. Products of neutrophils such as oxygen radicals and proteases can result in damage of the membrane (collateral damage) (7). Epithelial cells are also found to have an active role in inflammation. In pulmonary infections activated macrophages directly (IL-1, TNF-α) or indirectly (T-cells) stimulate nuclear factor 1φ (NF-kp) pathway of epithelial cells that consequently leads to increased production of chemokines-colony stimulating factors and adhesion molecules. Experimental studies have shown that inhibition of inflammatory molecules may have a protective role in ARDS (32). Some but not all studies indicated correlation between increased plasma or bronchoalveolar TNF-a and development of or mortality in ARDS (33, 34, 35). Recent genomic studies of cytokines and their receptors have shown that polymorphism in the genes for TNF-α is associated with increased mortality of ARDS, as well as ARDS susceptibility in some subgroups of patients according to the site of injury (36). Damage to epithelial and endothelial cells and production of inflammatory cytokines can lead to increased expression of tissue factor and stimulation of inhibitors of plasminogen activators. In addition levels of activated protein C (APC), antithrombin (AT) and tissue factor pathway inhibitor (TFPI) are found to be lower in sepsis due to decreased production and increased degradation (37) thus leading to increased fibrin production and micro-vascular thrombosis early in the course of ARDS (7).
Histology
Pathohistological changes in ARDS correspond to diffuse alveolar damage-DAD. Acute, exudative phase develops within first week and is characterized by interstitial and intraalveolar edema, capillary congestion, neutrophilic infiltrate, macrophages, erythrocytes and presence of hyaline membranes-eosino-philic structures which consist of cellular debris and proteins (albumin, fibrinogen and immunoglobulins). Elements of vascular microthrombosis are also present. Late, proliferative phase develops in first two weeks after insult. Proliferation of cuboid cells, fibroblasts, and myofibroblasts are seen with rare cellular infiltrate and interstitial deposition of collagen (38). DAD results in loss of the integrity of the alveolar-capillary barrier, exudation of protein-rich fluid across the barrier, pulmonary edema, and hypoxemia. Although described in 1976 by Katzeinstein (39) and considered to be a pathological substrate of ALI/ARDS presence of DAD was never included as one of the criteria in previous definitions since lung tissue is rarely available for pathological diagnosis. Furthermore, in patients with positive clinical criteria histological conformation of DAD is found in only about 50% on post mortem analysis (40, 41). Clinical and pathohistological definition correlates better in patients with extrapulmonary ARDS (40).
Diagnosis
Diagnosis of ALI is largely based on interpretation of chest radiograph that is consistent with pulmonary edema. Interpretation of chest X ray in critically ill may be difficult due to differences in quality, influence of mechanical ventilation as well as subjectivity of the interpreter. The interobserver agreement in applying radiographic criterion of ACCP definition was found to be moderate (kappa 0.55) (42). Cardiothoracic ratio and vascular pedicular width could be useful in differentiating cardiogenic from non-cardiogenic pulmonary edema (43). CT findings in ARDS correspond to ground glass opacity and zone dependent heterogeneous consolidations (44). Patients with extrapulmonary causes of ARDS present more often with symmetric evenly distributed ground glass opacification in contrast to ARDS associated with pulmonary risk factors where the infiltrates tend to be asymmetric with a mix of dorso-caudal consolidation and ground glass opacification (45). In recent years there has been an increasing use of ultrasound techniques in the ICU which are valuable in assessing cardiac function as well as presence of lung edema by lung ultrasonography (’comet tail sign’) (46). Preserved systolic function does not rule out cardiogenic edema since diastolic dysfunction (E/E’>15) is the common cause of cardiogenic edema. Echocar-diography also allows evaluation of pulmonary hypertension and functional status of the right ventricle. Excessive stretching of the ventricles induces secretion of Brain natriuretic peptide-BNP by myocytes. Values of 250 pg/mL are highly specific for diagnosis of non cardiogenic pulmonary edema while values of 950 pg/ dl and higher are suggestive of heart failure (Table 4.). Right heart failure which is often observed in ARDS can cause elevated values of BNP (300-600pg/dl) (47). In differential diagnosis there are conditions that can present as ARDS but require specific treatment (Table 5.). In the case of unclear etiology of ARDS surgical biopsy may have a crucial role in establishing diagnosis and initiation of appropriate therapy, especially in immunocompromised patients (table 5.)(50). In previous studies, open lung biopsy in ARDS cases of unclear etiology led to alteration of treatment in majority of cases (60-89%) and was not associated with increased morbidity (51-53). Treatment is focused on identification and management of predisposing condition such as adequate infection source control and supportive therapy. With the exception of low tidal volume strategy no other medical intervention or pharmacology treatment has been proven to reduce mortality in ARDS patients.
TABLE 4.
Differential diagnosis of cardiogenic and non-cardiogenic pulmonary edema
Abbreviations: EF-Ejection fraction; VPW-vascular pedicular width; CTR-Cardiothoracic ratio
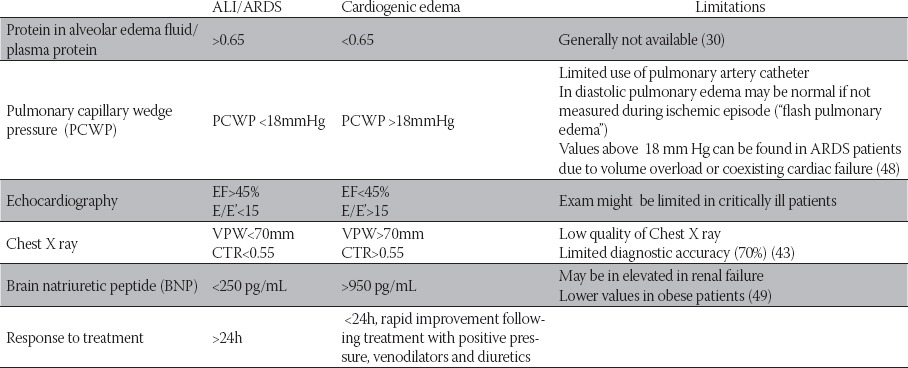
TABLE 5.
Differential diagnosis of ARDS; CBC complete blood count, BAL-bronchoalveolar lavage
Abbreviations: CBC-complete blood count; BAL-bronchoalveolar lavage

Mechanical ventilation
Lung protective ventilation using low tidal volumes 6ml/kg predicted body weight according to US National Institutes of Health Network for the Acute Respiratory Distress Syndrome (NIH ARDS Network) has led to a reduction of overall mortality from 39.8 to 31.0% in ARDS patients (8). Years of experimental studies indicated that using traditional tidal volumes of 10-15ml/kg can cause or worsen lung injury by direct mechanical stress, overdistension of healthy part of the lungs (volutrauma) (54-58), cyclic opening and closing of alveoli (atelectrauma)(59,60) and increasing production of pro-inflammatory cytokines (biotrauma) (61-64). Lung protection strategy is aimed at achieving SaO2 88-95% or PaO2 55-80mmHg and P plateau <25-30 mm Hg, with the lowest FiO2-PEEP values that often leads to retention of CO2 and acidosis. Positive end expiratory pressure (PEEP) opens atelectatic alveoli, decreases fraction of intrapulmonary shunt and improves oxygenation. On the other hand PEEP may cause distension of healthy part of the lungs and increase dead space fraction, decrease ventricular preload and cause hypotension. There is no evidence in difference in survival using high and low PEEP values (65). Use of low tidal volumes is shown to decrease concentration of inflammation markers and frequency of extrapulmonary organ failures compared to traditional ventilation modes (66). In case of inadequate oxygenation unconventional mechanical ventilation may be indicated. High frequency oscillation HFO uses rapid delivery (180 to 600 breaths in minutes, Fr 3-10HZ) of low tidal volumes (often below the values of anatomical dead-space). The concept of HFO is similar to low tidal volume ventilation. Experimental studies have shown lower level of lung injury and inflammation in HFO compared to conventional ventilation (63, 67). Small volumes prevent overdistension of alveoli while high mean pressure in airways prevents its collapse. Downsides of using HFO are risk of pneumothorax and hemodynamic instability due to decreased stroke volume. Preliminary studies failed to show benefit of survival in patients who were ventilated with HFO that could be due to a late initiation of HFO. Future studies should be focused on identifying patients who could benefit from unconventional mechanical ventilation and timing of initiation of HFO in these patients (68). In the cases of severe refractory hypoxemia ex-tracorporeal membrane oxygenation (ECMO) could be initiated. The benefit of ECMO remained to be confirmed in controlled clinical trials (Conventional ventilation or ECMO for Severe Adult Respiratory Failure -CESAR TRIAL) (69). Lung recruitment and prone position can improve oxygenation but effect is often transient, and there is no evidence of improved survival (7).
Noninvasive mechanical ventilation
Non invasive mechanical ventilation (NIV) may be used in early stages of ALI in patients without multiorgan failure and in whom there is no expectation of prolonged mechanical ventilation. Presence of shock and acidosis are associated with failure of NIV. All nonin-vasively ventilated ALI patients should be closely monitored to prevent potential delay in intubation (70, 71).
Fluid management
Due to an increased vascular permeability reduced fluid intake is beneficial to a gas exchange. On the other hand reduction of circulatory volume can impair tissue oxygenation. Conservative fluid strategy reduces days on mechanical ventilation and ICU length of stay but does not affect overall mortality or development of acute renal failure. Similarly, use of pulmonary arterial catheter was not found to be superior to central venous catheter (72). After initial fluid resuscitation fluid administration should be closely monitored and CVP should be maintained at 4 mmHg if shock is not present. Negative cumulative fluid balance at day 4 is associated with reduced mortality in acute lung injury (73).
Corticosteroids
Inflammatory changes in ALI as well as possible hyperimmune response and pathohistological changes in late ARDS give rationale for the use of corticosteroids in both early and late stages of ARDS. In the eighties high dose of corticosteroids were used for the treatment of ARDS but after reports of increased mortality related to their use, corticosteroids were used more cautiously. Corticosteroids have an indication in following clinical conditions that may present as ARDS: vasculitis (diffuse alveolar hemorrhage), acute eosino-philic pneumonia, acute interstitial pneumonia and acute bronchiolitis obliterans with organizing pneumonia. SARS experience has shown positive effect of corticosteroids on some infectious forms of ARDS. There is evidence that in early ARDS (inside first 72 h) use of corticosteroids may decrease days of mechanical ventilation, ICU length of stay and hospital mortality but this data needs to be confirmed in larger clinical trials (74). National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network study did not show difference in survival of patients with persistent ARDS (at least seven days) who did and did not receive corticosteroids, although there were evidence of improved gas exchange, respiratory compliance and ventilator free days (75). For those enrolled at least 14 days after the onset of ARDS the mortality rates in corticosteroid group were higher. Based on these results current guidelines do not recommend routine administration of corticosteroids in ARDS. Although previously associated with critical illness neuromyopathy and increased infection rates two recent meta-analysis found no relationship between use of corticosteroids and infection, neuromyopathy, or any major complications in ARDS patients (76, 77).
Pulmonary vasodilators
Use of semi-selective (nitric oxide, prostacyclin, prostaglandin E1) and non-selective (nitroprus-side, hydralazine) vasodilators can improve oxygenation and reduce pulmonary hypertension in severe refractory forms of hypoxia by reducing pulmonary vascular resistance and improving perfusion of well ventilated parts of lungs. In spite of obvious physiological effects clinical studies failed to confirm its effect on reducing overall mortality rates (7).
Surfactant replacement
Although in ARDS metabolism of surfactant is severely impaired use of surfactant did not improve survival in adults. There is evidence that some subgroups of ARDS patients such as those with pneumonia and aspiration could benefit from treatment with surfactant (78). Possible additional therapeutic targets include use of antioxidants, β adrenergic receptor agonists, ACE inhibitors and nutritional modifications as well as use of GM-CSF and activated protein C (44, 50). Other supportive measures include adequate nutrition, stress ulcer, DVT and decubital prophylaxis. Recent data show that intensive insulin therapy reduces the duration of mechanical ventilation, duration of ICU stay and 180-day mortality in ICU patients but there are no exclusive data on subpopulation of ALI patients. There is evidence that intensive insulin therapy reduces the incidence of critical illness polyneuro-and/or myopathy (77).
Prognosis
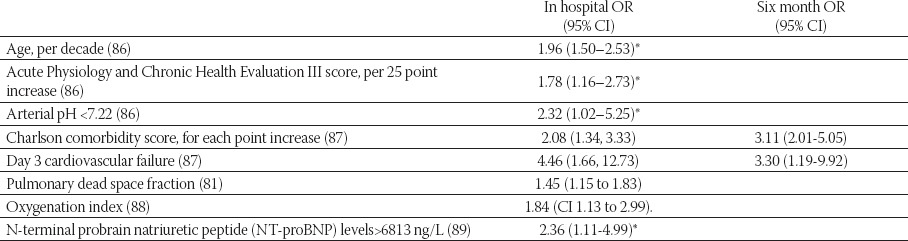
Severe impairment of alveolar epithelia and failure to improve in first week are associated with adverse outcome (79). Older patients with sepsis, liver diseases and MOF carry worse prognosis (80). Although severe impairment of pulmonary function is a hallmark of ARDS only two pulmonary features, oxygenation index and increased dead space fraction ventilation are shown to be of predictive value (81). The main cause of death is withdrawal of treatment due to irreversible MOF (82) and low quality of life in older patients (83) and patients with significant comorbidities. About 10% of patients will be mechanically ventilated for more than a month (44). In some patients acute phase may be complicated with fibrosing alveolitis and persisting hypoxemia, increased dead space ventilation and reduced compliance. First fibrotic changes may appear after 5-7 days (7). Obliteration of pulmonary capillaries and refractory hypoxia can cause severe pulmonary hypertension with right heart failure. In those who recover radiologic changes withdraw gradually and pulmonary function test recovers completely, sometimes with mild restriction, obstruction or decreased diffusion capacity that are usually asymptomatic (84). Histological resolution in ARDS survivors is not well known. Prospective studies have indicated that ARDS survivors have impaired quality of life at one year follow up. Main problems that ARDS survivors are facing are weight loss, deconditioning, cognitive and psychological problems as well as neuromuscular weakness (85).
TABLE 4.
Predictors of mortality in ARDS
*60-day mortality
CONCLUSION
Although survival of ARDS patients has been significantly improved in the last decades ARDS mortality rates are still high and survivors encounter significant physical and psychological impairments. Early treatment of predisposing conditions and the prevention of “second hit” in-hospital exposures are critical for prevention and treatment of this important complication of critical illness. Since ARDS patients represent etiologically and pathophysiologically a heterogeneous group of patients future studies should be focused on better defining subgroups of patients that could benefit from specific target therapies.
REFERENCES
- 1.Rubenfeld G.D, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Goss C.H, Brower R.G, Hudson L.D, et al. Incidence of acute lung injury in the United States. Crit. Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh D.G, Bigelow D.B, Petty T.L, et al. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 4.Petty T.L, Ashbaugh D.G. The adult respiratory distress syndrome. Clinical features, factors influencing prognosis and principles of management. Chest. 1971;60:233–239. doi: 10.1378/chest.60.3.233. [DOI] [PubMed] [Google Scholar]
- 5.Murray J.F, Matthay M.A, Luce J.M, et al. An expanded definition of the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 6.Bernard G.R, Artigas A, Brigham K.L, et al. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 7.Ware L.B, Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 8.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Bone R.C, Maunder R, Slotman G, et al. An early test of survival in patients with the adult respiratory distress syndrome. The PaO2/FIo2 ratio and its differential response to conventional therapy. Prostaglandin E1 Study Group. Chest. 1989;96:849–851. doi: 10.1378/chest.96.4.849. [DOI] [PubMed] [Google Scholar]
- 10.Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 11.Rice T.W, Wheeler A.P, Bernard G.R, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 12.Khemani R.G, Patel N.R, Bart R.D, 3rd, et al. Comparison of the pulse oximetric saturation/fraction of inspired oxygen ratio and the PaO2/fraction of inspired oxygen ratio in children. Chest. 2009;135:662–668. doi: 10.1378/chest.08-2239. [DOI] [PubMed] [Google Scholar]
- 13.Garber B.G, Hebert P.C, Yelle J.D, et al. Adult respiratory distress syndrome: a systemic overview of incidence and risk factors. Crit. Care Med. 1996;24:687–695. doi: 10.1097/00003246-199604000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson N.D, Frutos-Vivar F, Esteban A, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Crit. Care. 2007;11:R96. doi: 10.1186/cc6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthay M.A, Zimmerman G.A, Esmon C, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am. J. Respir. Crit. Care Med. 2003;167:1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 16.Hudson L.D, Milberg J.A, Anardi D, et al. Clinical risks for development of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 17.Gajic O, Moore S.B. Transfusion-related acute lung injury. Mayo Clin Proc. 2005;80:766–770. doi: 10.1016/S0025-6196(11)61531-0. [DOI] [PubMed] [Google Scholar]
- 18.Silliman C.C, Paterson A.J, Dickey W.O, et al. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion. 1997;37:719–726. doi: 10.1046/j.1537-2995.1997.37797369448.x. [DOI] [PubMed] [Google Scholar]
- 19.Iscimen R, Cartin-Ceba R, Yilmaz M, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36:1518–1522. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Perez E.R, Sprung J, Afessa B, et al. Intraoperative ventilator settings and acute lung injury after elective surgery: a nested case control study. Thorax. 2009;64:121–127. doi: 10.1136/thx.2008.102228. [DOI] [PubMed] [Google Scholar]
- 21.Gajic O, Dara S.I, Mendez J.L, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 22.Gajic O, Frutos-Vivar F, Esteban A, et al. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med. 2005;31:922–926. doi: 10.1007/s00134-005-2625-1. [DOI] [PubMed] [Google Scholar]
- 23.Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131:1308–1314. doi: 10.1378/chest.06-3048. [DOI] [PubMed] [Google Scholar]
- 24.Gajic O, Rana R, Winters J.L, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am. J. Respir. Crit. Care Med. 2007;176:886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.TenHoor T, Mannino D.M, Moss M. Risk factors for ARDS in the United States: analysis of the 1993 National Mortality Follow-back Study. Chest. 2001;119:1179–1184. doi: 10.1378/chest.119.4.1179. [DOI] [PubMed] [Google Scholar]
- 26.Moss M, Guidot D.M, Steinberg K.P, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28:2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Chen CY, Lee CH, Liu CY, et al. Clinical features and outcomes of severe acute respiratory syndrome and predictive factors for acute respiratory distress syndrome. J. Chinese Med. Association. 2005;68:4–10. doi: 10.1016/S1726-4901(09)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iribarren C, Jacobs D.R, Jr, Sidney S, et al. Cigarette smoking, alcohol consumption, and risk of ARDS: a 15-year cohort study in a managed care setting. Chest. 2000;117:163–168. doi: 10.1378/chest.117.1.163. [DOI] [PubMed] [Google Scholar]
- 29.Moss M, Bucher B, Moore F.A, et al. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. [PubMed] [Google Scholar]
- 30.Matthay M.A, Folkesson H.G, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol. Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 31.Bachofen M, Weibel E.R. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin. Chest. Med. 1982;3:35–56. [PubMed] [Google Scholar]
- 32.Mizgerd J.P. Acute Lower Respiratory Tract Infection. N. Engl. J. Med. 2008;358:716–727. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roumen R.M, Hendriks T, van der Ven-Jongekrijg J, et al. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann. Surg. 1993;218:769–776. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnelly T.J, Meade P, Jagels M, et al. Cytokine, complement, and endotoxin profiles associated with the development of the adult respiratory distress syndrome after severe injury. Crit. Care Med. 1994;22:768–776. doi: 10.1097/00003246-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Suter P.M, Suter S, Girardin E, et al. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am. Rev. Respir. Dis. 1992;145:1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- 36.Gong M.N, Zhou W, Williams P.L, et al. -308GA and TNFB polymorphisms in acute respiratory distress syndrome. Eur. Respir. J. 2005;26:382–389. doi: 10.1183/09031936.05.00000505. [DOI] [PubMed] [Google Scholar]
- 37.Schultz M.J, Haitsma J.J, Zhang H, et al. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia--a review. Crit. Care Med. 2006;34:871–877. [PubMed] [Google Scholar]
- 38.Castro C.Y. ARDS and diffuse alveolar damage: a pathologist’s perspective. Semin. Thorac. Cardiovasc. Surg. 2006;18:13–19. doi: 10.1053/j.semtcvs.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Katzenstein A.L, Bloor C.M, Leibow A.A. Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review. Am. J. Pathol. 1976;85:209–228. [PMC free article] [PubMed] [Google Scholar]
- 40.Esteban A, Fernandez-Segoviano P, Frutos-Vivar F, et al. Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann. Int. Med. 2004:141. doi: 10.7326/0003-4819-141-6-200409210-00009. [DOI] [PubMed] [Google Scholar]
- 41.de Hemptinne Q, Remmelink M, Brimioulle S, et al. ARDS: a clinicopathological confrontation. Chest. 2009;135:944–949. doi: 10.1378/chest.08-1741. [DOI] [PubMed] [Google Scholar]
- 42.Rubenfeld G.D, Caldwell E, Granton J, et al. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116:1347–1353. doi: 10.1378/chest.116.5.1347. [DOI] [PubMed] [Google Scholar]
- 43.Ely E.W, Smith A.C, Chiles C, et al. Radiologic determination of intravascular volume status using portable, digital chest radiography: a prospective investigation in 100 patients. Crit. Care Med. 2001;29:1502–1512. doi: 10.1097/00003246-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Wheeler A.P, Bernard G.R. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 45.Goodman L.R, Fumagalli R, Tagliabue P, et al. Adult respiratory distress syndrome due to pulmonary and extrapulmonary causes: CT, clinical, and functional correlations. Radiology. 1999;213:545–552. doi: 10.1148/radiology.213.2.r99nv42545. [DOI] [PubMed] [Google Scholar]
- 46.Lichtenstein D.A, Meziere G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rana R, Vlahakis N.E, Daniels C.E, et al. B-type natriuretic peptide in the assessment of acute lung injury and cardiogenic pulmonary edema. Crit. Care Med. 2006;34:1941–1946. doi: 10.1097/01.CCM.0000220492.15645.47. [DOI] [PubMed] [Google Scholar]
- 48.Ferguson N.D, Meade M.O, Hallett D.C, et al. High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Intensive Care Med. 2002;28:1073–1077. doi: 10.1007/s00134-002-1354-y. [DOI] [PubMed] [Google Scholar]
- 49.Daniels L.B, Maisel A.S. Natriuretic peptides. J. Am. Coll. Car-diol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 50.Leaver S.K, Evans T.W. Acute respiratory distress syndrome. BMJ. 2007;335:389–394. doi: 10.1136/bmj.39293.624699.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel S.R, Karmpaliotis D, Ayas N.T, et al. The role of open-lung biopsy in ARDS. Chest. 2004;125:197–202. doi: 10.1378/chest.125.1.197. [DOI] [PubMed] [Google Scholar]
- 52.Baumann H.J, Kluge S, Balke L, et al. Yield and safety of bedside open lung biopsy in mechanically ventilated patients with acute lung injury or acute respiratory distress syndrome. Surgery. 2008;143:426–433. doi: 10.1016/j.surg.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Charbonney E, Robert J, Pache J.C, et al. Impact of bedside open lung biopsies on the management of mechanically ventilated immunocompromised patients with acute respiratory distress syndrome of unknown etiology. J. Crit. Care. 2009;24:122–128. doi: 10.1016/j.jcrc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Webb H.H, Tierney D.F. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am. Rev. Respir. Dis. 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 55.Parker J.C, Townsley M.I, Rippe B, et al. Increased microvascular permeability in dog lungs due to high peak airway pressures. J. Appl. Physiol. 1984;57:1809–1816. doi: 10.1152/jappl.1984.57.6.1809. [DOI] [PubMed] [Google Scholar]
- 56.Dreyfuss D, Soler P, Basset G, et al. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am. Rev. Respir. Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 57.Dreyfuss D, Basset G, Soler P, et al. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am. Rev. Respir. Dis. 1985;132:880–884. doi: 10.1164/arrd.1985.132.4.880. [DOI] [PubMed] [Google Scholar]
- 58.Dreyfuss D, Soler P, Saumon G. Mechanical ventilation-induced pulmonary edema. Interaction with previous lung alterations. Am. J. Respir. Crit. Care Med. 1995;151:1568–1575. doi: 10.1164/ajrccm.151.5.7735616. [DOI] [PubMed] [Google Scholar]
- 59.Muscedere J.G, Mullen J.B, Gan K, et al. Tidal ventilation at low airway pressures can augment lung injury. Am. J. Respir. Crit. Care Med. 1994;149:1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 60.Corbridge T.C, Wood L.D, Crawford G.P, et al. Adverse effects of large tidal volume and low PEEP in canine acid aspiration. Am. Rev. Respir. Dis. 1990;142:311–315. doi: 10.1164/ajrccm/142.2.311. [DOI] [PubMed] [Google Scholar]
- 61.Tremblay L.N, Slutsky A.S. Ventilator-induced injury: from barotrauma to biotrauma. Proc. Assoc. Am. Physicians. 1998;110:482–488. [PubMed] [Google Scholar]
- 62.Tremblay L, Valenza F, Ribeiro SP, et al. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J. Clin. Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imai Y, Kawano T, Miyasaka K, et al. Inflammatory chemical mediators during conventional ventilation and during high frequency oscillatory ventilation. Am. J. Respir. Crit. Care Med. 1994;150:1550–1554. doi: 10.1164/ajrccm.150.6.7952613. [DOI] [PubMed] [Google Scholar]
- 64.Ranieri V.M, Suter P.M, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 65.Girard T.D, Bernard G.R. Mechanical ventilation in ARDS: a state-of-the-art review. Chest. 2007;131:921–929. doi: 10.1378/chest.06-1515. [DOI] [PubMed] [Google Scholar]
- 66.Slutsky A.S, Tremblay L.N. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am. J. Respir. Crit. Care Med. 1998;157:1721–1725. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- 67.Hamilton P, Onayemi A, Smyth J.A, et al. Comparison of conventional and high-frequency ventilation: oxygenation and lung pathology. J Appl Physiol. 1983;55:131–138. doi: 10.1152/jappl.1983.55.1.131. [DOI] [PubMed] [Google Scholar]
- 68.Chan K.P, Stewart T.E, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest. 2007;131:1907–1916. doi: 10.1378/chest.06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peek GJ, Clemens F, Elbourne D, et al. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv. Res. 2006;6:163. doi: 10.1186/1472-6963-6-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liesching T, Kwok H, Hill N.S. Acute applications of noninvasive positive pressure ventilation. Chest. 2003;124:699–713. doi: 10.1378/chest.124.2.699. [DOI] [PubMed] [Google Scholar]
- 71.Rana S, Jenad H, Gay P.C, et al. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit. Care. 2006;10:R79. doi: 10.1186/cc4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiedemann H.P, Wheeler A.P, Bernard G.R, et al. Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 73.Rosenberg A.L, Dechert R.E, Park P.K, et al. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J. Intensive Care Med. 2009;24:35–46. doi: 10.1177/0885066608329850. [DOI] [PubMed] [Google Scholar]
- 74.Meduri G.U, Golden E, Freire A.X, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 75.Steinberg K.P, Hudson L.D, Goodman R.B, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 76.Tang B.M, Craig J.C, Eslick G.D, et al. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit. Care Med. 2009;37:1594–1603. doi: 10.1097/CCM.0b013e31819fb507. [DOI] [PubMed] [Google Scholar]
- 77.Hermans G, De Jonghe B, Bruyninckx F, et al. Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database Syst. Rev. 2009 doi: 10.1002/14651858.CD006832.pub2. CD006832. [DOI] [PubMed] [Google Scholar]
- 78.Taut F.J, Rippin G, Schenk P, et al. A Search for subgroups of patients with ARDS who may benefit from surfactant replacement therapy: a pooled analysis of five studies with recombinant surfactant protein-C surfactant (Venticute) Chest. 2008;134:724–732. doi: 10.1378/chest.08-0362. [DOI] [PubMed] [Google Scholar]
- 79.Piantadosi C.A, Schwartz D.A. The acute respiratory distress syndrome. Ann. Intern. Med. 2004;141:460–470. doi: 10.7326/0003-4819-141-6-200409210-00012. [DOI] [PubMed] [Google Scholar]
- 80.Montgomery A.B, Stager M.A, Carrico C.J, et al. Causes of mortality in patients with the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1985;132:485–489. doi: 10.1164/arrd.1985.132.3.485. [DOI] [PubMed] [Google Scholar]
- 81.Nuckton T.J, Alonso J.A, Kallet R.H, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N. Engl. J. Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 82.Stapleton R.D, Wang B.M, Hudson L.D, et al. Causes and timing of death in patients with ARDS. Chest. 2005;128:525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 83.Suchyta M.R, Clemmer T.P, Elliott C.G, et al. Increased mortality of older patients with acute respiratory distress syndrome. Chest. 1997;111:1334–1339. doi: 10.1378/chest.111.5.1334. [DOI] [PubMed] [Google Scholar]
- 84.McHugh L.G, Milberg J.A, Whitcomb M.E, et al. Recovery of function in survivors of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1994;150:90–94. doi: 10.1164/ajrccm.150.1.8025779. [DOI] [PubMed] [Google Scholar]
- 85.Rubenfeld G.D, Herridge M.S. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 86.Gong M.N, Thompson B.T, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit. Care. Med. 2005;33:1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 87.Yilmaz M, Iscimen R, Keegan M.T, et al. Six-month survival of patients with acute lung injury: prospective cohort study. Crit. Care Med. 2007;35:2303–2307. doi: 10.1097/01.ccm.0000284505.96481.24. [DOI] [PubMed] [Google Scholar]
- 88.Seeley E, McAuley D.F, Eisner M, et al. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63:994–998. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bajwa E.K, Januzzi J.L, Gong M.N, et al. Prognostic value of plasma N-terminal probrain natriuretic peptide levels in the acute respiratory distress syndrome. Crit. Care Med. 2008;36:2322–2327. doi: 10.1097/CCM.0b013e318181040d. [DOI] [PMC free article] [PubMed] [Google Scholar]


