Abstract
The pedicle screw diameter, composite and design are variables that can affect the threshold of intraoperative electromyographic monitoring. Even though we know that larger diameter objects tend to have less resistance, no study documented the effect that this variable could have on pedicle screw resistance. Using high quality equipment, resistance and resistivity of ten pedicle screws (from four manufacturers) were calculated based on known constant current and measured voltage. Voltage was measured three times for each screw to determine intraobserver measurement variability. Resistance of all screws ranged from 1.4 to 3.9 mn (mean = 2.69±0.71 mn). The screw with largest diameter (7.75 mm) had lower resistance than screws with other diameters. Resistivity of screws ranged from 7.12 to 12.63 (mean = 9.9±1.82 μΩ•m) Based on the screw design, one manufacturer’s pedicle screws (A) had significantly lower resistivity compared to three other manufacturers (p<0.01). Larger diameter screws (7.75 mm in diameter) had lower resistance. Screw design (polyaxial or monoaxial) had no effect on its resistance. Screws of one manufacturer (A) showed lower resistivity compared to those manufactured by other three companies.
Keywords: pedicle screw, electrical resistance, electrical resistivity, intraoperative neuromonitoring
INTRODUCTION
In the past decade, pedicle screw systems have proven to provide the highest biomechanical stability in spinal instrumentation, which gives a surgeon greater flexibility to accommodate patient’s intrinsic anatomy. To achieve maximum fixation, the screw should be placed properly within the pedicle. Due to a high variability of pedicle geometry (1), the rates of pedicle cortical perforation have been reported to be between 5,4% and 40% (2-4). Clinically, this is relevant because an incorrect placement of a pedicle screw not only leads to suboptimal spinal stability and higher incidence of pseudoarthrosis (5), but also may lead to neurological irritation or nerve root injury.
An intraoperative electrical testing of pedicle screws is a widely accepted technique of minimizing intra-operative nerve root irritation or an injury during insertion of spinal instrumentation. A properly placed screw can be distinguished from those that perforate a pedicle wall by its minimum level (threshold) of the electrical current needed to elicit a compound muscle action potential (CMAP), On the other hand, stimulation thresholds have been shown to vary in several studies, The strong likelihood of a pedicle wall defect and a potential screw contact with a nerve root and/or the dura ranged from 4mA to 11mA (4-10).
Differences in CMAP threshold values may be attributed to a host of variables (screw, bone, nerve, muscle, subcutaneous fat tissue and skin), One variable that can affect the threshold is electrical conductivity of pedicle screws: their resistivity and resistance, Electrical resistivity (specific electrical resistance) is the property of an element that shows how strongly material opposes electrical current, High resistivity indicates that a material strongly opposes the movement of the electrical charge, Resistance is a material’s opposition to the flow of the current, which is affected by its length, diameter and resistivity, Resistance of a pedicle screw may vary with its length, diameter and resistivity of the material as well, This may affect electrical conduction during intraoperative neuromonitoring, To our knowledge, no earlier study evaluated effects of screw diameter, screw manufacturing and design on its intrinsic electrical properties.
MATERIALS AND METHODS
Ten titanium alloy (T16-Al4-V) pedicle screws from four different manufacturers (Table 1) commonly used in spine surgery were inserted into an aluminum block to provide a connection with the current source, A current meter (Kiethley 195 system digits multimeter, Kiethley Instruments, Inc,, Cleveland, Ohio, USA) was attached to the aluminum block with a current wire on one side, and through a surgical monopolar probe (WR Medical Electronics Company, Stillwater, MN, USA) on the other, The probe was manually kept in contact with a pedicle screw in order to test its resistance based on screw design, This created an electrical circuit (Figure 1).
TABLE 1.
Screws used in the study
*T i-6Al-4V alloy anodized thickness from ~50 nm to ~1μιη

FIGURE 1.

Diagram showing the electrical circuit used in this study.
Two voltage wires that measured a current potential were attached 28 mm apart along the length of a screw (the X1-X2 distance), using silver conductive epoxy (Chemtronics, Kennesaw, GA, USA) which has extremely low resistivity, A 100 mA current (I) was passed through a screw via current wire. Generated voltage was recorded using a voltmeter (HP34401A digital multimeter, Hewlett-Packard Company Test and Measurement Organization, Santa Clara, CA, USA). The resistance was calculated based on Ohm’s law (R=V/I); where R is resistance of an object (measured in ohms; Ω), V is a potential difference across an object (measured in volts) and I is a current through an object (measured in amperes). Each screw was tested three times to minimize an intraobserver error. Each screw had several diameters (Table 1).
In order to test the difference in conductivity at different screw sites based on screw design (monoaxial versus polyaxial), we attached a contact with mono-polar probe in three different locations on the screw surface; the screw top (the top of the screw crown surface), the inner surface (the inner site of the crown) and the screw shaft (a hexagonal screw base) (Figure 2). Resistivity of a screw was then calculated based on ρ = R· A /1, where ρ is resistivity of a material (measured in Ω•m), R is resistance of an object (measured in ohms), A is a cross sectional area (measured in m2), and I is a length (measured in meters).
FIGURE 2.
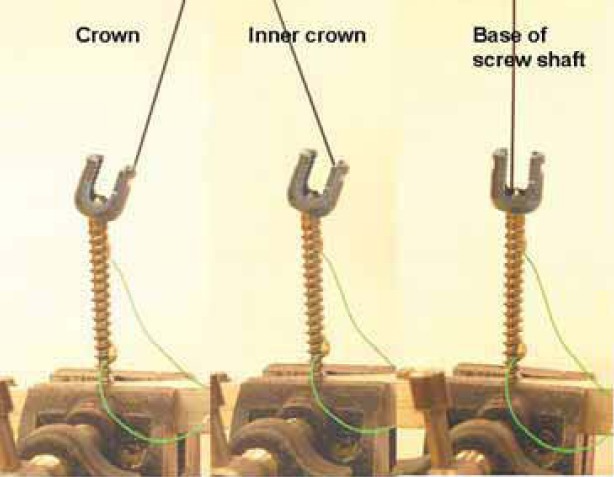
Three different locations of a monopolar probe attachment on a polyaxial screw (a screw top, an inner screw and a screw base).
Statistical analysis was done by using SAS 9.2 (SAS Institute, Cary NC) software and ANOVA. In order to see difference between groups of screws of different diameters we used Tukey grouping as the statistic method to categorize the data. Mean values of screw resistance within the same Tukey group are not statistically different. As it is shown in Table 4, there are 7 different categories of screw sizes, which based on their means are grouped in four groups: Group I (5.5 and 6.5 millimeter screws with not statistically different means: 3.5 and 3.2965 respectively); Group II (6.5 and 6.2 mm screws had no statistically different means: 3.2965 and 2.9250 respectively): Group III (7.5, 7, and 6.25 mm screws had no statistically different means from each other, but they had statistically different means from everyone in groups I, II and IV); and Group IV (7.75 mm screws had a mean of 1.41, statistically different from all other groups).
TABLE 2.
Measured screw voltage data

TABLE 3.
Calculated screw resistance values.

TABLE 4.
Screw diameter as a category

Note that the size 6.5 mm is in both groups I and II. So while size 6.2 is the same group as size 6.5, and the size 6.5 is the same as group 5.5, we cannot say that 6.2 is the same as 5.5, because 6.2 and 5.5 are not in the same group; they have statistically different means)
RESULTS
The obtained voltages and calculated resistance values for each screw are summarized in Table 2 and Table 3. Resistance of all screws ranged from 1.4 to 3.9 mΩ (mean = 2,69±0,71 mΩ). There was a strong negative linear correlation (r= /0.76-/, p<0.001) between resistance and a screw diameter (Figure 3). By treating each screw diameter as a Tukey category (a screw size was treated as a categorical variable, and not as an ordinal or a scale), 7.75-diameter screw (group IV in Table 4) had lower resistance than other diameter screws. A screw design (polyaxial versus monoaxial) and the location of a monopolar probe attachment had no effect on measured screw resistance (Figure 4). Resistivity of screws also varied from 7.12 to 12.63 (mean = 9.9±1.82 μΩ•m). Resistivity values of each screw diameter and manufacturer were summarized in Table 5. Manufacturer A screws had than screws from other three manufacturers ANOVA, p<0.01) (Figure 5). We found no statistically significant differences in screw resistivity among three other manufacturers (B, C and D) regardless of a screw diameter.
FIGURE 3.

Increased screw diameter decreased resistance as shown in the liner regression analysis graph
FIGURE 4.

Resistance in relationship to the monopolar probe and recording site on a screw.
FIGURE 5.

A significantly lower resistivity was measured in manufacturer A compared to other three manufacturers (ANOVA p<0.01).
DISCUSSION
In regards to resistance, our study showed that larger diameter screws with the same manufacturing process had lower resistance and more current flowed through it. However, a manufacturing process might alter the results as the 6.25mm diameter from one manufacturer (manufacturer A) showed as low resistance as the 7.0mm or 7.5mm diameter screws from other manufacturers (manufacturer B, C and D). (see group III in Table 4). Electrical resistivity of titanium is known to be 0.42 μΩ•m(11). In our study, resistivity of pedicle screws had higher ranges, from 7.12 to 12.63 (Table 5). Screws A showed a significantly lower resistivity when compared to screws of other three manufacturers (B, C and D). The difference in resistivity may be caused by difference in the process of anodization of screws (changing the voltage, electrolyte and temperature). Anodization of a titanium pedicle screw is a surface modification process that increases resistance to corrosion and a stability of a material, It is known that this process increases thickness of a titanium oxide layer on its surface and causes changes in a color of a screw and therefore potentially changes resistivity (11).
By measuring most of the same size screws (7x40mm), Anderson et al (12) demonstrated a resistance range from 0 to 36,4 Ω, both for titanium and stainless steel screws across all regions except the mobile crowns of polyaxial screws, Their higher resistance was explained by increased resistance across the mobile crown-shank connection (up to 36,4 Ω) or by high contact resistance between a screw and a measuring instrument, They also recommended placing the monopolar probe in contact with the hexagonal base of a screw shaft or directly on a screw shank below the crown in order to reduce a falsenegative result, In our study, we found no difference between different probe locations (outer and inner mobile crown versus a screw’s shank stimulation) and voltage through a screw, A probable reason for that is that the equipment used in our study was more accurate.
Furthermore, in our study, measured screw resistances were lower (1,4 to 3,9 mH) than in Anderson’s (12) study, We believe that the reason for that is that the contact resistance between a screw and a voltage wire was reduced by using highly electrical conductive elements such as silver epoxy in our study, During testing, we observed that a negligible voltage decrease occurred regardless of current strength (for currents as high as 10 Ampers, the voltage drop was less than 0,1 Volt), Other parameters with higher resistance that can interfere with an intraoperative spinal cord monitoring in vivo are: pedicle cortical thickness, conditions of a recording nerve, conductivity of a muscle and thickness of a subcutaneous fat layer when using percutaneous compound muscle action potential recording, (Figure 6), however, these factors were not examined in this study, More significant reduction in voltage will occur at the interface between a metal screw and a bone into which a screw is inserted, Bone and fat tissue (both perineural and subcutaneous) showed significantly higher resistivity among other human tissues due to low water content (the mean of 160 H«m in cortical bone and 38,50 H«m in fat tissue),(13, 14) With an intact pedicle, more current (>10mA) is needed to pass through a bone in order to be recorded in a peripheral nerve, Other potential pitfalls in neuromonitoring may be caused by an actual condition of a nerve root, Using direct stimulation to a nerve root after decompression, Holland et al (15) showed that significantly higher stimulus intensities were required to evoke myogenic responses from chronically compressed nerve roots compared with normal nerve roots, It is possible that a channel with lower resistance such as fluid in the operative field or a blood vessel next to a nerve root may conduct the electrical current to the nerve as well, With prolong nerve root compression, a perineural fat tissue may diminish and may not play an important role in conduction.
FIGURE 6.

Possible factors that can interfere with conductivity in intraoperative neuromonitoring.
Our study confirmed that resistance of pedicle screws with a larger diameter was lower, while resistivity varied depending on a screw manufacturer, By using a larger screw higher electrical current passes through it which might stimulate a nerve root earlier, Therefore, if higher threshold values are used intraoperatively (10 mA and above), there could be higher incidence of false positive measurements if larger diameter screws are used (7,75mm), A surgeon might accept even a lower threshold levels (below customary 10 mA) as a sign of an intact pedicle during spinal cord stimulation. Therefore, in a large diameter screws even lower threshold (below 10 mA) might not necessary indicate screw perforation through the pedicle cortex, and a possible nerve root injury. One has to keep in mind that manufacturing process might increase or decrease screw resistivity and ultimately determine whether these variables have an effect on an intra-operative spinal cord monitoring. Since the obtained values of resistance are very small (in milliohms), potential clinical implications are not easily predictable.
The question of clinical relevance of our data remains open and further research on this is needed. Based on the combined results of an animal study and a prospective clinical series some authors (5) recommended that threshold stimulus intensity higher than 8.0 mA is to be considered an indicator that a pedicle screw was entirely within the pedicle, while intensity below that threshold was considered to be an indicator for a potential pedicle wall defect due to screw perforation and a possible contact of a screw with a nerve root. These values are not absolute so direct palpation of the inner pedicle wall, intra-operative radiographs and direct visualization should be considered as well. In our institution, we use 11 mA as a threshold as recommended by Clements et al. (10) Because of these considerations and the fact that these threshold values are not absolute, although the difference in resistivity of larger diameter pedicle screw was statistically significant in our study, the clinical relevance is not strongly evident since current technology can not successfully detect such small differences in resistivity (milli-Ampers). These differences are merely an indicator of the difference in the quality and resistivity of the different screws used for spinal fusion. Future research should look into other factors that might affect threshold stimulus intensity such as thickness and resistance of a pedicle cortex around a pedicle screw as well as a subjective interpretation of recordings by an interpreter. During the intraoperative pedicle screw stimulation, values are not definitive as a threshold is identified only when a clear and relatively robust CMAP is obtained 50% of the time. Nevertheless a very high threshold is a good indicator of an appropriate pedicle screw placement.
REFERENCES
- 1.Zındrıck M.R, Wiltse L.L, Doornik A, Widell E.H, Knight G.W, Patwardhan A.G, Thomas J.C, Rothman S.L, Fields B.T. Analysis of the morphometric characteristics of the thoracic and lumbar pedicles. Spine. 1987;12:160–166. doi: 10.1097/00007632-198703000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Gertzbein S.D, Robbins S.E. Accuracy of pedicular screw placement in vivo. Spine. 1990;15(1):11–14. doi: 10.1097/00007632-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 3.George D.C, Krag M.H, Johnson C.C, Van Hal ME, Haugh L.D, Grobler LJ. Hole preparation techniques for transpedicle screws. Effect on pull-out strength from human cadaveric vertebrae. Spine. 1991;16(2):181–184. [PubMed] [Google Scholar]
- 4.Glassman S.D, Diamar J.R, Puno R.M, Johnson J.R, Shields C.B, Liden R.D. A prospective analysis of intraoperative electromyo-graphic monitoring of pedicle screw placement with computed tomographic scan confirmation. Spine. 1995;20:1375–1379. [PubMed] [Google Scholar]
- 5.Lenke L.G, Padberg A.M, Russo M.H, Bridwell K.H, Gelb D.E. Triggered electromyographic threshold for accuracy of pedicle screw placement. An animal model and clinical correlation. Spine. 1995;20(14):1585–1591. doi: 10.1097/00007632-199507150-00006. [DOI] [PubMed] [Google Scholar]
- 6.Raynor B.L, Lenke L.G, Bridwell K.H, Taylor B.A, Padberg A.M. Correlation between low triggered electromyographic thresholds and lumbar pedicle screw malposition: analysis of 4857 screws. Spine. 2007;32(24):2673–2678. doi: 10.1097/BRS.0b013e31815a524f. [DOI] [PubMed] [Google Scholar]
- 7.Maguire J, Wallace S, Madiga R, Leppanen R, Draper V. Evaluation of intrapedicular screw position using intraoperative evoked electromyography. Spine. 1995;20(9):1068–1074. doi: 10.1097/00007632-199505000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Calancie B, Madsen P, Lebwohl N. Stimulus-evoked EMG monitoring during transpedicular lumbosacral spine instrumentation: initial clinical results. Spine. 1994;19:2780–1786. doi: 10.1097/00007632-199412150-00008. [DOI] [PubMed] [Google Scholar]
- 9.Djurasovic M, Dimar J.R, 2nd, Glassman SD, Edmonds HL, Carreon LY. A prospective analysis of intraoperative electromyographic monitoring of posterior cervical screw fixation. J Spinal Disord Tech. 2005;18(6):515–518. doi: 10.1097/01.bsd.0000173315.06025.c6. [DOI] [PubMed] [Google Scholar]
- 10.Clements D.H, Morledge D.E, Martin W.H, Betz R.R. Evoked and spontaneous electromyography to evaluate lumbosacral pedicle screw placement. Spine. 1996;21(5):600–4. doi: 10.1097/00007632-199603010-00013. [DOI] [PubMed] [Google Scholar]
- 11.Lütjering G, Williams J.C. Titanium. New York: Springer; 2003. [Google Scholar]
- 12.Anderson D.G, Wierzbowski L.R, Schwartz D.M, Hilibrand A.S, Vaccaro A.R, Albert T.J. Pedicle screws with high electrical resistance: a potential source of error with stimulus-evoked EMG. Spine. 2002;27(14):1577–1581. doi: 10.1097/00007632-200207150-00018. [DOI] [PubMed] [Google Scholar]
- 13.Faes T.J.C, van der Meij H.A, de Munck J.C, Heethaar R M. The electric resistivity of human tissues (100 Hz-10 MHz): a meta-analysis of review studies. Physiol. Meas. 1999;20:R1–R10. doi: 10.1088/0967-3334/20/4/201. [DOI] [PubMed] [Google Scholar]
- 14.Geddes L.A, Baker L.E. The specific resistance of biologic material: A compendium of data for the biomedical engineer and physiologist. Med. Biol. Eng. 1967;5:271–293. doi: 10.1007/BF02474537. [DOI] [PubMed] [Google Scholar]
- 15.Holland N.R, Lukaczyk T.A, Riley L.H, 3rd, Kostuik J.P. Higher electrical stimulus intensities are required to activate chronically compressed nerve roots. Implications for intraoperative electro-myographic pedicle screw testing. Spine. 1998;23(2):224–272. doi: 10.1097/00007632-199801150-00014. [DOI] [PubMed] [Google Scholar]


