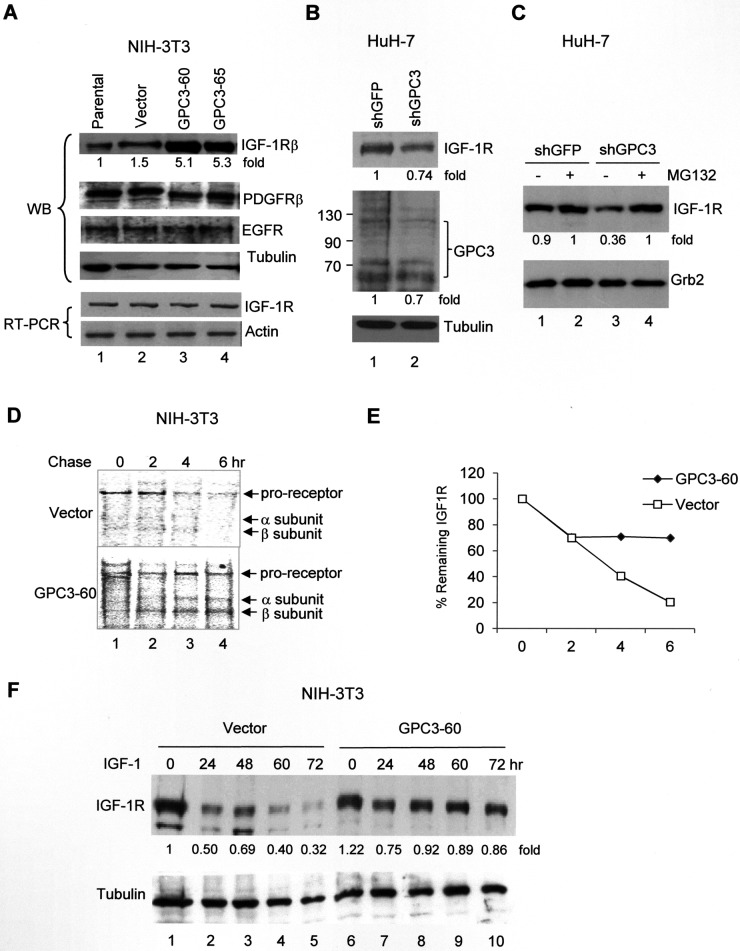
Figure 2. GPC3 decreases IGF-1-induced IGF-1R ubiquitination and degradation.
(A) Western blot analysis of IGF-1R in either parental NIH3T3 cells or stable clones overexpressing the vector alone or GPC3 (GPC3-60 and GPC3-65). Protein extracts (30 μg) were subjected to analysis with anti-IGF-1Rβ, PDGFRβ, EGFR, or tubulin antibodies. IGF-1R RNA levels were measured using RT-PCR, and actin was used as an internal control. (B) GPC3 knockdown decreases IGF-1R levels. HuH-7 cells were transfected with either shGFP or shGPC3. Western blot analysis revealed a decrease in both GPC3 and IGF-1R by shGPC3. The amount of tubulin was used as a control. (C) The proteasome inhibitor MG132 prevents IGF-1R degradation. HuH-7 cells were transfected with either shGFP or shGPC3 and treated with MG132. In the presence of MG132, shGPC3 could not decrease IGF-1R levels. The amount of Grb2 was used as a control. (D) Pulse-chase study. GPC3-60 cells were serum starved, pulsed with [35S]Cys-Met, stimulated with IGF-1, and chased for up to 6 hours. Immunoprecipitation was performed with an anti-IGF-1Rβ antibody. (E) Quantitation of the IGF-1R levels shown in panel (D) using densitometric analysis. (F) A time-course study for IGF-1-induced IGF-1R degradation. NIH3T3 stable lines overexpressing the vector alone or GPC3 (GPC3-60) were harvested for western blot analyses 0, 24, 48, 60, and 72 hrs after IGF-1 stimulation. The degradation of IGF-1R was prevented through the overexpression of GPC3 (p < 0.05, Fisher's exact test). Tubulin was used as a control. Quantitation after normalization by the quantity of tubulin or Grb2 was also expressed as a fold-change relative to the control experiment. All experiments were duplicated.