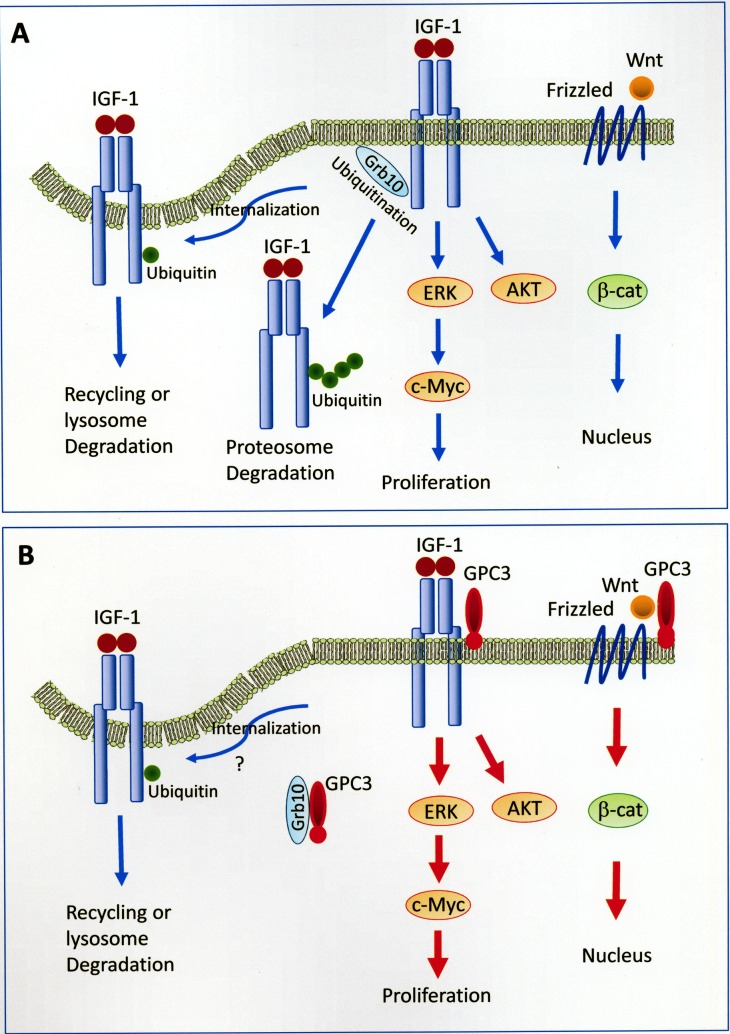
Figure 8. Models of GPC3-enhanced signal transduction.
(A) Activation of Wnt and IGF-1 signaling pathways without the presence of GPC3. Frizzled serves as a cell-surface receptor for Wnt, and the canonical Wnt pathway leads to the accumulation of β-catenin in the cytoplasm and its eventual translocation into the nucleus to act as a transcriptional coactivator. The binding of IGF-1 to IGF-1R induces the activation of both ERK and c-Myc, which leads to cell proliferation, and the AKT signaling pathway, which leads to protein synthesis and growth. The binding of IGF-1 to IGF-1R also leads to receptor ubiquitination, which requires the function of Grb10. Monoubiquitination serves as a regulatory signal for receptor internalization and endosomal sorting, while polyubiquitination targets proteins for degradation by the 26S proteasome [42, 43]. The mechanism for the translocation of polyubiquitinated receptor tyrosine kinase to the proteasome is currently not clear. (B) GPC3 activates β-catenin [19], ERK, AKT [44], and c-Myc [45] through cell-membrane receptors. GPC3 binds to and potentially sequestrates Grb10, thereby blocking IGF-1R ubiquitination and degradation in the proteasome. The status of receptor internalization in the presence of GPC3 was not examined in the present study. The mechanism for the cytoplasmic translocation of GPC3 is currently not clear.