Abstract
Background
New-onset atrial fibrillation (NOAF) occurs frequently in patients with acute myocardial infarction (AMI), and is associated with increased subsequent cardiovascular mortality. However, only a few studies directly evaluated the relationship of left ventricular ejection fraction (LVEF) or left atrium diameter (LAD) and NOAF following AMI.
Materials and Methods
MEDLINE®, EMBASE® and the Cochrane Library were carried out to find studies until January 2017. Pooled mean difference (MD) and 95% confidence interval (CI) were calculated to evaluate the value of LVEF and LAD in the prediction of NOAF after AMI. We performed sensitivity analyses to explore the potential sources of heterogeneity. Statistical analyses were carried out using the Revman 5.3.
Result
We included 10 qualifying studies comprising a total of 708 patients with NOAF and 6785 controls. Overall, decreased LVEF and increased LAD levels had a significant positive association with NOAF in patients with AMI. The MD in the LVEF levels between the patients with and those without NOAF was −4.91 units (95% Cl: −5.70 to −4.12), test for overall effect z-score = 12.18 (p < 0.00001, I2 = 35%). Moreover, in a subgroup analysis, the MD for LAD and NOAF was 2.55 units (95% Cl: 1.91 to 3.19), test for overall effect z-score = 7.80 (p < 0.00001, I2 = 57%).
Conclusions
Our meta-analysis demonstrated that both decreased LVEF and increased LAD levels were associated with greater risk of NOAF following AMI.
Keywords: atrial fibrillation, left ventricular ejection fraction, left atrium diameter, acute myocardial infarction
INTRODUCTION
Atrial fibrillation (AF) occurs commonly in hospitalized patients with acute myocardial infarction (AMI), with a reported incidence between 2% and 20% [1], and is closely associated with prolonged hospitalization, increased subsequent cardiovascular mortality in AMI patients [2–6]. The development of AF in the AMI setting is multiple factors, including older age, systemic inflammation, heart failure, acute ischemia, elevated left ventricular (LV) end-diastolic pressure, left atrial (LA) enlargement or infarction [7]. As well known, left ventricular ejection fraction (LVEF) serves as a significant prognostic marker of cardiac function, and left atrium diameter (LAD) responses to whether left atrial enlargement or not, both of those abnormalities are considered as a risk predictor for cardiovascular disease. However, to our knowledge, only a few studies directly evaluated the associations between LVEF or LAD and new-onset AF (NOAF) in patients with AMI. So we conducted this comprehensive meta-analysis to explore the impact of LVEF on NOAF following AMI by collecting data for previously published studies. Furthermore, the relationship of LAD and NOAF was assessed by a subgroup analysis.
MATERIALS AND METHODS
Literature search
Our study strictly complies with the guidelines of the meta-analysis of observational studies in epidemiology group (MOOSE) [8]. A comprehensive systematic search of MEDLINE®, EMBASE® and the Cochrane Library was carried out to find relevant studies until January 2017. Searches combined free-text and MeSH terms relating to “left ventricular ejection fraction” or “LVEF,” “atrial fibrillation” and “myocardial infarction” or “myocardial infarct”. Reference lists from the identified articles were manually examined for relevant new articles. Abstracts, unpublished reports, and non-English language articles were not included.
Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) the study had the baseline LVEF levels data based on with and without NOAF after AMI; and 2) the study used NOAF rates as an outcome. The exclusion criteria were: 1) history of AF and did not focus on AMI; 2) lacked of preprocedural LVEF levels; 3) Abstracts without the full text.
Identification of studies
We restricted our search to studies published in English. Abstracts and titles of related articles were initially scanned by a reviewer. Potentially relevant articles were then considered by at least 2 independent reviewers. Disagreements were resolved by discussion or upon consensus from a 3rd or 4th reviewer. Two reviewers agreed on the inclusionary or exclusionary status of 90% of the reviewed studies. Full texts of the selected articles were then screened by both authors for inclusion in the review. All disagreements were resolved by consensus.
Quality assessment and data extraction
Quality assessments were evaluated with the Newcastle-Ottawa Scale (NOS) list for nonrandomized studies. Each study was assessed in three aspects using this “star system”: the selection of the study groups; the comparability of the groups; and the ascertainment of the outcome of interest (Supplementary Tables 1 and 2).
Two blinded reviewers independently used a standardized data-extraction form to determine appropriately to extract data. We extracted data included the lead author's last name, the publication year, and the origin of the studied population; the study design; the characteristics of the studied population (sample size, age, sex, time of AF detection, and withdrawals and dropouts of patients); endpoint evaluations (definitions of NOAF and methods of AF detection); rates of NOAF; and means and SDs of LVEF in each group. Disagreements were resolved by consensus from another reviewer.
Statistical analysis
The association strength between LVEF or LAD and NOAF was measured by the mean difference (MD) and 95% confidence interval [CI). The significance of pooled MD was tested by z-test (P < 0.05 was considered significant). Heterogeneity was evaluated with Cochran's Q statistic and quality by I2 statistic. We premeditated that mild heterogeneity might be less than 30 percent of the variability in point estimates and the values of I2 exceeding 50% might express as significant heterogeneity [9], so we used the random-effects model for our study and between study variance, otherwise, with a fixed-effects model. To explore sources of heterogeneity, we performed several sensitivity analyses. Publication bias was also evaluated by inspecting funnel plots. All analyses were conducted with the use of Review Manager, version 5.3 (Revman, The Cochrane Collaboration; Oxford, UK).
RESULTS
Search results
The search yielded 546 research reports, of which 49 were excluded for having the same title or authors; 454 were excluded because they were laboratory studies, animal studies, review articles, or irrelevance to the current analysis. Of the remaining 33 studies, 21 studies did not assess the NOAF or AMI. 8 studies researched segmental LVEF levels and lacked of concrete LVEF data. 2 studies included the history of AF. One study just published by abstract. One study included patients after cardiac surgery. The foregoing studies were all excluded, and 10 observational studies [10–19] were finally included in our meta-analysis (Figure 1). As a result, 7493 patients were involved in our analysis: 708 patients in AF group and 6785 patients in without AF group.
Figure 1. Flow diagram of the trial-selection process.
AF = atrial fibrillation; AMI = acute myocardial infarction; LVEF = left ventricular ejection fraction.
Baseline characteristics and quality assessment
The NOS for assessing the quality of the 10 studies is shown in Table 1 and the scores ranged from 6–7. Table 2 presents the characteristics of each study. The average age of patients in the included studies ranged from 58 to 74 years and the rate of NOAF ranged from 7.44% to 20.7%.
Table 1. Evaluation of the quality of the 10 included studies by using the Newcastle-Ottawa Scale#.
| Factor | Study type | Selection | Comparability | Exposure or outcome | No. of star | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | ||||
| Cicek 2003 | Case-Control Study | * | * | * | * | * | * | * | 7 | |
| Aronson 2007 | Cohort Study | * | * | * | * | * | * | * | 7 | |
| Gedikli 2008 | Case-Control Study | * | * | * | * | * | * | * | 7 | |
| Bahouth 2009 | Cohort Study | * | * | * | * | * | * | * | 7 | |
| Hwang 2011 | Case-Control Study | * | * | * | * | * | * | * | 7 | |
| Aronson 2011 | Cohort Study | * | * | * | * | * | * | * | 7 | |
| Yoshizaki 2012 | Case-Control Study | * | * | * | * | * | * | * | 7 | |
| Dorje 2013 | Case-Control Study | * | * | * | * | * | * | * | 7 | |
| Guenancia 2014 | Case-Control Study | * | * | * | * | * | * | 6 | ||
| Zhang 2014 | Case-Control Study | * | * | * | * | * | * | 6 | ||
#The Newcastle-Ottawa Scale criteria are listed in supplementary files.
Table 2. Characteristics of the 10 studies included in the meta-analysis.
| Factor | Year | Study population | Patients, n | Male, n | Mean Age, yrs | New-onset AF rate, % | Time of AF detection | Timing of LVEF determination | Methods of AF detection | Method of revascularization |
|---|---|---|---|---|---|---|---|---|---|---|
| Cicek D, et al. | 2003 | Turkey | 100 | 77 | 59 | 19% | During hospitalization | N/A | ECG was monitored continuously | Thrombolytic (69%) |
| Aronson D, et al. | 2007 | Israel | 1209 | 936 | 62 | 11.3% | During the index hospitalization | Pre-interventional | Telemetry strips and ECGs | N/A |
| Gedikli O, et al. | 2008 | Turkey | 92 | 67 | 58 | 20.7% | During the first 7d after AMI | N/A | ECG was monitored continuously | N/A |
| Bahouth F, et al. | 2009 | Israel | 1920 | 1505 | 64 | 8.4% | At admission or later during the hospital stay | A median of 2 days from admission | Telemetry strips and ECGs | N/A |
| Hwang HJ, et al. | 2011 | South Korea | 401 | 294 | 61 | 8.2% | Within 24 h after AMI | Pre-interventional | Telemetry strips and ECGs | PTCA, CABG or medical treatment |
| Aronson D, et al. | 2011 | Israel | 1169 | 817 | 64 | 9.4% | During a follow-up period of 6 months. | A median of 2 days from admission | Telemetry strips and ECGs | N/A |
| Yoshizaki T, et al. | 2012 | Japan | 176 | 152 | 74 | 13.6% | During hospitalization | On day 5–7 of admission | ECG was monitored continuously | N/A |
| Dorje T, et al. | 2013 | China | 268 | 224 | 64 | 13.4% | During the AMI hospitalization | Pre-interventional | Telemetry strips and ECGs | N/A |
| Guenancia C, et al. | 2014 | France | 1123 | 779 | 79 | 8.1% | During the AMI hospitalization | On admission | Telemetry strips and ECGs | PCI (69%) Other (31%) |
| Zhang X, et al. | 2014 | China | 1035 | 693 | 65 | 7.44% | During the AMI hospitalization | Pre-interventional | ECG was monitored continuously | PCI (23.38%) Thrombolysis (1.30%) |
AF = atrial fibrillation; LVEF = left ventricular ejection fraction; ECG = electrocardiograph; N/A = not applicable; AMI = acute myocardial infarction; PTCA = percutaneous transluminal coronary angioplasty; CABG = coronary artery bypass grafting; PCI = percutaneous coronary intervention.
Quantitative data synthesis and heterogeneity analysis
Overall, decreased baseline LVEF levels had a significant positive association with NOAF in patients with AMI. The MD in the LVEF levels between the patients with and those without NOAF was −4.91 units (95% Cl: −5.70 to −4.12), test for overall effect z-score = 12.18 (p < 0.00001, I2 = 35%) (Figure 2). However, an asymmetric funnel plot showed the possible existence of publication bias (Figure 3). Because of small sample size, we could not explain the exact cause of heterogeneity in our meta-analysis.
Figure 2. Comparison of LVEF levels between AF and without AF groups in the 10 included studies.
CI = confidence interval; AF = atrial fibrillation; LVEF =left ventricular ejection fraction.
Figure 3. Funnel plot of the 10 included studies.
SE = standard error; MD = mean difference.
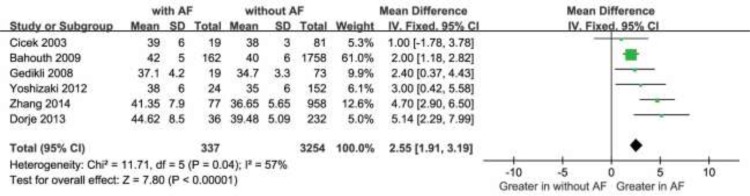
Moreover, The MD in a subgroup analysis for LAD levels between the patients with, and those without NOAF was 1.34 units (95% Cl: 1.04 to 1.64), test for overall effect z-score = 8.75 (p < 0.00001, I2 = 79%) (Figure 4). The heterogeneity test showed that there were significant differences between individual studies (p < 0.00001; I2 = 79%). We subsequently performed sensitivity analyses in order to identify the origin of this heterogeneity [20]. As shown in Figure 5, after excluding the studies by Aronson D et al. [19] the heterogeneity test showed less effects on the results (p < 0.00001, I2 = 57%), whereas the MD in the LAD levels between the patients with and without NOAF was 2.55units (95% Cl: 1.91 to 3.19), test for overall effect z-score = 7.80 (p < 0.00001). As known, the study by Aronson D et al. [19] had a longer follow-up period of 6 months, which was different from the remaining 6 studies, and this might be a possible source of heterogeneity.
Figure 4. Comparison of LAD levels between AF and without groups in the 7 included studies.
CI = confidence interval; AF = atrial fibrillation; LAD = left atrium diameter.
Figure 5. Comparison of LAD levels between AF and without groups in the remaining 6 included studies.
CI = confidence interval; AF = atrial fibrillation; LAD = left atrium diameter.
DISCUSSION
Interestingly, our meta-analysis demonstrated that lower LVEF levels were associated with NOAF occurrence after AMI. Furthermore, in a subgroup analysis, we also found that increased LAD levels related to greater risk of NOAF following AMI, although there was significant heterogeneity. Nonetheless, sensitivity analyses indicated that differences in follow-up period might account for the heterogeneity. Thus, present study might provide insights into mechanisms and lead to greater understanding of the risk factors for NOAF after AMI.
As well known, current evidence regarding the associations between NOAF and in-hospital or long-term outcomes in AMI patients is convincing. These outcomes include the length of hospital stay, heart failure, stroke, recurrent myocardial ischemia, major bleeding and increased mortality [5, 21–25]. Therefore, prediction of NOAF following hospitalization for AMI may reduce clinical adverse events [26]. In the setting of AMI, previous studies have demonstrated a number of risk factors for NOAF in AMI patients, such as old age, female gender, obesity, history of hypertension, history of stroke, higher Killip class or heart failure, hypotension, higher heart rate, higher CHADS2 score, increased peak creatinine kinase, C-reactive protein and N-terminal pro-brain natriuretic peptide levels [5, 6, 13, 16, 21, 23–25, 27, 28]. To our knowledge, this is the first meta-analysis directly evaluating the impact of LVEF and LAD on NOAF in patients with AMI.
Even though the exact mechanism for LVEF or LAD in AMI patients with NOAF was still unclear, several previous relevantly studies have contributed evidence to investigate it and provided potential responsible mechanisms. Aronson and colleagues reported that both LVEF and LAD were independently associated with NOAF, suggesting that increased LV filling pressures may contribute to the development of AF after AMI [19]. Numbers of studies have reported multitudinous pathologic mechanisms of AF following AMI, which could include abrupt changes such as increased LV filling pressure, deterioration of LV systolic functions, or direct ischemic insult of the atria [11, 22, 23, 29–32]. AMI often results in change LV filling dynamics, which may lead to advanced diastolic dysfunction. Subsequently, diastolic dysfunction may produce increased LA pressure and initiate LA remodeling, promoting the progression to AF [5, 7, 19]. In addition, experimental and clinical researches have demonstrated that increasing atrial pressure and/or causing acute atrial dilatation may act an important part in the development of AF in the AMI [5–7, 18, 29, 33]. Hence, it was not difficult to understand that left atrial enlargement that assessed by LAD was major predisposing factors for the development of AF [34–36]. Overall, all of above might indicate the potential mechanisms for the result of the present meta-analysis.
From the conclusion of this study, we could deduce that decreased LVEF and increased LAD levels might be associated with worse clinical prognosis in patient with NOAF following AMI. Early identification of patients with AMI who are at risk of AF attack is of particular importance. Hence, NOAF should be close monitoring for avoiding hemodynamic depression. Management of AF in patients with AMI should follow guideline recommendations [37–39]. It has been well established that oral anticoagulation is a proven therapy for stroke prevention in AF patients with high thromboembolic risk [21]. However, there is insufficient evidence to support prophylactic anticoagulant therapy for AMI patients with high risk of AF. Further studies specific to AF prevention in the patient with AMI are needed.
Our meta-analysis may provide worthy and reliable information regarding the relationships between LVEF, LAD, and NOAF in patients with AMI. However, there are still some potential limitations to this meta-analysis. First, the definitions of NOAF and methods of AF detection were not accordant, and some information on the timing of LVEF determination and the method of revascularization was not applicable, which might be subject to the source of potential bias. Second, most of the included studies did not directly research the impact of LVEF and/or LAD on NOAF after AMI, some potential confounders might have not entirely eliminated. Third, our analysis was based on observational studies, and the numbers of studies and patients were rather limited. Finally, the conclusions of the absence of publication bias were not always reliable. Therefore, the results of our analysis should be interpreted cautiously, and future investigations are needed to clarify the mechanisms of NOAF further.
CONCLUSIONS
In conclusion, our meta-analysis demonstrated that both decreased LVEF and increased LAD levels might be associated with greater risk of NOAF following AMI, which contributing compelling evidence that LVEF and LAD may be a useful marker in predicting NOAF in AMI patients.
SUPPLEMENTARY MATERIALS TABLES
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interests.
FUNDING
This work was partially supported by the National Natural Scientific Foundation (No. 81473471).
REFERENCES
- 1.Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. 2011;123:1587–1593. doi: 10.1161/CIRCULATIONAHA.110.986661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 3.Pai SM, Pai RG. Management of atrial fibrillation. N Engl J Med. 1992;327:1031. [PubMed] [Google Scholar]
- 4.Jabre P, Jouven X, Adnet F, Thabut G, Bielinski SJ, Weston SA, Roger VL. Atrial fibrillation and death after myocardial infarction: a community study. Circulation. 2011;123:2094–2100. doi: 10.1161/CIRCULATIONAHA.110.990192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, Solomon AJ. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. 2000;101:969–974. doi: 10.1161/01.cir.101.9.969. [DOI] [PubMed] [Google Scholar]
- 6.Asanin M, Perunicic J, Mrdovic I, Matic M, Vujisic-Tesic B, Arandjelovic A, Vasiljevic Z, Ostojic M. Prognostic significance of new atrial fibrillation and its relation to heart failure following acute myocardial infarction. Eur J Heart Fail. 2005;7:671–676. doi: 10.1016/j.ejheart.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30:1038–1045. doi: 10.1093/eurheartj/ehn579. [DOI] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 10.Aronson D, Boulos M, Suleiman A, Bidoosi S, Agmon Y, Kapeliovich M, Beyar R, Markiewicz W, Hammerman H, Suleiman M. Relation of C-reactive protein and new-onset atrial fibrillation in patients with acute myocardial infarction. Am J Cardiol. 2007;100:753–757. doi: 10.1016/j.amjcard.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Hwang HJ, Ha JW, Joung B, Choi EH, Kim J, Ahn MS, Lee MH, Jang Y, Chung N, Kim SS. Relation of inflammation and left atrial remodeling in atrial fibrillation occurring in early phase of acute myocardial infarction. Int J Cardiol. 2011;146:28–31. doi: 10.1016/j.ijcard.2009.05.065. [DOI] [PubMed] [Google Scholar]
- 12.Gedikli O, Orem C, Baykan M, Karahan C, Kucukosmanoglu M, Sahin S, Korkmaz L, Yilmaz H, Celik S. Association between serum C-reactive protein elevation and atrial fibrillation after first anterior myocardial infarction. Clin Cardiol. 2008;31:482–487. doi: 10.1002/clc.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guenancia C, Stamboul K, Garnier F, Beer JC, Touzery C, Lorgis L, Cottin Y, Zeller M. Obesity and new-onset atrial fibrillation in acute myocardial infarction: a gender specific risk factor. Int J Cardiol. 2014;176:1039–1041. doi: 10.1016/j.ijcard.2014.07.291. [DOI] [PubMed] [Google Scholar]
- 14.Yoshizaki T, Umetani K, Ino Y, Takahashi S, Nakamura M, Seto T, Aizawa K. Activated inflammation is related to the incidence of atrial fibrillation in patients with acute myocardial infarction. Intern Med. 2012;51:1467–1471. doi: 10.2169/internalmedicine.51.7312. [DOI] [PubMed] [Google Scholar]
- 15.Dorje T, Wang X, Shao M, Zhou J, Cui X, Zhang F, Qian J, Ge J. Plasma N-terminal pro-brain natriuretic peptide levels predict new-onset atrial fibrillation in patients with acute myocardial infarction. Int J Cardiol. 2013;168:3135–3137. doi: 10.1016/j.ijcard.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Li G, Zhao Z, Xu Y, Liu T. The value of CHADS2 score in predicting new-onset atrial fibrillation in Chinese patients with acute myocardial infarction. Int J Cardiol. 2014;176:1235–1237. doi: 10.1016/j.ijcard.2014.07.210. [DOI] [PubMed] [Google Scholar]
- 17.Cicek D, Camsari A, Pekdemir H, Kiykim A, Akkus N, Sezer K, Diker E. Predictive value of P-wave signal-averaged electrocardiogram for atrial fibrillation in acute myocardial infarction. Ann Noninvasive Electrocardiol. 2003;8:233–237. doi: 10.1046/j.1542-474X.2003.08311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahouth F, Mutlak D, Furman M, Musallam A, Hammerman H, Lessick J, Dabbah S, Reisner S, Agmon Y, Aronson D. Relationship of functional mitral regurgitation to new-onset atrial fibrillation in acute myocardial infarction. Heart. 2010;96:683–688. doi: 10.1136/hrt.2009.183822. [DOI] [PubMed] [Google Scholar]
- 19.Aronson D, Mutlak D, Bahouth F, Bishara R, Hammerman H, Lessick J, Carasso S, Dabbah S, Reisner S, Agmon Y. Restrictive left ventricular filling pattern and risk of new-onset atrial fibrillation after acute myocardial infarction. Am J Cardiol. 2011;107:1738–1743. doi: 10.1016/j.amjcard.2011.02.334. [DOI] [PubMed] [Google Scholar]
- 20.Liu T, Li G, Li L, Korantzopoulos P. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol. 2007;49:1642–1648. doi: 10.1016/j.jacc.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 21.Lau DH, Alasady M, Brooks AG, Sanders P. New-onset atrial fibrillation and acute coronary syndrome. Expert Rev Cardiovasc Ther. 2010;8:941–948. doi: 10.1586/erc.10.61. [DOI] [PubMed] [Google Scholar]
- 22.Sakata K, Kurihara H, Iwamori K, Maki A, Yoshino H, Yanagisawa A, Ishikawa K. Clinical and prognostic significance of atrial fibrillation in acute myocardial infarction. Am J Cardiol. 1997;80:1522–1527. doi: 10.1016/s0002-9149(97)00746-7. [DOI] [PubMed] [Google Scholar]
- 23.Crenshaw BS, Ward SR, Granger CB, Stebbins AL, Topol EJ, Califf RM. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO-I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30:406–413. doi: 10.1016/s0735-1097(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 24.Wong CK, White HD, Wilcox RG, Criger DA, Califf RM, Topol EJ, Ohman EM. New atrial fibrillation after acute myocardial infarction independently predicts death: the GUSTO-III experience. Am Heart J. 2000;140:878–885. doi: 10.1067/mhj.2000.111108. [DOI] [PubMed] [Google Scholar]
- 25.Pizzetti F, Turazza FM, Franzosi MG, Barlera S, Ledda A, Maggioni AP, Santoro L, Tognoni G, GISSI-3 Investigators Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI-3 data. Heart. 2001;86:527–532. doi: 10.1136/heart.86.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmiegelow MD, Pedersen OD, Kober L, Seibaek M, Abildstrom SZ, Torp-Pedersen C. Incidence of atrial fibrillation in patients with either heart failure or acute myocardial infarction and left ventricular dysfunction: a cohort study. BMC Cardiovasc Disord. 2011;11:19. doi: 10.1186/1471-2261-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren Y, Zeng RX, Li JJ, Guo LH, He DY, Li Y, Liao PD, Zhang MZ. Relation of C-reactive protein and new-onset atrial fibrillation in patients with acute myocardial infarction: A systematic review and meta-analysis. Int J Cardiol. 2015;190:268–270. doi: 10.1016/j.ijcard.2015.04.152. [DOI] [PubMed] [Google Scholar]
- 28.Gao X, Zeng R, Liao P, Zhu H, Zhang M. Relation of N-terminal pro-brain natriuretic peptide and new-onset atrial fibrillation in patients with acute coronary syndrome: a systematic review and meta-analysis. Scand J Clin Lab Invest. 2016;76:460–464. doi: 10.1080/00365513.2016.1199048. [DOI] [PubMed] [Google Scholar]
- 29.Eldar M, Canetti M, Rotstein Z, Boyko V, Gottlieb S, Kaplinsky E, Behar S. Significance of paroxysmal atrial fibrillation complicating acute myocardial infarction in the thrombolytic era. SPRINT and Thrombolytic Survey Groups. Circulation. 1998;97:965–970. doi: 10.1161/01.cir.97.10.965. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen OD, Abildstrom SZ, Ottesen MM, Rask-Madsen C, Bagger H, Kober L, Torp-Pedersen C, TRACE Study Investigators Increased risk of sudden and non-sudden cardiovascular death in patients with atrial fibrillation/flutter following acute myocardial infarction. Eur Heart J. 2006;27:290–295. doi: 10.1093/eurheartj/ehi629. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg RJ, Seeley D, Becker RC, Brady P, Chen ZY, Osganian V, Gore JM, Alpert JS, Dalen JE. Impact of atrial fibrillation on the in-hospital and long-term survival of patients with acute myocardial infarction: a community-wide perspective. Am Heart J. 1990;119:996–1001. doi: 10.1016/s0002-8703(05)80227-3. [DOI] [PubMed] [Google Scholar]
- 32.Hod H, Lew AS, Keltai M, Cercek B, Geft IL, Shah PK, Ganz W. Early atrial fibrillation during evolving myocardial infarction: a consequence of impaired left atrial perfusion. Circulation. 1987;75:146–150. doi: 10.1161/01.cir.75.1.146. [DOI] [PubMed] [Google Scholar]
- 33.Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997;96:1686–1695. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 34.Grigioni F, Avierinos JF, Ling LH, Scott CG, Bailey KR, Tajik AJ, Frye RL, Enriquez-Sarano M. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol. 2002;40:84–92. doi: 10.1016/s0735-1097(02)01922-8. [DOI] [PubMed] [Google Scholar]
- 35.Tani T, Tanabe K, Ono M, Yamaguchi K, Okada M, Sumida T, Konda T, Fujii Y, Kawai J, Yagi T, Sato M, Ibuki M, Katayama M, et al. Left atrial volume and the risk of paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2004;17:644–648. doi: 10.1016/j.echo.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Ariyarajah V, Malinski M, Khadem A, Harizi R, Wolfe K, Spodick DH. Relation of recurrence of atrial fibrillation after non-ST-elevation acute myocardial infarction to left atrial abnormality. Am J Cardiol. 2008;101:30–34. doi: 10.1016/j.amjcard.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 37.Lip GY, Windecker S, Huber K, Kirchhof P, Marin F, Ten Berg JM, Haeusler KG, Boriani G, Capodanno D, Gilard M, Zeymer U, Lane D, Document R, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS) Eur Heart J. 2014;35:3155–3179. doi: 10.1093/eurheartj/ehu298. [DOI] [PubMed] [Google Scholar]
- 38.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 39.Gorenek B, Pelliccia A, Benjamin EJ, Boriani G, Crijns HJ, Fogel RI, Van Gelder IC, Halle M, Kudaiberdieva G, Lane DA, Larsen TB, Lip GY, Lochen ML, et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS) Europace. 2017;19:190–225. doi: 10.1093/europace/euw242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.