Abstract
Reactive oxide species are the middle products of normal metabolism, and play a crucial role in cell signaling transduction. On the contrary, accumulation of excess reactive oxide species results in oxidative stress that often brings multifarious impairment to cells, including decrease of ATP level in cells, elevation of cytosolic Ca2+, DNA damage, dysfunction of biological function in lipid bilayer and so on. These effects will finally lead to all kinds of diseases. Tea polyphenols are widely considered as a kind of excellent antioxidant agents. It can be antioxidants by directly scavenging reactive oxide species or chelating transition metals, and indirectly upregulating the activity of antioxidant enzymes. In addition, tea polyphenols have also been observed a potent pro-oxidant capacity, which directly leads to the generation of reactive oxide species, and indirectly induces apoptosis and death of cancer cells. The underlying characters of its pro-oxidant activity in some diseases is not well understood. The present review we will discuss the dual character of tea polyphenols, both antioxidant and pro-oxidant properties, in some human diseases induced by oxidative stress.
Keywords: oxidative stress, tea polyphenols, antioxidant capacity, pro-oxidant capacity
INTRODUCTION
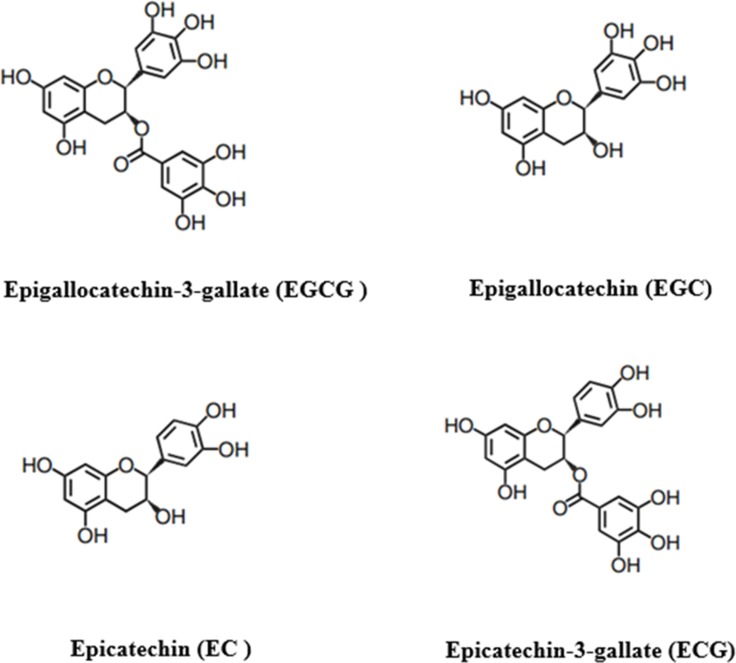
Tea is a popular consumed beverage worldwide [1]. According to the technology of manufacturing, tea can be classified into three major types: green tea, black tea, Oolong tea [2]. The different producing methods will lead to different chemical composition of dry leaves. Tea polyphenols, also known as catechins, is the floorboard of about 30 kinds of phenolic compounds, which are mainly including epigallocatechin-3-gallate (EGCG), epigallocatechin (EGC), epicatechin-3-gallate (ECG) and epicatechin (EC) [2] (Figure 1). And EGCG is the most abundant catechin, and may account for 50–70% of the catechins. The effective antioxidant capacity of tea polyphenols has been widely proved in lots of in vitro and in vivo studies [1, 3, 4]. This could be the critical reason that tea polyphenols, especially EGCG, have some important roles, such as anti-carcinogenic, anti-obesity, anti-inflammatory, anti-aging, anti-cancer, anti-virulence, anti-diabetic, anti-bacterial and neuro-protective effects [5–12].
Figure 1. The main phenolic compounds of tea polyphenols.
The recent study have indicated that many clinical diseases will occur with oxidative damage. As an excellent antioxidant, tea polyphenols can be used to deal with these diseases via resisting oxidative damage in the body [5–12]. However, some experiments showed that tea polyphenols also act as inhibitor in cancer cells through inducing the generation of reactive oxide species (ROS) and apoptosis, and impacting the cell signaling pathway [8, 13–19]. In the present review, we will mainly discuss the antioxidant capacity of tea polyphenols and its dual characters in regulating the redox state in cells, and pay attention to summarize the possible mechanisms of these effects. This review is aiming at stimulating more researches into the role of tea polyphenols in oxidation state, and the potential mechanism for cancer prevention and cure. An overall understanding of the chemical property and biological function of tea polyphenols will be essential for understanding their ultimate virtue in preventive chronic diseases, including cancers.
Free radicals and oxidative stress
Oxidative stress results from massive accumulation of reaction oxygen species (ROS) that is induced by a wide range of factors including radiation, pathogen invasion (hypersensitive reaction), ages, disease and heat stress [20–23] (Figure 2). Generally, ROS production (Table 1) is a physiological process that take place in every aerobic organism [24]. For example, cells release superoxide and hydrogen peroxide from mitochondria in the process of ATP formation [25]. However, development and effects of oxidative stress mainly depend on the capabilities of the organism, alone or with intervention from outside, to keep the dynamic balance of redox state. Thus, oxidative stress has been defined as a disturbance in the dynamically balance between the ROS generation and the antioxidant capacity [26], which leads to the production of an array of free radicals [24]. This often brings multifarious impairment to cells, including decrease of ATP level in cells, elevation of cytosolic Ca2+, DNA damage, dysfunction of biological function in lipid bilayer, decrease of the glutathione level, impairment of electron transport chain, regulation of gene expression through activation of redox-sensitive transcription factors, modulation of inflammatory responses, and change of crucial cell signaling pathways [5, 27–32]. These effects could lead to all kinds of diseases [21, 28–34].
Figure 2. The process of oxidative stress-induced diseases.
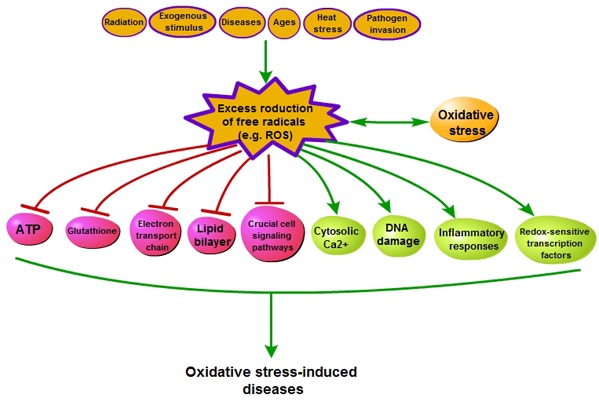
Table 1. Major reaction of oxygen species.
| Oxygen free radicals | RNS | Non-radical oxidant | Iron-oxygen complexes | Organic free radicals |
|---|---|---|---|---|
| O2 | NO· | H2O2 | Fe = O2+ | R-O |
| O2·− | NO2· | ONOOH | Fe = O3+ | R-OO |
| ·OH | HCLO | ·QH | ||
| HO2· | HOSCN |
Oxidative stress and human disease
Many studies indicate that ROS are involved in a good deal of physiological and pathphysiological process [5–12, 35]. The appropriate level of ROS plays a significant role as regulatory mediators in the cell signaling processes of differentiation, proliferation, apoptosis, immunity, defense against microorganism, melanogenesis and aging [36–40]. Conversely, the high level of ROS is dangerous for living organism as they are detrimental to the major cellular components. When the living organisms stay at oxidative stress state for a long time, the disease will occur in various system (Table 2).
Table 2. Diseases that have been linked to oxidative stress.
| Disease | Reference |
|---|---|
| Neurological Disease | |
| Alzheimer's Disease | 38, 49 |
| Parkinson's Disease | 32, 37, 83 |
| Huntington's Disease | 84 |
| Wilson Disease | 85 |
| Cancer | 33, 39, 50, 53, 61 |
| Aging | 21 |
| Vascular Disease | 13 58 |
| Pulmonary Disease | 69 |
| Diabets | 15 18 60 |
| Skin Disease | 88 |
| Chronic kidney Disease | 36, 89 |
| Inflammation | 27, 66, 68, 69, 72 |
| Obesity | 90 |
Oxidative stress and neurodegenerative disease
Several researches have shown that human brain is eccentrically sensitive to oxidative damage [36, 38] (Figure 3). Brain consumes an inordinate fraction (20%) of total oxygen consumption for its relatively small weight (2%), but it doesn't enrich antioxidant defenses [28]. Antioxidant activity in brain is lower than that in other tissues [41], for example, about 10% less than liver [42]. However, neuronal biochemical composition is mainly sensitive to ROS, which is due that it contains abundant unsaturated lipids that are labile to peroxidation and oxidative modification [43].
Figure 3. Pathogenesis of oxidative stress-induced neurodegenerative diseases.
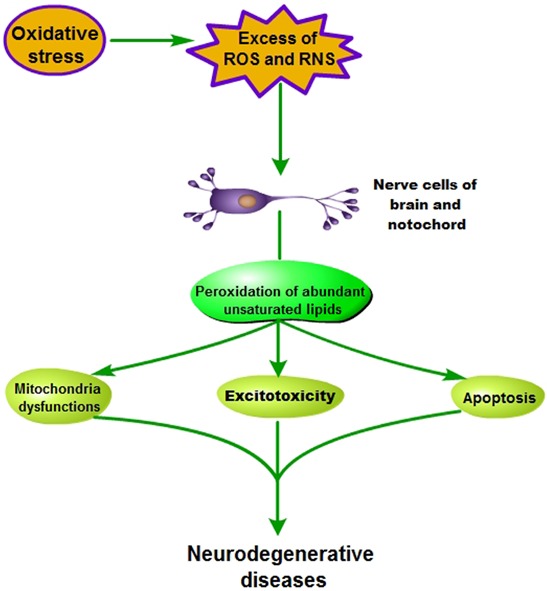
With the oxidative stress forming, human nerve cells of brain and notochord lead to either functional loss or dysfunction perception, and further mitochondria dysfunctions, excitotoxicity and apoptosis were observed during neurodegenerative diseases [37, 38, 41]. Thus, oxidative stress, inducing production of a plenty of ROS and reactive nitrogen species (RNS), is implicated as the pathogenesis of many neurodegenerative diseases, such as Parkinson's disease, Alzheimer's disease, mutiple sclerosis and amyolotrophic lateral sclerosis [44].
Oxidative stress and carcinogenesis
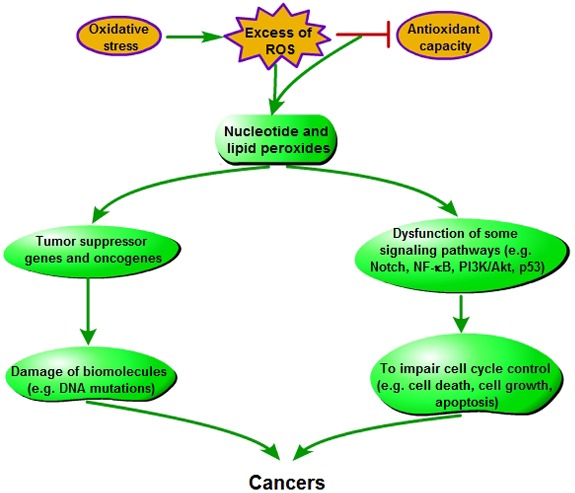
Oxidative stress derived from excess ROS production can lead to carcinogenesis in normal cells (Figure 4). ROS inducing nucleotide and lipid peroxides will directly and indirectly aggravate damage of biomolecules, including DNA (such as mtDNA and genomic DNA) in chain reactions [28]. Besides impairing the genes that are related to cell cycle control or DNA damage pathway [45], ROS-induced DNA damage gives rise to mutations involved in tumor suppressor genes or oncogens [34]. If these damages can not be properly repaired, DNA mutations will occur, which further induces cancer in the end. This is an oncogenic mechanism of oxidative stress. Several previous clinical studies have also implicated ROS exposition with an increased risk of cancer [43, 46]. Moreover, researches on various types of cancer show a possible link between the low activity of superoxide dismutase and the high level of hydroxylated DNA base [47–50].
Figure 4. Pathogenesis of oxidative stress-induced carcinogenesis.
In addition, it has been definitively recognized that apoptosis plays an important role in controlling the tumor expansion [51]. Many studies indicated that cancers, including lymphomas, p53-mutation carcinmas and some hormone-dependent tumors (such as breast, prostate, ovarian, pancreatic and colon cancers), have the closely association with the inhibiton of apoptosis [52]. The activities of several genes known to be influential in cancer progression have been indentified as modulating cell death, especially Bcl-2, fas, and p53 [53]. Meanwhile, the inhibiton of apoptosis in tumor progression can involve some signaling pathways, such as Notch, nuclear factor-κB (NF-κB), protein kinase B (Akt) and p53 [51, 54, 55]. Researchers indicated that ROS could activate the PI3K-dependent Akt counteracted by phosphatase through inhibiting phosphatase and tensin homolog deleted from chromosome 10 (PTEN), and then Akt may foster tumorigenesis [54, 55]. Akt is an inhibitor of apoptosis via triggering the NF-κB activity and regulating pro-apoptotic molecules (such as caspase-9 and Bcl-2). Moreover, Akt affects the nuclear translocation of ubiquitin ligase MDM2, which inhibits p53-mediated apoptosis [56]. It also has been reported that there is cross-talk between Notch-1 and another major cell growth and apoptotic regulatory pathway, and the downregulation of Notch-1 resulted in the increasing cell growth inhibition and apoptosis [51].
Oxidative stress and inflammation
The persistent oxidative stress can lead to chornic inflammation [57] (Figure 5). The relative mechanisms involve the activation of many transcription factors, such as NF-κB, activator protein-1 (AP-1), p53 and Nrf2, which will lead to the expression of more than 500 genes (including inflammatory cytokines), and finally trigger inflammation [46]. During oxidative stress, ROS may promote the release of damage-associated molecular pattern molecules (DAMPs) in the damaged or apoptotic cells, which stimulates toll-like receptors (TLRs) signaling, such as TLR4, in immune cells. This will induce the production of inflammatory factors, and trigger TLR4-mediated inflammation [54]. However, during inflammation, mast cells and leukocytes are recruited to the damage site. This will lead to a ‘respiratory burst’ derived from increasing oxygen uptake, and then ROS is further released and accumulated at the damage site [55, 56]. Thus, there is a vicious circle between oxidative stress and inflammation. These also demonstrate that oxidative stress and inflammation are closely related pathophysiological processes, and both are stimultaneously found in many pathological conditions [39, 57, 58].
Figure 5. Pathogenesis of oxidative stress-induced inflammation.
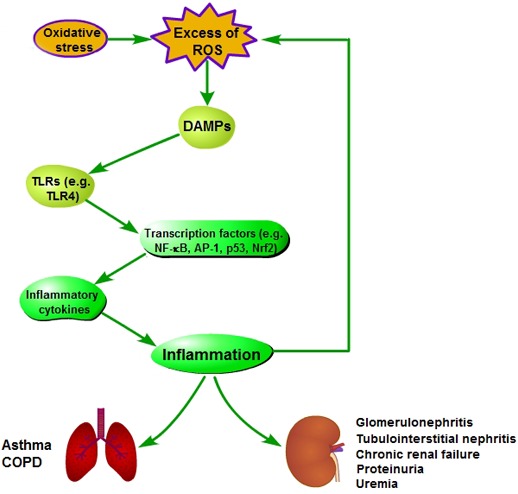
The inflammation derived from oxidative stress in different tissues and organisms will induce different diseases. Inflammatory lung diseases, such as asthma and chronic obstructive pulmonary disease (COPD), are characterized by systemic and local chronic inflammation and oxidative stress [59]. Oxidative stress and inflammation also play an important role in lots of renal diseases, such as glomerulonephritis, tubulointerstitial nephritis, chronic renal failure, proteinuria and uremia [60, 61].
Oxidative stress and some other diseases
Oxidative stress relates to the generation and development of some other diseases. Obesity may induce systemic oxidative stress, and oxidative stress is associated with an irregular production of adipokines that will further induce the development of metabolic syndrome [62]. Oxidative stress also plays a key role in the pathogensis of diabetes and its complications, and then insulin resistance and beta-cell dysfunction that are two central events in the pathophysiology of type 2 diabetes have been linked to redox imbalance [18, 63, 64]. In addition, the level of oxidative stress is relative with aging [65]. Oxidative stress has been found to implicate with age-related macular degeneration and cataracts by altering various cell types in the human eye [66], and excess ROS production can lead to cross-link and aggreation of the crystalline proteins in the lens, which will cause the formation of cataracts [67]. Besides these, nephrotoxicity resulted from some drugs is mainly due to oxidative stress via lipid peroxidation in kidney [68].
Tea polyphenols and diseases triggered by oxidative stress
Green tea consumed within a balance diet is able to improve the organism redox status, rescue cells from oxidative damage, and limit the risk of various degenerative diseases associated to oxidative stress [15, 69–71]. The in vitro and in vivo studies indicate that polyphenols are derived from tea may have the bioactivity to affect the pathogenesis of several chornic disease [4, 12, 71]. Tea polyphenols curing diseases has been partially attributed to the antioxidative capacity. And, in all tea polyphenols, EGCG is mainly responsible for antioxidant capacity.
Many studies show that administration of tea polyphenols can limit carcinogenesis, neurodegenerative diseases, inflammation, aging and renal disease [15, 19, 66, 67, 72]. Most of these diseases are associated with the damage of DNA, proteins and lipids caused by oxidative stress. Tea polyphenols can help to limit this damage via two ways. One is that it can directly act on ROS, and the other is that it may stimulate endogenous defence system [73].
The mechanism of tea polyphenols curing diseases triggered by oxidative stress
Tea polyphenols can relieve the oxidative stress by scavenging ROS and generating more stable phenolic radicals in vivo and in vitro (Figure 6). The ability of EGCG scavenging radicals is mainly derived from the D ring in the galloyl group of its structure [17]. Following analysis of electron paramagnetic resonance spectroscopy, the .OH and O2– are scavenged by EGCG mainly through oxidating the D ring of galloyl group [22, 73]. In addition, tea polyphenols also have indirect antioxidant effects. Many studies have shown that treatment of tea polyphenols may increase the levels of phase II antioxidant enzymes, such as glutathione peroxidase and reductase, glutathione S-transferase, catalase, quinone reductase, and superoxide dismutase in different organs (including liver, small intestine, lung, skin, brain, prostate and oral cavity) of rodents [74–78]. Administration of tea polyphenols can decrease the level of 8-oxoguanine that is a reliable marker for DNA damage induced by oxidative stress, and inhibit DNA oxidative damage via reducing the expression of cytochormes P450 [79–82]. Therefore, tea polyphenols may cure oxidative stress-relative diseases via increasing antioxidant capacity that can relieve the oxidative damage [25, 26, 83–85].
Figure 6. The mechanism of tea polyphenols curing diseases triggered by oxidative stress.
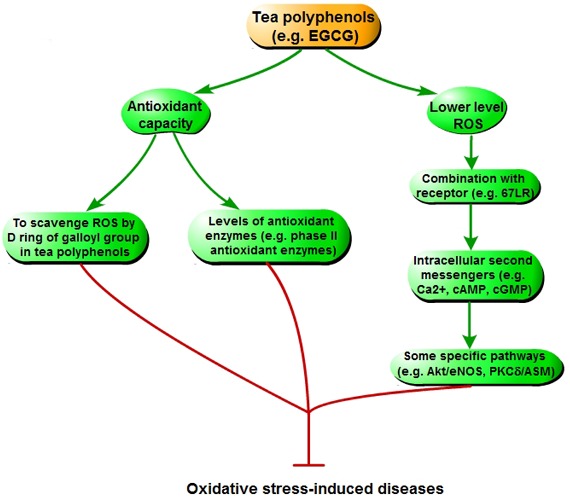
Moreover, there are some other indirect ways of tea polyphenols inhibiting disease induced by oxidative stress. In cell cuture experiment, EGCG is mainly found in the cytosol [86], which indicates that biological function of EGCG may occur through EGCG metabolites or interaction between EGCG and intracelluar molecules. EGCG producing the lower level of ROS, including hydrogen peroxide, will act as a messenger molecule for downstream signaling pathway [87]. However, via combining with a specific receptor (67 kD laminin receptor, 67LR), EGCG also increases some other intracellular second messengers, such as Ca2+, cAMP, and cGMP [88–90]. These messengers, espeicially cGMP, can activate some specific pathways, such as protein kinase B/endothelial nitric oxide synthase (Akt/eNOS) and protein kinase Cδ/acidic sphingomyelinase (PKCδ/ASM). This inhibits oxidative stress, and cures the relative diseases (including cardiovascular disease, cancer and neurodegenerative disease) [25].
Some special pathways involved in tea polyphenols inhibiting cancers
Tea and tea extractive regulating some pathways can also inhibit tumorigenesis in oral, esophageal, forestomach, stomach, intestinal, colon, skin, liver, bladder, prostate and breast cancers [3, 91–93] (Figure 7). (1) Tea polyphenols can induce apoptosis and cell cycle arrest of cancer cells via regulating caspase-3 activation, nuclear condensation, and the expression of Bax, Bcl-2, p21 and p27 (p27Kip1). (2) Tea polyphenols, especially EGCG, can modulate NF-κB, MAPKs, proteasome, epidermal growth factor receptor (EGFR)-mediated, and insulin-like growth factor-I-mediated signaling pathway in cancers. (3) The expressions of some proteins, including AP-1, cyclooxygenase-2 (COX-2), vascular endothelial growth factor (VEGF), matrix metalloproteinase (MMP) and urokinase-plasminogen activator (uPA), are inhibited by tea polyphenols in cancers.
Figure 7. Some special mechanisms that tea polyphenols inhibit cancers, except increasing antioxidant capacity.
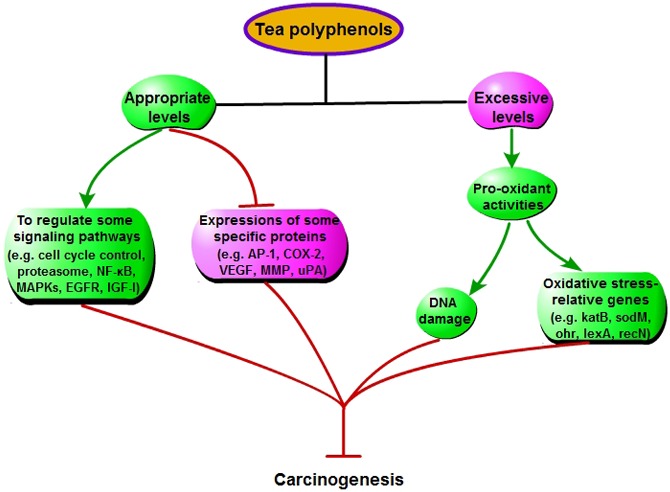
Pro-oxidant activities of tea polyphenols
Although tea polyphenols have generally been regarded as antioxidant, some evidence for its pro-oxidant property is interesting. Tea polyphenols are unstable, and undergo autooxidative reactions resulting in ROS production in vivo and in vitro (like H2O2) [94, 95]. Some reports have shown that tea polyphenols are the apoptosis-inducing agent presenting in the popular beverage [96], increase phosphorylation of histone 2A.X (γH2A.X) that is a marker of oxidative DNA damage, and enhance the expression of oxidative stress-relative genes, such as katB, sodM, ohr, lexA and recN. This can be an important mechanism that tea polyphenols inducing the death of cells via the pro-oxidant property may inhibit carcinogenesis [3] (Figure 7). In the in vitro experiments, tea polyphenols, such as EGCG and EGC, inhibiting prostate cancer cells is a dose-dependent and pH-value-dependent process [97, 98]. Besides these, the recent study have also shown that, compared with normal cells, cancer cells are more sensitive to pro-oxidant activity of tea polyphenols [99].
All the time, tea polyphenols are generally regarded as health promotion agents. However, the high dose of tea polyphenols triggering pro-oxidant activities exists the potential toxic effects in normal hepatocyte and livers, and this cytotoxicity is time- and dose-dependent [100, 101]. Thus, there is a hepatoxicity risk of tea polyphenols in humans.
The different effect of tea polyphenols derived from different teas on antioxidant status
Recent studies have shown that tea polyphenols derived from different teas have different effect on oxidative stress-induced diseases [102–104]. Nowadays, there are three main types of tea consumed by human, including green tea, Oolong tea and black tea. Oolong tea is manufactured by a partial oxidation of the leaf, which is intermediate between the processes for green and black tea [105]. Green and black teas have strong antioxidant property in in vivo and in vitro studies, and green tea has higher antioxidant capacity than fermented teas, such as Oolong tea and black tea [106, 107]. Based on Ferric reducing antioxidant power assay, the lower antioxidant capacity in fermented teas can be due to the decreased content of polyphenols in the fermentation process [108]. However, by using oxygen-radical absorbance capacity assay and Trolox equivalent antioxidant capacity assay in the in vitro studies, black tea extract has comparable antioxidant capacity to green tea extract [109, 110]. The further study showed that black tea extract could protect tissues against oxidative damage derived from lipid peroxidation, and are more effective than green tea extract in scavenging superoxide anion [111]. In addition, Oolong tea extract has higher antioxidant capacity and lipoxygenase inhibitory activity than black tea extract [105], but the biological effect of Oolong tea remains unclear.
The different effect of polyphenols derived from three teas on antioxidant status could be associated with the concentration of monomeric and polymers polyphenols. Black tea contains lower amounts of monomeric polyphenols and higher concentrations of polymers, compared with green tea [112, 113]. Oolong tea are partially oxidized, which leads to an intermediate tea with a lower concentration of polymeric polyphenols and higher concentrations of EGCG, compared with black tea [113].
CONCLUSIONS
Tea is a popular, socially accepted, safe and healthy beverage that is widely consumed around the world. Tea is beneficial to prevent and cure a variety of diseases related to oxidative stress. As one of mainly active compounds in tea, tea polyphenols act as an excellent antioxidant through regulating both endogenous and exogenous antioxidant mechanism, which have been clearly demonstrated in the in vitro and in vivo studies. In addition, some studies have indicated that tea polyphenols have pro-oxidant activity, which can reduce the risk of some types of cancer via ROS-mediated cancer cell death. Although there are a plenty of important results of tea polyphenols protecting the body via regulating the redox status, some further studies, such as its specific mechanism of anti-chronic diseases, need to be done. And the in vivo evidences about the mechanism of its anti-cancer activity should also be further afforded.
ACKNOWLEDGMENTS AND FUNDING
Special thanks to Professor De Wu of Sichuan Agricultural University and Dr. Judy Deng of Oklahoma State University for revising this manuscript. This work was supported by the grant from the China Agriculture Research System (CARS-35), the grant from Major Project of Education Department of Sichuan Province (17ZA0311), and the fund from the research program of “Sheng Yang” students’ association (B2016010).
Footnotes
CONFLICTS OF INTEREST
None.
REFERENCES
- 1.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–50. doi: 10.1016/0091-7435(92)90041-f. https://doi.org/10.1016/0091-7435(92)90041-F [DOI] [PubMed] [Google Scholar]
- 2.Stoner GD, Mukhtar H. Polyphenols as cancer chemopreventive agents. J Cell Biochem. 1995;59:169–80. doi: 10.1002/jcb.240590822. https://doi.org/10.1002/jcb.240590822 [DOI] [PubMed] [Google Scholar]
- 3.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. https://doi.org/10.1016/j.abb.2010.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–84S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 5.Yang CS, Lambert JD, Sang S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch Toxicol. 2009;83:11–21. doi: 10.1007/s00204-008-0372-0. https://doi.org/10.1007/s00204-008-0372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin JK, Lin-Shiau SY. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol Nutr Food Res. 2006;50:211–7. doi: 10.1002/mnfr.200500138. https://doi.org/10.1002/mnfr.200500138 [DOI] [PubMed] [Google Scholar]
- 7.Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets. 2007;7:135–44. doi: 10.2174/187152907780830905. https://doi.org/10.2174/187152907780830905 [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya U, Mukhopadhyay S, Giri AK. Comparative antimutagenic and anticancer activity of three fractions of black tea polyphenols thearubigins. Nutr Cancer. 2011;63:1122–32. doi: 10.1080/01635581.2011.605985. https://doi.org/10.1080/01635581.2011.605985 [DOI] [PubMed] [Google Scholar]
- 9.Yin H, Deng Y, Wang H, Liu W, Zhuang X, Chu W. Tea polyphenols as an antivirulence compound Disrupt Quorum-Sensing Regulated Pathogenicity of Pseudomonas aeruginosa. Sci Rep. 2015;5:17987. doi: 10.1038/srep16158. https://doi.org/10.1038/srep16158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Li L, Kim SH, Hagerman AE, Lü J. Anti-Cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm Res. 2009;26:2066–80. doi: 10.1007/s11095-009-9932-0. https://doi.org/10.1007/s11095-009-9932-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukai K, Ishigami T, Hara Y. Antibacterial activity of tea polyphenols against phytopathogenic bacteria. Agric Biol Chem. 1991;55:1895–7. [Google Scholar]
- 12.Mo J, Chen Y, Huang L, Zhang H, Li J, Zhou W. Neuroprotective effect of tea polyphenols on oxyhemoglobin induced subarachnoid hemorrhage in mice. Oxid Med Cell Longev. 2013;2013:743938. doi: 10.1155/2013/743938. https://doi.org/10.1155/2013/743938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med. 2008;5:338–49. doi: 10.1038/ncpcardio1211. https://doi.org/10.1038/ncpcardio1211 [DOI] [PubMed] [Google Scholar]
- 14.Pierrou S, Broberg P, O’Donnell RA, Pawlowski K, Virtala R, Lindgvist E, Richter A, Wilson SJ, Angco G, Möller S, Bergstrand H, Koopmann W, Wieslander E, et al. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:577–86. doi: 10.1164/rccm.200607-931OC. https://doi.org/10.1164/rccm.200607-931OC [DOI] [PubMed] [Google Scholar]
- 15.Sabu MC, Smitha K, Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol. 2002;83:109–16. doi: 10.1016/s0378-8741(02)00217-9. https://doi.org/10.1016/S0378-8741(02)00217-9 [DOI] [PubMed] [Google Scholar]
- 16.Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (–)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–6. [PubMed] [Google Scholar]
- 17.Severino JF, Goodman BA, Kay CW, Stolze K, Tunega D, Reichenauer TG, Pirker KF. Free radicals generated during oxidation of green tea polyphenols: electron paramagnetic resonance spectroscopy combined with density functional theory calculations. Free Radic Biol Med. 2009;46:1076–88. doi: 10.1016/j.freeradbiomed.2009.01.004. https://doi.org/10.1016/j.freeradbiomed.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 18.Rains JL, Jain. SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–75. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen CL, Samathanam C, Graham S, Dagda RY, Chyu MC, Dunn DM. Green tea polyphenols and 1-α-OH-vitamin D3 attenuate chronic inflammation-induced myocardial fibrosis in female rats. J Med Food. 2012;15:269–77. doi: 10.1089/jmf.2011.0163. https://doi.org/10.1089/jmf.2011.0163 [DOI] [PubMed] [Google Scholar]
- 20.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–94. doi: 10.1093/aob/mcf118. https://doi.org/10.1093/aob/mcf118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. https://doi.org/10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- 22.Ikeda M, Kodama H, Fukuda J, Shimizu Y, Murata M, Kumagai J, Tanaka T. Role of radical oxygen species in rat testicular germ cell apoptosis induced by heat stress. Biol Reprod. 1999;61:393–9. doi: 10.1095/biolreprod61.2.393. https://doi.org/10.1095/biolreprod61.2.393 [DOI] [PubMed] [Google Scholar]
- 23.Bhatnagar A. Biochemical mechanism of irreversible cell injury caused by free radical-initiated reaction. Mol Cell Biochem. 1994;137:9–16. doi: 10.1007/BF00926034. https://doi.org/10.1007/BF00926034 [DOI] [PubMed] [Google Scholar]
- 24.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–50. doi: 10.1042/BST0351147. https://doi.org/10.1042/BST0351147 [DOI] [PubMed] [Google Scholar]
- 25.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelial-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. https://doi.org/10.1038/327524a0 [DOI] [PubMed] [Google Scholar]
- 26.Bodamyali T, Stevens CR, Blake DR, Winyard PG. Reactive oxygen/nitrogen species and acute inflammation: a physiological process. In: Winyard PG, Blake DR, Evans CH, editors. Free radicals and inflammation. Birkhäuser Basel; 2000. https://doi.org/10.1007/978-3-0348-8482-2 [Google Scholar]
- 27.Fu PP, Xia Q, Hwang HM, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. https://doi.org/10.1016/j.jfda.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Váli L, Hahn O, Kupcsulik P, Drahos A, Sárváry E, Szentmihályi K, Pallai Z, Kurucz T, Sípos P, Blázovics A. Oxidative stress with altered element content and decreased ATP level of erythrocytes in hepatocellular carcinoma and colorectal liver metastases. Eur J Gastroenterol Hepatol. 2008;20:393–8. doi: 10.1097/MEG.0b013e3282f495c7. https://doi.org/10.1097/MEG.0b013e3282f495c7 [DOI] [PubMed] [Google Scholar]
- 29.Nicotera P, McConkey D, Svensson SA, Bellomo G, Orrenius S. Correlation between cytosolic Ca2+ concentration and cytotoxicity in hepatocytes exposed to oxidative stress. Toxicology. 1988;52:55–63. doi: 10.1016/0300-483x(88)90196-5. https://doi.org/10.1016/0300-483X(88)90196-5 [DOI] [PubMed] [Google Scholar]
- 30.Liang R, Zhang JP, Skibsted LH. Evaluation of physical integrity of lipid bilayer under oxidative stress: application of fluorescence microscopy and digital image processing. Methods Mol Biol. 2015;1208:111–21. doi: 10.1007/978-1-4939-1441-8_9. https://doi.org/10.1007/978-1-4939-1441-8_9 [DOI] [PubMed] [Google Scholar]
- 31.Miura K, Ishii T, Sugita Y, Bannai S. Cystine uptake and glutathione level in endothelial cells exposed to oxidative stress. Am J Physiol. 1992;262:C50–8. doi: 10.1152/ajpcell.1992.262.1.C50. [DOI] [PubMed] [Google Scholar]
- 32.Abdulwahid Arif I, Ahmad Khan H. Environmental toxins and Parkinson's disease: putative roles of impaired electron transport chain and oxidative stress. Toxicol Ind Health. 2010;26:121–8. doi: 10.1177/0748233710362382. https://doi.org/10.1177/0748233710362382 [DOI] [PubMed] [Google Scholar]
- 33.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. https://doi.org/10.1016/j.cbi.2005.12.009 [DOI] [PubMed] [Google Scholar]
- 34.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. https://doi.org/10.2337/diabetes.48.1.1 [DOI] [PubMed] [Google Scholar]
- 35.Johar S, MacCarthy PA, Shah AM. Oxidative stress and cardiovascular disease. In: Singh KK, editor. Oxidative stress, disease and cancer. World Scientific; 2006. https://doi.org/10.1142/9781860948046_0016 [Google Scholar]
- 36.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008;74:S4–9. doi: 10.1038/ki.2008.516. https://doi.org/10.1038/ki.2008.516 [DOI] [PubMed] [Google Scholar]
- 37.Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Prog Neurobiol. 2013;106–107:589–97. doi: 10.1016/j.pneurobio.2013.04.004. https://doi.org/10.1016/j.pneurobio.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zana M, Janka Z, Kálmán J. Oxidative stress: a bridge between Down's syndrome and Alzheimer's disease. Neurobiol Aging. 2007;28:648–76. doi: 10.1016/j.neurobiolaging.2006.03.008. https://doi.org/10.1016/j.neurobiolaging.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 39.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. https://doi.org/10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Todorova I, Simeonova G, Kyuchukova D, Dinev D, Gadjeva V. Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp Clin Path. 2005;12:190–4. https://doi.org/10.1007/s00580-005-0547-5 [Google Scholar]
- 41.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. https://doi.org/10.2174/157015909787602823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floyd RA, Carney JM. Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol. 1992;32:S22–7. doi: 10.1002/ana.410320706. https://doi.org/10.1002/ana.410320706 [DOI] [PubMed] [Google Scholar]
- 43.Praticò D. Oxidative imbalance and lipid peroxidation in Alzheimer's disease. Drug Dev Res. 2002;56:446–51. https://doi.org/10.1002/ddr.10097 [Google Scholar]
- 44.Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. https://doi.org/10.1016/j.biopha.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 45.Cejas P, Casado E, Belda-Iniesta C, De Castro J, Espinosa E, Redondo A, Sereno M, García-Cabezas MA, Vara JA, Domínguez-Cáceres A, Perona R, González-Barón M. Implications of oxidative stress and cell membrane lipid peroxidation in human cancer (Spain) Cancer Causes Control. 2004;15:707–19. doi: 10.1023/B:CACO.0000036189.61607.52. https://doi.org/10.1023/B:CACO.0000036189.61607.52 [DOI] [PubMed] [Google Scholar]
- 46.Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–90. doi: 10.1089/ars.2008.2270. https://doi.org/10.1089/ARS.2008.2270 [DOI] [PubMed] [Google Scholar]
- 47.Yuzhalin AE, Kutikhin AG. Inherited variations in the SOD and GPX gene families and cancer risk. Free Radic Res. 2012;46:581–99. doi: 10.3109/10715762.2012.658515. https://doi.org/10.3109/10715762.2012.658515 [DOI] [PubMed] [Google Scholar]
- 48.Kang D, Lee KM, Park SK, Berndt SI, Peters U, Reding D, Chatterjee N, Welch R, Chanock S, Huang WY, Hayes RB. Functional variant of manganese superoxide dismutase (SOD2 V16A) polymorphism is associated with prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer study. Cancer Epidemiol Biomarkers Prev. 2007;16:1581–6. doi: 10.1158/1055-9965.EPI-07-0160. https://doi.org/10.1158/1055-9965.EPI-07-0160 [DOI] [PubMed] [Google Scholar]
- 49.Knight JA, Onay UV, Wells S, Li H, Shi EJ, Andrulis IL, Ozcelik H. Genetic variants of GPX1 and SOD2 and breast cancer risk at the Ontario site of the Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2004;13:146–9. doi: 10.1158/1055-9965.epi-03-0164. https://doi.org/10.1158/1055-9965.EPI-03-0164 [DOI] [PubMed] [Google Scholar]
- 50.Satomi A, Murakami S, Hashimoto T, Ishida K, Matsuki M, Sonoda M. Significance of superoxide dismutase (SOD) in human colorectal cancer tissue: correlation with malignant intensity. J Gastroenterol. 1995;30:177–82. doi: 10.1007/BF02348662. https://doi.org/10.1007/BF02348662 [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2011;106:2503–13. doi: 10.1002/cncr.21904. https://doi.org/10.1002/cncr.21904 [DOI] [PubMed] [Google Scholar]
- 52.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. https://doi.org/10.1016/j.cell.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lockshin RA, Zakeri Z. Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol Cell Biol. 2001;2:545–50. doi: 10.1038/35080097. https://doi.org/10.1038/35080097 [DOI] [PubMed] [Google Scholar]
- 54.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. https://doi.org/10.1016/j.cell.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–42. doi: 10.1038/sj.onc.1209097. https://doi.org/10.1038/sj.onc.1209097 [DOI] [PubMed] [Google Scholar]
- 56.Matés JM, Segura JA, Alonso FJ, Márquez J. Oxidative stress in apoptosis and cancer: an update. Arch Toxicol. 2012;86:1649–65. doi: 10.1007/s00204-012-0906-3. https://doi.org/10.1007/s00204-012-0906-3 [DOI] [PubMed] [Google Scholar]
- 57.Cottone S, Lorito MC, Riccobene R, Nardi E, Mulè G, Buscemi S, Geraci C, Guarneri M, Arsena R, Cerasola G. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J Nephrol. 2008;21:175–9. [PubMed] [Google Scholar]
- 58.Vaziri ND, Rodríguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–93. doi: 10.1038/ncpneph0283. https://doi.org/10.1038/ncpneph0283 [DOI] [PubMed] [Google Scholar]
- 59.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;429:195–207. doi: 10.1016/s0014-2999(01)01320-6. https://doi.org/10.1016/S0014-2999(01)01320-6 [DOI] [PubMed] [Google Scholar]
- 60.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. https://doi.org/10.1152/physrev.00018.2001 [DOI] [PubMed] [Google Scholar]
- 61.Galle J. Oxidative stress in chronic renal failure. Nephrol Dial Transplant. 2001;16:2135–42. doi: 10.1093/ndt/16.11.2135. https://doi.org/10.1093/ndt/16.11.2135 [DOI] [PubMed] [Google Scholar]
- 62.Calhau C, Santos A. Oxidative stress in the metabolic syndrome. In: Soares R, Costa C, editors. Oxidative stress, inflammation and angiogenesis in the metabolic syndrome. Springer Netherlands; 2009. https://doi.org/10.1007/978-1-4020-9701-0 [Google Scholar]
- 63.Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C, Ghirlanda G. Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud. 2010;7:15–25. doi: 10.1900/RDS.2010.7.15. https://doi.org/10.1900/RDS.2010.7.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright E, Jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract. 2006;60:308–14. doi: 10.1111/j.1368-5031.2006.00825.x. https://doi.org/10.1111/j.1368-5031.2006.00825.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neki NS. Oxidative stress and aging. Bangladesh J Med Sci. 2015;14:221–7. https://doi.org/10.3329/bjms.v14i3.23468 [Google Scholar]
- 66.Santosa S, Jones PJ. Oxidative stress in ocular disease: does lutein play a protective role? CMAJ. 2005;173:861–2. doi: 10.1503/cmaj.1031425. https://doi.org/10.1503/cmaj.1031425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyer CH, Sekundo W. Nutritional supplementation to prevent cataract formation. Dev Ophthalmol. 2005;38:103–19. doi: 10.1159/000082771. https://doi.org/10.1159/000082771 [DOI] [PubMed] [Google Scholar]
- 68.Massicot F, Lamouri A, Martin C, Pham-Huy C, Heymans F, Warnet JM, Godfroid JJ, Claude JR. Preventive effects of two PAF-antagonists, PMS 536 and PMS 549, on cyclosporin-induced LLC-PK1 oxidative injury. J Lipid Mediat Cell Signal. 1997;15:203–14. doi: 10.1016/s0929-7855(96)00555-x. https://doi.org/10.1016/S0929-7855(96)00555-X [DOI] [PubMed] [Google Scholar]
- 69.Minuzzo M, Ceribelli M, Pitarque-Marti M, Borrelli S, Erba E, DiSilvio A, D’lncalci M, Mantovani R. Selective effects of the anticancer drug Yondelis (ET-743) on cell-cycle promoters. Mol Pharmacol. 2005;68:1496–503. doi: 10.1124/mol.105.013615. https://doi.org/10.1124/mol.105.013615 [DOI] [PubMed] [Google Scholar]
- 70.Andrade JP, Assunção M. Protective effects of chronic green tea consumption on age-related neurodegeneration. Curr Pharm Des. 2012;18:4–14. doi: 10.2174/138161212798918986. https://doi.org/10.2174/138161212798918986 [DOI] [PubMed] [Google Scholar]
- 71.Mandel SA, Amit T, Weinreb O, Youdim MB. Understanding the broad-spectrum neuroprotective action profile of green tea polyphenols in aging and neurodegenerative diseases. J Alzheimers Dis. 2011;25:187–208. doi: 10.3233/JAD-2011-101803. https://doi.org/10.3233/JAD-2011-101803 [DOI] [PubMed] [Google Scholar]
- 72.Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. https://doi.org/10.1080/1040869059096 [DOI] [PubMed] [Google Scholar]
- 73.Shi X, Ye J, Leonard SS, Ding M, Vallyathan V, Castranova V, Rojanasakul Y, Dong Z. Antioxidant properties of epicatechin-3-gallate and its inhibition of Cr(VI)-induced DNA damage and Cr(IV)- or TPA-stimulated NF-kappaB activation. Mol Cell Biochem. 2000;206:125–32. doi: 10.1023/a:1007012403691. https://doi.org/10.1023/A:1007012403691 [DOI] [PubMed] [Google Scholar]
- 74.Lee SF, Liang YC, Lin JK. Inhibition of 1,2,4-benzenetriol-generated active oxygen species and induction of phase II enzymes by green tea polyphenols. Chem Biol Interact. 1996;98:283–301. doi: 10.1016/0009-2797(95)03652-0. https://doi.org/10.1016/0009-2797(95)03652-0 [DOI] [PubMed] [Google Scholar]
- 75.O’Sullivan J, Sheridan J, Mulcahy H, Tenniswood M, Morrissey C. The effect of green tea on oxidative damage and tumour formation in Lobund-Wistar rats. Eur J Cancer Prev. 2008;17:489–501. doi: 10.1097/CEJ.0b013e3282f0c04e. https://doi.org/10.1097/CEJ.0b013e3282f0c04e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan SG, Katiyar SK, Agarwal R, Mukhtar H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: possible role in cancer. Cancer Res. 1992;52:4050–2. [PubMed] [Google Scholar]
- 77.Chandra Mohan KV, Hara Y, Abraham SK, Nagini S. Comparative evaluation of the chemopreventive efficacy of green and black tea polyphenols in the hamster buccal pouch carcinogenesis model. Clin Biochem. 2005;38:879–86. doi: 10.1016/j.clinbiochem.2005.06.011. https://doi.org/10.1016/j.clinbiochem.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 78.Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine. 2002;9:232–8. doi: 10.1078/0944-7113-00119. https://doi.org/10.1078/0944-7113-00119 [DOI] [PubMed] [Google Scholar]
- 79.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349:431–4. doi: 10.1038/349431a0. https://doi.org/10.1038/349431a0 [DOI] [PubMed] [Google Scholar]
- 80.Anderson RF, Fisher LJ, Hara Y, Harris T, Mak WB, Melton LD, Packer JE. Green tea catechins partially protect DNA from (·)OH radical-induced strand breaks and base damage through fast chemical repair of DNA radicals. Carcinogenesis. 2001;22:1189–93. doi: 10.1093/carcin/22.8.1189. https://doi.org/10.1093/carcin/22.8.1189 [DOI] [PubMed] [Google Scholar]
- 81.Chen CH, Liu TZ, Chen CH, Wong CH, Chen CH, Lu FJ, Chen SC. The efficacy of protective effects of tannic acid, gallic acid, ellagic acid, and propyl gallate against hydrogen peroxide-induced oxidative stress and DNA damages in IMR-90 cells. Mol Nutr Food Res. 2007;51:962–8. doi: 10.1002/mnfr.200600230. https://doi.org/10.1002/mnfr.200600230 [DOI] [PubMed] [Google Scholar]
- 82.Feng Q, Torii Y, Uchida K, Nakamura Y, Hara Y, Osawa T. Black tea polyphenols, theaflavins, prevent cellular DNA damage by inhibiting oxidative stress and suppressing cytochrome P450 1A1 in cell cultures. J Agric Food Chem. 2002;50:213–20. doi: 10.1021/jf010875c. https://doi.org/10.1021/jf010875c [DOI] [PubMed] [Google Scholar]
- 83.Kweon MH, Adhami VM, Lee JS, Mukhtar H. Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J Biol Chem. 2006;281:33761–72. doi: 10.1074/jbc.M604748200. https://doi.org/10.1074/jbc.M604748200 [DOI] [PubMed] [Google Scholar]
- 84.Thakur VS, Gupta K, Gupta S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis. 2012;33:377–84. doi: 10.1093/carcin/bgr277. https://doi.org/10.1093/carcin/bgr277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol Oncol. 2007;1:19–25. doi: 10.1016/j.molonc.2007.01.001. https://doi.org/10.1016/j.molonc.2007.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang DH. Oxidative stress, DNA damage, and breast cancer. AACN Clin Issues. 2002;13:540–9. doi: 10.1097/00044067-200211000-00007. https://doi.org/10.1097/00044067-200211000-00007 [DOI] [PubMed] [Google Scholar]
- 87.Engel RH, Evens AM. Oxidative stress and apoptosis: a new treatment paradigm in cancer. Front Biosci. 2006;11:300–12. doi: 10.2741/1798. https://doi.org/10.2741/1798 [DOI] [PubMed] [Google Scholar]
- 88.Umeda D, Yano S, Yamada K, Tachibana H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J Biol Chem. 2008;283:3050–8. doi: 10.1074/jbc.M707892200. https://doi.org/10.1074/jbc.M707892200 [DOI] [PubMed] [Google Scholar]
- 89.Lee JH, Song DK, Jung CH, Shin DH, Park J, Kwon TK, Jang BC, Mun KC, Kim SP, Suh SI, Bae JH. (−)-Epigallocatechin gallate attenuates glutamate-induced cytotoxicity via intracellular Ca modulation in PC12 cells. Clin Exp pharmacol physiol. 2004;31:530–536. doi: 10.1111/j.1440-1681.2004.04044.x. https://doi.org/10.1111/j.1440-1681.2004.04044.x [DOI] [PubMed] [Google Scholar]
- 90.Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem. 2007;282:30143–9. doi: 10.1074/jbc.M702390200. https://doi.org/10.1074/jbc.M702390200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 92.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–22. doi: 10.1158/0008-5472.CAN-04-2840. https://doi.org/10.1158/0008-5472.CAN-04-2840 [DOI] [PubMed] [Google Scholar]
- 93.Hussain T, Gupta S, Adhami VM, Mukhtar H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int J Cancer. 2005;113:660–9. doi: 10.1002/ijc.20629. https://doi.org/10.1002/ijc.20629 [DOI] [PubMed] [Google Scholar]
- 94.Sang S, Lee MJ, Hou Z, Ho CT, Yang CS. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J Agric Food Chem. 2005;53:9478–84. doi: 10.1021/jf0519055. https://doi.org/10.1021/jf0519055 [DOI] [PubMed] [Google Scholar]
- 95.Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys. 2008;476:107–12. doi: 10.1016/j.abb.2008.01.028. https://doi.org/10.1016/j.abb.2008.01.028 [DOI] [PubMed] [Google Scholar]
- 96.Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol In Vitro. 2004;18:555–61. doi: 10.1016/j.tiv.2003.12.012. https://doi.org/10.1016/j.tiv.2003.12.012 [DOI] [PubMed] [Google Scholar]
- 97.Liu X, Li J, Wang Y, Li T, Zhao J, Zhang C. Green tea polyphenols function as prooxidants to inhibit Pseudomonas aeruginosa and induce the expression of oxidative stress-related genes. Folia Microbiol (Praha) 2013;58:211–7. doi: 10.1007/s12223-012-0198-2. https://doi.org/10.1007/s12223-012-0198-2 [DOI] [PubMed] [Google Scholar]
- 98.Khan N, Bharali DJ, Adhami VM, Siddiqui IA, Cui H, Shabana SM, Mousa SA, Mukhtar H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis. 2014;35:415–23. doi: 10.1093/carcin/bgt321. https://doi.org/10.1093/carcin/bgt321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li GX, Chen YK, Hou Z, Xiao H, Jin H, Lu G, Lee MJ, Liu B, Guan F, Yang Z, Yu A, Yang CS. Pro-oxidative activities and dose-response relationship of (−)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis. 2010;31:902–10. doi: 10.1093/carcin/bgq039. https://doi.org/10.1093/carcin/bgq039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galati G, Lin A, Sultan AM, O’Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med. 2006;40:570–80. doi: 10.1016/j.freeradbiomed.2005.09.014. https://doi.org/10.1016/j.freeradbiomed.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 101.Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331–41. doi: 10.1007/s00228-008-0610-7. https://doi.org/10.1007/s00228-008-0610-7 [DOI] [PubMed] [Google Scholar]
- 102.Heber D, Zhang Y, Yang J, Ma JE, Henning SM, Li Z. Green tea, black tea, and oolong tea polyphenols reduce visceral fat and inflammation in mice fed high-fat, high-sucrose obesogenic diets. J Nutr. 2014;144:1385–93. doi: 10.3945/jn.114.191007. https://doi.org/10.3945/jn.114.191007 [DOI] [PubMed] [Google Scholar]
- 103.Mehrabian S. The Study of Antioxidant and Anticarcinogenic Green Tea and Black Tea. Pak J Biol Sci. 2007;10:989–91. doi: 10.3923/pjbs.2007.989.991. https://doi.org/10.3923/pjbs.2007.989.991 [DOI] [PubMed] [Google Scholar]
- 104.Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of Cancer Prevention by Green and Black Tea Polyphenols. Anticancer Agents Med Chem. 2006;6:389–406. doi: 10.2174/187152006778226468. https://doi.org/10.2174/187152006778226468 [DOI] [PubMed] [Google Scholar]
- 105.Villaño D, Lettieri-Barbato D, Guadagni F, Schmid M, Serafini M. Effect of acute consumption of oolong tea on antioxidant parameters in healthy individuals. Food Chem. 2012;132:2102–6. doi: 10.1016/j.foodchem.2011.11.109. https://doi.org/10.1016/j.foodchem.2011.12.064 [DOI] [PubMed] [Google Scholar]
- 106.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–84S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 107.Sun S, Pan S, Ling C, Miao A, Pang S, Lai Z, Chen D, Zhao C. Free radical scavenging abilities in vitro and antioxidant activities in vivo of black tea and its main polyphenols. J Med Plants Res. 2012;6:114–21. https://doi.org/10.5897/jmpr11.1308 [Google Scholar]
- 108.Benzie IF, Szeto YT. Total antioxidant capacity of teas by the Ferric reducing/antioxidant power assay. J Agric Food Chem. 1999;47:633–6. doi: 10.1021/jf9807768. https://doi.org/10.1021/jf9807768 [DOI] [PubMed] [Google Scholar]
- 109.Rechner AR, Wagner E, Van Buren L, Van De Put F, Wiseman S, Rice-Evans CA. Black tea represents a major source of dietary phenolics among regular tea drinkers. Free Radic Res. 2009;36:1127–35. doi: 10.1080/1071576021000006707. https://doi.org/10.1080/1071576021000006707 [DOI] [PubMed] [Google Scholar]
- 110.Bancirova M. Comparison of the antioxidant capacity and the antimicrobial activity of black and green tea. Food Res Int. 2010;43:1379–82. https://doi.org/10.1016/j.foodres.2010.04.020 [Google Scholar]
- 111.Halder J, Bhaduri AN. Protective role of black tea against oxidative damage of human red blood cells. Biochem Biophys Res Commun. 1998;244:903–7. doi: 10.1006/bbrc.1998.8366. https://doi.org/10.1006/bbrc.1998.8366 [DOI] [PubMed] [Google Scholar]
- 112.Sharma V, Rao LJ. A thought on the biological activities of black tea. Crit Rev Food Sci Nutr. 2009;49:379–404. doi: 10.1080/10408390802068066. https://doi.org/10.1080/10408390802068066 [DOI] [PubMed] [Google Scholar]
- 113.Kuhnert N, Drynan JW, Obuchowicz J, Clifford MN, Witt M. Mass spectrometric characterization of black tea thearubigins leading to an oxidative cascade hypothesis for thearubigin formation. Rapid Commun Mass Spectrom. 2010;24:3387–404. doi: 10.1002/rcm.4778. https://doi.org/10.1002/rcm.4778 [DOI] [PubMed] [Google Scholar]