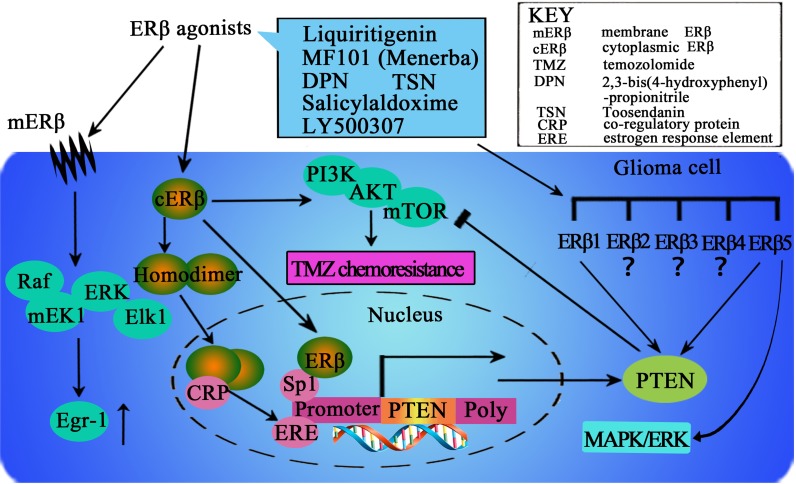
Figure 1. The potential mechanisms mediated by ERβ in the intracellular signaling events in glioma cells.
Increasing ERβ protein expression and nuclear translocation could be important for activation of the ERβ pathway. In addition, ERβ could stimulate Egr-1 transcription via the MEK1/Erk/Elk-1 cascade in glioma cells. Furthermore, ERβ agonist could enhance temozolomide sensitivity of glioma cells by inhibiting the PI3K/AKT/mTOR pathway. Ligand binding with ERβ could induce conformational changes that facilitate receptor dimerization to homodimers (ERβ/ERβ), translocation of dimers to the nucleus, and binding with co-regulatory proteins (CRP). This supra-molecular assembly thus may interact with the estrogen response element (ERE) in the promoter region of target genes. Furthermore, ERβ could also bind to the promoter region of PTEN through Sp1 and increase PTEN transcription. The presence of various ERβ isoforms could add further complexity to the action of ERβ, which should be considered in clarifying the effects of ERβ in human glioma. However, it remains unclear which isoforms primarily exert anticancer effects in human glioma, and the distinct function of each ERβ isoform is unknown. More efforts should be directed toward clarifying the activation and agonist-induced dynamics of ERβ/ERβ homodimers and the distinct function of each ERβ isoform to design more potent ligands that selectively activate ERβ.