Abstract
Purpose: This purpose of this prospective study was to use a continuous glucose monitoring (CGM) system to evaluate the suitability of our institution’s glucose management protocol after cardiovascular surgery and to clarify the impact of glycemic variability on postoperative complications.
Methods: In all, 76 patients who underwent elective cardiovascular surgery and were monitored perioperatively using a CGM system were evaluated. Postoperative glucose management consisted of continuous intravenous insulin infusion (CIII) in the intensive care unit, and subcutaneous insulin injections (SQII) after oral food intake started. CIII and subcutaneous injections were initiated when blood glucose level exceeded 150 mg/dL. CGM data were used to analyze perioperative glycemic variability and association with postoperative complications.
Results: Target glucose levels (71–180 mg/dL) were achieved during 97.1 ± 5.5% and 86.4 ± 19.0% of the continuous insulin infusion and subcutaneous injection periods, respectively. Major postoperative complications were surgical site infections, found in 6.6% of total patients, and atrial fibrillation, found in 44% of patients with off-pump coronary artery bypass grafting. High glycemic variability during SQII was associated with increased risk for both complications.
Conclusion: Data analysis revealed that our glucose management protocol during CIII was adequate. However, the management protocol during SQII required improvement.
Keywords: cardiac surgery, operative morbidity, glycemic variability, continuous glucose monitoring, glucose management protocol.
Introduction
The significance of blood glucose management during adult cardiac surgery has been well documented. Hyperglycemia after cardiovascular surgery is known to be a risk factor for wound infection1–3) and operative mortality.1,4–6) Although intensive insulin therapy is effective in achieving normoglycemia (81–108 mg/dL) in critically ill patients,7) it often leads to hypoglycemic events and increased mortality in these patients.8,9) Several recent studies have shown that hyperglycemia and hypoglycemia during surgery, including cardiovascular surgery, are associated with poor outcomes, including the increased risk of adverse events such as surgical site infections (SSI),1,2,10) postoperative atrial fibrillation (POAF)11) and coronary artery events,12) increased duration of hospital stay,1,13) and increased mortality.3,5,14–16) Although high blood glucose variability is associated with poor outcomes in critically ill patients,12–15) the relationship between blood glucose variability and complications after cardiovascular surgery has not been adequately clarified.17)
The Society of Thoracic Surgeons (STS) recommends that postoperative blood glucose level (BGL) be maintained below 180 mg/dL during adult cardiac surgery.18) They further advocate the use of continuous intravenous insulin infusion (CIII) in the intensive care unit (ICU) and transitioning to subcutaneous insulin injections (SQII) upon discharge from the ICU. Our institute has developed a glucose management protocol for ICU patients following cardiovascular surgery based on the STS guidelines and the Portland protocol.1) The notable differences in our institution’s protocol are the criteria for starting CIII (BGL ≥150 mg/dL) and changing insulin administration. Based on the NICE-SUGAR study, which reported that hypoglycemia during CIII led to adverse events,8,9) we decided that insulin administration should be reduced by half if the patient’s blood glucose was 80–99 mg/dL and dropped ≥50 mg/dL since the last measurement.
We also utilize a continuous glucose monitoring (CGM) system to monitor glucose levels in these high-risk patients. Such CGM systems are increasingly used in inpatient settings.19–22) The feasibility and precision of the CGM system have been demonstrated in patients undergoing coronary artery bypass grafting (CABG) surgery23) and cardiac surgery.24) The CGM system has the ability to measure subcutaneous glucose levels every 5 minutes and can, therefore, be expected to evaluate glucose variability and the suitability of perioperative blood glucose management more precisely than intermittent blood glucose analysis, which is generally performed at 1- or 2-hour intervals in the ICU. Furthermore, CGM data can be analyzed separately and compared among different periods such as preoperative, intraoperative, CIII, and SQII periods. Such nuanced analysis could be effective in revealing new risk factors for postoperative complications that cannot be detected using conventional intermittent blood glucose analysis with fewer data points.
The aim of this study was to use CGM data to evaluate the suitability of our institution’s glucose management protocol after cardiovascular surgery and to clarify the impact of glycemic variability on postoperative complications such as SSI and POAF.
Materials and Methods
Patients: All patients who underwent elective cardiovascular surgery between May 2013 and October 2015 at Niigata University Medical and Dental Hospital were included in this prospective, single-center study approved by the Institutional Ethics Committee of Niigata University (approval number: 1475). After obtaining informed written consent from all patients before the study, the patients were monitored perioperatively with the CGM system. Intraoperative blood glucose management was conducted by the anesthesiologist. Postoperative transfusion management comprised 5% dextrose in water; catecholamines were administered based on the judgment of surgeons or ICU physicians.
Glucose Management Protocol: In the ICU, blood glucose measurements were obtained at 2-hour intervals and blood glucose management was based on an instructional protocol adapted from the Portland protocol1) and STS guidelines.18) At BGL ≥150 mg/dL, CIII was initiated using 50 units of regular human insulin in 50 mL of saline administered using a syringe driver at an initial rate of 1 unit/h. The target BGL was 71–180 mg/dL. At BGL, 100–149 mg/dL, CIII was continued at the same dose. If BGL increased to 150–199 mg/dL, 200–249 mg/dL, 250–299 mg/dL, and ≥300 mg/dL, the insulin dose was increased to 0.5 units/h, 1.0 units/h, 1.5 units/h, 2.0 units/h, and 2.5 units/h, respectively. If BGL was 80–99 mg/dL and decreased below 50 mg/dL, the insulin dose was reduced by half. If BGL was below 80 mg/dL, CIII was stopped. If hypoglycemia (defined as BGL ≤70 mg/dL) occurred during CIII, 40 mL of 20% dextrose was administered intravenously. When patients could ingest food, CIII was stopped and a sliding-scale-guided SQII was started based on BGL measured at 6-hour intervals (before each major meal and before sleep). At a BGL of 150–199 mg/dL, 200–249 mg/dL, 250–300 mg/dL, or >300 mg/dL, 2 units, 4 units, 6 units, 8 units, or 10 units, respectively, of regular human insulin were injected subcutaneously. If satisfactory blood glucose management was not achieved, a diabetic specialist was consulted.
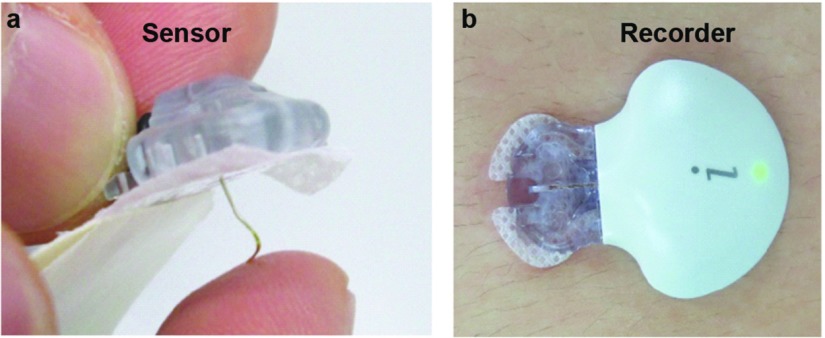
CGM system: The study used a CGM system comprising a soft sensor (Enlite sensor; Medtronic, Northridge, CA, USA) and a small, lightweight, waterproof recorder (iPRO2; Medtronic) (Fig. 1).20,25) The sensor is placed subcutaneously in the anterior abdominal wall and records the subcutaneous glucose concentration every 5 minutes. The recorder records these signals for up to 7 days, after which the data are uploaded to a computer and retrospectively analyzed. BGLs were also initially measured at least four times a day for CGM data calibration.26,27) CGM recording was started 2 days before operation and continued 4 days after operation. In addition, during the CGM examination (at approximately 3 days post-surgery), oral diabetes medication was not resumed for any patients. Calibrated CGM data were visually assessed for glucose variability. CGM data were compared among the different perioperative periods: preoperative state, intraoperative state, CIII period, and SQII period.
Fig. 1. Components of the CGM system: (a) A soft sensor (Enlite) and (b) a small, lightweight, waterproof recorder (iPro2). CGM: continuous glucose monitoring.
Statistical analyses: CGM data were statistically analyzed using Excel 2016 (Microsoft Corporation, Redmond, WA, USA). We used the standard deviation of CGM data as a marker of blood glucose variability.15) Furthermore, the efficiency of the glycemic management protocol was determined by measuring the fraction of time when BGLs were ≤70 mg/dL, 71–179 mg/dL (desired target), and ≥180 mg/dL. Continuous variables were reported as mean ± SD values. Paired t-test, Mann–Whitney U-test, and Fisher’s exact test were used to evaluate statistical significance. A p value below 0.05 was considered to indicate statistical significance.
Results
Patient characteristics: Of the 83 patients enrolled, 7 were excluded; 2 did not have CGM data, 2 were excluded for hypokalemia requiring glucose-insulin therapy, and 3 were excluded because CIII was not required (BGL <150 mg/dL in ICU). The patient characteristics are presented in Table 1. Patients had a mean age of 66.6 years and mean body mass index (BMI) of 22.7 kg/m2, 55% had a smoking history, 46% had hypertension, 39% had dyslipidemia, 25% had diabetes mellitus, 17% had chronic obstructive pulmonary disease, and 22% had severe renal dysfunction, with 9% requiring hemodialysis.
Table 1. Patient characteristics.
| Total (n = 76) | |
|---|---|
| General characteristics | |
| Age (years) | 66.6 ± 9.4 |
| Sex (male) | 45 (59%) |
| BMI (kg/m2) | 22.7 ± 3.4 |
| Smoking history | 42 (55%) |
| Current | 6 (8%) |
| Past | 36 (47%) |
| Never | 34 (45%) |
| Hypertension | 35 (46%) |
| Dyslipidemia | 33 (43%) |
| Diabetes mellitus | 19 (25%) |
| HbA1c (%) | 6.0 ± 0.8 |
| COPD | 13 (17%) |
| Severe renal dysfunction (GFR <30) | 17 (22%) |
| Hemodialysis | 7 (9 %) |
| Ejection fraction (%) | 62.4 ± 13.7 |
| Preoperative medications | |
| ARB | 35 (46%) |
| ACEI | 20 (26%) |
| SAB | 2 (3%) |
| CCB | 35 (46%) |
| β-blocker | 41 (54%) |
| DPP-4 inhibitor | 8 (11%) |
| α-glucosidase inhibitor | 6 (8%) |
| Glinide | 3 (4%) |
| Biguanide | 2 (3%) |
| Sulfonyl urea | 1 (1%) |
| Thiazolidine | 1 (1%) |
| Insulin | 3 (4%) |
| Statin | 33 (43%) |
| Procedure | |
| CPB use | 56 (74%) |
| Valve surgery | |
| Aortic | 13 (17%) |
| Mitral | 2 (3%) |
| Aortic root | 2 (3%) |
| Combined | 27 (36%) |
| AVR + CABG | 3 (4%) |
| MVP + CABG | 5 (7%) |
| Major thoracic vascular surgery | |
| Ascending aorta | 2 (3%) |
| Arch | 1 (1%) |
| Thoracoabdominal aorta | 1 (1%) |
| OPCAB | 20 (26%) |
| Operative time (m) | 452.2 ± 133.9 |
| CPB time (m) | 268.6 ± 88.7 |
| Cardiac arrest time (m) | 181.6 ± 65.9 |
BMI: body mass index; ARB: angiotensin II receptor blocker; ACEI: angiotensin-converting enzyme inhibitor; SAB: selective aldosterone blocker; CCB: calcium channel blocker; HbA1c: hemoglobin A1c; DPP: dipeptidyl peptidase; COPD: chronic obstructive pulmonary disease; GFR: glomerular filtration rate; CPB: cardiopulmonary bypass; CABG: coronary artery bypass graft; OPCAB: off-pump coronary artery bypass; AVR: aortic valve replacement; MVP: mitral valve plasty
Surgical procedures: In all, 56 of 76 (74%) patients required extracorporeal circulation and had aortic and/or mitral valve surgery with or without combined CABG, or major thoracic vascular surgery. In total, 20 of the 76 (26%) patients underwent off-pump CABG (OPCAB) without the assistance of cardiopulmonary bypass and extracorporeal circulation. The mean operative time was 452 ± 134 minutes, cardiopulmonary bypass time was 269 ± 88 minutes, and cardiac arrest time was 182 ± 66 minutes (Table 1).
Glycemic management: CIII was administered over 37.3 ± 13.6 hours at an average insulin administration rate of 1.1 ± 0.6 units/h. The mean ICU length was 1.5 ± 1.0 days. SQII was then administered for 117.4 ± 11.4 hours. Table 2 shows the summary of perioperative CGM data and the percentage of time in the three groups divided by CGM value. The target glucose level (71–180 mg/dL) was achieved during 97.1 ± 5.5% of the CIII period; glucose levels were below 70 mg/dL during 0.1 ± 0.6% and over 180 mg/dL during 2.8 ± 5.2% of this period. The target glucose level was achieved during 86.4 ± 19.0% of the SQII period; glucose levels were below 70 mg/dL during 0.6 ± 2.6% and over 180 mg/dL during 13.0 ± 18.2% of this period. There was no difference in the minimum CGM values between CIII and SQII periods (90.7 ± 12.5 mg/dL vs. 89.2 ± 17.4 mg/dL, p = 0.46), whereas the maximum CGM values in the SQII period were significantly higher than in the CIII period (219.8 ± 63.5 mg/dL vs. 179.1 ± 30.5 mg/dL, p <0.01). Similarly, the mean CGM value during the SQII period was significantly higher than that in the CIII period (141.3 ± 25.0 mg/dL vs. 127.5 ± 8.2 mg/dL, p <0.01), as was the standard deviation of the CGM value during SQII compared to CIII (28.8 ± 16.3 mg/dL vs. 19.5 ± 8.1 mg/dL, p <0.01).
Table 2. Summary of perioperative CGM data.
| CGM value | Preoperative | Intraoperative | Postoperative | p value | |
|---|---|---|---|---|---|
| During CIII | During SQII | (CIII vs. SQII) | |||
| Mean (mg/dL) | 107.1 ± 19.3 | 143.9 ± 36.1 | 127.5 ± 8.2 | 141.3 ± 25.0 | <0.01* |
| SD (mg/dL) | 21.2 ± 8.4 | 29.1 ± 20.8 | 19.5 ± 8.1 | 28.8 ± 16.3 | <0.01* |
| Max (mg/dL) | 168.4 ± 38.6 | 191.4 ± 64.0 | 179.1 ± 30.5 | 219.8 ± 63.5 | <0.01* |
| Min (mg/dL) | 67.9 ± 16.0 | 96.7 ± 21.1 | 90.7 ± 12.5 | 89.2 ± 17.4 | 0.46 |
| ≤70 mg/dL | 0.1 ± 0.6 (%) | 0.6 ± 2.6 (%) | <0.01* | ||
| 71–179 mg/dL | 97.1 ± 5.5 (%) | 86.4 ± 19.0 (%) | <0.01* | ||
| ≥180 mg/dL | 2.8 ± 5.2 (%) | 13.0 ± 18.2 (%) | <0.01* | ||
*Value is statistically significant. p value: Paired t-test; CGM: continuous glucose monitoring; CIII: continuous intravenous insulin infusion; SQII: subcutaneous insulin infusion; SD: standard deviation
Complications: There were no hospital deaths after surgery. Two patients (2.6%) experienced stroke. One of the twenty patients who underwent OPCAB developed atrial fibrillation prior to surgery; this patient was therefore excluded from all comparative analyses. Eight (44%) of the remaining 19 OPCAB patients had POAF after an average of 2.5 days (range: 2–6 days) after surgery. Notably, POAF developed during the SQII period in all eight patients. POAF was successfully managed using antiarrhythmic drugs. Among the variables associated with POAF, heart rate was significantly lower in the POAF group (64 ± 5 bpm vs. 70 ± 7 bpm; p = 0.049; Table 3).
Table 3. Independent predictors of POAF and SSI.
| POAF (+) | POAF (−) | p value | Analysis | SSI (+) | SSI (−) | p value | Analysis | |
|---|---|---|---|---|---|---|---|---|
| (n = 8) | (n = 11) | (n = 5) | (n = 71) | |||||
| Age (years) | 73.1 ± 6.9 | 65.4 ± 8.9 | 0.07 | MHU | 65.2 ± 6.1 | 66.7 ± 9.6 | 0.675 | MHU |
| Sex (male) | 7 (88%) | 8 (73%) | 0.60 | Fisher | 4 (80%) | 41 (58%) | 0.64 | Fisher |
| Heart rate (bpm) | 64 ± 5 | 70 ± 7 | 0.049* | MHU | ||||
| BMI (kg/m2) | 23.0 ± 4.2 | 24.7 ± 2.1 | 0.34 | MHU | 25.0 ± 1.8 | 22.6 ± 3.5 | 0.053 | MHU |
| PR interval (msec) | 0.16 ± 0.02 | 0.17 ± 0.01 | 0.16 | MHU | ||||
| β-blocker use | 7 (88%) | 10 (91%) | >0.99 | Fisher | ||||
| Hypertension | 3 (38%) | 9 (82%) | 0.07 | Fisher | 3 (60%) | 32 (45%) | 0.66 | Fisher |
| Dyslipidemia | 6 (75%) | 8 (73%) | >0.99 | Fisher | 2 (40%) | 31 (44%) | >0.99 | Fisher |
| Diabetes mellitus | 5 (63%) | 4 (36%) | 0.37 | Fisher | 3 (60%) | 16 (23%) | 0.096 | Fisher |
| HbA1c (%) | 6.4 ± 0.9 | 6.3 ± 0.7 | 0.57 | MHU | 7.1 ± 1.3 | 5.9 ± 0.7 | 0.02* | MHU |
| COPD | 1 (13%) | 2 (18%) | >0.99 | Fisher | 1 (20%) | 12 (17%) | >0.99 | Fisher |
| Smoking history | 6 (75%) | 9 (82%) | >0.99 | Fisher | 4 (80%) | 38 (54%) | 0.37 | Fisher |
| Hemodialysis | 1 (20%) | 1 (9%) | >0.99 | Fisher | 1 (20%) | 6 (8%) | 0.39 | Fisher |
| Severe renal dysfunction (GFR <30) | 2 (25%) | 3 (27%) | >0.99 | Fisher | 2 (40%) | 15 (21%) | 0.31 | Fisher |
| Ejection fraction (%) | 57.1 ± 10.3 | 59.3 ± 6.7 | 0.57 | MHU | 50.9 ± 18.2 | 63.3 ± 13.1 | 0.74 | MHU |
| IMA use | 3 (60%) | 25 (35%) | 0.35 | Fisher | ||||
| CPB use | 4 (80%) | 52 (73%) | >0.99 | Fisher | ||||
| Operative time (m) | 413 ± 116 | 375 ± 48 | 0.53 | MHU | 471 ± 127 | 451 ± 135 | 0.53 | MHU |
| CPB time (m) | 299 ± 47 | 264 ± 91 | 0.35 | MHU | ||||
| CA time (m) | 227 ± 40 | 178 ± 66 | 0.09 | MHU | ||||
| ICU length (day) | 1.1 ± 0.4 | 1 ± 0 | 0.53 | MHU | 2.4 ± 1.5 | 1.5 ± 0.9 | 0.15 | MHU |
| CIII period (h) | 32.0 ± 14.8 | 37.6 ± 7.0 | 0.13 | MHU | 41.4 ± 11.4 | 37.0 ± 13.7 | 0.19 | MHU |
| RHI administration | ||||||||
| Mean (units/h) | 1.0 ± 0.5 | 0.7 ± 0.2 | 0.18 | MHU | 1.4 ± 0.7 | 1.1 ± 0.6 | 0.34 | MHU |
| Max (units/h) | 1.9 ± 0.8 | 1.3 ± 0.3 | 0.14 | MHU | 2.4 ± 1.0 | 2.0 ± 0.9 | 0.28 | MHU |
| SQII period (h) | 124.9 ± 7.8 | 121.5 ± 7.5 | 0.38 | MHU | 119.2 ± 19.6 | 117.3 ± 10.8 | 0.60 | MHU |
*Value is statistically significant. POAF: postoperative atrial fibrillation; SSI: surgical site infection; MHU: Mann–Whitney U-test; Fisher: Fisher’s exact test; BMI: body mass index; COPD: chronic obstructive pulmonary disease; GFR: glomerular filtration rate; IMA: internal mammary artery; CPB: cardiopulmonary bypass; CA: cardiac arrest; ICU: intensive care unit; CIII: continuous intravenous insulin infusion; RHI: regular human insulin; SQII: subcutaneous insulin infusion
As part of postoperative management, cefazolin (2 g/day) was administered to all patients for 3 days. Five patients (6.6%) experienced SSI after an average of 13 days (range: 9–19 days) after surgery; four patients had deep sternal infections and one had mediastinitis. All SSIs were caused by Staphylococcus epidermidis and were managed using negative pressure wound therapy and daptomycin. Among the variables associated with SSI, hemoglobin A1c (HbA1c) was significantly higher in the SSI group (7.1 ± 1.3% vs. 5.9 ± 0.7%; p = 0.02; Table 3).
Table 4 shows the association between CGM data on postoperative morbidity. There were no significant differences in the mean and standard deviation of CGM data during the preoperative, operative, and CIII periods, or between patients with and without SSI. However, the maximum, mean, and standard deviation values in the CGM data were significantly higher in SSI patients during SQII. Similarly, there were no significant differences in the mean and standard deviation of CGM data during the preoperative, operative, and CIII periods, or between patients with and without POAF. However, the maximum and standard deviation values in the CGM data were significantly higher in POAF patients during SQII.
Table 4. Effect of CGM data on postoperative morbidity.
| POAF (+) | POAF (−) | p value | Analysis | SSI (+) | SSI (−) | p value | Analysis | |
|---|---|---|---|---|---|---|---|---|
| (n = 8) | (n = 11) | (n = 5) | (n = 71) | |||||
| Preoperative | ||||||||
| Mean (mg/dL) | 123.9 ± 15.8 | 110.5 ± 25.5 | 0.18 | MHU | 121.3 ± 22.1 | 106.1 ± 18.9 | 0.09 | MHU |
| SD (mg/dL) | 26.7 ± 8.2 | 24.3 ± 9.0 | 0.49 | MHU | 25.6 ± 16.9 | 20.9 ± 7.5 | 0.55 | MHU |
| Max (mg/dL) | 207.4 ± 33.3 | 175.5 ± 39.8 | 0.08 | MHU | 200.6 ± 67.8 | 166.1 ± 35.3 | 0.20 | MHU |
| Min (mg/dL) | 82.0 ± 22.9 | 63.3 ± 17.7 | 0.051 | MHU | 72.2 ± 20.3 | 67.6 ± 15.8 | 0.49 | MHU |
| Intraoperative | ||||||||
| Mean (mg/dL) | 124.3 ± 19.4 | 115.4 ± 19.3 | 0.49 | MHU | 170.3 ± 51.8 | 142.1 ± 34.5 | 0.19 | MHU |
| SD (mg/dL) | 9.1 ± 4.6 | 8.6 ± 4.7 | 0.54 | MHU | 43.2 ± 21.5 | 28.0 ± 20.5 | 0.12 | MHU |
| Max (mg/dL) | 146.0 ± 38.1 | 129.3 ± 20.8 | 0.34 | MHU | 232.2 ± 64.8 | 188.5 ± 63.4 | 0.11 | MHU |
| Min (mg/dL) | 106.4 ± 14.1 | 98.2 ± 22.7 | 0.30 | MHU | 101.2 ± 21.4 | 96.4 ± 21.2 | 0.53 | MHU |
| CIII period | ||||||||
| Mean (mg/dL) | 127.7 ± 7.3 | 126.6 ± 9.0 | 0.53 | MHU | 131.5 ± 3.8 | 127.2 ± 8.4 | 0.20 | MHU |
| SD (mg/dL) | 20.3 ± 9.3 | 15.8 ± 6.4 | 0.30 | MHU | 21.6 ± 9.8 | 19.3 ± 8.0 | 0.51 | MHU |
| Max (mg/dL) | 175.3 ± 31.2 | 162.6 ± 18.0 | 0.53 | MHU | 199.8 ± 49.3 | 177.7 ± 28.7 | 0.29 | MHU |
| Min (mg/dL) | 87.5 ± 17.2 | 92.9 ± 12.3 | 0.52 | MHU | 95.0 ± 16.4 | 90.4 ± 12.2 | 0.50 | MHU |
| SQII period | ||||||||
| Mean (mg/dL) | 160.1 ± 35.8 | 144.3 ± 18.3 | 0.24 | MHU | 170.3 ± 36.5 | 139.2 ± 22.9 | 0.01* | MHU |
| SD (mg/dL) | 45.2 ± 22.4 | 25.3 ± 9.1 | 0.048* | MHU | 41.0 ± 16.2 | 27.9 ± 16.1 | 0.04* | MHU |
| Max (mg/dL) | 284.9 ± 80.8 | 214.6 ± 32.0 | 0.02* | MHU | 280.8 ± 68.2 | 215.4 ± 61.3 | 0.02* | MHU |
| Min (mg/dL) | 81.1 ± 17.9 | 97.7 ± 16.1 | 0.08 | MHU | 99.6 ± 11.4 | 88.4 ± 17.6 | 0.19 | MHU |
*Value is statistically significant. CGM: continuous glucose monitoring; SSI: surgical site infection; POAF: postoperative atrial fibrillation; MHU: Mann–Whitney U-test; SD: standard deviation; CIII: continuous intravenous insulin infusion; SQII: subcutaneous insulin infusion
Discussion
In this study, we report the glycemic management and CGM of 76 patients who underwent cardiovascular surgery requiring ICU management. Our modified institutional protocol for glycemic management achieved target glucose levels in 97% of the CIII period and 86% of the SQII period. The protocol was associated with a very low incidence (0.1–0.6%) of low glucose levels during the postoperative period. These results demonstrate the efficacy of the glucose management protocol and the feasibility of using the CGM system to closely analyze perioperative BGLs following cardiovascular surgery.
The CGM system used in our study offered detailed insight into the accuracy and variability in glycemic control during different postoperative periods. These variations in glucose levels are likely missed during intermittent blood glucose measurement by self-monitoring of blood glucose (SMBG).11) In particular, CGM data revealed that the maximum, mean, and standard deviation values of glucose levels were higher during SQII than during CIII. It should, however, be noted that glucose levels exceeded the target level in 2.8% of the CIII period and 13% of the SQII period, indicating that glucose management using the insulin sliding scale during SQII requires further optimization.
We were also able to generate a large amount of CGM data that allowed investigation of the association between perioperative CGM values and postoperative morbidities. It is well known that glycemic variability increases the risk for postoperative complications after cardiac surgery.2,16,28) and negatively affects the prognosis in critically ill patients.29,30) Although some studies have indicated that the maximum, average, and variability of postoperative BGLs could affect SSI,28) the importance of glycemic control during different postoperative periods was not clear. Our study revealed that blood glucose control was adequate in the CIII period, but suboptimal in the SQII period. Furthermore, the higher maximum and standard deviation values in the CGM data during SQII were associated with POAF occurrence. Various factors have been reported as causes of POAF,31,32 and there are some reports that β-blockers are effective for preventing POAF after cardiovascular surgery.33,34 Furthermore, the presence of high HbA1c levels (i.e., >7% or >8.6%35)) and/or hyperglycemia after cardiac surgery has been shown to be associated with POAF.36) We observed no association between POAF and HbA1c values, possibly because HbA1c levels were relatively low (<7%) in our study population. These results suggest that postoperative CGM data analysis could be useful in predicting several postoperative complications13) with higher accuracy than HbA1c.12) The influence of blood glucose variability on POAF has not been clarified in these patients. Using basic research data, Zhang et al.37) showed that hypoglycemia and hyperglycemia may prolong the Q-T interval and cause arrhythmia in diabetic patients. These data indicate a causal connection between glycemic variability and POAF. Further basic and clinical research studies are needed to clarify the underlying mechanism of POAF resulting from glycemic variability.
The apparently superior glycemic control during CIII compared to SQII could be due to several reasons. First, BGLs were measured more frequently during CIII (every 2 hours) compared to SQII (every 6 hours), potentially allowing finer glycemic control. Second, intravenous infusion in CIII could have aided glycemic control more than subcutaneous injection in SQII. Third, food ingestion during SQII could have contributed to glycemic variability. Furthermore, the SQII protocol may require additional optimization. For example, a basal-bolus insulin regimen with glargine once daily and glulisine before meals combined with sliding-scale regular insulin administered four times daily in patients with type 2 diabetes mellitus undergoing general surgery improved glycemic control and reduced hospital complications compared with sliding-scale regular insulin only.38) This strategy is also expected to lower glycemic variability and reduce postoperative complications such as SSI and POAF. Moreover, a real-time CGM system, recently noted for its ability to further reduce glycemic variability and improve outcomes,39) might be effective for glycemic control during SQII.
There are several limitations to this study. First, it was a single-center study, using a single type of CGM system. Therefore, extrapolation of results from this study to other populations and CGM devices should be performed with caution. Second, we recorded CGM data for only 7 days due to restrictions imposed by the CGM recorder. Since we started CGM data measurement preoperatively, the data could only be collected for a portion of the postoperative SQII period. More precise analysis of glycemic variability may be possible with CGM measurement over longer postoperative periods.
Conclusion
CGM data analysis revealed that our current institutional protocol of glucose management during CIII was adequate. However, glucose management during SQII using sliding-scale insulin administration requires further optimization. High glycemic variability during SQII was related to a higher incidence of SSI and POAF. Optimization of the SQII protocol to improve glycemic control would likely reduce the rate of several postoperative complications. Furthermore, CGM analysis shows promise for predicting postoperative complications.
Acknowledgment
No external funding was received for this study. We thank Drs. Ayoko Nagasawa, Hideyuki Kabasawa, Kazuhiko Hanzawa, and Osamu Namura from Niigata University Graduate School of Medical and Dental Science for their support with this study. Editorial support, in the form of medical writing based on the authors’ detailed directions, collating author comments, copyediting, fact checking, and referencing, was provided by Cactus Communications.
Disclosure Statement
There are no conflicts of interest.
References
- 1).Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract 2004; 10 Suppl 2: 21–3. [DOI] [PubMed] [Google Scholar]
- 2).Zerr KJ, Furnary AP, Grunkemeier GL, et al. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg 1997; 63: 356-61. [DOI] [PubMed] [Google Scholar]
- 3).Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 2015; 261: 97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Ascione R, Rogers CA, Rajakaruna C, et al. Inadequate blood glucose control is associated with in-hospital mortality and morbidity in diabetic and nondiabetic patients undergoing cardiac surgery. Circulation 2008; 118: 113-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Doenst T, Wijeysundera D, Karkouti K, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 2005; 130: 1144. [DOI] [PubMed] [Google Scholar]
- 6).Jones KW, Cain AS, Mitchell JH, et al. Hyperglycemia predicts mortality after CABG: postoperative hyperglycemia predicts dramatic increases in mortality after coronary artery bypass graft surgery. J Diabetes Complicat 2008; 22: 365-70. [DOI] [PubMed] [Google Scholar]
- 7).van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345: 1359-67. [DOI] [PubMed] [Google Scholar]
- 8).NICE-SUGAR Study Investigators. Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283-97. [DOI] [PubMed] [Google Scholar]
- 9).NICE-SUGAR Study Investigators. Finfer S, Liu B, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367: 1108-18. [DOI] [PubMed] [Google Scholar]
- 10).Ogawa S, Okawa Y, Sawada K, et al. Continuous postoperative insulin infusion reduces deep sternal wound infection in patients with diabetes undergoing coronary artery bypass grafting using bilateral internal mammary artery grafts: a propensity-matched analysis. Eur J Cardiothorac Surg 2016; 49: 420-6. [DOI] [PubMed] [Google Scholar]
- 11).Tatsuishi W, Adachi H, Murata M, et al. Postoperative hyperglycemia and atrial fibrillation after coronary artery bypass graft surgery. Circ J 2015; 79: 112-8. [DOI] [PubMed] [Google Scholar]
- 12).Su G, Mi S, Tao H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol 2011; 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Mendez CE, Mok KT, Ata A, et al. Increased glycemic variability is independently associated with length of stay and mortality in noncritically ill hospitalized patients. Diabetes Care 2013; 36: 4091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Dossett LA, Cao H, Mowery NT, et al. Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg 2008; 74: 679-85; discussion 685. [DOI] [PubMed] [Google Scholar]
- 15).Egi M, Bellomo R, Stachowski E, et al. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 2006; 105: 244-52. [DOI] [PubMed] [Google Scholar]
- 16).Stamou SC, Nussbaum M, Carew JD, et al. Hypoglycemia with intensive insulin therapy after cardiac surgery: predisposing factors and association with mortality. J Thorac Cardiovasc Surg 2011; 142: 166-73. [DOI] [PubMed] [Google Scholar]
- 17).Punke MA, Goepfert MS, Kluge S, et al. Perioperative glycemic control with a computerized algorithm versus conventional glycemic control in cardiac surgical patients undergoing cardiopulmonary bypass with blood cardioplegia. J Cardiothorac Vasc Anesth 2014; 28: 1273-7. [DOI] [PubMed] [Google Scholar]
- 18).Lazar HL, McDonnell M, Chipkin SR, et al. The society of thoracic surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009; 87: 663-9. [DOI] [PubMed] [Google Scholar]
- 19).Keenan DB, Mastrototaro JJ, Voskanyan G, et al. Delays in minimally invasive continuous glucose monitoring devices: a review of current technology. J Diabetes Sci Technol 2009; 3: 1207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Boom DT, Sechterberger MK, Rijkenberg S, et al. Insulin treatment guided by subcutaneous continuous glucose monitoring compared to frequent point-of-care measurement in critically ill patients: a randomized controlled trial. Crit Care 2014; 18: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Schierenbeck F, Franco-Cereceda A, Liska J. Accuracy of 2 different continuous glucose monitoring systems in patients undergoing cardiac surgery. J Diabetes Sci Technol 2017; 11: 108-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Wilinska ME, Hovorka R. Glucose control in the intensive care unit by use of continuous glucose monitoring: what level of measurement error is acceptable? Clin Chem 2014; 60: 1500-9. [DOI] [PubMed] [Google Scholar]
- 23).Aust H, Dinges G, Nardi-Hiebl S, et al. Feasibility and precision of subcutaneous continuous glucose monitoring in patients undergoing CABG surgery. J Cardiothorac Vasc Anesth 2014; 28: 1264-72. [DOI] [PubMed] [Google Scholar]
- 24).Siegelaar SE, Barwari T, Hermanides J, et al. Microcirculation and its relation to continuous subcutaneous glucose sensor accuracy in cardiac surgery patients in the intensive care unit. J Thorac Cardiovasc Surg 2013; 146: 1283-9. [DOI] [PubMed] [Google Scholar]
- 25).Holzinger U, Warszawska J, Kitzberger R, et al. Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care 2010; 33: 467-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Boyne MS, Silver DM, Kaplan J, et al. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 2003; 52: 2790-4. [DOI] [PubMed] [Google Scholar]
- 27).Rossetti P, Bondia J, Vehí J, et al. Estimating plasma glucose from interstitial glucose: the issue of calibration algorithms in commercial continuous glucose monitoring devices. Sensors (Basel) 2010; 10: 10936-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Jeon CY, Furuya EY, Berman MF, et al. The role of pre-operative and post-operative glucose control in surgical-site infections and mortality. PLoS ONE 2012; 7: e45616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Melduni RM, Schaff HV, Bailey KR, et al. Implications of new-onset atrial fibrillation after cardiac surgery on long-term prognosis: a community-based study. Am Heart J 2015; 170: 659-68. [DOI] [PubMed] [Google Scholar]
- 30).Risnes I, Abdelnoor M, Almdahl SM, et al. Mediastinitis after coronary artery bypass grafting risk factors and long-term survival. Ann Thorac Surg 2010; 89: 1502-9. [DOI] [PubMed] [Google Scholar]
- 31).Erdil N, Gedik E, Donmez K, et al. Predictors of postoperative atrial fibrillation after on-pump coronary artery bypass grafting: is duration of mechanical ventilation time a risk factor? Ann Thorac Cardiovasc Surg 2014; 20: 135-42. [DOI] [PubMed] [Google Scholar]
- 32).Wang M, Chen M, Ao H, et al. The effects of different BMI on blood loss and transfusions in Chinese patients undergoing coronary artery bypass grafting. Ann Thorac Cardiovasc Surg 2017; 23: 83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Sezai A, Shiono M. The role of β-blockers in cardiac perioperative management. Ann Thorac Cardiovasc Surg 2014; 20: 261-6. [DOI] [PubMed] [Google Scholar]
- 34).Nagaoka E, Arai H, Tamura K, et al. Prevention of atrial fibrillation with ultra-low dose landiolol after off-pump coronary artery bypass grafting. Ann Thorac Cardiovasc Surg 2014; 20: 129-34. [DOI] [PubMed] [Google Scholar]
- 35).Halkos ME, Puskas JD, Lattouf OM, et al. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2008; 136: 631-40. [DOI] [PubMed] [Google Scholar]
- 36).Tatsuishi W, Adachi H, Murata M, et al. Postoperative hyperglycemia and atrial fibrillation after coronary artery bypass graft surgery. Circ J 2015; 79: 112-8. [DOI] [PubMed] [Google Scholar]
- 37).Zhang Y, Han H, Wang J, et al. Impairment of human ether-á-go-go-related gene (HERG) K+ channel function by hypoglycemia and hyperglycemia. Similar phenotypes but different mechanisms. J Biol Chem 2003; 278: 10417-26. [DOI] [PubMed] [Google Scholar]
- 38).Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34: 256-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Polonsky WH, Peters AL, Hessler D. The Impact of Real-Time Continuous Glucose Monitoring in Patients 65 Years and Older. J Diabetes Sci Technol 2016; 10: 892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]