Abstract
Thymomas with ring calcifications are very rare and quaint style. Herein, we presented our three cases of thymomas with ring calcifications and reviewed totally 10 cases including 7 cases of previous English literatures. The median age was 53 years. Myasthenia gravis was a complication in 40%. The median maximal diameter was 50 mm. They were diagnosed as pathological type B or had type B component. Based on World Health Organization (WHO) classification, 20%, 60%, and 20% cases were stage I, stage II, and stage III, respectively. Seven ring calcifications were within tumors (inner type) and two cases were outside tumors (outer type). The other had a thymoma arising in the calcic wall of a calcified thymic cyst (miscellaneous type). Four other anterior mediastinal tumors with ring calcification had been reported. We need pathological examinations for a definitive diagnosis. Surgeons should plan surgery because of the possibility of invasive thymomas, or other malignant tumors.
Keywords: thymoma, ring, egg shell, calcification
Introduction
Calcifications occur sometimes in thymomas and are easily detected radiologically.1,2) The frequency of calcifications in thymomas ranges from 10% to 40%, and the pattern of calcifications is usually classified as stippled or nodular. However, thymomas with ring calcifications are very rare and quaint style. We have experienced three cases of thymomas showing ring calcification. A search of reported cases was made in PubMed, Google, and Yahoo (up to May 2016) to identify relevant cases of thymoma with ring calcification in the English literature using the terms “thymoma,” “ring,” “egg shell,” “round,” and “rim calcification,” and seven cases had been reported previously. Here, we analyzed the clinicopathological features of thymomas with ring calcifications.
Case Reports
Case 1
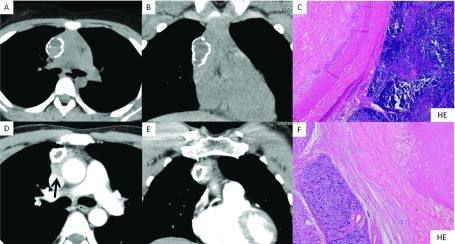
A 12-year-old girl had complained of limb weakness and was referred to the Department of Pediatrics in our hospital. Acetylcholine receptor antibody (AChRA) levels were elevated to 160 nmol/mL. Chest computed tomography (CT) showed that the anterior mediastinal tumor measured 50 × 48 × 20 mm with ring calcification (Figs. 1A and 1B). She had been diagnosed as myasthenia gravis (MG) with Myasthenia Gravis Foundation of America (MGFA) class IIa and treated with distigmine. She underwent extended thymectomy through median sternotomy. And she suffered from acute respiratory failure due to a myasthenia crisis in the operating room after the operation. She recovered and discharged on postoperative day 22. In 12 months, her AChRA level had decreased to 60 nmol/mL without steroid therapy. Finally, we diagnosed the tumor as a stage II type B1 thymoma according to World Health Organization (WHO) classification. There were calcification, hyaline degeneration, and fibrosis in a ring formation which contained cholesterol crystals inside the lesion (Fig. 1C). She had been followed postoperatively for 31 months without recurrence.
Fig. 1. (A) Horizontal view and (B) (frontal view): Chest CT showing anterior mediastinal tumor measuring 50 × 48 × 20 mm with ring calcification. (C) Type B1 thymoma (right side) outside the ring calcification (left side). (D) Horizontal view and (E) frontal view: Enhanced chest CT revealed the anterior mediastinal tumor measuring 50 × 40 × 20 mm with ring calcification, which was suspected to have invaded the superior vena cava (arrow). (F) Type B2 thymoma (left side) outside of the ring calcification (right side).
Case 2
A 40-year-old Bangladeshi male complained of ptosis and was admitted to an institution. His blood examination revealed elevation of AChRA to 15.0 nmol/mL. He was diagnosed as MG with MGFA class I. Chest CT revealed that the anterior mediastinal tumor measured 50 × 40 × 20 mm with ring calcification, which was suspected to have invaded to the superior vena cava (SVC; Figs. 1D and 1E). We performed extended thymectomy through median sternotomy. Because the tumor had invaded both the SVC and the pericardium and had strongly adhered to the right upper lobe of the lung, we performed combined partial resection and angioplasty of the SVC using a 0.1 mm Gore-tex patch and also performed combined resection of the right partial lung. He was administered warfarin and was uneventfully discharged on postoperative day 13. Finally, we diagnosed the tumor as a stage III type B2 thymoma according to WHO classification due to invasion to the SVC and pericardium. He underwent postoperative radiotherapy to his mediastinum. He had been followed postoperatively for 20 months without recurrence. Calcification, hyaline degeneration, and fibrosis formed a ring structure, which contained cholesterol crystals in the ring calcification (Fig. 1F).
Case 3
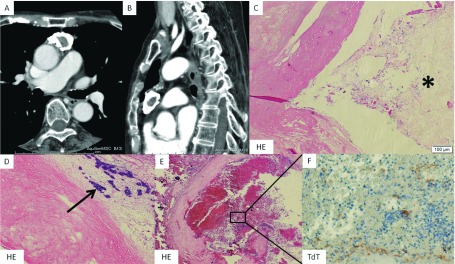
A 74-year-old female complained of progressive limb weakness and was admitted to the institution mentioned above. Her blood examination revealed elevation of AChRA to 170 nmol/L. She had suffered from acute respiratory failure and required intubation and tracheostomy. She was diagnosed as MG with MGFA class V. Chest CT showed an anterior mediastinal tumor measuring 64 × 32 × 18 mm with ring calcification (Figs. 2A and 2B). Mechanical ventilation was ceased owing to treatment with intravenous immune globulin and oral steroidal therapy, and her AChRA decreased to 21 nmol/L. After she recovered, we performed thoracoscopic extended thymectomy. The postoperative course was uneventful, and she was discharged on postoperative day 17. Finally, we diagnosed a stage II type B1 thymoma according to WHO classification. She had been followed postoperatively for 7 months without recurrence. Calcification, hyaline degeneration, and fibrosis were observed in a ring formation, which contained cholesterol crystals and a bare tumor in the inside lesion. A partly bleeding necrotic lesion was in the ring calcification. Terminal deoxynucleotidyl transferase staining showed tumor cells in the bleeding necrotic lesion (Figs. 2C–2F).
Fig. 2. (A) Horizontal view and (B) sagittal view: Chest CT showing anterior mediastinal tumor measuring 64 × 32 × 18 mm with ring calcification. (C) Cholesterol crystals inside the ring calcification (asterisk). (D) Bare tumor is inside the ring calcification (arrow). (E) Partly bleeding necrosis is in the ring calcification. (F) Terminal deoxynucleotidyl transferase stain showing tumor cells in the bleeding necrotic lesion.
Comment
Calcification in thymomas is not homogeneous; it is mostly stippled or nodular. Harris reported that the type, location, size, or other characteristics of thymus gland calcifications were not relevant features in clinical and radiologic diagnosis of thymoma.3) However, thymomas with ring calcifications are very rare and quaint style. Seven cases of thymomas with ring calcifications had been reported.3–9) This is the first report to analyze the clinicopathological features of thymomas with ring calcifications.
A total of 10 cases of thymomas with ring calcifications, including our three cases, are summarized in Table 1. Four cases each were female and male, and the other two were not specified. The median age was 53 years, and our pediatric case was extremely rare. The clinical symptoms were mostly related to MG, and other symptoms were dyspnea and chronic coughing. Their complication rate of MG was 40% in total. Our all three cases were accompanied with MG, meanwhile remaining six of seven reported cases were not accompanied with MG. Their complication rate of MG with thymoma was between 20% and 40%, and the rate of MG with type B thymoma was 35%–49%.10–13) Although the complication rate with MG could not simply compare due to the small number of cases, it was approximated rate. In other reports, the median maximal diameter of usual thymomas was 57–68 mm, and our case series was 50 mm, which was smaller than usual thymomas.10,12,13) However, these cases were greater than or equal to 40 mm. Pathologically, only one case had type AB, and the other eight had type B (Type B1:B2:B3 = 4:3:1 cases). All thymomas with ring calcification had pathologically type B or type B component, and type B1 and B2 were more frequent. Nakajima et al. reported that 63% of thymomas had equal to or more advanced than stage II (WHO classification), and Weis et al. reported that about 55% of type B1 and 70% of type B2 thymomas had equal to or more advanced than stage II.10,12) In this literature, 80% had thymomas equal to or more advanced than stage II, which meant that they were frequently invasive thymomas. Calcifications were pathologically found in more than half of the thymomas of type B2 and B3, and found more frequently in invasive thymomas than in noninvasive thymomas.14,15) These case series showed similar features. The follow-up period was between 6 and 50 months (median: 20 months) in five cases, all of which received extended thymectomy, and they had survived without recurrence during these periods. As these prognosis data were showed only short-term results, we need longer-term prognosis.
Table 1. Clinicopathological findings of thymomas with ring calcifications.
| Author/year | Age/sex | MG/MGFA class | Presentation | Tumor size (mm) | Pathological type | WHO stage | Calcification type | Inside of the calcification | Surgical procedure | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 2013 | 12 F | Yes IIa | Muscle weakness | 50 × 50 × 20 | B1 | II | Inner | Cholesterin crystal | Extended thymectomy | 31 months alive |
| Case 2 2014 | 47 M | Yes I | Ptosis | 50 × 40 × 20 | B2 | III | Inner | Cholesterin crystal | Extended thymectomy | 20 months alive |
| Case 3 2015 | 74 F | Yes IV | Muscle weakness | 64 × 32 × 18 | B1 | II | Inner | Cholesterin crystal and bare tumor | Extended thymectomy | 7 months alive |
| H Chen 20094) | 62 M | No | Abnormal shadow | 145 × 92 × 33 | B1 | III | Inner | Gritty consistency | Extended thymectomy | 50 months alive |
| F Siraj 20105) | 35 F | Yes IIa | Muscle weakness | 120 × 40 × 30 | B3 | II | Inner | Unspecified | Extended thymectomy | 6 months alive |
| L Yu 20116) | 46 M | No | Dyspnea | 50 × 40 | B1 | I | Outer | Tumor and yellowish liquid | Partial resection | Unspecified |
| K Harris 20113) | Unspecified | No | Unspecified | 40 × 30 | B2 | II | Inner | Tumor | Partial resection | Unspecified |
| H Elouazzani 20117) | Unspecified | No | Chronic cough | 150 × 80 × 60 | AB | II | Tumor arising in the calcic wall | Cholesterol | Partial resection | Unspecified |
| A Low 20138) | 83 F | No | Unspecified | Unspecified | Unspecified | I | Outer | Tumor | Unspecified | Unspecified |
| A Sano 20149) | 59 M | No | Abnormal shadow | 40 | B2 | II | Inner | Tumor | Thymectomy | Unspecified |
MG: myasthenia gravis; MGFA: Myasthenia Gravis Foundation of America; WHO: World Health Organization
In this study, we detected three types of ring calcification in the point of calcic location. Seven cases were within tumors (inner type), and two cases were outside tumors (outer type). Inner types were more common than outer ones. Outer type was non-invasive thymoma, and inner type was invasive thymoma. Elouazzani et al. had reported an extremely rare case of a thymoma arising in the calcic wall (miscellaneous type), consisting of multilocular thymic cysts which were partially lined by small cuboidal cells with severe chronic inflammation.7) Until now, the processes of intratumoral calcification had been proposed, but the mechanism of forming a ring calcification was still unclear. Here we focused on the relationship between calcification and its inner structure. In this study, the inner structure of the calcification contained only cholesterol crystals in three cases (37.5%), cholesterol crystals and tumors in two cases (25%), and tumors in three cases (37.5%) of the eight cases, apart from two unspecified cases. The pathological mechanism of calcification was reported in various tissues. As a common process, dystrophic calcification can occur in the presence of degenerative or necrotic tissues, entrapment of pre-existing calcified scar tissue or granulomatous tissue, and mucus-producing areas adjacent to tumor cells.16) However, this theory had no application to thymomas, which contained tumors inside the ring calcification without necrotic tissue. We could explain the ring calcification as follows. Calcium deposition had occurred in the fibrotic capsule of the thymoma, which assumed a ring formation due to its prolonged course. Outer types were of a relatively less advanced than stage II due to slow growth. Inner types may take an additional course. The calcic change could have brought on intratumoral ischemic and nutritional disorder, resulting in necrosis and degeneration, which depended on the degree, and could attract more calcification as dystrophic calcification. Inner types could suggest that an ancient outer calcified capsule was incorporated in the tumor in the form of tumor growth.
We also searched for other mediastinal tumors with ring calcification in the same way. We found four other cases of anterior mediastinal tumors with ring calcification apart from thymomas, which included two cases of multilocular thymic cysts, a pericardial cyst, and a squamous cell carcinoma.17–20) Ring calcification was not specific to thymomas. A pathological examination is necessary to get a definitive diagnosis. When surgeons encounter these calcified tumors, they should plan surgery because of the possibility of invasive thymomas, or other malignant tumors.
Conclusion
All thymomas with ring calcification had pathologically type B or type B component. We suggested that calcium deposition had occurred in the fibrotic capsule of the thymoma, which assumed a ring formation due to its prolonged course. Because ring calcification is not specific to thymomas, we need pathological examinations for a definitive diagnosis. Surgeons should plan surgery because of the possibility of invasive thymomas, or other malignant tumors.
Disclosure Statement
No conflict of interests.
References
- 1).Tomiyama N, Müller NL, Ellis SJ, et al. Invasive and noninvasive thymoma: distinctive CT features. J Comput Assist Tomogr 2001; 25: 388-93. [DOI] [PubMed] [Google Scholar]
- 2).Jeong YJ, Lee KS, Kim J, et al. Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis? AJR Am J Roentgenol 2004; 183: 283-9. [DOI] [PubMed] [Google Scholar]
- 3).Harris K, Elsayegh D, Azab B, et al. Thymoma calcification: is it clinically meaningful? World J Surg Oncol 2011; 9: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Chen HK, Huang WT, Eng HL, et al. Ossifying thymoma clinically presenting with peripheral T-cell lymphocytosis. Ann Thorac Surg. 2009; 88: e5-7. [DOI] [PubMed] [Google Scholar]
- 5).Siraj F, Dhawan S, Jain D. Invasive thymoma with osseous metaplasia and cystic change in a case of myasthenia gravis: a rare presentation. Gen Thorac Cardiovasc Surg 2011; 59: 583-6. [DOI] [PubMed] [Google Scholar]
- 6).Yu L, Shi E, Gu T. Calcified thymoma: an egg in the chest. J Thorac Cardiovasc Surg 2011; 142: 216-8. [DOI] [PubMed] [Google Scholar]
- 7).Elouazzani H, Zouaidia F, Jahid A, et al. Calcified multilocular thymic cyst associated with thymoma: a case report. J Med Case Rep 2011; 5: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Low A, Abbas A, Medford AR. A ring calcified benign thymoma in a patient with asbestos exposure. QJM 2013; 106: 371-2. [DOI] [PubMed] [Google Scholar]
- 9).Sano A, Kawashima M. Thymoma with ring calcification. Ann Thorac Surg 2014; 98: 2202-4. [DOI] [PubMed] [Google Scholar]
- 10).Nakajima J, Okumura M, Yano M, et al. Myasthenia gravis with thymic epithelial tumour: a retrospective analysis of a Japanese database. Eur J Cardiothorac Surg 2016; 49: 1510-5. [DOI] [PubMed] [Google Scholar]
- 11).Lucchi M, Ricciardi R, Melfi F, et al. Association of thymoma and myasthenia gravis: oncological and neurological results of the surgical treatment. Eur J Cardiothorac Surg 2009; 35: 812-6; discussion 816. [DOI] [PubMed] [Google Scholar]
- 12).Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol 2015; 10: 367-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Roden AC, Yi ES, Jenkins SM, et al. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J Thorac Oncol 2015; 10: 691-700. [DOI] [PubMed] [Google Scholar]
- 14).Tomiyama N, Johkoh T, Mihara N, et al. Using the World Health Organization classification of thymic epithelial neoplasms to describe CT findings. AJR Am J Roentgenol 2002; 179: 881-6. [DOI] [PubMed] [Google Scholar]
- 15).Jung KJ, Lee KS, Han J, et al. Malignant thymic epithelial tumors: CT-pathologic correlation. AJR Am J Roentgenol 2001; 176: 433-9. [DOI] [PubMed] [Google Scholar]
- 16).Hara H, Iwabuchi K, Shinada J, et al. Pulmonary adenocarcinoma with heterotopic bone formation. Pathol Int 2000; 50: 910-3. [DOI] [PubMed] [Google Scholar]
- 17).Osaki T, Nakagawa M. Multilocular mediastinal cyst with rim calcification: report of a case. Surg Today 2008; 38: 52-5. [DOI] [PubMed] [Google Scholar]
- 18).Sugimoto S, Misao T, Nakano H, et al. Mediastinal cyst with rim calcification. Jpn J Thorac Cardiovasc Surg 2004; 52: 261-3. [DOI] [PubMed] [Google Scholar]
- 19).Zhao F, Wang C, Song XL. Calcified pericardial cyst—a case report and the roentgenologic and pathologic differentiation from other calcified mediastinal cysts. Thorac Cardiovasc Surg 1984; 32: 193-5. [PubMed] [Google Scholar]
- 20).Hennessy O. Calcified squamous cell carcinoma of mediastinum. Available at http://radiopaedia.org/cases/calcified-squamous-cell-carcinoma-of-mediastinum. Accessed Jan 14, 2015.