Abstract
Two natural nicotinamide-based coenzymes (NAD and NADP) are indispensably required by the vast majority of oxidoreductases for catabolism and anabolism, respectively. Most NAD(P)-dependent oxidoreductases prefer one coenzyme as an electron acceptor or donor to the other depending on their different metabolic roles. This coenzyme preference associated with coenzyme imbalance presents some challenges for the construction of high-efficiency in vivo and in vitro synthetic biology pathways. Changing the coenzyme preference of NAD(P)-dependent oxidoreductases is an important area of protein engineering, which is closely related to product-oriented synthetic biology projects. This review focuses on the methodology of nicotinamide-based coenzyme engineering, with its application in improving product yields and decreasing production costs. Biomimetic nicotinamide-containing coenzymes have been proposed to replace natural coenzymes because they are more stable and less costly than natural coenzymes. Recent advances in the switching of coenzyme preference from natural to biomimetic coenzymes are also covered in this review. Engineering coenzyme preferences from natural to biomimetic coenzymes has become an important direction for coenzyme engineering, especially for in vitro synthetic pathways and in vivo bioorthogonal redox pathways.
Keywords: Coenzyme engineering, Nicotinamide-based coenzymes, NAD, NADP, Protein engineering, Synthetic biology, Biomimetic coenzymes
1. Introduction
Protein engineering is the process of modifying the amino acid sequence of proteins toward desired properties, including improved substrate spectrum [1], [2], product selectivity [3], [4], enzyme activity [5], thermostability [6], [7], [8], and solvent tolerance [8]. Protein engineering has been a powerful tool in biotechnology to generate a vast number of enhanced or novel enzymes for industrial applications and has played a crucial role in advancing synthetic biology [9].
Synthetic biology is an emerging discipline that applies engineering principles for the design and assembly of biological components toward synthetic biological entities with an ultimate goal of cost-effective biomanufacturing [10]. The purpose of synthetic biology is to design and construct novel biological pathways, organisms and devices or to redesign the existing natural biological systems, in order to understand the complexity of biological systems and to improve various applications [11]. The most important application of synthetic biology may be the low-cost production of new drugs, chemicals, biomaterials, and bioenergy [12], [13], [14], [15], [16], [17], [18]. Synthetic biology can influence many other scientific and engineering fields as well as various aspects of daily life and society [17]. It can be classified into in vivo and in vitro synthetic biology [19]. In vivo synthetic biology mainly focuses on fundamental biological research facilitated by the use of synthetic DNA and genetic circuits on typical model microorganisms, such as Escherichia coli, Bacillus subtilis and Saccharomyces cerevisiae [14]. It is a current predominant research area because living organisms can self-duplicate without major concerns of the biocatalyst preparation, possibly due to a biotechnology paradigm based on thousands of years of fermentation. In contrast, in vitro synthetic biology, sometimes referred to as cell-free synthetic biology, is based on reconstituted enzyme mixtures or cell lysates in one pot for the ultimate purpose of biomanufacturing [20], [21], [22], [23], [24]. Strictly speaking, in vitro synthetic biology is slightly different from cell-free synthetic biology, where the former is based on the reconstitution of (purified) enzymes, coenzymes and/or other abiotic components (for example, using benzyl viologen as electron mediator for in vitro biohydrogen generation [25]), and the latter is mainly based on the cell lysates of one or multiple cell cultures. The in vitro synthetic biology platform has some distinctive advantages, such as high product yield, fast reaction rate, highly engineering flexibility, and high tolerance in toxic environments [19], [20], [21], [26]. Recently, the first industrial biomanufacturing example of the cost-effective production of myo-inositol from starch has been demonstrated in China [27].
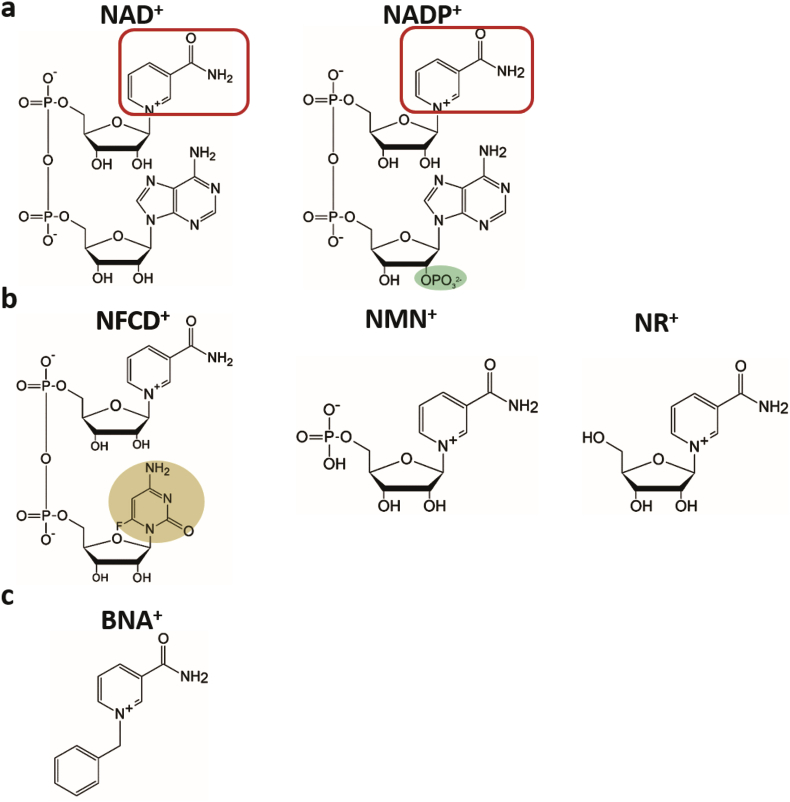
Oxidoreductases are the largest group of enzymes in the Enzyme Commission nomenclature. These enzymes account for nearly 30% (1801/6300) of active enzyme classes according to Brenda database (http://brenda-enzymes.info/) [28]. Coenzymes are usually required in these oxidoreductase-catalyzed reactions to transport electron, hydride, hydrogen, oxygen, or other atoms or small molecules in different enzymatic pathways [29], [30]. Typical coenzymes are nicotinamide adenine dinucleotide (NAD)/nicotinamide adenine dinucleotide phosphate (NADP), ubiquinone (CoQ), and flavin mononucleotide (FMN)/flavin adenine dinucleotide (FAD). Nicotinamide-based coenzymes for the transport and storage of electrons in the form of hydride groups are the most important, because 80% of characterized oxidoreductases need NAD as a coenzyme, and 10% of them need NADP as a coenzyme [30]. NAD and NADP are two types of ubiquitous pyridine nucleotide coenzymes that differ only by the additional 2′-phosphate group esterified to the adenosine monophosphate moiety of NADP (Fig. 1a). Because the phosphate group of NADP is sufficiently distant spatially and covalently from the chemically active nicotinamide moiety (red rounded rectangle in Fig. 1a), nearly all oxidoreductases exhibit a strong preference for one to the other for implementing different metabolic roles [31].
Fig. 1.
Structures of nicotinamide-based coenzymes and biomimetic nicotinamide coenzymes. a) Two natural coenzymes, NAD+ and NADP+, in which the chemical groups in the open red rounded rectangles represent where the redox reaction occurs; these chemical groups are the same in all coenzymes. b) Biomimetic coenzymes derived from natural coenzymes, nicotinamide flucytosine dinucleotide (NFCD+), nicotinamide mononucleotide (NMN+), and nicotinamide mononucleoside (NR+); the chemical group in the shaded area indicates the structural difference between NFCD+ and NAD+. c) Synthetic biomimetic coenzyme 1-benzyl nicotinamide (BNA+).
Changing the coenzyme preference of oxidoreductases is an important area of protein engineering. It has also been recognized as an important tool for in vitro and in vivo synthetic biology projects. For in vitro synthetic biology and cascade biocatalysis projects, coenzyme preference is usually switched from NADP to NAD because the price of NADP is much higher than that of NAD (e.g., $200 per g for NADH (Sigma N8129), $6000 per g for NADPH (Sigma N5130), $140 per g for NAD+ (Sigma N7004) and $1000 per g for NADP+ (Sigma N5755)). Additionally, NAD is more stable than NADP [2], [32], [33]. Furthermore, more NADH-regeneration enzymes in vitro are available than NADPH-regeneration enzymes [29], [34]. For in vivo synthetic biology projects, the switch of coenzyme preference can be conducted in both directions from NAD to NADP or from NADP to NAD to balance coenzyme availability and increase metabolic pathway efficiency [35], [36], [37], [38], [39]. Coenzyme engineering from natural to biomimetic nicotinamide-based coenzymes (Fig. 1b and c) may further decrease the production cost for in vitro synthetic biology because the cost and stability of biomimics are much better than those of natural coenzymes [40], [41]. Engineered enzymes with specificities on biomimetic nicotinamide coenzymes can be used to develop bioorthogonal redox systems in vivo without interfering with native biochemical processes [42], [43], [44].
In this review, we focus on the methods of coenzyme engineering regarding switching the nicotinamide-based coenzyme preferences of oxidoreductases and the application of the mutant enzymes with different coenzyme preferences in product-oriented synthetic biology. The latest advances in the general design of coenzyme engineering and high-throughput screening methods for directed evolution are highlighted. Coenzyme preference changes from natural to biomimetic coenzymes could be extremely important, especially to in vitro synthetic biology such as biohydrogen and bioelectricity generation from oligosaccharides [25], [45], [46], [47], [48], [49], [50], [51].
2. Coenzyme engineering methods of nicotinamide-based coenzymes
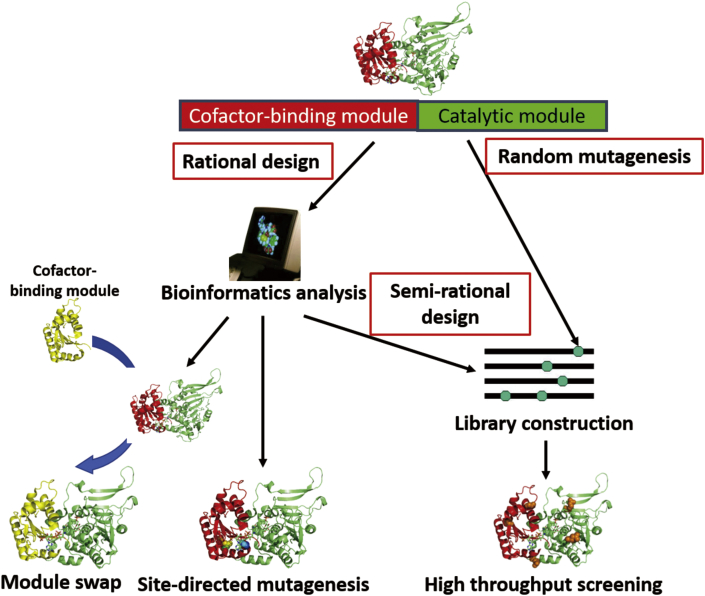
Coenzyme engineering that changes enzymatic coenzyme preferences has three major methods: rational design, semi-rational design and random mutagenesis (Fig. 2) [52], [53]. Table 1 presents some representatives of product-oriented coenzyme engineering for in vivo and in vitro synthetic biology using these engineering methods. Rational design is a knowledge-based method that requires prior information on structure and/or function. Specific residues are used to replace the residues in specific sites of the targeted enzymes by site-directed mutagenesis, hoping to obtain mutants with the desired properties. Semi-rational design is also a knowledge-based approach that creates a mutant library by site-saturation mutagenesis (where all or a fraction of the 20 natural amino acids are tested) at specific residues. Random mutagenesis is a knowledge-free approach that generates a mutant library by error-prone PCR or gene shuffling for whole-gene randomization. The last two approaches always require an extra step for the screening or selection of the mutated enzymes possessing desired properties from the mutant library. Chica et al. proposed a flowchart regarding the selection of the appropriate enzyme engineering approach based on the availability of experimental tools as well as the prior knowledge of structure and function [53]. Because most NAD(P)-based oxidoreductases share a highly conserved coenzyme-binding motif -- the Rossmann fold, which is the first identified conserved protein domain based on sequence alignments and crystal structures [54], [55], rational design and semi-rational design that creates ‘small but smart’ libraries based on this conserved motif are more widely used in coenzyme engineering projects than random mutagenesis that renders a large size of mutant library.
Fig. 2.
Scheme of coenzyme engineering methods, including rational design, semi-rational design and random mutagenesis.
Table 1.
List of product-oriented coenzyme engineering on natural nicotinamide coenzymes NAD(P).
| Enzyme | Source | Specificity | Mutations | Product | Increasing effect | Reference |
|---|---|---|---|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase | Corynebacterium glutamicum | NADH→NADPH | D35G/L36R/P192S | Lysine | ∼60% higher yield | [120] |
| NADH oxidase 2 | Streptococcus mutans | NADH→NADPH | V193R/V194H | 2-heptanone | NA | [121] |
| 1,5-anhydro-d-fructose reductase | Sinorhizobium Morelense | NADPH→NADH | A13G | 1,5-anhydro-D-mannitol | NA | [122] |
| Imine reductase | Streptomyces sp. GF3587 | NADPH→NADH | K40A | 2-methylpyrolidine | ∼64% higher conversion | [123] |
| Ketol-acid reductoisomerase | Escherichia coli | NADPH→NADH | A71S/R76D/S78D/Q110V | 2-methylpropan-1-ol (isobutanol) | 3-fold higher titer | [39] |
| Xylose reductase | Pichia stipitis | NADPH→NADH | R276H | Ethanol | ∼20% higher yield | [91] |
| Xylose reductase | Candida tenuis | NADPH→NADH | K274R/N276D | Ethanol | ∼42% higher yield | [124] |
| 6-phosphogluconate dehydrogenase | Thermotoga maritima | NADP+→NAD+ | N32E/R33I/T34I | Electricity | ∼25% higher maximum power density and current density | [125] |
| 6-phosphogluconate dehydrogenase | Geobacillus stearothermophilus | NADP+→NAD+ | N33D/R34Y/K38L | Polyhydroxybutyrate | NA | [126] |
| Glucose 6-phosphate dehydrogenase | Geobacillus stearothermophilus | NADP+→NAD+ | A47D | Polyhydroxybutyrate | NA | [126] |
| Glucose 6-phosphate dehydrogenase | Thermotoga maritima | NADP+→NAD+ | S33E/R65M/T66S | Hydrogen | NA | Unpublished |
NA: Not Available.
2.1. Rational design
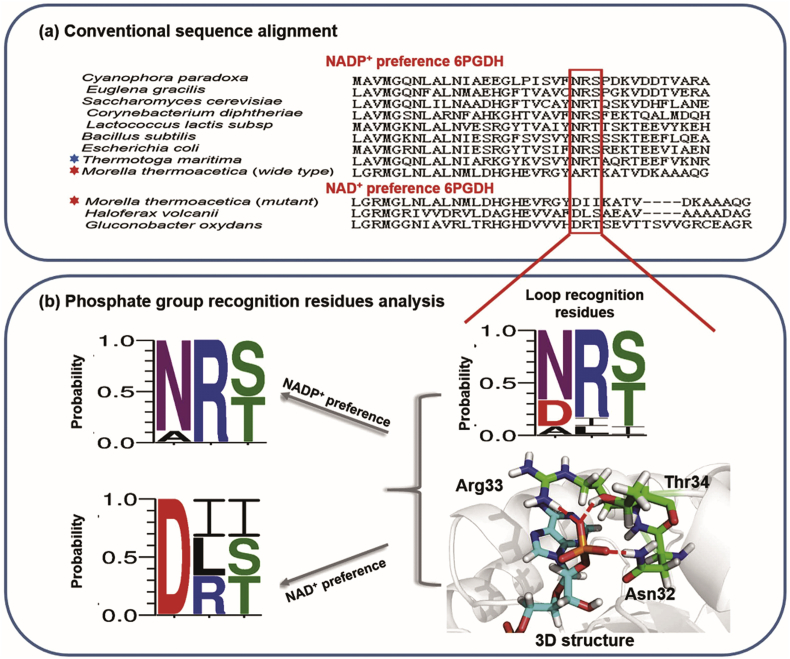
Rational design is the oldest protein engineering tool to switch the coenzyme preference of oxidoreductases. It mutates specific amino acid residues to certain other residues using site-directed mutagenesis based on the structures of NAD(P)-enzyme complexes and related catalytic mechanisms. Generally, coenzyme engineering by rational design starts with the identification of residues near coenzyme-binding sites [56], [57], residues binding with the 2′ phosphate group [58] or adenosine-binding pocket [59], or residues essential for catalytic activity [2], [60], [61], [62], [63]. Chen et al. performed an amino acid sequence alignment of the coenzyme-binding motifs of NADP- and NAD-preferred 6-phosphogluconate dehydrogenases (6PGDH) (Fig. 3a) [2]. The loop region amino acids (in red box of Fig. 3a) are responsible for the interaction between enzymes and 2′-phosphate of NADP. The alignment of the loop region indicates that NAD-preferred 6PGDH wild-type enzymes and mutants share a highly conservative, acidic aspartate residue at the N-terminal end of this loop region (site 32), while NADP-preferred 6PGDHs have three highly conserved amino acid residues at sites 32, 33, and 34 (Asn32, Arg33 and Ser/Thr34) (Fig. 3b). When these key amino acid residues responsible for the binding of the 2′-phosphate group of NADP+ were modified by site-directed mutagenesis on a 6PGDH from Thermotoga maritima, the best mutant N32E/R33I/T34I exhibited a ratio of 96 for catalytic efficiency (kcat/Km) on NAD+ to NADP+, which is a ∼64,000-fold reversal of the coenzyme selectivity from NADP+ to NAD+. Among these residues, arginine 33 plays a critical role in NADP+ binding by contributing a positively charged planar residue that interacts primarily with the 2′-phosphate of NADP+. Changing this arginine to aspartate or glutamate is often used to shift coenzyme preference from NADP to NAD [64], [65], [66]. Cui et al. (2015) developed a novel computational strategy to alter the coenzyme preference that enhances the hydrogen-bond interaction between an enzyme and a coenzyme. This novel computational strategy only required the structure of the target enzyme without other homologous enzymes. By using this rational design method, Gluconobacter oxydans Gox2181, which belongs to the short-chain dehydrogenases/reductases superfamily (SDR superfamily), was engineered to show a much higher enzymatic activity with NADPH as its coenzyme through the two-site mutation of Q20R and D43S [67].
Fig. 3.
a) Amino acid sequence alignment of the coenzyme-binding motif of various 6PGDH enzymes. The residues comprising the loop region and responsible for coenzyme recognition are boxed. Red stars represent M. thermoacetica wild-type NADP+-preferred 6PGDH and the NAD+-preferred 6PGDH mutant. The blue star indicates T. maritima 6PGDH studied in this research. (b) Sub-alignments of key amino acid residues playing an important role in 2′-phosphate interaction. The colors in the sequence logo refer to hydrophobic (black), positive charge (blue), negative charge (red) and polar (green) residues (This figure is a courtesy from Ref. [2]).
Module swapping is another rational design method to switch coenzyme preference by replacing the original coenzyme binding pocket with a new one from homologous enzymes [68]. For example, Yaoi et al. changed the coenzyme preference of an isocitrate dehydrogenase by replacing the NADP-binding pocket with a homogeneous NAD-binding pocket [69]. Similarly, the coenzyme preferences of a β-isopropylmalate dehydrogenase [70] and a short-chain dehydrogenase [71] have been reversed using this strategy.
2.2. Semi-rational design
Semi-rational design is a powerful method to switch the coenzyme preference by site-saturated mutagenesis of some critical amino acid residues deduced from bioinformatics analysis followed by the screening of mutant libraries. Coenzyme engineering of an E. coli ketol acid reductoisomerase (KARI) from NADP to NAD is a typical example of semi-rational design from Arnold's laboratory [39]. Five amino acids in the Rossmann fold of this KARI were determined based on previous work [72], sequence alignment and structure of the cofactor binding pocket. Five individual libraries of each amino acid were constructed by site-saturation mutagenesis and were screened for variants exhibiting a higher ratio of NADH to NADPH activities. A library was constructed by combining all beneficial mutations as well as the wild-type residues. The best variant, which had four mutation sites, exhibited much higher activity on NADH to NADPH, resulting in a 54,000-fold change in the ratio of the catalytic efficiency (kcat/Km) on NADH to NADPH compared with the wild-type enzyme [39]. Later, the same group proposed a general semi-rational approach to switch the coenzyme preference of KARI from NADPH to NADH by integrating previous results of an engineered NADH-dependent mutant of E. coli KARI, available KARI crystal structure information, and a comprehensive sequence alignment of KARI [61]. With this approach, the specific patterns of amino acid residue replacement in the β2αB loop showed a positive effect on reversing the coenzyme specificity of KARI. The approach included the following steps: (1) identification of the loop, (2) determination of the β2αB-loop length and mutation based on the loop length by site-directed mutagenesis and site-saturated mutagenesis to achieve the coenzyme switch; and (3) improvement of the overall activity on NADH via random mutagenesis [61]. Recently, this group has developed a structure-guided, semi-rational strategy for reversing enzymatic nicotinamide-based coenzyme specificity of all NAD(P)-utilizing enzymes [31] based on the increased number of protein crystal structures with high resolution and homogeneous oxidoreductase sequences with different coenzyme preferences. This strategy is comprised of three steps: structural analysis of enzymes, design and screening of focused mutant libraries to reverse cofactor preference, and recovery of catalytic efficiency. Unlike the case of KARI engineering involving random mutagenesis of the entire gene [61], the recovery of catalytic efficiency in this strategy is based on the predicted positions in the amino acid sequence with dramatically increased probabilities of harboring compensatory mutations. This strategy has shown the efficacy of inverting the coenzyme preference of four structurally diverse NADP-dependent enzymes: glyoxylate reductase, cinnamyl alcohol dehydrogenase, xylose reductase, and iron-containing alcohol dehydrogenase. The analytical components of this approach have been fully automated and available in the form of a user-friendly online tool named Cofactor Specificity Reversal-Structural Analysis and Library Design (CSR-SALAD) [31].
2.3. Random mutagenesis
Random mutagenesis of the entire DNA sequence may be the last solution to change the enzyme properties without relying on the crystal or modeling structure of the target protein [73], [74]. This method is rarely used for shifting the coenzyme preference between NADP and NAD because coenzyme-binding domains are highly conserved at some specific residues close to coenzyme-binding sites. However, this method may be of particular importance for screening mutants that can work on biomimetic coenzymes, whose structures largely differ from NADP and NAD (Fig. 1). Random mutagenesis is also useful when compensatory mutations are remote from the cofactor-binding sites [31].
2.4. Directed evolution based on high-throughput screening (HTS)
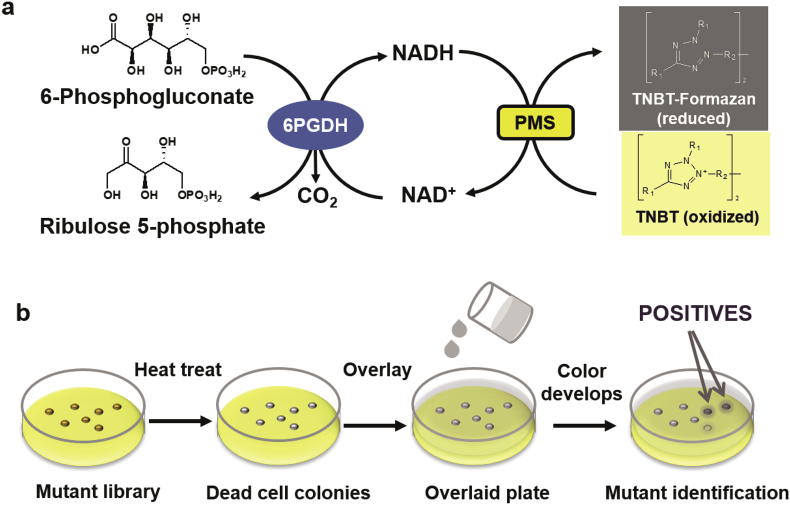
A high-throughput screening method is urgently required to identify positive mutants from the library constructed by site-saturated mutagenesis or random mutagenesis. The use of 96-well microplate screening based on the absorbency of NAD(P)H at 340 nm or coenzyme-linked colorimetric assay is straightforward to measure the activities of dehydrogenases [33], [61], [75]. However, the microplate-based screening is labor-intensive, time-consuming and may require expensive automated machines [31], [76]. It will be of great significance to develop a simple and effective HTS method to determine the coenzyme preference change of oxidoreductases. Recently, Zhang's group developed a Petri-dish double layer-based screening method to identify mutants of thermophilic 6-phosphogluconate dehydrogenase (6PGDH) from Moorella thermoacetica with reversed coenzyme preference from NADP+ to NAD+ [1]. The colonies of a 6PGDH mutant library were treated by heat to deactivate intracellular mesophilic dehydrogenases and reductive compounds (i.e., NADPH and NADH) and to disrupt the cell membrane. A second semi-solid layer was added by pouring the melted agarose solution containing tetranitroblue tetrazolium (TNBT), phenazine methosulfate (PMS), NAD+, and 6-phosphogluconate. In the second layer, 6PGDH catalyzes the hydration of 6-phosphogluconate, coproducing NADH from NAD+. In the presence of PMS and NADH, the colorless redox dye TNBT was reduced to black TNBT-formazan (Fig. 4A). As a result, 6PGDH mutants with improved activity on NAD+ can be identified by naked eyes (Fig. 4B). Positive mutants were recovered by direct extraction of the plasmid from dead-cell colonies followed by plasmid transformation into E. coli TOP10 [1]. Using this method, our laboratory has also switched the coenzyme preference of T. maritima glucose 6-phosphate dehydrogenase (G6PDH) from NADP+ to NAD+ (submitted for publication).
Fig. 4.
Scheme of double layer-based screening. a) Mechanism of the colorimetric assay. 6PGDH catalyzes the oxidation of 6-phosphogluconate to ribulose 5-phosphate and CO2, and the reduction of NAD+ to NADH. In the presence of PMS, NADH transfers its hydride and reduces the colorless redox dye TNBT to black color TNBT formazan. b) The process of the double layer-based screening method. The mutant library was treated by heat and overlaid by a second agarose layer with reagents. The colonies with a darker color and halo were identified as positive mutants.
3. Applications of coenzyme engineering in in vivo synthetic biology
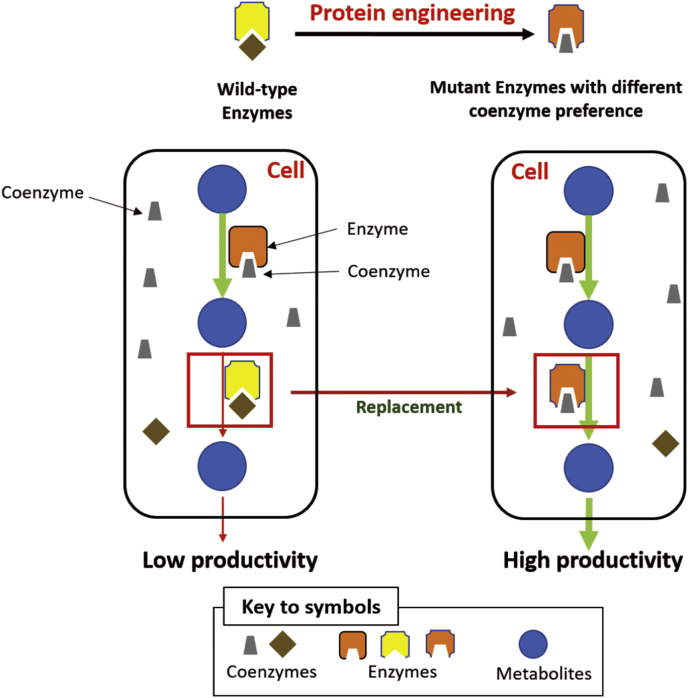
In vivo synthetic biology and metabolic engineering are widely investigated for their potentials in the production of biofuels, amino acids, alcohols, natural products, and antibiotics [77], [78]. Because NAD and NADP have different roles in catabolism and anabolism, respectively, their supply and consumption, as well as their balance, are essential for engineered organisms. Synthetic pathways that failed to match the coenzyme supply and consumption will possibly result in low product yields and slow volumetric productivity. For example, Liao's isobutanol synthesis pathway has a NADH-generation pathway to produce an isobutanol precursor followed by a NADPH-consumption step for the formation of isobutanol [79], [80]. Fig. 5 illustrates a case in which one coenzyme is more prevalent in the cell organelle than the other. One enzyme in the pathway prefers the high-abundant coenzyme, while the other enzyme prefers the low-abundant coenzyme, coenzyme imbalance occurs, leading to the low-efficiency biosynthesis of the desired product. To balance different coenzymes, several approaches can be taken: (1) Increase of oxygen supply can balance the energy flux, yet may still result in a lower-than-theoretical product yield. (2) Introduction of a transhydrogenase [81] that catalyzes the reversible transfer of a hydride ion between NADH and NADP+. Nevertheless, the transhydrogenase may not always shift the hydride ion in the desired direction [82]. Additionally, introduction of new cellular components may increase the metabolic burden of the cells or may direct the energy flux toward undesired directions. (3) Replacement of native enzymes with those harboring a different coenzyme specificity [83], [84]. However, finding a sequence with specific desired properties can be difficult, particularly when few members of a protein family have been characterized [62]. (4) The best strategy is changing the coenzyme specificity of the oxidoreductases in the pathway by protein engineering and then introducing the mutant enzyme into the cells for the replacement of the wild-type enzyme to solve the problem of coenzyme imbalance. Unlike in vitro synthetic biology in which the coenzyme engineering from NADP to NAD is preferred because of the cost issue, in vivo synthetic biology can change the coenzyme preference in both directions from NADP to NAD and from NAD to NADP [85]. In this section, we introduce some examples concerning the improvement of the productivity of microbial cell factories by changing the enzyme's coenzyme preference.
Fig. 5.
Engineering the coenzyme preference of oxidoreductases in a metabolic pathway by protein engineering in vitro followed by the replacement of the wild-type enzyme with the mutant enzyme to solve the problem of coenzyme un-matching.
3.1. From NAD to NADP
Amino acids represent one of the largest classes of fermentative products, whose production closely correlates with the availability of NADPH. For example, the synthesis of one mole of lysine requires 4 mol of NADPH in Corynebacterium glutamicum. To produce more NADPH from glycolysis for the synthesis of lysine, Bommareddy et al. changed the coenzyme specificity of a native NAD-dependent glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from C. glutamicum to NADP by rational protein design (D35G/L36T/T37K/P192S). The mutant GAPDH-containing C. glutamicum strain shows approximately 60% improvement in lysine production than the wild-type strain [86]. In another example, a recombinant S. cerevisiae strain containing Pichia stipitis xylose reductase (PsXR) and xylitol dehydrogenase (PsXDH) genes can convert xylose to ethanol, along with the unfavorable excretion of xylitol due to intercellular redox imbalance caused by the different coenzyme specificity between NADPH-preferring XR and NAD+-dependent XDH. Watanabe et al. succeeded in generating several PsXDH mutants with a reversal of coenzyme specificity toward NADP+ by multiple site-directed mutagenesis of the coenzyme-binding domain. A quadruple mutant (D207A/I208R/F209S/N211R) showed a more than 4500-fold increase of kcat/Km on NADP+ than the wild-type enzyme, reaching a comparable value with the kcat/Km on NAD+ of the wild-type enzyme [87]. These researchers further constructed a recombinant yeast coexpressing NADPH-preferring PsXR and NADP+-dependent PsXDH, and the resultant recombinant yeast showed increased ethanol production and decreased xylitol excretion [35], [88].
3.2. From NADP to NAD
Isobutanol can be produced from glucose by recombinant E. coli through the modified biosynthesis of branched-chain amino acids (BCAAs) pathway [61], [62], [79], [80]. The pathway generates two pyruvates and two NADH per glucose molecule via glycolysis but consumes two equivalents of NADPH per isobutanol molecule synthesized, where NADPH is consumed by ketol-acid reductoisomerase (KARI) and alcohol dehydrogenase (ADH). The fermentation of this recombinant strain was operated aerobically or micro-aerobically to activate the pentose phosphate pathway (PPP) or the tricarboxylic acid (TCA) cycle for sufficient NADPH supply. Nevertheless, anaerobic conditions are preferred for large-scale biofuel production due to lower operating costs (e.g., cooling, mixing and aeration) as well as higher product yields [89]. Under anaerobic conditions, isobutanol production by engineered E. coli showed a limited supply of NADPH because of the shutdown of PPP or TCA cycle [37], [39]. Bastian et al. investigated the construction of a NADH-dependent pathway using NADH-preferring engineered E. coli KARI and ADH to produce high-yield isobutanol under anaerobic conditions. The introduction of this NADH-dependent pathway enabled anaerobic isobutanol production at a theoretical yield [39]. Similarly, the NADH-dependent pathway containing PsXDH and PsXR was also introduced into S. cerevisiae [90], [91]. PsXR was engineered to use NADH by the mutation of R276H. The expression of the PsXR/R276H mutant and wild-type (WT) PsXDH in S. cerevisiae can lead to a 20% increase in ethanol production and a 52% decrease in xylitol excretion compared with the WT strain.
4. Applications of coenzyme engineering for in vitro synthetic biology
In vitro synthetic biology is an emerging biomanufacturing platform with advantages such as a high product yield, improved energy conversion efficiency, fast reaction rates, and broad reaction conditions [92]. This platform has shown great potential on the production of bioenergy (e.g., hydrogen and electricity), pharmaceuticals (e.g., heparin), and biochemicals (i.e., α-ketoglutarate, myo-inositol, isobutanol, fructose 1,6-biphosphate, polyhydroxybutyrate, and (R)-phenylethanol) [25], [27], [93], [94], [95], [96], [97], [98], [99]. The pathway design principle of the in vitro synthetic biology platform requires balances between the coenzyme supply and consumption as well as their types [100]. The product cost of this platform is crucial for manufacturing biocommodities [12], [26], and can be reduced by the utilization of less costly coenzymes and the addition of coenzyme regeneration systems. NAD is preferable to NADP for in vitro synthetic biology because of its lower price [33], [68], higher stability [101], and more regeneration methods [29], [34]. In this section, we highlight several examples of in vitro synthetic (enzymatic) biosystems (ivSEB) involving coenzyme engineering from NADP to NAD. Cascade biocatalysis by engineered oxidoreductases with NADH or biomimetic cofactors along with coenzyme regeneration are not covered here and are referred elsewhere [41], [102], [103].
Biohydrogen is believed to be the best future transportation fuel. Hydrogen can be produced by ivSEBs from advanced water splitting energized by starch, sucrose and cellodextrins with a theoretical yield of 12 mol H2 per mol of hexose and water [25], [47], [48], breaking the Thauer limit of 4 mol of H2 per mol of glucose [104], [105]. In these ivSEBs, glucose 6-phosphate (G6P) is generated from ATP-free enzymatic phosphorylation of glucan (i.e., starch) and regenerated from the non-oxidative pentose phosphate pathway (PPP) and partial gluconeogenesis pathway. Two cascade dehydrogenases, G6PDH and 6PGDH, oxidize G6P to ribulose 5-phosphate (Ru5P) and simultaneously reduce two NADP+ molecules to two NADPH molecules, which are converted into hydrogen with the help of a hydrogenase or even a biomimetic electron-transport chain containing an abiotic electron mediator [25]. Economic analysis suggests that the replacement of NADP+ with NAD+ shows great impact on the cost decrease of in vitro hydrogen production by changing the coenzyme preference of G6PDH and 6PGDH from NADP+ to NAD+ [106]. Chen et al. changed the coenzyme preference of hyperthermophilic T. maritima 6PGDH from NADP+ to NAD+ by rational design [2]. The best mutant shows ∼64,000-fold reversal of the coenzyme preference from NADP+ to NAD+, resulting in 25% higher current density of the 6PGDH-diaphorase electricity production system [2]. Additionally, we further engineered T. maritima G6PDH to change its coenzyme preference from NADP+ to NAD+. The best mutant shows a more than 262-fold reversal of the coenzyme preference from NADP+ to NAD+ (submitted for publication). By coupling the G6PDH and 6PGDH mutants into the hydrogen production pathway, we achieved the highest in vitro hydrogen production rate of 530 mmol H2/L/h at 80 °C from starch (submitted for publication). Polyhydroxybutyrate (PHB) is a type of biodegradable polyester. It can be produced by microbes in response to physiological stress [107] or engineered E. coli harboring Streptomyces aureofaciens PHB biosynthesis genes [108]. Recently, Opgenorth et al. designed an in vitro pentose-bifido-glycolysis (PBG) cycle to breakdown glucose for PHB synthesis. Through the PBG cycle, one mole of glucose can be converted into 2 mol of acetyl-CoA with 4 mol of NAD(P)H and 2 mol of CO2. To prevent the accumulation of NADPH due to coenzyme imbalance, G6PDH and 6PGDH involved in the PBG cycle were engineered to change the coenzyme preference from NADP+ to NAD+. Engineered dehydrogenases were used to regulate the efficiency of the pathway by incorporation with NADH oxidase, NADP+-dependent wide-type G6PDH and 6PGDH, exhibiting a more than two-fold improvement of the product yield [94]. Sieber and coworkers designed an ATP-free ivSEB to produce pyruvate from glucose with two NADH molecules per glucose molecule; pyruvate can then be converted to ethanol and isobutanol, consuming the 2 mol of NADH per 2 mol of ethanol and one mole of isobutanol molecule, respectively [109]. The NADH-generating enzymes are glucose dehydrogenase (GDH) and glyceraldehyde dehydrogenase (AIDH). However, AIDH has a very low activity on NAD+ compared with NADP+. To minimize the reaction complexity, the designed pathway was further consolidated to use the coenzyme NADH as the only electron carrier, and AlDH was engineered by directed evolution to have an 8-fold higher activity on NAD+ [110].
5. Biomimetic coenzyme engineering
To further decease the coenzyme costs in vitro, the best solution is the replacement of natural coenzymes with low-cost biomimetic ones [40], [68]. Biomimetic coenzymes, such as nicotinamide mononucleotide (NMN), nicotinamide mononucleoside (NR) (Fig. 1b) and 1-benzyl nicotinamide (BNA) (Fig. 1c), are not only less costly but also have better stability [41], [68]. NMN and NR are precursors of NAD(P) and are much smaller in size than NAD(P) (Fig. 1b), and BNA is a typical biomimetic nicotinamide coenzyme. Few wild-type redox enzymes have been reported to have promiscuous activities on NMN, including liver alcohol dehydrogenase [111] and glutamic dehydrogenase [112]. Scott and coworkers have engineered Pyrococcus furiosus alcohol dehydrogenase to act on NMN, but the enzyme activity remains very low [113]. Fish et al. found that the pyrophosphate and adenosine groups in NAD(P) are not essential for the hydride transfer for some flavin-containing oxidoreductases, and proposed the use of BNA chloride to replace NAD(P) [114]. Clark and Fish collaborated to show that an engineered flavin-containing P450 mutant with two amino acid changes can utilize BNA [115]. Additionally, another group showed that engineered P450 utilizes zinc dust rather than natural coenzymes as an electron source [116], [117]. In 2011, Zhao and coworkers presented a bio-orthogonal system that catalyzed the oxidative decarboxylation of l-malate with a dedicated biomimetic coenzyme, nicotinamide flucytosine dinucleotide (NFCD, Fig. 1b). The redox enzymes were engineered using site-saturation mutagenesis of the key amino acid sites [42], and the balance of this biomimetic coenzyme was achieved through a designed enzymatic pathway containing two engineered enzymes, both of which can use NFCD as coenzymes. This research opened the window to engineer bio-orthogonal redox systems for various applications in in vivo synthetic biology.
Despite several papers that pertain to the engineering of NAD/NADP preference of oxidoreductases exist [92], [100], [118] (Table 1), and some general rules have been proposed for coenzyme engineering [31], [61], [67], coenzyme engineering utilizing biomimetic coenzymes remains in its early stage due to the significant differences in structures and sizes among natural and biomimetic coenzymes (Fig. 1) [113]. This direction is becoming one of the top R&D priorities of in vitro synthetic biology [106].
6. Conclusions
Due to variations among different types of coenzymes, the imbalance of coenzyme supply and consumption, as well as coenzyme cost and stability issues, coenzyme engineering is one of the most important areas of protein engineering, with great application to in vivo and in vitro synthetic biology projects. With the increasing number of protein crystal structures with high-resolution and homogeneous oxidoreductase sequences and the development of novel high-throughput screening methods, the semi-rational design of switching coenzyme preferences between NAD and NADP is maturing. Coenzyme engineering utilizing biomimics is becoming more prevalent because such biomimics are more stable and less costly than natural ones [40], [68]. It is increasingly acceptable that in vitro synthetic biology platforms could become a cornerstone of advanced biomanufacturing 4.0 for the cost-competitive biomanufacturing of low-value biocommodities and new foods [119].
Acknowledgements
This study was mainly supported by the Key Research Program of the Chinese Academy of Sciences (Grant No. ZDRW-ZS-2016-3), 1000-youth program of China to CY and the National Natural Science Foundation of China (Grant No. 31600636). Funds were partially provided by the DOE EERE award (DE-EE0006968) to YPZ.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Chun You, Email: you_c@tib.cas.cn.
Yi-Heng Percival Zhang, Email: zhang_yh@tib.cas.cn.
References
- 1.Huang R., Chen H., Zhong C., Kim J.E., Zhang Y.-H.P. High-throughput screening of coenzyme preference change of thermophilic 6-phosphogluconate dehydrogenase from NADP+ to NAD+ Sci Rep. 2016;6:32644. doi: 10.1038/srep32644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H., Zhu Z., Huang R., Zhang Y.-H.P. Coenzyme engineering of a hyperthermophilic 6-phosphogluconate dehydrogenase from NADP+ to NAD+ with its application to biobatteries. Sci Rep. 2016;6:36311. doi: 10.1038/srep36311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntosh J.A., Coelho P.S., Farwell C.C., Wang Z.J., Lewis J.C., Brown T.R. Enantioselective intramolecular C-H amination catalyzed by engineered cytochrome P450 enzymes in vitro and in vivo. Angew Chem Int Ed Engl. 2013;52:9309–9312. doi: 10.1002/anie.201304401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agudo R., Roiban G.D., Reetz M.T. Achieving regio- and enantioselectivity of P450-catalyzed oxidative CH activation of small functionalized molecules by structure-guided directed evolution. Chembiochem. 2012;13:1465–1473. doi: 10.1002/cbic.201200244. [DOI] [PubMed] [Google Scholar]
- 5.van Leeuwen J.G., Wijma H.J., Floor R.J., van der Laan J.M., Janssen D.B. Directed evolution strategies for enantiocomplementary haloalkane dehalogenases: from chemical waste to enantiopure building blocks. Chembiochem. 2012;13:137–148. doi: 10.1002/cbic.201100579. [DOI] [PubMed] [Google Scholar]
- 6.You C., Huang Q., Xue H., Xu Y., Lu H. Potential hydrophobic interaction between two cysteines in interior hydrophobic region improves thermostability of a family 11 xylanase from Neocallimastix patriciarum. Biotechnol Bioeng. 2010;105:861–870. doi: 10.1002/bit.22623. [DOI] [PubMed] [Google Scholar]
- 7.Blum J.K., Ricketts M.D., Bommarius A.S. Improved thermostability of AEH by combining B-FIT analysis and structure-guided consensus method. J Biotechnol. 2012;160:214–221. doi: 10.1016/j.jbiotec.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Reetz M.T., Soni P., Fernandez L., Gumulya Y., Carballeira J.D. Increasing the stability of an enzyme toward hostile organic solvents by directed evolution based on iterative saturation mutagenesis using the B-FIT method. Chem Commun (Camb) 2010;46:8657–8658. doi: 10.1039/c0cc02657c. [DOI] [PubMed] [Google Scholar]
- 9.Foo J.L., Ching C.B., Chang M.W., Leong S.S. The imminent role of protein engineering in synthetic biology. Biotechnol Adv. 2012;30:541–549. doi: 10.1016/j.biotechadv.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Wellhausen R., Oye K.A. 2007 atlanta conference on science, technology and innovation policy. 2007. Intellectual property and the commons in synthetic Biology: strategies to facilitate an emerging technology; pp. 1–2. [Google Scholar]
- 11.Osbourn A.E., O'Maille P.E., Rosser S.J., Lindsey K. Synthetic biology. New Phytol. 2012;196:671–677. doi: 10.1111/j.1469-8137.2012.04374.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y.-H.P., Myung S., You C., Zhu Z., Rollin J.A. Toward low-cost biomanufacturing through in vitro synthetic biology: bottom-up design. J Mater Chem. 2011;21:18877–18886. [Google Scholar]
- 13.Chang M.C., Keasling J.D. Production of isoprenoid pharmaceuticals by engineered microbes. Nat Chem Biol. 2006;2:674–681. doi: 10.1038/nchembio836. [DOI] [PubMed] [Google Scholar]
- 14.Stephanopoulos G. Synthetic biology and metabolic engineering. ACS Synth Biol. 2012;1:514–525. doi: 10.1021/sb300094q. [DOI] [PubMed] [Google Scholar]
- 15.Cheng A.A., Lu T.K. Synthetic biology: an emerging engineering discipline. Annu Rev Biomed Eng. 2012;14:155–178. doi: 10.1146/annurev-bioeng-071811-150118. [DOI] [PubMed] [Google Scholar]
- 16.Haseloff J., Ajioka J. Synthetic biology: history, challenges and prospects. J R Soc Interface. 2009;6(Suppl 4):S389–S391. doi: 10.1098/rsif.2009.0176.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrianantoandro E., Basu S., Karig D.K., Weiss R. Synthetic biology: new engineering rules for an emerging discipline. Mol Syst Biol. 2006:2. doi: 10.1038/msb4100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinemann M., Panke S. Synthetic biology–putting engineering into biology. Bioinformatics. 2006;22:2790–2799. doi: 10.1093/bioinformatics/btl469. [DOI] [PubMed] [Google Scholar]
- 19.Forster A.C., Church G.M. Synthetic biology projects in vitro. Genome Res. 2007;17:1–6. doi: 10.1101/gr.5776007. [DOI] [PubMed] [Google Scholar]
- 20.Dudley Q.M., Karim A.S., Jewett M.C. Cell-free metabolic engineering: biomanufacturing beyond the cell. Biotechnol J. 2015;10:69–82. doi: 10.1002/biot.201400330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgman C.E., Jewett M.C. Cell-free synthetic biology: thinking outside the cell. Metab Eng. 2012;14:261–269. doi: 10.1016/j.ymben.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rupp S. Next-generation bioproduction systems: cell-free conversion concepts for industrial biotechnology. Eng Life Sci. 2013;13:19–25. [Google Scholar]
- 23.Morgado G., Gerngross D., Roberts T.M., Panke S. Adv Biochem Eng Biotechnol. Springer; Berlin, Heidelberg: 2016. Synthetic biology for cell-free biosynthesis: fundamentals of designing novel in vitro multi-enzyme reaction networks; pp. 1–30. [DOI] [PubMed] [Google Scholar]
- 24.Fessner W.D. Systems Biocatalysis: development and engineering of cell-free “artificial metabolisms” for preparative multi-enzymatic synthesis. N Biotechnol. 2015;32:658–664. doi: 10.1016/j.nbt.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Kim E.J., Wu C.H., Adams M.W., Zhang Y.-H.P. Exceptionally high rates of biological hydrogen production by biomimetic in vitro synthetic enzymatic pathways. Chemistry. 2016;22:16047–16051. doi: 10.1002/chem.201604197. [DOI] [PubMed] [Google Scholar]
- 26.You C., Zhang Y.-H.P. Biomanufacturing by in vitro biosystems containing complex enzyme mixtures. Process Biochem. 2017;52:106–114. [Google Scholar]
- 27.You C., Shi T., Li Y., Han P., Zhou X., Zhang Y.-H.P. An in vitro synthetic biology platform for the industrial biomanufacturing of myo-inositol from starch. Biotechnol Bioeng. 2017;14:1855–1864. doi: 10.1002/bit.26314. [DOI] [PubMed] [Google Scholar]
- 28.Schomburg I., Jeske L., Ulbrich M., Placzek S., Chang A., Schomburg D. The BRENDA enzyme information system–From a database to an expert system. J Biotechnol. 2017 doi: 10.1016/j.jbiotec.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Wichmann R., Vasic-Racki D. Adv Biochem Engin/Biotechnol. Springer; Berlin, Heidelberg: 2005. Cofactor regeneration at the lab scale; pp. 225–260. [DOI] [PubMed] [Google Scholar]
- 30.Wu H., Tian C., Song X., Liu C., Yang D., Jiang Z. Methods for the regeneration of nicotinamide coenzymes. Green Chem. 2013;15:1773. [Google Scholar]
- 31.Cahn J.K.B., Werlang C.A., Baumschlager A., Brinkmann-Chen S., Mayo S.L., Arnold F.H. A general tool for engineering the NAD/NADP cofactor preference of oxidoreductases. ACS Synth Biol. 2017;6:326–333. doi: 10.1021/acssynbio.6b00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banta S., Swanson B.A., Wu S., Jarnagin A., Anderson S. Alteration of the specificity of the cofactor-binding pocket of Corynebacterium 2,5-diketo-D-gluconic acid reductase A. Protein Eng. 2002;15:131–140. doi: 10.1093/protein/15.2.131. [DOI] [PubMed] [Google Scholar]
- 33.Woodyer R., van der Donk W.A., Zhao H. Relaxing the nicotinamide cofactor specificity of phosphite dehydrogenase by rational design. Biochemistry. 2003;42:11604–11614. doi: 10.1021/bi035018b. [DOI] [PubMed] [Google Scholar]
- 34.Weckbecker A., Groger H., Hummel W. Adv Biochem Eng Biotechnol. Springer; Berlin, Heidelberg: 2010. Regeneration of nicotinamide coenzymes: principles and applications for the synthesis of chiral compounds; pp. 195–242. [DOI] [PubMed] [Google Scholar]
- 35.Matsushika A., Watanabe S., Kodaki T., Makino K., Inoue H., Murakami K. Expression of protein engineered NADP+-dependent xylitol dehydrogenase increases ethanol production from xylose in recombinant Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2008;81:243–255. doi: 10.1007/s00253-008-1649-1. [DOI] [PubMed] [Google Scholar]
- 36.Bengtsson O., Hahn-Hägerdal B., Gorwa-Grauslund M.F. Xylose reductase from Pichia stipitis with altered coenzyme preference improves ethanolic xylose fermentation by recombinant Saccharomyces cerevisiae. Biotechnol Biofuels. 2009;2:9. doi: 10.1186/1754-6834-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasegawa S., Uematsu K., Natsuma Y., Suda M., Hiraga K., Jojima T. Improvement of the redox balance increases L-valine production by Corynebacterium glutamicum under oxygen deprivation conditions. Appl Environ Microbiol. 2012;78:865–875. doi: 10.1128/AEM.07056-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamakawa H., Ikushima S., Yoshida S. Ethanol production from xylose by a recombinant Candida utilis strain expressing protein-engineered xylose reductase and xylitol dehydrogenase. Biosci Biotechnol Biochem. 2011;75:1994–2000. doi: 10.1271/bbb.110426. [DOI] [PubMed] [Google Scholar]
- 39.Bastian S., Liu X., Meyerowitz J.T., Snow C.D., Chen M.M., Arnold F.H. Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli. Metab Eng. 2011;13:345–352. doi: 10.1016/j.ymben.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Paul C.E., Arends I.W.C.E., Hollmann F. Is simpler better? Synthetic nicotinamide cofactor analogues for redox chemistry. ACS Catal. 2014;4:788–797. [Google Scholar]
- 41.Paul C.E., Hollmann F. A survey of synthetic nicotinamide cofactors in enzymatic processes. Appl Microbiol Biotechnol. 2016;100:4773–4778. doi: 10.1007/s00253-016-7500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji D., Wang L., Hou S., Liu W., Wang J., Wang Q. Creation of bioorthogonal redox systems depending on nicotinamide flucytosine dinucleotide. J Am Chem Soc. 2011;133:20857–20862. doi: 10.1021/ja2074032. [DOI] [PubMed] [Google Scholar]
- 43.Ji D., Wang L., Liu W., Hou S., Zhao K.Z. Synthesis of NAD analogs to develop bioorthogonal redox system. Sci China Chem. 2013;56:296–300. [Google Scholar]
- 44.Wang L., Ji D., Liu Y., Wang Q., Wang X., Zhou Y.J. Synthetic cofactor-linked metabolic circuits for selective energy transfer. ACS Catal. 2017;7:1977–1983. [Google Scholar]
- 45.Rollin J.A., Martin del Campo J., Myung S., Sun F., You C., Bakovic A. High-yield hydrogen production from biomass by in vitro metabolic engineering: mixed sugars coutilization and kinetic modeling. Proc Natl Acad Sci U. S. A. 2015;112:4964–4969. doi: 10.1073/pnas.1417719112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myung S., Rollin J., You C., Sun F., Chandrayan S., Adams M.W. In vitro metabolic engineering of hydrogen production at theoretical yield from sucrose. Metab Eng. 2014;24:70–77. doi: 10.1016/j.ymben.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Ye X., Wang Y., Hopkins R.C., Adams M.W.W., Evans B.R., Mielenz J.R. Spontaneous high-yield production of hydrogen from cellulosic materials and water catalyzed by enzyme cocktails. ChemSusChem. 2009;2:149–152. doi: 10.1002/cssc.200900017. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y.-H.P., Evans B.R., Mielenz J.R., Hopkins R.C., Adams M.W.W. High-yield hydrogen production from starch and water by a synthetic enzymatic pathway. PLoS One. 2007;2:e456. doi: 10.1371/journal.pone.0000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Z., Zhang Y.-H.P. Use of nonimmobilized enzymes and mediators achieved high power densities in closed biobatteries. Energy Sci Eng. 2015;3:490–497. [Google Scholar]
- 50.Zhu Z., Zhang Y.-H.P. In vitro metabolic engineering of bioelectricity generation by the complete oxidation of glucose. Metab Eng. 2017;39:110–116. doi: 10.1016/j.ymben.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Z., Wang Y., Minteer S.D., Zhang Y.-H.P. Maltodextrin-powered enzymatic fuel cell through a non-natural enzymatic pathway. J Power Sources. 2011;196:7505–7509. [Google Scholar]
- 52.Li Y. Beyond protein engineering: its applications in synthetic biology. Enzyme Eng. 2012;1:1000e103. [Google Scholar]
- 53.Chica R.A., Doucet N., Pelletier J.N. Semi-rational approaches to engineering enzyme activity: combining the benefits of directed evolution and rational design. Curr Opin Biotechnol. 2005;16:378–384. doi: 10.1016/j.copbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Lesk A.M. NAD-binding domains of dehydrogenases. Curr Opin Struct Biol. 1995;5:775–783. doi: 10.1016/0959-440x(95)80010-7. [DOI] [PubMed] [Google Scholar]
- 55.Rao S.T., Rossmann M.G. Comparison of super-secondary structures in proteins. J Mol Biol. 1973;76:241–256. doi: 10.1016/0022-2836(73)90388-4. [DOI] [PubMed] [Google Scholar]
- 56.Wulf H., Mallin H., Bornscheuer U.T. Protein engineering of a thermostable polyol dehydrogenase. Enzyme Microb Technol. 2012;51:217–224. doi: 10.1016/j.enzmictec.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Bubner P., Klimacek M., Nidetzky B. Structure-guided engineering of the coenzyme specificity of Pseudomonas fluorescens mannitol 2-dehydrogenase to enable efficient utilization of NAD(H) and NADP(H) FEBS Lett. 2008;582:233–237. doi: 10.1016/j.febslet.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Rosell A., Valencia E., Ochoa W.F., Fita I., Pares X., Farres J. Complete reversal of coenzyme specificity by concerted mutation of three consecutive residues in alcohol dehydrogenase. J Biol Chem. 2003;278:40573–40580. doi: 10.1074/jbc.M307384200. [DOI] [PubMed] [Google Scholar]
- 59.Morikawa S., Nakai T., Yasohara Y., Nanba H., Kizaki N., Hasegawa J. Highly active mutants of carbonyl reductase S1 with inverted coenzyme specificity and production of optically active alcohols. Biosci Biotechnol Biochem. 2014;69:544–552. doi: 10.1271/bbb.69.544. [DOI] [PubMed] [Google Scholar]
- 60.Rosado L.A., Caceres R.A., de Azevedo W.F., Basso L.A., Santos D.S. Role of Serine140 in the mode of action of Mycobacterium tuberculosis β-ketoacyl-ACP reductase (MabA) BMC Res Notes. 2012;5:526. doi: 10.1186/1756-0500-5-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brinkmann-Chen S., Flock T., Cahn J.K.B., Snow C.D., Brustad E.M., McIntosh J.A. General approach to reversing ketol-acid reductoisomerase cofactor dependence from NADPH to NADH. Proc Nat Acad Sci U. S. A. 2013;110:10946–10951. doi: 10.1073/pnas.1306073110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brinkmann-Chen S., Cahn J.K., Arnold F.H. Uncovering rare NADH-preferring ketol-acid reductoisomerases. Metab Eng. 2014;26:17–22. doi: 10.1016/j.ymben.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Geertz-Hansen H.M., Blom N., Feist A.M., Brunak S., Petersen T.N. Cofactory: sequence-based prediction of cofactor specificity of Rossmann folds. Proteins Struct Funct Bioinf. 2014;82:1819–1828. doi: 10.1002/prot.24536. [DOI] [PubMed] [Google Scholar]
- 64.Levy H.R., Vought V.E., Yin X., Adams M.J. Identification of an arginine residue in the dual coenzyme-specific glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides that plays a key role in binding NADP+ but not NAD+ Arch Biochem Biophys. 1996;326:145–151. doi: 10.1006/abbi.1996.0058. [DOI] [PubMed] [Google Scholar]
- 65.Li L., Cook P.F. The 2′-phosphate of NADP Is responsible for proper orientation of the nicotinamide ring in the oxidative decarboxylation reaction catalyzed by sheep liver 6-phosphogluconate dehydrogenase. J Biol Chem. 2006;281:36803–36810. doi: 10.1074/jbc.M604609200. [DOI] [PubMed] [Google Scholar]
- 66.Tetaud E., Hanau S., Wells J.M., Le Page R.W.F., Adams M.J., Arkison S. 6-Phosphogluconate dehydrogenase from Lactococcus lactis: a role for arginine residues in binding substrate and coenzyme. Biochem J. 1999;338:55–60. [PMC free article] [PubMed] [Google Scholar]
- 67.Cui D., Zhang L., Jiang S., Yao Z., Gao B., Lin J. A computational strategy for altering an enzyme in its cofactor preference to NAD(H) and/or NADP(H) Febs J. 2015;282:2339–2351. doi: 10.1111/febs.13282. [DOI] [PubMed] [Google Scholar]
- 68.Rollin J.A., Tam T.K., Zhang Y.-H.P. New biotechnology paradigm: cell-free biosystems for biomanufacturing. Green Chem. 2013;15:1708–1719. [Google Scholar]
- 69.Yaoi T., Miyazaki K., Oshima T., Komukai Y., Go M. Conversion of the coenzyme specificity of isocitrate dehydrogenase by module replacement. J Biochem. 1996;119:1014–1018. doi: 10.1093/oxfordjournals.jbchem.a021316. [DOI] [PubMed] [Google Scholar]
- 70.Miller S.P., Lunzer M., Dean A.M. Direct demonstration of an adaptive constraint. Science. 2006;314:458–461. doi: 10.1126/science.1133479. [DOI] [PubMed] [Google Scholar]
- 71.Takase R., Mikami B., Kawai S., Murata K., Hashimoto W. Structure-based conversion of the coenzyme requirement of a short-chain dehydrogenase/reductase involved in bacterial alginate metabolism. J Biol Chem. 2014;289:33198–33214. doi: 10.1074/jbc.M114.585661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rane M.J., Calvo K.C. Reversal of the nucleotide specificity of ketol acid reductoisomerase by site-directed mutagenesis identifies the NADPH binding site1. Arch Biochem Biophys. 1997;338:83–89. doi: 10.1006/abbi.1996.9802. [DOI] [PubMed] [Google Scholar]
- 73.Kim J.E., Huang R., Chen H., You C., Zhang Y.-H.P. Facile construction of random gene mutagenesis library for directed evolution without the use of restriction enzyme in Escherichia coli. Biotechnol J. 2016;11:1142–1150. doi: 10.1002/biot.201600121. [DOI] [PubMed] [Google Scholar]
- 74.Chusacultanachai S., Yuthavong Y. Random mutagenesis strategies for construction of large and diverse clone libraries of mutated DNA fragments. Methods Mol Biol. 2004;270:319–334. doi: 10.1385/1-59259-793-9:319. [DOI] [PubMed] [Google Scholar]
- 75.Mayer K.M., Arnold F.H. A colorimetric assay to quantify dehydrogenase activity in crude cell lysates. J Biomol Screen. 2002;7:135–140. doi: 10.1177/108705710200700206. [DOI] [PubMed] [Google Scholar]
- 76.Liu W., Hong J., Bevan D.R., Zhang Y.-H.P. Fast identification of thermostable beta-glucosidase mutants on cellobiose by a novel combinatorial selection/screening approach. Biotechnol Bioeng. 2009;103:1087–1094. doi: 10.1002/bit.22340. [DOI] [PubMed] [Google Scholar]
- 77.Demain A.L. Pickles, pectin, and penicillin. Annu Rev Microbiol. 2004;58:1–42. doi: 10.1146/annurev.micro.58.030603.123757. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X., Jantama K., Moore J.C., Shanmugam K.T., Ingram L.O. Production of L -alanine by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol. 2007;77:355–366. doi: 10.1007/s00253-007-1170-y. [DOI] [PubMed] [Google Scholar]
- 79.Atsumi S., Wu T.Y., Eckl E.M., Hawkins S.D., Buelter T., Liao J.C. Engineering the isobutanol biosynthetic pathway in Escherichia coli by comparison of three aldehyde reductase/alcohol dehydrogenase genes. Appl Microbiol Biot. 2010;85:651–657. doi: 10.1007/s00253-009-2085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Atsumi S., Hanai T., Liao J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature. 2008;451:86–89. doi: 10.1038/nature06450. [DOI] [PubMed] [Google Scholar]
- 81.Gameiro P.A., Laviolette L.A., Kelleher J.K., Iliopoulos O., Stephanopoulos G. Cofactor balance by nicotinamide nucleotide transhydrogenase (NNT) coordinates reductive carboxylation and glucose catabolism in the tricarboxylic acid (TCA) cycle. J Biol Chem. 2013;288:12967–12977. doi: 10.1074/jbc.M112.396796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nissen T.L., Anderlund M., Nielsen J., Villadsen J., Kielland-Brandt M.C. Expression of a cytoplasmic transhydrogenase in Saccharomyces cerevisiae results in formation of 2-oxoglutarate due to depletion of the NADPH pool. Yeast. 2001;18:19–32. doi: 10.1002/1097-0061(200101)18:1<19::AID-YEA650>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 83.Martinez I., Zhu J., Lin H., Bennett G.N., San K.Y. Replacing Escherichia coli NAD-dependent glyceraldehyde 3-phosphate dehydrogenase (GAPDH) with a NADP-dependent enzyme from Clostridium acetobutylicum facilitates NADPH dependent pathways. Metab Eng. 2008;10:352–359. doi: 10.1016/j.ymben.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Takeno S., Murata R., Kobayashi R., Mitsuhashi S., Ikeda M. Engineering of Corynebacterium glutamicum with an NADPH-generating glycolytic pathway for L-lysine production. Appl Environ Microbiol. 2010;76:7154–7160. doi: 10.1128/AEM.01464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ehsani M., Fernández M.R., Biosca J.A., Dequin S. Reversal of coenzyme specificity of 2,3-butanediol dehydrogenase from Saccharomyces cerevisae and in vivo functional analysis. Biotechnol Bioeng. 2009;104:381–389. doi: 10.1002/bit.22391. [DOI] [PubMed] [Google Scholar]
- 86.Bommareddy R.R., Chen Z., Rappert S., Zeng A.P. A de novo NADPH generation pathway for improving lysine production of Corynebacterium glutamicum by rational design of the coenzyme specificity of glyceraldehyde 3-phosphate dehydrogenase. Metab Eng. 2014;25:30–37. doi: 10.1016/j.ymben.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 87.Watanabe S., Kodaki T., Makino K. Complete reversal of coenzyme specificity of xylitol dehydrogenase and increase of thermostability by the introduction of structural zinc. J BiolChem. 2005;280:10340–10349. doi: 10.1074/jbc.M409443200. [DOI] [PubMed] [Google Scholar]
- 88.Matsushika A., Watanabe S., Kodaki T., Makino K., Sawayama S. Bioethanol production from xylose by recombinant Saccharomyces cerevisiae expressing xylose reductase, NADP+-dependent xylitol dehydrogenase, and xylulokinase. J Biosci Bioeng. 2008;105:296–299. doi: 10.1263/jbb.105.296. [DOI] [PubMed] [Google Scholar]
- 89.Huang W.D., Zhang Y.-H.P. Analysis of biofuels production from sugar based on three criteria: thermodynamics, bioenergetics, and product separation. Energy Environ Sci. 2011;4:784–792. [Google Scholar]
- 90.Petschacher B., Nidetzky B. Altering the coenzyme preference of xylose reductase to favor utilization of NADH enhances ethanol yield from xylose in a metabolically engineered strain of Saccharomyces cerevisiae. Microb Cell Fact. 2008;7:9. doi: 10.1186/1475-2859-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe S., Abu Saleh A., Pack S.P., Annaluru N., Kodaki T., Makino K. Ethanol production from xylose by recombinant Saccharomyces cerevisiae expressing protein-engineered NADH-preferring xylose reductase from Pichia stipitis. Microbiology. 2007;153:3044–3054. doi: 10.1099/mic.0.2007/007856-0. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y.-H.P. Simpler Is better: high-Yield and potential low-cost biofuels production through cell-free synthetic pathway biotransformation (SyPaB) ACS Catal. 2011;1:998–1009. [Google Scholar]
- 93.Xu Y., Masuko S., Takieddin M., Xu H., Liu R., Jing J. Chemoenzymatic synthesis of homogeneous ultralow molecular weight heparins. Science. 2011;334:498–501. doi: 10.1126/science.1207478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Opgenorth P.H., Korman T.P., Bowie J.U. A synthetic biochemistry module for production of bio-based chemicals from glucose. Nat Chem Biol. 2016;12:393–395. doi: 10.1038/nchembio.2062. [DOI] [PubMed] [Google Scholar]
- 95.Moustafa H.M.A., Kim E.J., Zhu Z., Wu C.H., Zaghloul T.I., Adams M.W.W. Water splitting for high-yield hydrogen production energized by biomass xylooligosaccharides catalyzed by an enzyme cocktail. Chemcatchem. 2016;8:2898–2902. [Google Scholar]
- 96.Zhu Z., Sun F., Zhang X., Zhang Y.-H.P. Deep oxidation of glucose in enzymatic fuel cells through a synthetic enzymatic pathway containing a cascade of two thermostable dehydrogenases. Biosens Bioelectron. 2012;36:110–115. doi: 10.1016/j.bios.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Zhu Z., Kin Tam T., Sun F., You C., Zhang Y.-H.P. A high-energy-density sugar biobattery based on a synthetic enzymatic pathway. Nat Commun. 2014;5:3026. doi: 10.1038/ncomms4026. [DOI] [PubMed] [Google Scholar]
- 98.Beer B., Pick A., Sieber V. In vitro metabolic engineering for the production of α-ketoglutarate. Metab Eng. 2017;40:5–13. doi: 10.1016/j.ymben.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 99.Johannes T.W., Woodyer R.D., Zhao H. Efficient regeneration of NADPH using an engineered phosphite dehydrogenase. Biotechnol Bioeng. 2007;96:18–26. doi: 10.1002/bit.21168. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y.-H.P. Production of biocommodities and bioelectricity by cell-free synthetic enzymatic pathway biotransformations: challenges and opportunities. Biotechnol Bioeng. 2010;105:663–677. doi: 10.1002/bit.22630. [DOI] [PubMed] [Google Scholar]
- 101.Wu J.T., Wu L.H., Knight J.A. Stability of NADPH: effect of various factors on the kinetics of degradation. Clin Chem. 1986;32:314–319. [PubMed] [Google Scholar]
- 102.Löw S.A., Löw I.M., Weissenborn M.J., Hauer B. Enhanced ene-reductase activity through alteration of artificial nicotinamide cofactor substituents. ChemCatChem. 2016;8:911–915. [Google Scholar]
- 103.Knaus T., Paul C.E., Levy C.W., de Vries S., Mutti F.G., Hollmann F. Better than nature: nicotinamide biomimetics that outperform natural coenzymes. J Am Chem Soc. 2016;138:1033–1039. doi: 10.1021/jacs.5b12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thauer K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maeda T., Sanchez-Torres V., Wood T.K. Hydrogen production by recombinant Escherichia coli strains. Microb Biotechnol. 2012;5:214–225. doi: 10.1111/j.1751-7915.2011.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y.-H.P. Production of biofuels and biochemicals by in vitro synthetic biosystems: opportunities and challenges. Biotechnol Adv. 2015;33:1467–1483. doi: 10.1016/j.biotechadv.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 107.Ackermann J-u, Müller S., Lösche A., Bley T. Methylobacterium rhodesianum cells tend to double the DNA content under growth limitations and accumulate PHB. J Biotechnol. 1995;39:9–20. [Google Scholar]
- 108.Mahishi L.H., Tripathi G., Rawal S.K. Poly(3-hydroxybutyrate) (PHB) synthesis by recombinant Escherichia coli harbouring Streptomyces aureofaciens PHB biosynthesis genes: effect of various carbon and nitrogen sources. Microbiol Res. 2003;158:19–27. doi: 10.1078/0944-5013-00161. [DOI] [PubMed] [Google Scholar]
- 109.Guterl J.K., Garbe D., Carsten J., Steffler F., Sommer B., Reisse S. Cell-free metabolic engineering: production of chemicals by minimized reaction cascades. ChemSusChem. 2012;5:2165–2172. doi: 10.1002/cssc.201200365. [DOI] [PubMed] [Google Scholar]
- 110.Steffler F., Guterl J.-K., Sieber V. Improvement of thermostable aldehyde dehydrogenase by directed evolution for application in synthetic cascade biomanufacturing. Enz Microb Technol. 2013;53:307–314. doi: 10.1016/j.enzmictec.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 111.Plapp B.V., Sogin D.C., Dworschack R.T., Bohlken D.P., Woenckhaus C., Jeck R. Kinetics and native and modified liver alcohol dehydrogenase with coenzyme analogs: isomerization of enzyme-nicotinamide adenine dinucleotide complex. Biochemistry. 1986;25:5396–5402. doi: 10.1021/bi00367a008. [DOI] [PubMed] [Google Scholar]
- 112.Fisher H.F., McGregor L.L. The ability of reduced nicotinamide mononucleotide to function as a hydrogen donor in the glutamic dehydrogenase reaction. Biochem Biophys Res Commun. 1969;34:627–632. doi: 10.1016/0006-291x(69)90784-0. [DOI] [PubMed] [Google Scholar]
- 113.Campbell E., Meredith M., Minteer S.D., Banta S. Enzymatic biofuel cells utilizing a biomimetic cofactor. Chem Commun. 2012;48:1898–1900. doi: 10.1039/c2cc16156g. [DOI] [PubMed] [Google Scholar]
- 114.Lo H.C., Leiva C., Buriez O., Kerr J.B., Olmstead M.M., Fish R.H. Bioorganometallic chemistry. 13. regioselective reduction of NAD+ models, 1-benzylnicotinamde triflate and beta-nicotinamide ribose-5'-methyl phosphate, with in situ generated [Cp*Rh(Bpy)H]+: structure –activity relationships, kinetics, and mechanistic aspects in the formation of the 1,4-NADH derivatives. Inorg Chem. 2001;40:6705–6716. doi: 10.1021/ic010562z. [DOI] [PubMed] [Google Scholar]
- 115.Ryan J.D., Fish R.H., Clark D.S. Engineering cytochrome P450 enzymes for improved activity towards biomimetic 1,4-NADH cofactors. Chembiochem. 2008;9:2579–2582. doi: 10.1002/cbic.200800246. [DOI] [PubMed] [Google Scholar]
- 116.Nazor J., Schwaneberg U. Laboratory evolution of P450 BM-3 for mediated electron transfer. ChemBioChem. 2006;7:638–644. doi: 10.1002/cbic.200500436. [DOI] [PubMed] [Google Scholar]
- 117.Nazor J., Dannenmann S., Adjei R.O., Fordjour Y.B., Ghampson I.T., Blanusa M. Laboratory evolution of P450 BM3 for mediated electron transfer yielding an activity-improved and reductase-independent variant. Protein Eng Des Sel. 2008;21:29–35. doi: 10.1093/protein/gzm074. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y.-H.P., Huang W.-D. Constructing the electricity-carbohydrate-hydrogen cycle for a sustainability revolution. Trends Biotechnol. 2012;30:301–306. doi: 10.1016/j.tibtech.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Y.-H.P., Sun J., Ma Y. Biomanufacturing: history and perspective. J Ind Microbiol Biotechnol. 2017;44:773–784. doi: 10.1007/s10295-016-1863-2. [DOI] [PubMed] [Google Scholar]
- 120.Bommareddy R.R., Chen Z., Rappert S., Zeng A.P. A de novo NADPH generation pathway for improving lysine production of Corynebacterium glutamicum by rational design of the coenzyme specificity of glyceraldehyde 3-phosphate dehydrogenase. Metab Eng. 2014;25:30–37. doi: 10.1016/j.ymben.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 121.Petschacher B., Staunig N., Müller M., Schürmann M., Mink D., De Wildeman S. Cofactor specificity engineering of Streptococcus mutans NADH oxidase 2 for NAD(P)+ regeneration in biocatalytic oxidations. Comput Struct Biotechnol J. 2014;9:1–11. doi: 10.5936/csbj.201402005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dambe T.R., Kühn A.M., Brossette T., Giffhorn F., Scheidig A.J. Crystal structure of NADP (H)-dependent 1,5-anhydro-d-fructose reductase from Sinorhizobium morelense at 2.2 Å resolution: construction of a NADH-accepting mutant and its application in rare sugar synthesis. Biochemistry. 2006;45:10030–10042. doi: 10.1021/bi052589q. [DOI] [PubMed] [Google Scholar]
- 123.Gand M., Thöle C., Müller H., Brundiek H., Bashiri G., Höhne M. A NADH-accepting imine reductase variant: immobilization and cofactor regeneration by oxidative deamination. J Biotechnol. 2016;230:11–18. doi: 10.1016/j.jbiotec.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 124.Petschacher B., Nidetzky B. Altering the coenzyme preference of xylose reductase to favor utilization of NADH enhances ethanol yield from xylose in a metabolically engineered strain of Saccharomyces cerevisiae. Microb Cell Fact. 2008;7:9. doi: 10.1186/1475-2859-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen H., Zhu Z., Huang R., Zhang Y.-H.P. Coenzyme engineering of a hyperthermophilic 6-phosphogluconate dehydrogenase from NADP+ to NAD+ with its application to biobatteries. Sci Rep. 2016;6:36311. doi: 10.1038/srep36311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Opgenorth P.H., Korman T.P., Bowie J.U. A synthetic biochemistry module for production of bio-based chemicals from glucose. Nat Chem Biol. 2016;12:393–395. doi: 10.1038/nchembio.2062. [DOI] [PubMed] [Google Scholar]