Abstract
Aging is a process characterized by accumulating degenerative damages, resulting in the death of an organism ultimately. The main goal of aging research is to develop therapies that delay age-related diseases in human. Since signaling pathways in aging of Caenorhabditis elegans (C. elegans), fruit flies and mice are evolutionarily conserved, compounds extending lifespan of them by intervening pathways of aging may be useful in treating age-related diseases in human. Natural products have special resource advantage and with few side effect. Recently, many compounds or extracts from natural products slowing aging and extending lifespan have been reported. Here we summarized these compounds or extracts and their mechanisms in increasing longevity of C. elegans or other species, and the prospect in developing anti-aging medicine from natural products.
Keywords: Aging, Natural products, Anti-aging, Drug screening
Introduction
Since realizing the inevitability of death, the fear of death and pursuit of immortality might have preoccupied with human beings. In the Epic of Gilgamesh, Gilgamesh (the Sumerian king of Uruk) was obsessed in pursuit of immortality herbal. About 200 BC, Qin Shi Huang (the first emperor of a unified China) feared death and desperately sought the fabled elixir of life. A more recent story was the Spanish explorer Ponce de Leon who was looking for the mythical fountain of youth. Unexpectedly, all these human activities of pursuing for immortality were failed. We now know that there is no such elixir of immortality placed in somewhere by god and waited for human to find it.
On the other hand, early medical practice was developed in Babylon, Egypt, Greece, India, and China. Along with the development of biology, chemistry, physics and math, the west medical tradition developed into modern medical science. Great success has been achieved in prevention and treatment of disease. Consequently, the longevity of human has been greatly extended. The aged population is growing rapidly in modern world. Aging is the most risk factor for many age-associated diseases, such as neurodegenerative disease, diabetes, stroke, and cancer. The aged people are often suffering from one or multiple aging associated diseases, which brings enormous social and economic burden. While current medicine is focused on treatment of individual disease, the aging people recovered for one disease would probably suffer from other disease soon later.
Two thousand years ago, a systematic theory and practice to achieve healthy aging with core idea of “preventive treatment of disease” was proposed in Huang Di Nei Jing (one of the most important classical texts of traditional Chinese medicine). Current geroscience research have revealed key molecular processes that underlie biological aging [1], and that delaying aging process could delay the onset and progress of age-associated diseases and the disability of aging people [2]. As the modern version of “the preventative treatment of disease”, anti-aging medicine could be the most effective way to combat the age-associated diseases and the disability of aging people. Currently, many compounds with anti-aging activity have been discovered. A large portion of these compounds are natural products. Therefore, we summarized these natural products or extracts that are reported to have anti-aging effects. We also discussed the prospect and challenges of natural products in development of anti-aging medicine.
Current Progress in Aging Research
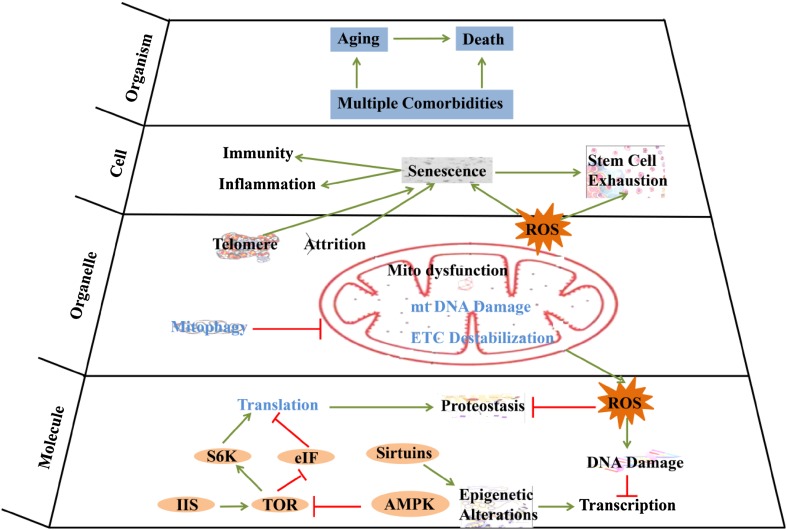
Biological process was relying on the delicate interaction of biomolecules. These building blocks of organism were selected during the origin of life, and were imperfect and intrinsic to generation of damage in every biological process, such as in DNA replication, epigenetic modification, transcription and translation, protein post-translational modification, protein fold, and metabolic process. For some of the damages were endangering species survival, their correction mechanisms were evolved by natural selection, such as DNA repair, protein unfolding response, antioxidant mechanism, detoxification, autophagy, and proteasome. The failure of these protection processes would cause the occurrence of aging and pathological phenotypes, while enhancing these protection processes would delay aging and related phenotypes. Here we summarized how the dynamic interactions between various damages and errors occurred in biological process and their evoked response of correction mechanisms contribute to genome stability, proteostasis and metabolic homeostasis, to cellular homeostasis and finally to aging process (Figs. 1, 2). For the detailed mechanisms of aging, we refer to the reviews elsewhere [1, 3–8].
Fig. 1.
Aging mechanisms in different hierarchies
Fig. 2.
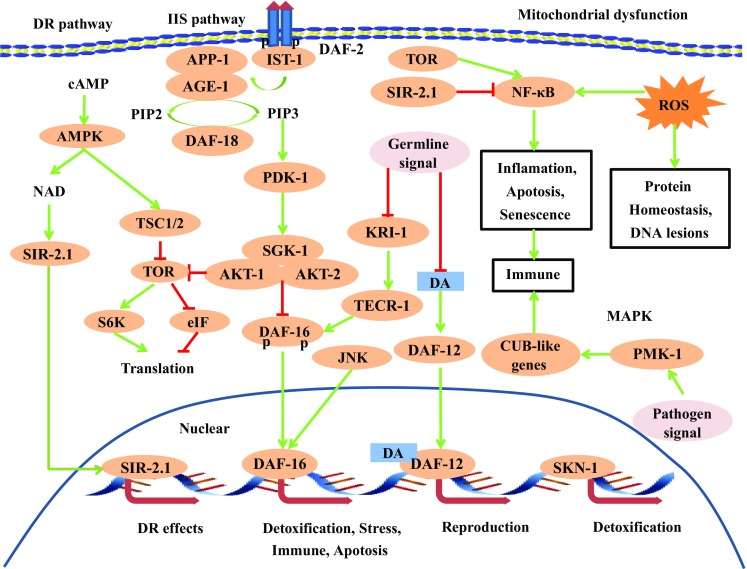
Signaling networks in aging. Dietary restriction (DR), insulin/IGF-1-like signaling (IIS), germline, MAPK and mitochondrial dysfunction pathway networks in aging
Genome Stability and Aging
Accumulation of genome damage is one of the major causes of aging [9]. The intrinsic threats to DNA integrity, including DNA replication errors, spontaneous hydrolytic reactions, and reactive oxygen species (ROS), together with exogenous physical (e.g. UV/IR radiation), chemical and biological agents (e.g. virus) cause various genetic lesions, such as point mutations, translocations, chromosomal gains and losses, telomere shortening, and gene disruption. About 70,000 lesions per day were estimated to happen in each normal human cell [10]. Accordingly, a complex repair mechanisms, such as base excision repair (BER), nucleotide excision repair (NER), transcription-coupled repair (TCR), homologous recombination, nonhomologous end-joining (NHEJ), and telomere elongation have been evolved in the organism. The deletion of genes for BER were lethal in mice [11], while mutations affecting NER and TCR were associated with numerous disorders and accelerated aging [12–14]. Mice with defected in NHEJ were subjected to early onset of aging [15]. The discovery of the causality between telomere shortening and cell replication limits, has led to the generation of telomere theory of aging [16]. Patients with inherited telomere syndrome presents greater overall telomere attrition and premature aging [16]. Compounds with improving telomerase activity or suppressing telomere shortening play distinct roles in anti-aging [17].
The epigenetic changes are one of the hallmarks of aging, including alterations in transcription factor binding, histone marks, DNA methylation, and nucleosome positioning [18]. These epigenetic changes can either happen spontaneously or modulated by environmental stimuli, nutrient signaling, and metabolic state, via multiple enzymatic systems including DNA methyltransferases, histone acetylases, deacetylases, methylases, demethylases, and other protein complex. These epigenetic changes can cause aberrant transcription and noncoding RNA expression and impair DNA integrity, affect cellular function and stress resistance, heavily influence the progression of aging. Diet or environment and genetic influencing epigenetic information could alter aging process [19]. Humans and mice with genetic defects in genome maintenance present accelerated aging symptoms, while enhancing DNA maintenance could delay aging [20].
Proteostasis and Aging
Errors happen on proteins including abnormally synthesized proteins, protein unfolding, abnormal cleavage, undesirable posttranslational modifications, can cause protein self-assembling into toxic oligomeric structures or aggregation into cytosolic inclusions. These damaged proteins can be recognized by chaperones or heat shock proteins and delivered to degradation by the ubiquitin/proteasome system or the lysosomes/autophagy. Increased protein damages would compromise endo-reticulum (ER) homeostasis, lead to increased synthesis of ER chaperones and reduced protein translation to maintain proteostasis, this response is called the unfolding protein response (UPR) [21]. The ability to maintain the protein homeostasis decline with age, many age-related diseases, such as Alzheimer’s disease, Parkinson’s disease, and ALS are associated with intracellular accumulation of abnormal proteins in the form of protein inclusions and aggregates [22]. Chaperone defective could lead to accelerated aging [23], while activation of the master regulator of the heat-shock response, the transcription factor HSF-1, could upregulate heat-shock proteins and increase longevity in C. elegans and mice [24, 25].
Metabolic Homeostasis and Aging
Metabolism provides energy for cell activity, molecules attending signaling transmission, and building block of cell components. Genome instability, proteostasis failure, and environmental influence could lead to abnormal energy supply and metabolite production, such as excessive free oxygen radicals and toxic molecules. Free oxygen radicals including reactive oxygen species (ROS) and diffusible hydrogen peroxide (H2O2), could lead to accumulated oxidative damages, such as carbonylation, oxidized methionine, glycation, aggregation of proteins and DNA damage, and contribute to aging and age-related diseases [26]. This process was proposed by the famous free radical theory of aging. Many compounds increase longevity or improve age-related diseases via scavenging free radicals, such as resveratrol, astaxanthin and gallic acid [27–29].
JNK, a MAP kinase family member, activated by oxidative stress increases longevity in fruit flies and worms [30, 31]. Reduced function of electron transport chain (ETC) could dramatic extend the lifespan of C. elegans and Drosophila [32, 33]. Recently research shows that mitophagy modulates bioenergetics and survival in the neurodegenerative disease by reducing redox and damage [34].
The regulation of metabolism is closely coupled with nutrient sensing pathways, including insulin-like growth factor (IGF) signaling (IIS) pathway [35], target of rapamycin (TOR) signaling [36], adenosine monophosphate activated protein kinase (AMPK) pathway [37], and sirtuins [38]. These signaling pathways sense nutrient or metabolites to regulate the level of glucose, amino acid, cAMP and nicotinamide adenine dinucleotide (NAD+). These pathways regulate growth, metabolic and aging process. Genetic or pharmacological intervention of their components can extend lifespan and delay age-associated dysregulation [8].
Cellular Homeostasis and Aging
Failure to maintain genome stability, proteostasis and metabolic homeostasis will lead to imbalance of cellular homeostasis and cellular senescence. Genome instability could lead to abnormality of nuclear structure, while excessive protein aggregation could cause ER malfunction. Genome damage, defective proteins, and excessive production of ROS could impair mitochondria. Mitochondria damage could induce rescue mechanisms: mitochondrial biogenesis, mitochondria specific unfolded protein response and mitophagy (macroautophagy that targets deficient mitochondria for proteolytic degradation) [39]. Recently research shows that mitophagy modulates bioenergetics and survival in the neurodegenerative disease by reducing redox and damage [34]. The increased damage and reduced repair response are important to aging process.
Senescent cells secret signaling molecules enriched in proinflammatory cytokines and matrix metalloproteinases, which could attract mast cells to clear the senescent cells through macrophage. But deficient clearance of senescent cells will induce inflammation, impair adjacent cells and tissue function, and lead to stem cell exhaustion, and finally contribute to aging [40]. Either genetic or pharmacological elimination of senescent cells could delay age-related pathologies [41, 42].
Natural Products with Anti-aging Activity
To date, there are about 5, 400 scientific research/review articles published under the terms of “anti-aging” and “anti-ageing” terms (obtained from Web of Science, May 2017; keywords restricted to the topics: anti-aging and anti-ageing, at the search domain of Science & Technology). These reports revealed more than 300 compounds with anti-aging activity. Here we summarized the compounds or natural product extracts with explicit anti-aging activity, including 185 compounds from natural products (Table 1), 55 complex or extracts from natural products (Table 2), 62 from clinical drugs (of which more than 50% are also from natural products or natural products analogues, Table 3), 35 from synthesized chemicals (Table 4). Some of them received popular interest and under vigorous investigation, present anti-aging activities in multiple aging models, such as resveratrol [28, 43–53], α-lipoic acid [54–56], astaxanthin [29, 57–59], catechin [60–62], curcumin [63–65], fucoxanthin [66, 67], spermidine [68, 69], metformin [70–72], caffeine [73–75], and rapamycin [76–84], all show anti-aging activity in both D. melanogaster and C. elegans, as well as in other aging models (Table 1). There are 39 compounds present anti-aging activity in two aging models, 32 of them with anti-aging activity in C. elegans. 19 of the 39 compounds are antioxidant (including acacetin, antcin M, agmatine, baicalein, caffeic acid, carnosine, chlorogenic acid, coenzyme Q10, dimethyl sulfide, gallic acid, gluconate, glycerol, hesperidin, icariin, lactate, oleanolic acid, minocycline, vitamin E, and vitexin). Compound betaine, catalpol, (−)-epicatechin, huperzine A and polydatin regulate inflammation. 11 compounds act through energy sensing pathway, including acetic acid, α-ketoglutarate, D-glucosamine, epigallocatechin gallate, nordihydroguaiaretic acid, oligonol, polydatin, rosmarinic acid, sesamin, aspirin, and tetrahydrocurcumin. There are 14, 9, and 109 natural products with anti-aging activity reported only in mice or rat, fruit fly, and C. elegans, respectively, while 14 compounds present anti-aging activities in other aging models, such as mammalian cells and S. cerevisiae. Among the 109 compounds with anti-aging activity in C. elegans, 18 with antioxidative activity, five regulating IIS pathway, four regulating AMPK, four regulating mTOR signaling, 10 regulating SIR-2.1, six regulating SKN-1/Nrf2 pathway, seven regulating JNK-1, 16 with unknown mechanisms, and about half of 109 compounds revealed to regulate multiple signaling pathways.
Table 1.
Compounds from natural products with anti-aging activities
| CAS | Chemicals | Structure | Source | Anti-aging activity and proposed anti-aging mechanism | |
|---|---|---|---|---|---|
| With anti-aging activities in a variety of aging models | |||||
| 501-36-0 | Resveratrol |
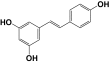
|
Polygonum cuspidatum Sieb.et Zucc. | In mice: 4.7% increase in mean lifespan; increasing insulin sensitivity, reducing insulin-like growth factor-1 (IGF-I) levels, increasing AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) activity, increasing mitochondrial number, and improving motor function [44, 46–51] In D. melanogaster: extends mean lifespan of females fed the low sugar–high protein diet by ∼15.0%, fed the high-fat diet by ∼10.0%; modulating genetic pathways that can reduce cellular damage [45] In C. elegans: 18.0% increase in mean lifespan; regulating AMPK, SIR-2.1, autophagy, and proteasomal degradation [28, 52, 53] In cell: increasing NAD(+) and the activity of AMPK and Sirt1, inhibiting PDE4, JAK2/STAT3 [89–92] In S. cerevisiae: 70.0% increase in mean lifespan; regulating Sir2 and SNF1 [93, 94] In Nothobranchius guentheri: antioxidant [95] |
|
| 62-46-4 | α-Lipoic acid |
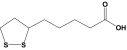
|
Cell metabolite | In SAMP8 mice: improving memory and oxidative stress in extremely old SAMP8 mice, but decreasing lifespan [56] In D. melanogaste: 12.0% increase in mean lifespan and antioxidant [54] In C. elegans: 24.0% increase in mean lifespan and antioxidant, enhancing chemotaxis index [55] |
|
| 472-61-7 | Astaxanthin |
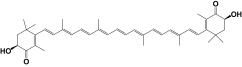
|
Carotenoid | In d-galactose-induced brain aging in rats: antioxidant, upregulating BDNF expression [58, 59] In D. melanogaster: antioxidant [57] In C. elegans: 29.0% increase in mean lifespan and regulating DAF-16 [29] |
|
| 154-23-4 | Catechin |
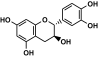
|
Green tea, cocoa, grapes, and apples | In senescence-accelerated (SAMP10) mice: preventing memory regression and DNA oxidative damage [62] In D. melanogaster: 16.0% increase in mean lifespan and antioxidant [61, 96] In C. elegans: 13.0% increase in mean lifespan and antioxidant, regulating DAF-2, AKT-2, MEV-1, and NHR-8; decreasing insulin-like growth factor-1 [60] |
|
| 458-37-7 | Curcumin |
|
Curcuma longa L. | In C57BL6/N mice: antioxidant, increasing collagen and AGEs [64] In D. melanogaster: 25.8% increase in mean lifespan and antioxidant [65] In C. elegans: 25.0% increase in mean lifespan and antioxidant [63] |
|
| 3351-86-8 | Fucoxanthin |
|
Natural substances in human diet | In hairless mice: lessening UVB-induced epidermal hypertrophy, VEGF, and MMP-13 expression [67] In D. melanogaster: 33.0% increase in mean lifespan and antioxidant [66] In C. elegans: 14.0% increase in mean lifespan and antioxidant [66] |
|
| 124-20-9 | Spermidine |
|
Natural polyamine | In D. melanogaster: 30.0% increase in mean lifespan and autophagy [68] In C. elegans: 15.0% increase in mean lifespan and autophagy [68] In S. cerevisiae: autophagy [68, 69] |
|
| With anti-aging activities in two aging models | |||||
| 480-44-4 | Acacetin |
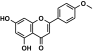
|
Naturally occurring flavonoid | In D. melanogaster: decreasing APP protein expression, BACE-1 activity, and Aβ production [97] In C. elegans: 27.3% increase in mean lifespan and upregulating SOD-3 and GST-4 [98] |
|
| 64-19-7 | Acetic acid |
|
Vinegars | In C. elegans: 23.0% increase in mean lifespan and regulating insulin/IGF-1 pathway [99] In S. cerevisiae: stimulating growth signaling pathways, increasing oxidative stress and replication stress [100] |
|
| 1005344-44-4 | Antcin M |
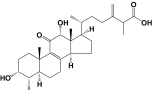
|
Antrodia cinnamomea | In cell: antioxidant, regulating Nrf2 and SIRT-1 [101] In C. elegans from oxidative stress: ~10.0% increase in mean lifespan and antioxidant [101] |
|
| 306-60-5 | Agmatine |
|
Generated by arginine decarboxylase | In male sprague–dawley rats: suppressing age-related elevation in nitric oxide synthase activity in the dentate gyrus of the hippocampus and prefrontal cortex [102] In C. elegans: 16.0% increase in mean lifespan and needs further research [103] |
|
| 328-50-7 | α-Ketoglutarate |
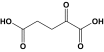
|
Tricarboxylic acid cycle intermediate | In mice: decreasing TBARS level and the activity of superoxide dismutase, increasing glutathione peroxidase activity [104] In C. elegans: 50.0% increase in mean lifespan and inhibiting ATP synthase and TOR signaling [105] |
|
| 491-67-8 | Baicalein |
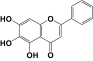
|
Scutellaria baicalensis Lamiaceae | In C. elegans: 24.0% increase in mean lifespan and antioxidant, regulating SKN-1 [106] In PC12 cell: suppressing mitochondria dysfunction and apoptosis [107] |
|
| 107-43-7 | Betaine |
|
Nitrogen containing metabolite | In aged rats: upregulating IKK/MAPKs, attenuating NF-κB activation [108, 109] In C. elegans: 9.0% increase in mean lifespan and needs further research [103] |
|
| 331-39-5 | Caffeic acid |
|
Tomatoes, carrots, strawberries, blueberries and wheat | In Sprague–Dawley rats and intra-cerebroventricular streptozotocin induced experimental dementia in rats: antioxidant, restoring cholinergic functions [110, 111] In C. elegans: 11.0% increase in mean lifespan and regulating OSR-1, SEK-1, SIR-2.1, UNC-43, and DAF-16 [112] |
|
| 305-84-0 | Carnosine |
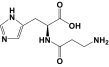
|
Endogenous dipeptide | In aged rats: preventing oxidative stress and apoptosis [113, 114] In D. melanogaster: 26.0% increase in mean lifespan and antioxidant [115, 116] |
|
| 2415-24-9 | Catalpol |
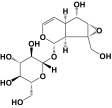
|
Rehmannia glutinosa | In senescent mice induced by d-galactose: improving cholinergic function, reducing inflammatory cytokines; In rats: rebalancing E2 and P4 levels in aged rats [117, 118] In C. elegans: 28.5% increase in mean lifespan and antioxidant, regulating SKN-1/Nrf and DAF-16 [119] |
|
| 327-97-9 | Chlorogenic acid |
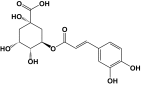
|
Coffee and tea | In d-galactose-induced mice: antioxidant, reducing tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) protein levels [120] In C. elegans: 20.1% increase in mean lifespan and antioxidant, regulating IIS pathway [121] |
|
| 303-98-0 | Coenzyme Q10 |
|
Mitochondrial respiratory chain component | In mice: ameliorating age-related impairment, reducing protein oxidation [122] In C. elegans: 18.0% increase in mean lifespan and scavenging reactive oxygen species [123] |
|
| 3416-24-8 | d-Glucosamine |
|
Hexosamine pathway | In mice: 6.0% increase in mean lifespan; enhancing expression of several murine amino-acid transporters, increasing amino-acid catabolism [124] In C. elegans: 11.0% increase in mean lifespan and mimicking a low-carbohydrate diet by regulating AMPK and SKN-1 [124] |
|
| 75-18-3 | Dimethyl sulfide |
|
Metabolite of marine algae or fermentative bacteria | In D. melanogaster: 24.2% increase in mean lifespan and antioxidant [17] In C. elegans: 24.3% increase in mean lifespan and antioxidant [17] |
|
| 490-46-0 | (−)-Epicatechin |
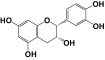
|
Cocoa | In obese diabetic mice: antioxidant, improving skeletal muscle stress output, reducing systematic inflammation and serum LDL cholesterol [61] In D. melanogaster: ~8.0% increase in mean lifespan and needs further research [61] |
|
| 989-51-5 | Epigallo-catechin gallate |
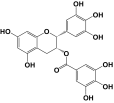
|
Tea polyphenols | In d-galactose-induced mice: increasing oxidative stress and the expression of EGFR proteins [125] In C. elegans: 13.0% increase in mean lifespan and antioxidant, regulating IIS pathway [126] |
|
| 149-91-7 | Gallic acid |
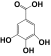
|
Beverages (red wines and green teas), plant leaves (beriberry) | In senescence accelerated mice: antioxidant [127] In C. elegans: 25.0% increase in mean lifespan and antioxidant [27] |
|
| 527-07-1 | Gluconate |
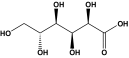
|
Sugars metabolite | In D. melanogaster: 22.0% increase in mean lifespan and antioxidant [128] In lacking nitrogen on C. elegans: 16.0% increase in mean lifespan and antioxidant [103] |
|
| 56-81-5 | Glycerol |
|
Sugars metabolite | In lacking nitrogen on C. elegans: 21.0% increase in mean lifespan and needs further research [103] In rotifer: 50.0% increase in mean lifespan; increasing resistance to starvation, heat, oxidation, and osmotic stress, but not UV stress [129] |
|
| 520-26-3 | Hesperidin |
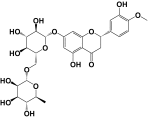
|
Citrus genus | In Murine model of sepsis: antioxidant [130] In S. cerevisiae: 37.0% increase in mean lifespan and antioxidant, regulating Sir2, UTH1 [131] |
|
| 489-32-7 | Icariin |
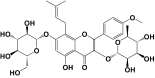
|
Herba epimedii | In mice: inducting antioxidant protein superoxide dismutase (SOD) activity, decreasing oxidative marker malondialdehyde (MDA) [132] In C. elegans: 20.7% increase in mean lifespan and regulating IIS pathway [133] |
|
| 74-79-3 | Arginine |
|
Amino acid | In C. elegans: 27.0% increase in oxidative stress; 370% in heat stress and antioxidant, regulating insulin/IGF signaling pathway [103, 134] In Megalobrama amblycephala: antioxidant [134] |
|
| 50-21-5 | Lactate |
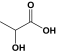
|
Metabolite | In D. melanogaster: 15.0% increase in mean lifespan and antioxidant [128] In C. elegans: 6.0% increase in mean lifespan and antioxidant [103] |
|
| 500-38-9 | Nordi-hydroguaiaretic acid |
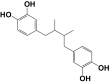
|
Creosote plant (Larrea tridentata: Zygophyllaceae) | In mice:12.0% increase in mean lifespan; decreasing the absorption or increasing the utilization of calories [135–138] In Mosquito: 64.0% increase in mean lifespan and needs further research [137] |
|
| 508-02-1 | Oleanolic acid |
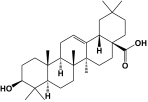
|
Olea europaea, Viscum album L., and Aralia chinensis L. | In d-galactose-induced mice: anti-oxidative, anti-glycative, and anti-apoptotic [139] In C. elegans: 16.6% increase in mean lifespan and antioxidant, regulating DAF-16 [140] |
|
| 851983-55-6 | Oligonol |
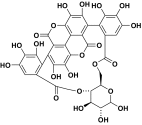
|
Grape seed or lychee fruit | In mice: Regulating AMPK, SIRT1, autophagy, and increasing cell proliferation [141, 142] In C. elegans: regulating AMPK and autophagy [141] |
|
| 27208-80-6 | Polydatin |
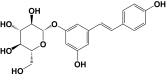
|
Grape juice | In mice: anti-oxidative, anti-inflammatory, and anti-apoptotic [143] In C. elegans: 30.0% increase in mean lifespan and regulating DAF-2, SIR-2.1, SKN-1, SOD-3, and DAF-16 [144] |
|
| 537-15-5 | Rosmarinic acid |
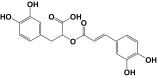
|
Subfamily Nepetoideae of the Lamiaceae | In aging mice: antioxidant [145] In C. elegans: 10.0% increase in mean lifespan and regulating SIR-2.1, OSR-1, SEK-1, UNC-43, and DAF-16 [112] |
|
| 607-80-7 | Sesamin |
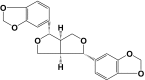
|
Sesame seeds | In D. melanogaster: 12.0% increase in mean lifespan and antioxidant [146] In C. elegans:14.0% increase in mean lifespan and regulating DAF-2, SKN-1, PMK-1, and DAF-16 [147] |
|
| 36062-04-1 | Tetra-hydrocurcumin |
|
Biotransformed metabolite of curcumin contained in turmeric of Indian curry | In mice: 12.0% increase in mean lifespan; attenuating oxidative stress, hypertension, vascular dysfunction, and baroreflex dysfunction [148–151] In D. melanogaster: ~28.0% increase in mean lifespan and regulating Sir2 and FoxO [152] |
|
| 1143-70-0 | Urolithin A |
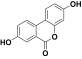
|
Pomegranate fruit, nuts and berries | In mouse models of age-related decline of muscle function: improving exercise capacity [153] In C. elegans: 45.4% increase in mean lifespan and regulating mitochondrial function, mitophagy [153] |
|
| 3681-93-4 | Vitexin |
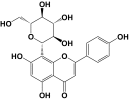
|
Vigna angularis | In d-galactose-aged mice: antioxidant [154] In C. elegans: 17.0% increase in mean lifespan and antioxidant [155] |
|
| With anti-aging activities in rats or mice | |||||
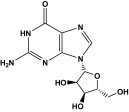
| 118-00-3 | Guanosine |
|
Endogenous nucleoside | In Wistar rats: antioxidant [156] | |
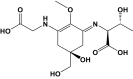
| 70579-26-9 | Porphyra-334 |
|
Red alga Porphyra rosengurttii | In mice skin: antioxidant, Hsp70 [157] | |
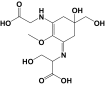
| 73112-73-9 | Shinorine |
|
Red alga Porphyra rosengurttii | In mice skin: antioxidant, Hsp70 [157] | |
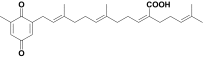
| 70363-87-0 | Sargaquinoic acid |
|
Sargassum sagamianum | In mice skin: inducing apoptosis [158] | |
| 70363-89-2 | Sargachromenol |
|
Sargassum sagamianum | In mice skin: inducing apoptosis [158] | |
| 1094-61-7 | β-Nicotinamide mononucleotide |
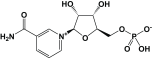
|
Turnover of the oxidized form of nicotinamide adenine dinucleotide (NAD+) | In rats: increasing NAD+ level [159] | |
| 1339070-29-9 | TA-65 |
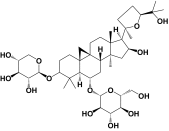
|
Root of Astragalus membranaceus | In mice: activating telomerase [160] | |
| 34157-83-0 | Celastrol |
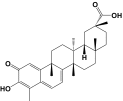
|
Traditional Chinese medicinal herbs of the Celastraceae family | In transgenic mouse model of amyotrophic lateral sclerosis: 13.0% increase in mean lifespan and regulating HSP70, blocking neuronal cell death [161] | |
| 57-00-1 | Creatine |
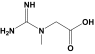
|
Natural ergogenic compound | In mice: 9.0% increase in mean lifespan and upregulating genes implicated in neuronal growth, neuroprotection, and learning [162] | |
| 42553-65-1 | Crocin |
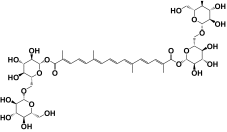
|
Kashmiri saffron (Crocus sativus) | In mice: 44.0% increase in mean lifespan and impacting on hematological parameters [163] | |
| 61276-17-3 | Acteoside |
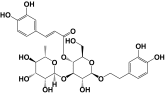
|
Roots of Incarvillea younghusbandii Sprague | In senescent mouse model induced by a combination of d-gal and AlCl3: decreasing nitric oxide, the activity of nitric oxide synthase and the expression of caspase-3 [164] | |
| 11096-26-7 | Erythropoietin | Glycoprotein | Glycoprotein hormone | In rats: antioxidant, regulating ERK/Nrf2-ARE [165] | |
| 62499-27-8 | Gastrodin |
|
A number of plants and herbs | In vascular dementia rats induced by chronic ischemia: antioxidant, regulating ADH7, GPX2, GPX3 and NFE2L2 [166] | |
| 22427-39-0 | Ginsenoside Rg1 |
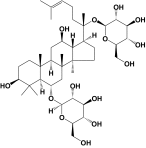
|
Panax ginseng | In d-galactose-induced mice: antioxidant, regulating the level of proinflammatory cytokines and telomerase system, activating the Wnt/β-catenin signaling [167, 168] | |
| With anti-aging activities in Drosophila melanogaster | |||||
| 87-89-8 | Chiro-inositol |
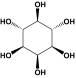
|
Inositol family | 16.7% increase in mean lifespan and antioxidant, regulating dFOXO [169] | |
| 526-95-4 | Gluconic acid |
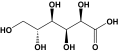
|
Glucose catabolism | 22.0% increase in mean lifespan and antioxidant [128] | |
| Glycoside acteoside |
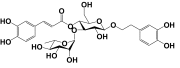
|
Roots of Incarvillea younghusbandii Sprague | 15.0% increase in mean lifespan and antioxidant [170] | ||
| 127-40-2 | Lutein |
|
Major carotenoids in most fruits and vegetables | 11.0% increase in mean lifespan and antioxidant [171] | |
| 1004313-10-3 | S,S-Trolox-carnosine |
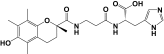
|
Trolox acylated derivatives | 36.0% increase in mean lifespan and antioxidant [116] | |
| 4670-05-7 | Theaflavins |
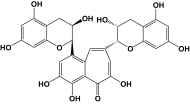
|
Black tea | 10.0% increase in mean lifespan and antioxidant [172] | |
| 353-09-3 | β-Guani-dinopropionic acid |
|
Metabolites | 13.0% in female, 90% in male increase in mean lifespan and regulating AMPK-Atg1-autophagy signaling [173] | |
| 19545-26-7 | Wortmannin |
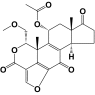
|
Penicillium funiculosum | 5.0% increase in mean lifespan and inhibiting PI3K [174] | |
| 139-85-5 | 3,4-Dihydro-xybenzaldehyde |
|
Sasa senanensis leaves | 23.0% increase in mean lifespan and inhibiting the 2-oxoglutarate binding sites of prolyl 4-hydroxylase [175] | |
| With anti-aging activities in Caenorhabditis elegans | |||||
| 57-91-0 | β-Estradiol |
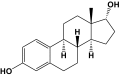
|
Hormone | 7.0% increase in mean lifespan and antioxidant [176] | |
| 1406-65-1 | Chlorophyll |
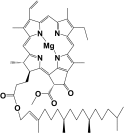
|
Green vegetables | 25.0% increase in mean lifespan and antioxidant [177] | |
| 730-08-5 | Dipeptide Tyr-Ala |
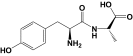
|
Hydrolyzed maize protein | 12.4% increase in mean lifespan and antioxidant [178] | |
| 934822-64-7 | Ferulsinaic acid |
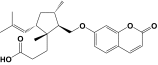
|
Sesquiterpene coumarins from the genus Ferula | 20.0% increase in mean lifespan and antioxidant [179] | |
| 446-72-0 | Genistein |
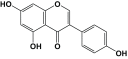
|
Vigna angularis | 27.9% increase in mean lifespan and antioxidant [180, 181] | |
| Quercetin 3-O-β-d-glucopyranoside-(4 → 1)-β-d-glucopyranoside |
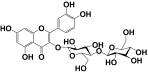
|
Onion | 12.4% increase in mean lifespan and antioxidant [182] | ||
| 69-72-7 | Salicylic acid |
|
Plant hormone | 14.0% increase in mean lifespan and antioxidant [183] | |
| 72514-90-0 | Specioside |
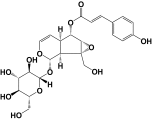
|
Stereospermum suaveolens | 15.5% increase in mean lifespan and antioxidant [184] | |
| 480-18-2 | Taxifolin |
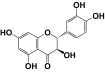
|
Citrus fruits and onion | 51.0% increase in mean lifespan and antioxidant [185] | |
| 3081-61-6 | Theanine |
|
Camellia sinensis | ~5.0% increase in mean lifespan and antioxidant [186] | |
| 6829-55-6 | Tocotrienols |
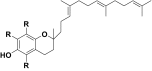
|
Vitamin E members | ~20.0% increase in mean lifespan and antioxidant [187] | |
| 53188-07-1 | Trolox |
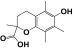
|
Vitamin E analog | 31.0% increase in mean lifespan and antioxidant [185] | |
| 528-48-3 | Fisetin |
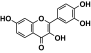
|
Apples, onions and grapes and many more herbal edibles | 6.0% increase in mean lifespan of thermal stress and antioxidant, regulating DAF-16 [188] | |
| 215112-16-6 | 4-Hydroxy-E-globularinin |
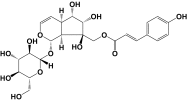
|
Premna integrifolia | 18.8% increase in mean lifespan and antioxidant, regulating DAF-16 [189] | |
| 521-48-2 | Iso-xanthohumol |
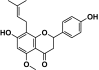
|
Humulus lupulus L. | 10.2% increase in mean lifespan and antioxidant, regulating DAF-16 [190] | |
| 520-18-3 | Kaempferol |
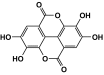
|
Apples, onions and grapes and many more herbal edibles | 10.0% increase in mean lifespan and antioxidant,regulating DAF-16 [188] | |
| 117-39-5 | Quercetin |
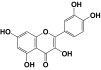
|
Onions, apples, and broccoli as well as in red wine, tea, and extracts of Ginkgo biloba | 15.0% increase in mean lifespan and antioxidant, regulating DAF-16 [191] | |
| 50932-19-9 | Verminoside |
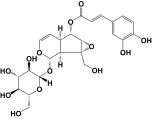
|
Stereospermum suaveolens | 20.8% increase in mean lifespan and antioxidant, regulating DAF-16 [192] | |
| 113558-15-9 | Icariside II |
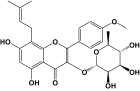
|
Icariin active metabolite | 20.0% increase in mean lifespan and regulating IIS signaling [133] | |
| 99-20-7 | Trehalose |
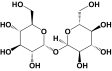
|
Disaccharide of glucose | 32.0% increase in mean lifespan and regulating IIS signaling [193] | |
| 32911-62-9 | Withanolide A |
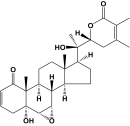
|
Ayurvedic | 29.7% increase in mean lifespan and regulating IIS pathway and neural activity [194] | |
| 501-94-0 | Tyrosol |
|
Extra virgin olive oil | 10.8% increase in mean lifespan and regulating IIS pathway and heat shock response [195–197] | |
| 4339-71-3 | Piceatannol |
|
Grapes and white tea | ~18.3% increase in mean lifespan and regulating IIS pathway and SIR-2.1 [198] | |
| 52-89-1 | Cysteine |
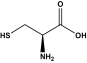
|
Amino acids | 16.0% increase in mean lifespan and regulating AMPK and DAF-16 [103] | |
| 6537-80-0 | Chicoric acid |
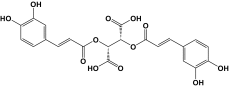
|
Caffeoyl derivative | 21.0% increase in mean lifespan and regulating AMPK [199] | |
| 328-42-7 | Oxaloacetate |
|
Citric acid cycle metabolite | 25.0% increase in mean lifespan and regulating AMPK [200] | |
| 29700-22-9 | Oxy-resveratrol |
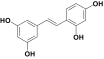
|
Isomer of hydroxylated resveratrol | 31.1% increase in mean lifespan and regulating calorie restriction, AMPK, and SIR-2.1 [201] | |
| β-Dihydro-agarofuran-type sesquiterpenes |
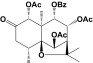
|
Seeds of Celastrus monospermus | 38.0% increase in mean lifespan and rapamycin mimetics [202] | ||
| 13095-47-1 | (R)-2-Hydro-xyglutarate |
|
Oncometabolite | 43.0% increase in mean lifespan and inhibiting ATP synthase and mTOR signaling [203] | |
| 13095-48-2 | (S)-2-Hydroxyglutarate |
|
Oncometabolite | 32.0% increase in mean lifespan and inhibiting ATP synthase and mTOR signaling [203] | |
| 765-01-5 | 10-Hydroxy-2-decenoic acid |
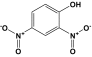
|
Major lipid component of Royal Jelly | 10.0% increase in mean lifespan and regulating dietary restriction and mTOR signaling [204] | |
| Ascr#2 |
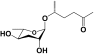
|
Pheromone | 14.0% increase in mean lifespan and regulating SIR-2.1 [205] | ||
| Ascr#3 |
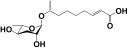
|
Pheromone | 14.0% increase in mean lifespan and regulating SIR-2.1 [205] | ||
| 1740-19-8 | Dehydro-abietic acid |
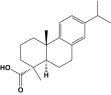
|
P. densiflora, P. sylvestris, Abies grandis | 15.5% increase in mean lifespan and regulating SIR-2.1 [206] | |
| 7783-06-4 | Hydrogen sulfide |
|
Naturally produced in animal cells | 74.0% increase in mean lifespan and antioxidant, regulating SIR-2.1 [207, 208] | |
| 932-30-9 | Salicylamine |
|
Phenolic amines | 56.0% increase in mean lifespan and regulating SIR-2.1 and ETS-7 [209] | |
| 481-39-0 | Juglone |
|
Roots, leaves, woods and fruits of Juglandaceae walnut trees | 29.0% increase in mean lifespan and regulating SIR-2.1 and DAF-16 [210] | |
| Deutero-haemin-AlaHisThrValGluLys | Peptides | Porphyrin-peptide | 19.1% increase in mean lifespan and regulating SIR-2.1and DAF-16 [211] | ||
| 53-84-9 | Nicotinamide adenine dinucleotide |
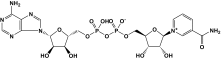
|
Tricarboxylic acid cycle intermediate | 15.0% increase in mean lifespan and regulating SIR-2.1 and DAF-16 [212] | |
| 149-61-1 | Malate |
|
Tricarboylic acid (TCA) cycle metabolite | 14.0% increase in mean lifespan and regulating dietary restriction, SIR-2.1, and DAF-16 [213] | |
| 70-47-3 | Asparagine |
|
Amino acid | 5.0% increase in mean lifespan and regulating SKN-1 [103] | |
| 2050-87-5 | Diallyl trisulfide |
|
Garlic | 12.6% increase in mean lifespan and regulating SKN-1 [214] | |
| 481-42-5 | Plumbagin |
|
Plumbago zeylanica L. | 15.0% increase in mean lifespan and regulating SKN-1 [215] | |
| 77-59-8 | Tomatidine |
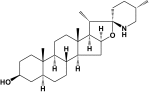
|
Unripe tomato fruits, leaves and stems | 7.0% increase in mean lifespan and regulating SKN-1/Nrf2 pathway, mitophagy [216] | |
| 21593-77-1 | S-Allylcysteine |
|
Allium sativum L. | 17.0% increase in mean lifespan and antioxidant, regulating SKN-1 [217] | |
| 2281-22-3 | S-Allylmercapto-cysteine |
|
Allium sativum L. | 20.9% increase in mean lifespan and antioxidant, regulating SKN-1 [217] | |
| 61-90-5 | Leucine |
|
Amino acids | 16.0% increase in mean lifespan and regulating SKN-1and DAF-16 [103] | |
| 62333-08-8 | Isolappaol A |
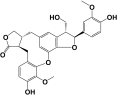
|
A. lappa seeds | 11.0% increase in mean lifespan and regulating JNK-1 and DAF-16 [218] | |
| 64855-00-1 | Lappaol C |
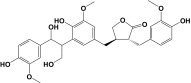
|
A. lappa seeds | 12.0% increase in mean lifespan and regulating JNK-1 and DAF-16 [218] | |
| 69394-17-8 | Lappaol F |
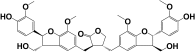
|
A. lappa seeds | 13.0% increase in mean lifespan and regulating JNK-1 and DAF-16 [218] | |
| 580-72-3 | Matairesinol |
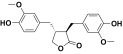
|
Arctium lappa | 25.0% increase in mean lifespan and regulating JNK-1 and DAF-16 [218] | |
| 7770-78-7 | Arctigenin |
|
Arctium lappa | 14.0% increase in mean lifespan and antioxidant, regulating JNK-1 and DAF-16 [218] | |
| 20362-31-6 | Arctiin |
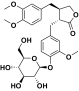
|
Arctium lappa | 15.0% increase in mean lifespan and antioxidant, regulating JNK-1 and DAF-16 [218] | |
| 484-68-4 | Pinitol |
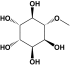
|
Fine wood, alfalfa, and legumes | 13.0% increase in mean lifespan and regulating JNK, S6K, and DAF-16 [169] | |
| 56-41-7 | Alanine |
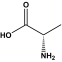
|
Amino acid | 11.0% increase in mean lifespan and regulating AAK-2, SKN-1, and DAF-16 [103] | |
| 56-87-1 | Lysine |
|
Amino acids | 8.0% increase in mean lifespan and regulating AAK-2, SKN-1, and DAF-16 [103] | |
| 338-69-2 | d-Alanine |
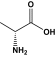
|
Amino acids | 16.0% increase in mean lifespan and regulating AAK-2, SIR-2.1, and DAF-16 [103] | |
| 56-85-9 | Glutamine |
|
Amino acids | 16.0% increase in mean lifespan and regulating EAT-2, AAK-2, and SKN-1 [103] | |
| 87-44-5 | β-Caryophyllene |
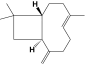
|
Edible plants | 22.0% increase in mean lifespan and regulating SIR-2.1, SKN-1 and DAF-16 [219] | |
| 60-18-4 | Tyrosine |
|
Amino acids | 10.0% increase in mean lifespan and regulating SIR-2.1, SKN-1, and DAF-16 [103] | |
| 107-95-9 | β-Alanine |
|
Amino acid | 13.0% increase in mean lifespan and regulating AAK-2, SIR-2.1, SKN-1, and DAF-16 [103] | |
| Acacetin 7-O-α-l-rhamnopyranosyl (1–2) β-d-xylopyranoside |
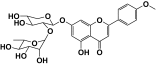
|
Premna integrifolia | 39.0% increase in mean lifespan and regulating EAT-2, SIR-2.1, SKN-1, HSF-1, MEV-1, and DAF-16 [220] | ||
| 15502-74-6 | Arsenite |
|
Natural and anthropogenic sources | (10 μM) 10.0% increase in mean lifespan, (>100 μM) 12.0% decrease and antioxidant, regulating SKN-1, MTL-2, TIN-9, and DAF-16 [221, 222] | |
| 625-72-9 | d-β-Hydroxybutyrate |
|
Ketone body | 26.0% increase in mean lifespan and regulating AAK-2, SIR-2.1, SKN-1, and DAF-16; inhibiting histone deacetylase [223] | |
| 71-00-1 | Histidine |
|
Amino acids | 12.0% increase in mean lifespan and regulating EAT-2, AAK-2, SIR-2.1, SKN-1, BEC-1, HIF-1, GAS-1, IFE-2, GCN-2, and DAF-16 [103] | |
| 37159-97-0 | Proline |
|
Amino acids | 19.0% increase in mean lifespan and regulating EAT-2, AAK-2, SIR-2.1, SKN-1, BEC-1, and DAF-16 [103] | |
| 56-45-1 | Serine |
|
Amino acids | 22.0% increase in mean lifespan and regulating EAT-2, AAK-2, SIR-2.1, SKN-1, HIF-1, BEC-1, and DAF-16 [103] | |
| 73-22-3 | Tryptophan |
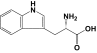
|
Amino acids | 14.0% increase in mean lifespan and regulating EAT-2, AAK-2, SIR-2.1, SKN-1, BEC-1, GCN-2, and DAF-16 [103] | |
| 1405-87-4 | Bacitracin |
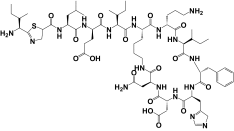
|
Bacillus subtilisvar Tracy | 59.0% increase in mean lifespan and regulating CBP-1, improving proteotoxcity [224] | |
| 142-42-7 | Fumarate |
|
Tricarboxylic acid (TCA) cycle metabolite | 16.0% increase in mean lifespan and increasing the amount of oxidized NAD and FAD cofactors [213] | |
| 63-68-3 | Methionine |
|
Amino acids | 14.0% increase in mean lifespan and regulating mitochondrial unfolded protein response [103] | |
| 62-75-9 | N-Nitrosodimethylamine |
|
Ubiquitously distributed organic xenobiotic compounds | 6.0% increase in mean lifespan and reducing transcription of many stress response genes [225] | |
| 25166-14-7 | 2,3-Dehydrosily-bin A/B |
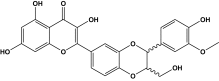
|
Potential active components of silymarin | 16.1% increase in mean lifespan and antioxidant, regulating FGT-1, improving proteotoxic stress [226] | |
| 476-66-4 | Ellagic acid |
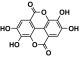
|
Strawberry and raspberry | ~10.0% increase in mean lifespan and antioxidant, CR mimetics, antimicrobial [27] | |
| 1259-86-5 | Glau-carubinone |
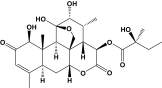
|
Different species of the tropical plant family Simaroubaceae | ~80.0% increase in mean lifespan and promoting mitochondrial metabolism, reducing body fat [227] | |
| 529-44-2 | Myricetin |
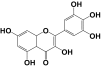
|
Tea, different vegetables, onions, berries, grapes and medical plants | 34.3% increase in mean lifespan and regulating DAF-16; enhanced quality of life during aging [63, 228] | |
| 106758-54-7 | Otophylloside B |
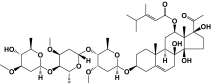
|
Cynanchum otophyllum | 11.3% increase in mean lifespan and regulating DAF-2, SIR-2.1, CLK-1, and DAF-16 [229] | |
| 14937-32-7 | Pentagalloyl glucose |
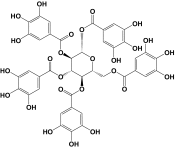
|
Eucalyptus leaves | 18.0% increase in mean lifespan and regulating dietary restriction, IIS pathway, SIR-2.1 and mitochondrial electron transport chain [230] | |
| 7512-17-6 | N-Acetyl-glucosamine |
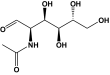
|
Hexosamine Pathway Metabolite | 50.0% increase in mean lifespan and enhancing autophagy, ER-associated protein degradation, and proteasomal activity [231] | |
| Quercetin 3′-O-β-d-glucopyranoside |
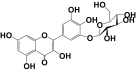
|
Onion | 20.9% increase in mean lifespan and regulating DAF-2, OLD-1, OSR-1,and AEK-1 [182] | ||
| 50-70-4 | Sorbitol |
|
S. cerevisiae | 35.0% increase in mean lifespan and regulating DR and osmotic response [232] | |
| 1401-55-4 | Tannic acid |
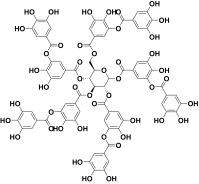
|
Grapes and green tea | 19.0% increase in mean lifespan and regulating TGF-β, p38 MAPK pathways, and DAF-12 [233, 234] | |
| 77-92-9 | Citrate |
|
Tricarboxylic acid cycle intermediate | 13.0% increase in mean lifespan and inducing ER stress response [103] | |
| 107-35-7 | Taurine |
|
Nitrogen containing metabolites | 11.0% increase in mean lifespan and inducing ER stress response [103] | |
| 38748-32-2 | Triptolide |
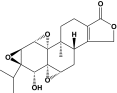
|
Tripterygium wilfordii | 20.1% increase in mean lifespan and antioxidant, regulating HSP16.2 and SOD-3 [235] | |
| 67-97-0 | Vitamin D3 |
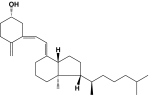
|
Vitamins | 39.0% increase in mean lifespan and regulating SKN-1, IRE-1, XBP-1, DAF-12, and proteostasis [236] | |
| 57-88-5 | Cholesterol |
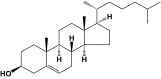
|
Cyclo-pentanoper-hydro-phenanthrene ring | Regulating cholesterol-binding protein NSBP-1and DAF-16 [237] | |
| 949004-12-0 | Dafachronic acid |
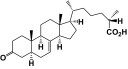
|
Bile acid-like steroid | 17.0% increase in mean lifespan and “antiaging” in the germ-line longevity pathway [238] | |
| 145-13-1 | Pregnenolone |
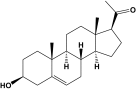
|
Hormonal steroids | 20.0% increase in mean lifespan and relating to germline-defective regulated longevity [239] | |
| 1315285-41-6 | Royalactin | Glycoprotein | Royal jelly | 34.0% increase in mean lifespan and regulating EGF signaling [240] | |
| 104594-70-9 | Caffeic acid phenethyl ester |
|
Propolis | 9.0% increase in mean lifespan and regulating DAF-16 [241] | |
| 64-17-5 | Ethanol |
|
Metabolites | Serving as a carbon and energy source and/or by inducing a stress response [242] | |
| N-γ-(l-Glutamyl)-l-seleno-methionine |
|
Garlic as and primary metabolic product of SeMet | Antioxidant, regulating sele-noprotein TRXR-1 [243] | ||
| 74-81-7 | Caprylate |
|
Metabolites | In lacking nitrogen on C. elegans: 7.0% increase in mean lifespan and needs further research [51] | |
| 6893-26-1 | d-Glutamate |
|
Amino acids | 18.0–114.0% increase in mean lifespan and needs more research [103] | |
| 10257-28-0 | Galact-opyranose |
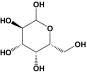
|
Sugars metabolites | In lacking nitrogen on C. elegans: 6.0% increase in mean lifespan and needs more research [103] | |
| 56-40-6 | Glycine |
|
Amino acids | 10.0% increase in mean lifespan and needs more research [103] | |
| 6027-13-0 | Homocysteine |
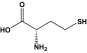
|
Nitrogen containing metabolites | 13.0% increase in mean lifespan and needs more research [103] | |
| 87-89-8 | Inositol |
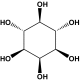
|
Metabolites | In lacking nitrogen on C. elegans: 17.0% increase in mean lifespan and needs more research [103] | |
| 320-77-4 | Isocitrate |
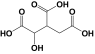
|
TCA cycle intermediate | 13.0% increase in mean lifespan and needs more research [103] | |
| 7004-09-3 | Isoleucine |
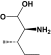
|
Amino acids | 3.0% increase in mean lifespan and needs more research [103] | |
| 70-26-8 | Ornithine |
|
Amino acids | 8.0% increase in mean lifespan and needs more research [103] | |
| 138-08-9 | Phosphoenol-pyruvate |
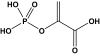
|
Metabolites | In lacking nitrogen on C. elegans: 12.0% increase in mean lifespan and needs more research [103] | |
| 98-98-6 | Picolinic acid |
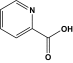
|
Endogenous metabolite of the kynurenine pathway | 7.0% increase in mean lifespan and needs further research [103] | |
| 10257-32-6 | Ribopyranose |
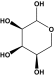
|
Sugars metabolites | In lacking nitrogen on C. elegans: 9.0% increase in mean lifespan and needs more research [103] | |
| 56-14-4 | Succinate |
|
TCA cycle intermediates | 11.0% increase in mean lifespan and needs more research [103] | |
| 72-19-5 | Threonine |
|
Amino acids | 8.0% increase in mean lifespan and needs more research [103] | |
| 72-18-4 | Valine |
|
Amino acids | 13.0% increase in mean lifespan and needs more research [103] | |
| 58-86-6 | Xylose |
|
Sugars metabolites | In lacking nitrogen on C. elegans: 6.0% increase in mean lifespan and needs more research [103] | |
| With anti-aging activities in other aging models | |||||
| 32619-42-4 | Oleuropein |
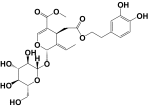
|
Olea europea leaf | In cell: 15.0% increase in mean lifespan; and increasing proteasome-mediated degradation rates, retaining proteasome function and Nrf2/heme oxygenase-1 pathway [244] | |
| 84605-18-5 | Cyclo-astragenol |
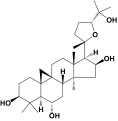
|
Astragalus membranaceus | In PC12 cells and primary neurons: inducing telomerase activity and cAMP response element binding (CREB) [245] | |
| 528-58-5 | Cyanidin |
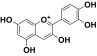
|
Fruits and vegetables | In cell: antioxidant, decreasing expressions of nuclear factor-kappaB, cyclooxygenase-2, and nitric oxide synthase [246] | |
| 88095-77-6 | Dieckol |
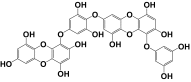
|
Eckloina cava | In radiation-induced cell damages: protecting effects on UV-B [247] | |
| 1229519-12-3 | HDTIC-1 |
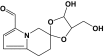
|
Herb Astragalus membranaceus var. mongholicus | In cell: antioxidant, improving proliferation, inhibiting glycation end product formation, slowing down telomere shortening rate [248, 249] | |
| 1229519-13-4 | HDTIC-2 |
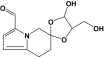
|
Herb Astragalus membranaceus var. mongholicus | In cell: antioxidant, improving proliferation, inhibiting glycation end product formation, slowing down telomere shortening rate [248, 249] | |
| 87798-94-5 | Quercetin caprylate |
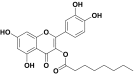
|
Quercetin derivative | In cell: antioxidant, proteasome activator [250] | |
| 501334-35-6 | Collemin A |
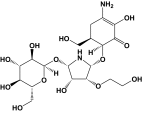
|
Lichenized ascomycete Collema cristatum | In cell and human skin: preventing pyrimidine dimer formation and UV-B induced erythema [251] | |
| 87425-34-1 | Nolinospiroside F |
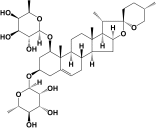
|
Ophiopogon japonicus | In S. cerevisiae: 23.0% increase in mean lifespan and antioxidant [252, 253] | |
| 57103-57-8 | (−)-Glyceollin I |
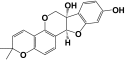
|
Soybeans | In S. cerevisiae: calorie restriction mimetic [254] | |
| 487-52-5 | Butein |
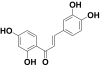
|
Toxicodendron vernicifluum | In S cerevisia: 31.0% increase in mean lifespan and regulating Sir2 [255] | |
| 1341-23-7 | Nicotinamide riboside |
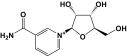
|
NAD(+) precursor | In S. cerevisiae: 20.0% increase in mean lifespan and increasing net NAD(+) synthesis and Sir2 function [252, 253] | |
| 434-13-9 | Lithocholic acid |
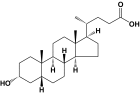
|
Major bile acids excreted by mammals | In S. cerevisiae: 100.0% increase in mean lifespan and modulating housekeeping longevity assurance processes [256, 257] | |
| 57-94-3 | Curare |
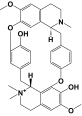
|
Chondrodendron tomentosum, Menispermaceae or Strychnos | In Asplanchna brightwelli: 34.0% increase in mean lifespan and needs further research [258] | |
Table 2.
Natural product extracts with anti-aging activities
| Complex or extracts | Source | Anti-aging activity and proposed anti-aging mechanism |
|---|---|---|
| With anti-aging activities in two aging models | ||
| Green tea extract | Green tea | In mice: 7.0% increase in mean lifespan and antioxidant [44] In D. melanogaster: 16.0% increase in mean lifespan and antioxidant [96] |
| Korean mistletoe water extract | Viscum album coloratum | In D. melanogaster: 20.0% increase in mean lifespan and regulating Sir2 [259] In C. elegans: 10.0% increase in mean lifespan and antioxidant [259] |
| With anti-aging activities in rats or mice | ||
| A-type proanthocyanidins-rich cranberry extract | Cranberry | In mice: antioxidant [260] |
| Fungus Phellinus sp. polysaccharide | Fungus Phellinus sp. | In mice: antioxidant [261] |
| Polysaccharides of Dicliptera chinensis (L.) Juss | Dicliptera chinensis (L.) Juss | In mice: scavenging free radical and antioxidant [262] |
| Polysaccharides of Urtica | Urtica | In d-galactose-induced mice: antioxidant [263] |
| Cocoa polyphenolic extract | Acticoa powder | In rats: 11.0% increase in mean lifespan and retarding age-related brain impairments [264] |
| Nigella Sativa fixed oil | Nigella Sativa | In mice: reducing lipid peroxidation, Bax/Bcl2, and caspase-3 [265] |
| Exopolysaccharides of Agrocybe | Agrocybe cylindracea | In d-galactose-induced mice: antioxidant, reducing the contents of malonaldehyde (MDA) and total cholesterol (TC) [266] |
| Neem leaves extract | Neem | In UVB-irradiated NHDFs, hairless mice: increasing TGF-β1, decreasing AP-1, ROS, and MAPK [267] |
| With anti-aging activities in Drosophila melanogaster | ||
| APPLE polyphenols | Apple | 10.0% increase in mean lifespan and antioxidant [268] |
| Cocoa | ~14.0% increase in mean lifespan and antioxidant [269] | |
| Cordyceps sinensis oral liquid | Traditional Chinese medicine | 32.0% increase in mean lifespan and antioxidant [270] |
| Cynomorium songaricum Rupr | Traditional Chinese medicine | 15.0% increase in mean lifespan and antioxidant [271] |
| Emblica officinalis (fruit) | Emblica officinalis | 6.0% increase in mean lifespan and antioxidant [272] |
| Rhizome powder of Rhodiola rosea | Rhodiola rosea | 17.0% increase in mean lifespan and antioxidant [273] |
| Curcuma longa (rhizome) | Curcuma longa | 18.0% increase in mean lifespan and antioxidant [272] |
| Oregano and cranberry extracts | Oregano and cranberry | ~43.0% in male and ~62.0% in female (full diet +2% OC) increase in mean lifespan and partly through DR-independent pathways [274] |
| Cinnamon extract | Cinnamon | 17.0% in male, 37.0% in female increase in mean lifespan and regulating insulin signaling [275] |
| Ludwigia octovalvis extract | Ludwigia octovalvis | 24.0% increase in mean lifespan and regulating AMPK [276] |
| Jujube fruit | Jujube | 11.1% increase in mean lifespan and regulating FoxO [277] |
| Black tea extract | Black tea | 21.4% increase in mean lifespan and inhibiting the ageing-related accumulation of iron [278] |
| Cranberry anthocyanin extract | Cranberry | 10.0% increase in mean lifespan and up-regulation of SOD1 and down-regulation of MTH, InR, TOR and PEPCK [279] |
| Rosa damascena extract | Rosa damascena | 32.0% increase in mean lifespan and increasing sensitivity to heat [280] |
| With anti-aging activities in Caenorhabditis elegans | ||
| Acanthopanax sessiliflorusstem stem extract | Acanthopanax sessiliflorusstem stem | 16.8% increase in mean lifespan and antioxidant [281] |
| Angelica sinensis peptides | Angelica sinensis | ~20.0% increase in mean lifespan and antioxidant [282] |
| Apple procyanidins | Apple | 12.1% increase in mean lifespan and antioxidant [283] |
| Blueberry polyphenols | Blueberry | 28.0% increase in mean lifespan and antioxidant [284] |
| Extract from seed of Platycladus orientalis | Platycladus orientalis | 24.5% increase in mean lifespan and antioxidant [285] |
| Ginko biloba extract | Ginko biloba | 8.0–25.0% increase in mean lifespan and antioxidant [286] |
| HonTsai Tai extract | HonTsai Tai | 8.0% increase in mean lifespan and antioxidant [287] |
| KPG-7 | Herb mixture | 12.0% increase in mean lifespan and antioxidant [288] |
| Panax notoginseng Polysaccharides | Panax notoginseng | 21.0% increase in mean lifespan and antioxidant [289] |
| Tenebrio molitor extracts | Tenebrio molitor | 30.6% increase in mean lifespan and antioxidant [290] |
| Erchen wan | Traditional Chinese medicine | 22.0% increase in mean lifespan and antioxidant [291] |
| Huanshao dan | Traditional Chinese medicine | 38.0% increase in mean lifespan and antioxidant [291] |
| Liuwei dihuang wan | Traditional Chinese medicine | 13.0% increase in mean lifespan and antioxidant [291] |
| Shengmai yin | Traditional Chinese medicine | 47.0% increase in mean lifespan and antioxidant [291] |
| Shiquan dabu wan | Traditional Chinese medicine | 15.0% increase in mean lifespan and antioxidant [291] |
| Bletilla striata polysaccharide | Bletilla striata | ~20.0% increase in mean lifespan and regulating IIS pathway [292] |
| Ethylacetate fraction from Ribes fasciculatum | Ribes fasciculatum | 16.3% increase in mean lifespan and regulating IIS pathway and SIR-2.1 [293] |
| Peptides from sesame cake | Sesame cake | 15.6% increase in mean lifespan and regulating SKN-1signaling [294] |
| Astragalus membranaceus polysaccharide | Astragalus membranaceus | 24.0% increase in mean lifespan and regulating DAF-16 [295] |
| Garlic extract | Garlic extract | 21.0% increase in mean lifespan and regulating DAF-16 [296] |
| Reishi mushroom polysaccharide | Reishi mushroom | ~20.0% increase in mean lifespan and regulating TIR-1 and DAF-16 [99] |
| Royal Jelly | Honeybee | 18.0% increase in mean lifespan and DAF-16 dependent [297, 298] |
| Ayurvedic polyherbal extract | Ayurvedic | 16.1% increase in mean lifespan and regulating DAF-2, SKN-1, SOD-3, GST-4, and DAF-16 [299] |
| Damnacanthus officinarum leaf extract | Damnacanthus officinarum | 19.0% increase in mean lifespan and regulating neuroprotective activity [300] |
| Deuterohemin peptide | Peptides | 21.0% increase in mean lifespan and antioxidant, regulating DR [301] |
| Eleutherococcus senticosus root extract | Eleutherococcus senticosus | 16.0% increase in mean lifespan and antioxidant, regulating DAF-16 [302] |
| Lowbush cranberry | Lowbush cranberry | 22.0% increase in mean lifespan and altering mechanosensory neuron aging [303] |
| Mulberry leaf polyphenols | Mulberry leaf | 23.0% increase in mean lifespan and regulating DAF-12, PHA-4, NHR-80, and DAF-16 [304] |
| Dauer-inducing Pheromone | Worms | 27.0% increase in mean lifespan and needs more research [305] |
| With anti-aging activities in other aging models | ||
| Annurca apple extracts | Annurca apple | In S. cerevisiae: antioxidant, antiapoptotic [306] |
| Red algal extracts | Red algal | In Brachionus manjavacas: 9.0% increase in mean lifespan and needs further research [307] |
Table 3.
Clinical medicine with anti-aging activities
| CAS | Chemicals | Structure | Clinical application | Anti-aging activity and proposed anti-aging mechanism |
|---|---|---|---|---|
| With anti-aging activities in a variety of aging models | ||||
| 58-08-2 | Caffeine |
|
Psychoactive drug | In rats: antioxidant, alleviating neuroinflammation and neurodegeneration [75] In zebrafish: 29.4% increase in mean lifespan and regulating proteostasis [74] In C. elegans: 29.4% increase in mean lifespan and regulating IIS pathway and proteostasis [73] |
| 657-24-9 | Metformin |
|
Treatment of type 2 diabetes and polycystic ovary syndrome | In rats: altering erythrocyte redox status [70] In D. melanogaster: has not effect on fecundity or lifespan and activating AMPK, reducing lipid stores [72] In C. elegans: 40.0% increase in mean lifespan and regulating AMPK, LKB1, and SKN-1 [71] |
| 53123-88-9 | Rapamycin |
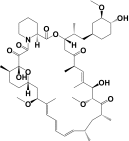
|
Used to coat coronary stents, prevent organ transplant rejection and to treat a rare lung disease called lymphangioleiomyomatosis | In mice: 14.0% increase in mean lifespan for females and 9% for males and reducing mTOR activity [76–82] In D. melanogaster: 13.0% increase in mean lifespan and regulating TORC1 branch of the TOR pathway, through alterations to both autophagy and translation [83] In C. elegans: 19.0% increase in mean lifespan and regulating TOR, SKN-1 and DAF-16 [84] |
| With anti-aging activities in two aging models | ||||
| 50-78-2 | Aspirin |
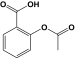
|
Used to treat pain, fever, and inflammation | In genetically heterogeneous male mice: 8.0% increase in mean lifespan and needs further research [135] In C. elegans: 30.0% increase in mean lifespan and antioxidant, regulating AMPK and insulin-like signaling pathway [183, 308] |
| 2086-83-1 | Berberine |
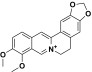
|
Used to treat bacillary dysentery and gastroenteritis | In aged mice: suppressing neuroinflammation, reducing vascular stiffness in aged mice through suppression of TRPV4 [309, 310] In D. melanogaster: 46.0% increase in mean lifespan and inhibiting kynurenine (KYN) formation from tryptophan (TRP) [311] |
| 102518-79-6 | Huperzine A |
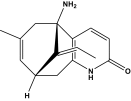
|
Treatment for neurological conditions such as Alzheimer’s disease | In d-galactose-induced mice: inhibiting DAMPs-mediated NF-κB nuclear localization and activation [312] In C. elegans: 14.0% increase in mean lifespan and needs further research [285] |
| 10118-90-8 | Minocycline |
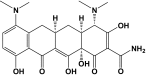
|
Antibiotic | In D. melanogaster: 63.0% increase in mean lifespan and antioxidant [313] In C. elegans: 29.0% increase in mean lifespan and antioxidant [176] |
| 114-86-3 | Phenformin |
|
Antidiabetic | In mice: 21.0% increase in mean lifespan and decreasing the body weight, slowing down the age-related decline of the reproductive function in female rats [314] In C. elegans: 29.0% increase in mean lifespan and needs further research [315] |
| 59-02-9 | Vitamin E |
|
Vitamins | In rats: reducing the oxidative stress increased in old age [316, 317] In C. elegans: 23.0% increase in mean lifespan and antioxidant [318] |
| With anti-aging activities in rats or mice | ||||
| 692-13-7 | Buformin |
|
Antidiabetic | In rats: 7.0% increase in mean lifespan in female and decreasing the body weight, slowing down the age-related decline of the reproductive function in female rats [314] |
| 73-31-4 | Melatonin |
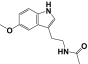
|
Regulating sleep and wakefulness | In male Wistar: restoring rSocs1 rhythms and levels in various tissues [319] |
| 155974-00-8 | Ivabradine |
|
Used for the symptomatic management of stable heart related chest pain and heart failure | In C57BL/6 J mice: 6.0% increase in mean lifespan and reducing heart rate [320] |
| 56180-94-0 | Acarbose |
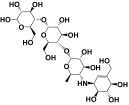
|
Antidiabetic | In SAMP8 mice and male mice: 22.0% increase in mean lifespan and changing in the insulin system and the levels of BDNF, IGF-1R, and the pre-synaptic proteins Syt1 and Stx1 [321, 322] |
| 51384-51-1 | Metoprolol |
|
Used to treat high blood pressure, chest pain due to poor blood flow to the heart, and a number of conditions involving an abnormally fast heart rate | In mice: 10.0% increase in mean lifespan and needs further research [323] |
| 99200-09-6 | Nebivolol |
|
Treatment of hypertension | In mice: 6.4% increase in mean lifespan and needs further research [323] |
| With anti-aging activities in Drosophila melanogaster | ||||
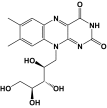
| 13123-37-0 | Riboflavin |
|
Vitamin | 14.1% increase in mean lifespan and increasing SOD1 and CAT, inhibiting LF [324] |
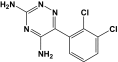
| 84057-84-1 | Lamotrigine |
|
Anticonvulsant | 17.0% increase in mean lifespan and reducing locomotor activity and metabolic rate [325] |
| 1716-12-7 | 4-Phenyl-butyrate |
|
Used to treat urea cycle disorder | 40.0% increase in mean lifespan and increasing histone acetylation [326] |
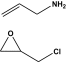
| 52757-95-6 | Sevelamer |
|
Used to treat hyperphosphatemia in patients with chronic kidney disease | 16.0% increase in mean lifespan and regulating cellular and organismic phosphate levels [327] |
| 79902-63-9 | Simvastatin |
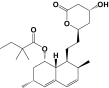
|
Hypolipidemic | 25.0% increase in mean lifespan and decreasing specific protein prenylation [328] |
| 871700-17-3 | Trametinib |
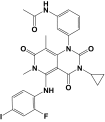
|
Anti-cancer | 12.0% increase in mean lifespan and inhibiting Ras-Erk-ETS signaling [329] |
| 58880-19-6 | Trichostatin A |
|
Antifungal antibiotic | 27.0% increase in mean lifespan and changing the level of histone acetylation, influencing the expression of hsp22 gene [330] |
| 57-27-2 | Morphine |
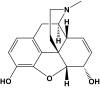
|
Treatment of acute pain and chronic pain | 22.0% increase in mean lifespan and needs further research [331] |
| With anti-aging activities in Caenorhabditis elegans | ||||
| 14028-44-5 | Amoxapine |
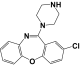
|
Antidepressant | 33.0% increase in mean lifespan and antioxidant [176] |
| 298-57-7 | Cinnarizine |
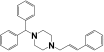
|
Treatment of vertigo, motion sickness, and vomiting | 15.0% increase in mean lifespan and antioxidant [176] |
| 59865-13-3 | Cyclosporin A |
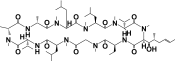
|
Immunosuppressants | 18.0% increase in mean lifespan and antioxidant [176] |
| 427-51-0 | Cyproterone acetate |
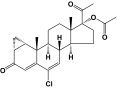
|
Antiandrogen and progestogen | 23.0% increase in mean lifespan and antioxidant [176] |
| 17230-88-5 | Danazol |
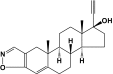
|
Treatment of endometriosis | 13.0% increase in mean lifespan and antioxidant [176] |
| 127-33-3 | Demeclocycline hydrochloride |
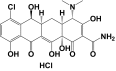
|
Antibiotic | 16.0% increase in mean lifespan and antioxidant [176] |
| 564-25-0 | Doxycycline |
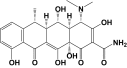
|
Antibiotic | 18.0% increase in mean lifespan and antioxidant [176] |
| 10592-13-9 | Doxycycline hydrochloride |
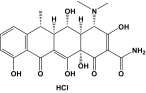
|
Antibiotic | 18.0% increase in mean lifespan and antioxidant [176] |
| 119431-25-3 | Eliprodil |
|
NMDA antagonist, treatment of acute ischemic stroke | 16.0% increase in mean lifespan and antioxidant [176] |
| 23256-50-0 | Guanabenz acetate |
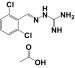
|
Antihypertensive | 12.0% increase in mean lifespan and antioxidant [176] |
| 29110-48-3 | Guanfacine hydrochloride |
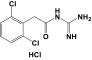
|
Treatment of hyperactivity | 15.0% increase in mean lifespan and antioxidant [176] |
| 27833-64-3 | Loxapine succinate |
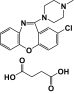
|
Antipsychotic | 43.0% increase in mean lifespan and antioxidant [176] |
| 57149-08-3 | Naftopidil di-hydrochloride |
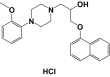
|
Antihypertensive | 14.0% increase in mean lifespan and antioxidant [176] |
| 54527-84-3 | Nicardipine hydrochloride |
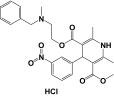
|
Used to treat high blood pressure and angina | 23.0% increase in mean lifespan and antioxidant [176] |
| 39562-70-4 | Nitrendipine |
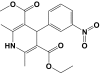
|
Used in the treatment of primary (essential) hypertension to decrease blood pressure and can reduce the cardiotoxicity of cocaine | 25.0% increase in mean lifespan and antioxidant [176] |
| 894-71-3 | Nortriptyline hydrochloride |
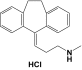
|
Tricyclic antidepressant | 21.0% increase in mean lifespan and antioxidant [176] |
| 60607-34-3 | Oxatomide |
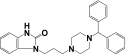
|
Anti-allergic | 25.0% increase in mean lifespan and antioxidant [176] |
| 130-61-0 | Thioridazine hydrochloride |
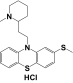
|
Antipsychotic | 31.0% increase in mean lifespan and antioxidant [176] |
| 2068-78-2 | Vincristine sulfate |
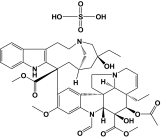
|
Anti-cancer | 12.0% increase in mean lifespan and antioxidant [176] |
| 97-59-6 | Allantoin |
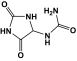
|
Used to treat gastric ulcer, duodenal bulb ulcer, chronic gastritis | 21.9% increase in mean lifespan and caloric restriction mimetics [332] |
| 169590-42-5 | Celecoxib |
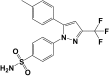
|
COX-2 selective nonsteroidal anti-inflammatory drug (NSAID) It is used to treat the pain and inflammation of osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, acute pain | 19.0% increase in mean lifespan and inhibiting insulin-like signaling [333] |
| 99-66-1 | Valproic acid |
|
Used to treat epilepsy and bipolar disorder and to prevent migraine headaches | 35.0% increase in mean lifespan and regulating IIS pathway [334] |
| 103-90-2 | Acetaminophen |
|
Used to treat pain and fever | 49.0% increase in mean lifespan and regulating CBP-1 [224] |
| 69-52-3 | Ampicillin |
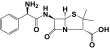
|
Antibiotic | 34.0% increase in mean lifespan and antimicrobial [284] |
| 41859-67-0 | Bezafibrate |
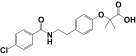
|
Treatment of hypertriglyceridemia | 13.0% increase in mean lifespan and regulating NHR-49/PPARalpha-dependent manner [335] |
| 637-07-0 | Clofibrate |
|
Lipid-lowering agent used for controlling the high cholesterol and triacylglyceride level in the blood | 16.0% increase in mean lifespan and regulating NHR-49/PPARalpha-dependent manner [335] |
| 49562-28-9 | Fenofibrate |
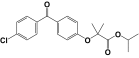
|
Used to reduce cholesterol levels in people at risk of cardiovascular disease | 19.0% increase in mean lifespan and regulating NHR-49/PPARalpha-dependent manner [335] |
| 127-48-0 | Trimethadione |
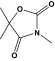
|
Anticonvulsant | 47.0% increase in mean lifespan and regulating neuromuscular activity [336] |
| 42971-09-5 | Vinpocetine |
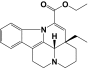
|
Treatment of cerebrovascular disorders and age-related memory impairment | 15.0% increase in mean lifespan and regulating PDE1 [176] |
| 59-30-3 | Folic acid |
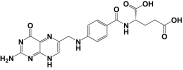
|
Used to treat anemia caused by folic acid deficiency | 27.0% increase in mean lifespan and antioxidant, regulating SIR-2.1, SKN-1, and DAF-16 [337] |
| 77-67-8 | Ethosuximide |
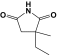
|
Used to treat absence seizures | 17.0% increase in mean lifespan and disrupting sensory function, regulating DAF-16 [336, 338, 339] |
| 50264-69-2 | Lonidamine |
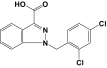
|
Anti-cancer | 8.0% increase in mean lifespan and promoting longevity in a pmk-1sensitive manner by increasing formation of ROS [340] |
| 50-55-5 | Reserpine |
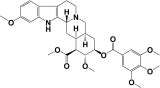
|
Antipsychotic, and antihypertensive | 31.0% increase in mean lifespan and antioxidant, modulating acetylcholine release [341, 342] |
| 13292-46-1 | Rifampicin |
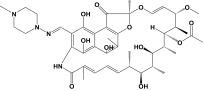
|
Antibiotic | 56.0% increase in mean lifespan and reducing advanced glycation end products and activating DAF-16 [343] |
| 6998-60-3 | Rifamycin SV |
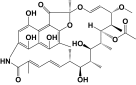
|
Antibiotic | 21.0% increase in mean lifespan and reducing advanced glycation end products and activating DAF-16 [343] |
| 723-46-6 | Sulfa-methoxazole |
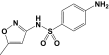
|
Antibiotic | 34.0% increase in mean lifespan and increasing lipid peroxidation oxidative stress [344] |
| 56-75-7 | Chloram-phenicol |
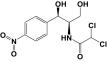
|
Antibiotic | 16.0% increase in mean lifespan and needs further research [345] |
| With anti-aging activities in other aging models | ||||
| 53-06-5 | Cortisone |
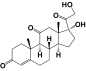
|
Used to reduce inflammation and attendant pain and swelling at the site of the injury | In Asplanchna brightwelli: 21.0% increase in mean lifespan and stabilizing lysosomal membranes, or altering resource allocation by the rotifers [346] |
| 35891-70-4 | Myriocin |
|
Antibiotic ISP-1 and thermozymocidin | In S. cerevisiae: activating the Snf1/AMPK pathway, down-regulating the protein kinase A (PKA) and target of rapamycin complex 1 (TORC1) pathways [347] |
Table 4.
Synthetic compounds with anti-aging activities
| CAS | Chemicals | Structure | Anti-aging activity and proposed anti-aging mechanism |
|---|---|---|---|
| With anti-aging activities in two aging models | |||
| 51-28-5 | 2,4-Dinitrophenol |
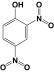
|
In mice: 7.0% increase in mean lifespan; enhancing tissue respiratory rates, improving serological glucose, triglyceride and insulin levels, decreasing reactive oxygen species levels and tissue DNA and protein oxidation, as well as reduced body weight [348] In D. melanogaster: 20.0% increase in mean lifespan; increasing the rate of oxygen consumption by isolated mitochondria and tissue homogenates, decreasing the activity of alcohol dehydrogenase [349] |
| With anti-aging activities in mice | |||
| 91-53-2 | Ethoxyquin |
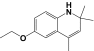
|
In C3H mice: 18.0% increase in mean lifespan in male, 20.0% in female and antioxidant [350] |
| 1001645-58-4 | SRT1720 |
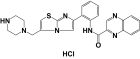
|
In mice: 9.0% increase in mean lifespan and inhibiting proinflammatory gene expression [351] |
| With anti-aging activities in Drosophila melanogaster | |||
| 307297-39-8 | Epitalon |
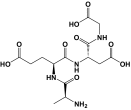
|
17.0% increase in mean lifespan and antioxidatant [352] |
| 34592-47-7 | Thiazolidine carboxylic acid |
|
31.0% increase in mean lifespan and antioxidatant [353] |
| 133550-30-8 | AG-490 |
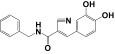
|
18.0% increase in mean lifespan and activating ERK1/2 signaling [354] |
| 4431-00-9 | Aurintri-carboxylic acid |
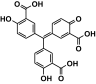
|
15.0% increase in mean lifespan and regulating p66ShcA [355] |
| 91742-10-8 | HA-1004 (dihydrochloride) |
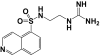
|
18.0% increase in mean lifespan and inhibiting protein kinase [354] |
| 103745-39-7 | HA-1077 (Fasudil) |
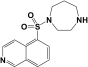
|
15.0% increase in mean lifespan and inhibiting protein kinase [354] |
| 5108-96-3 | Pyrrolidine dithiocarbamate |
|
16.0% increase in mean lifespan and inhibiting NF-κB [356] |
| With anti-aging activities in Caenorhabditis elegans | |||
| 75529-73-6 | Amperozide hydrochloride |
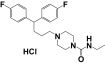
|
38.0% increase in mean lifespan and antioxidatant [176] |
| 193611-72-2 | BRL 15572 |
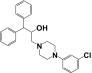
|
10.0% increase in mean lifespan and antioxidatant [176] |
| 433695-36-4 | BRL 50481 |
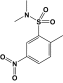
|
18.0% increase in mean lifespan and antioxidatant [176] |
| 145915-58-8 | DAPH (4,5-dianilino-phthalimide) |
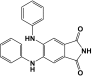
|
15.0% increase in mean lifespan and antioxidatant [176] |
| 53177-12-1 | EUK-8 |
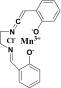
|
54.0% increase in mean lifespan and antioxidant [357] |
| 81065-76-1 | EUK-134 |
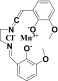
|
54.0% increase in mean lifespan and antioxidant [357] |
| 98299-40-2 | Hexahydro-sila-diphenidol |
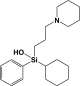
|
15.0% increase in mean lifespan and antioxidatant [176] |
| 142273-20-9 | Kenpaullone |
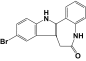
|
27.0% increase in mean lifespan and antioxidatant [176] |
| 83846-83-7 | Ketanserin tartrate |
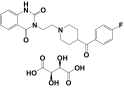
|
13.0% increase in mean lifespan and antioxidatant [176] |
| 13614-98-7 | Minocycline hydrochloride |
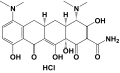
|
43.0% increase in mean lifespan and antioxidatant [176] |
| 66104-23-2 | Pergolide methanesulfonate |
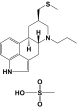
|
37.0% increase in mean lifespan and antioxidatant [176] |
| 497-27-8 | 4-Phenyl-3-Furoxan-carbonitrile |
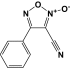
|
30.0% increase in mean lifespan and antioxidatant [176] |
| 58-33-3 | Promethazine hydrochloride |
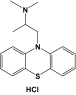
|
32.0% increase in mean lifespan and antioxidatant [176] |
| 7681-67-6 | Propionyl-promazine hydrochloride |
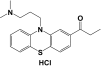
|
20.0% increase in mean lifespan and antioxidatant [176] |
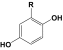
| Trans-3,5-dimethoxy-4-fluoro-4-hydroxystilbene |
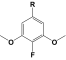
|
3.6% increase in mean lifespan and antioxidatant [358] | |
| Trans-2,4,5-trihydroxystilbene |
|
5.4% increase in mean lifespan and antioxidatant [358] | |
| 78416-81-6 | Trequinsin hydrochloride |
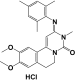
|
27.0% increase in mean lifespan and antioxidatant [176] |
| 274-85-1 | 1,2,4-Triazolo[1,5-a]pyridine |
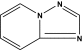
|
12.0% increase in mean lifespan and antioxidant [359] |
| 138090-06-9 | (R,R)-cis-Diethyl- tetrahydro-2,8-chrysenediol |
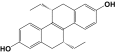
|
7.0% increase in mean lifespan and increasing stress resistance [176] |
| 2390-54-7 | Thioflavin T |
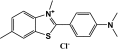
|
60.0% increase in mean lifespan and regulating HSF-1 and SKN-1 [360] |
| 175698-05-2 | 3,3-Diethyl-2-pyrrolidinone |
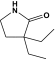
|
31.0% increase in mean lifespan and regulating neuromuscular activity [336] |
| 631-64-1 | Dibromoacetic acid |
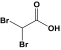
|
15.0% increase in mean lifespan and inducing protective stress response [225] |
| 82-76-8 | N-Phenyl periacid(ANSA) |
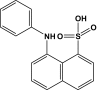
|
22.7% increase in mean lifespan and increasing aging related pharyngeal pumping rate [63] |
| 51314-51-3 | Benzimidazole derivative M084 |
|
19.10% increase in mean lifespan; regulating IIS pathway, AMPK, SIR-2.1, SKN-1, mitochondrial electron transport chain, and mitochondrial unfolded protein response [361–364] |
| With anti-aging activities in Asplanchna brightwelli | |||
| 111-17-1 | 3,3′-Thiodipropionic acid |
|
16.0% increase in mean lifespan and increasing lipid peroxides [365] |
Among the 55 complex or extracts from natural products, 8, 14 and 29 of them were tested in mice, fruit fly and C. elegans, respectively. A majority of these extracts present antioxidative activity.
Among the 62 clinical medicine with anti-aging activity, three (rapamycin, metformin, caffeine) present anti-aging activities in three aging models, six (aspirin, berberine, huperzine A, minocycline, phenformin, and vitamin E) in two aging models, two (buformin and melatonin) in rats, four (ivabradine, acarbose, metoprolol, and nebivolol) in mice, 8 in D. melanogaster, 37 in C. elegans, cortisone in Asplanchna brightwelli and myriocin in S. cerevisiae, respectively. Interestingly, the anti-aging mechanisms of the most drugs are different from their clinical applications.
We also summarized 35 synthetic compounds with explicit anti-aging activity (Table 4). 2,4-Dinitrophenol presents anti-aging activities in mice and fruit fly, ethoxyquin and SRT1720 in mice. Seven and 24 compounds present anti-aging activity in fruit fly and C. elegans, respectively. 3,3′-thiodipropionic acid with anti-aging activity in Asplanchna brightwelli. Twenty-one of the 35 compounds present antioxidative activity.
In total, there are 212 and 46 compounds present anti-aging activity in C. elegans and fruit fly, respectively, indicating C. elegans and fruit fly are the most popular aging models for anti-aging screening. Those compounds present anti-aging activity in both C. elegans and fruit fly are worth to be further investigated in mammalian models.
Prospects of Discovering Anti-aging Molecules from Natural Products
Many clinical medicines are derived from natural products. But in the past two decades, pharmaceutical companies have been enthusing the drug development strategy of high-throughput screening (HTS) and combinatorial synthesis of enormous synthetic libraries of small molecules. Natural products were largely neglected for unsuitable for HTS of targeted protein assay and difficult in compound isolation and synthesis. But the achievement of new lead discovery and new drug approval was disappointing [85]. Compared with synthetic compounds, natural products are secondary metabolite, evolutionarily optimized with biologically relevant chemical space and preferred ligand binding motif, are not only biologically active, but with a high degree of bioavailability, suitable for functional and phenotypic assays [86]. Recent innovation in techniques for structural elucidation, metabolomics for profiling and isolation, and metagenomics or gene manipulation for synthetic pathways has facilitated to explore the enormous biodiversity on earth, including plant, microorganism and marine organism [87]. Engineered production of natural products from uncultivated species could extremely expand the chemical space of natural products by synthetic biology [88]. Moreover, modern computer-assisted drug design could utilize natural-product-derived fragments to computationally infer the biomolecular targets and activities of natural products and fragment-based de novo design. As summarized in above, currently discovered agents with anti-aging activity, majority of them are natural products. Therefore, natural products are invaluable sources and provide great promise for developing anti-aging medicine.
Acknowledgements
This work was supported by the Natural Science Foundation of China (81671405 and 81370453), Natural Science Foundation of Yunnan province (2013FA045 and 2015FB172), and Open Funds of Guangdong Key Laboratory of Marine Materia Medica.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Fontana L, Partridge L, Longo VD. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.M. Kaeberlein. F1000Prime Rep. 5, 5 (2013) [DOI] [PMC free article] [PubMed]
- 3.Riera CE, Merkwirth C, De Magalhaes Filho CD, Dillin A. Annu. Rev. Biochem. 2016;85:35–64. doi: 10.1146/annurev-biochem-060815-014451. [DOI] [PubMed] [Google Scholar]
- 4.Campisi J. Annu. Rev. Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Otin C, Galluzzi L, Freije JM, Madeo F, Kroemer G. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Sen P, Shah PP, Nativio R, Berger SL. Cell. 2016;166:822–839. doi: 10.1016/j.cell.2016.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenyon CJ. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 9.Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, Fraifeld VE. Ageing Res. Rev. 2013;12:661–684. doi: 10.1016/j.arr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Lindahl T, Barnes DE. Cold Spring Harb. Symp. Quant. Biol. 2000;65:127–133. doi: 10.1101/sqb.2000.65.127. [DOI] [PubMed] [Google Scholar]
- 11.Tebbs RS, Flannery ML, Meneses JJ, Hartmann A, Tucker JD, Thompson LH, Cleaver JE, Pedersen RA. Dev. Biol. 1999;208:513–529. doi: 10.1006/dbio.1999.9232. [DOI] [PubMed] [Google Scholar]
- 12.de Vries A, van Oostrom CT, Hofhuis FM, Dortant PM, Berg RJ, de Gruijl FR, Wester PW, van Kreijl CF, Capel PJ, van Steeg H, Verbeek SJ. Nature. 1995;377:169–173. doi: 10.1038/377169a0. [DOI] [PubMed] [Google Scholar]
- 13.de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, Weeda G, van der Horst GT, van Leeuwen W, Themmen AP, Meradji M, Hoeijmakers JH. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- 14.Murai M, Enokido Y, Inamura N, Yoshino M, Nakatsu Y, van der Horst GT, Hoeijmakers JH, Tanaka K, Hatanaka H. Proc. Natl. Acad. Sci. USA. 2001;98:13379–13384. doi: 10.1073/pnas.231329598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel H, Lim DS, Karsenty G, Finegold M, Hasty P. Proc. Natl. Acad. Sci. USA. 1999;96:10770–10775. doi: 10.1073/pnas.96.19.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackburn EH, Epel ES. J. Lin. Sci. 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 17.X.L. Guan, P.F. Wu, S. Wang, J.J. Zhang, Z.C. Shen, H. Luo, H. Chen, L.H. Long, J.G. Chen, F. Wang. Aging Cell (2016)
- 18.Oberdoerffer P, Sinclair DA. Nat. Rev. Mol. Cell Biol. 2007;8:692–702. doi: 10.1038/nrm2238. [DOI] [PubMed] [Google Scholar]
- 19.Pal S, Tyler JK. Sci. Adv. 2016;2:e1600584. doi: 10.1126/sciadv.1600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker DJ, Dawlaty MM, Wijshake T, Jeganathan KB, Malureanu L, van Ree JH, Crespo-Diaz R, Reyes S, Seaburg L, Shapiro V, Behfar A, Terzic A, van de Sluis B, van Deursen JM. Nat. Cell Biol. 2013;15:96–102. doi: 10.1038/ncb2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ron D, Walter P. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 22.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Annu. Rev. Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 23.Marques C, Guo W, Pereira P, Taylor A, Patterson C, Evans PC, Shang F. Faseb J. 2006;20:741–743. doi: 10.1096/fj.05-5080fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. Cell. 2012;148:322–334. doi: 10.1016/j.cell.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swindell WR, Masternak MM, Kopchick JJ, Conover CA, Bartke A, Miller RA. Mech. Ageing Dev. 2009;130:393–400. doi: 10.1016/j.mad.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo DK, Shadel GS. Cell. 2011;144:11–12. doi: 10.1016/j.cell.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Saul N, Pietsch K, Sturzenbaum SR, Menzel R, Steinberg CE. J. Nat. Prod. 2011;74:1713–1720. doi: 10.1021/np200011a. [DOI] [PubMed] [Google Scholar]
- 28.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 29.Yazaki K, Yoshikoshi C, Oshiro S, Yanase S. Oxid. Med. Cell Longev. 2011;2011:596240. doi: 10.1155/2011/596240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. Proc. Natl. Acad. Sci. USA. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang MC, Bohmann D, Jasper H. Dev. Cell. 2003;5:811–816. doi: 10.1016/S1534-5807(03)00323-X. [DOI] [PubMed] [Google Scholar]
- 32.Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 33.Rogina B, Reenan RA, Nilsen SP, Helfand SL. Science. 2000;290:2137–2140. doi: 10.1126/science.290.5499.2137. [DOI] [PubMed] [Google Scholar]
- 34.A. Shaik, A. Schiavi, N. Ventura. Cell Cycle (2016) [DOI] [PMC free article] [PubMed]
- 35.Kenyon C. Philos. Trans. R. Soc. Lond. B. 2011;366:9–16. doi: 10.1098/rstb.2010.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCormick MA, Tsai SY, Kennedy BK. Philos. Trans. R. Soc. Lond. B. 2011;366:17–27. doi: 10.1098/rstb.2010.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salminen A, Kaarniranta K, Kauppinen A. Ageing Res. Rev. 2016;28:15–26. doi: 10.1016/j.arr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Kida Y, Goligorsky MS. Can. J. Cardiol. 2016;32:634–641. doi: 10.1016/j.cjca.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang K, Klionsky DJ. Autophagy. 2011;7:297–300. doi: 10.4161/auto.7.3.14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodier F, Campisi J. J. Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Mech. Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C, Wheeler CT, Alberico T, Sun X, Seeberger J, Laslo M, Spangler E, Kern B, de Cabo R, Zou S. Age (Dordr.) 2013;35:69–81. doi: 10.1007/s11357-011-9332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tung BT, Rodriguez-Bies E, Thanh HN, Le-Thi-Thu H, Navas P, Sanchez VM, Lopez-Lluch G. Aging Clin. Exp. Res. 2015;27:775–783. doi: 10.1007/s40520-015-0366-8. [DOI] [PubMed] [Google Scholar]
- 48.Ringholm S, Olesen J, Pedersen JT, Brandt CT, Halling JF, Hellsten Y, Prats C, Pilegaard H. Exp. Gerontol. 2013;48:1311–1318. doi: 10.1016/j.exger.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, Liu L. Hum. Reprod. 2013;28:707–717. doi: 10.1093/humrep/des437. [DOI] [PubMed] [Google Scholar]
- 51.Cui Y, Zhang B, Zhang R, Li C, Zhao Y, Ren Y, Yang J. Wei Sheng Yan Jiu. 2013;42(995–998):1003. [PubMed] [Google Scholar]
- 52.Collins JJ, Evason K, Kornfeld K. Exp. Gerontol. 2006;41:1032–1039. doi: 10.1016/j.exger.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 53.Regitz C, Fitzenberger E, Mahn FL, Dussling LM, Wenzel U. Eur. J. Nutr. 2016;55:741–747. doi: 10.1007/s00394-015-0894-1. [DOI] [PubMed] [Google Scholar]
- 54.Bauer JH, Goupil S, Garber GB, Helfand SL. Proc. Natl. Acad. Sci. USA. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown MK, Evans JL, Luo Y. Pharmacol. Biochem. Behav. 2006;85:620–628. doi: 10.1016/j.pbb.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Farr SA, Price TO, Banks WA, Ercal N, Morley JE. J Alzheimers Dis. 2012;32:447–455. doi: 10.3233/JAD-2012-120130. [DOI] [PubMed] [Google Scholar]
- 57.Huangfu J, Liu J, Sun Z, Wang M, Jiang Y, Chen ZY, Chen F. J. Agric. Food Chem. 2013;61:7800–7804. doi: 10.1021/jf402224w. [DOI] [PubMed] [Google Scholar]
- 58.Kuraji M, Matsuno T, Satoh T. J. Clin. Biochem. Nutr. 2016;59:79–85. doi: 10.3164/jcbn.15-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu W, Wang X, Xiang Q, Meng X, Peng Y, Du N, Liu Z, Sun Q, Wang C, Liu X. Food Funct. 2014;5:158–166. doi: 10.1039/C3FO60400D. [DOI] [PubMed] [Google Scholar]
- 60.Saul N, Pietsch K, Menzel R, Sturzenbaum SR, Steinberg CE. Mech. Ageing Dev. 2009;130:477–486. doi: 10.1016/j.mad.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Si H, Fu Z, Babu PV, Zhen W, Leroith T, Meaney MP, Voelker KA, Jia Z, Grange RW, Liu D. J. Nutr. 2011;141:1095–1100. doi: 10.3945/jn.110.134270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unno K, Takabayashi F, Yoshida H, Choba D, Fukutomi R, Kikunaga N, Kishido T, Oku N, Hoshino M. Biogerontology. 2007;8:89–95. doi: 10.1007/s10522-006-9036-8. [DOI] [PubMed] [Google Scholar]
- 63.K. Cuanalo-Contreras, K.W. Park, A. Mukherjee, L. Millan-Perez Pena, C. Soto. Biochem. Biophys. Res. Commun. (2016) [DOI] [PubMed]
- 64.Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, Seals DR. Exp. Gerontol. 2013;48:269–276. doi: 10.1016/j.exger.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen LR, Xiao F, Yuan P, Chen Y, Gao QK, Parnell LD, Meydani M, Ordovas JM, Li D, Lai CQ. Age (Dordr.) 2013;35:1133–1142. doi: 10.1007/s11357-012-9438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lashmanova E, Proshkina E, Zhikrivetskaya S, Shevchenko O, Marusich E, Leonov S, Melerzanov A, Zhavoronkov A, Moskalev A. Pharmacol. Res. 2015;100:228–241. doi: 10.1016/j.phrs.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Urikura I, Sugawara T, Hirata T. Biosci. Biotechnol. Biochem. 2011;75:757–760. doi: 10.1271/bbb.110040. [DOI] [PubMed] [Google Scholar]
- 68.Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Nat. Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 69.Beach A, Titorenko VI. Subcell. Biochem. 2013;69:153–167. doi: 10.1007/978-94-007-6889-5_9. [DOI] [PubMed] [Google Scholar]
- 70.Garg G, Singh S, Singh AK, Rizvi SI. Rejuvenation Res. 2017;20:15–24. doi: 10.1089/rej.2016.1826. [DOI] [PubMed] [Google Scholar]
- 71.Onken B, Driscoll M. PLoS ONE. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slack C, Foley A, Partridge L. PLoS ONE. 2012;7:e47699. doi: 10.1371/journal.pone.0047699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sutphin GL, Bishop E, Yanos ME, Moller RM, Kaeberlein M. Longev. Healthspan. 2012;1:9. doi: 10.1186/2046-2395-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cruz FF, Leite CE, Kist LW, de Oliveira GM, Bogo MR, Bonan CD, Campos MM, Morrone FB. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017;194:28–36. doi: 10.1016/j.cbpc.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Ullah F, Ali T, Ullah N, Kim MO. Neurochem. Int. 2015;90:114–124. doi: 10.1016/j.neuint.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Rendon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fok WC, Chen Y, Bokov A, Zhang Y, Salmon AB, Diaz V, Javors M, Wood WH, 3rd, Zhang Y, Becker KG, Perez VI, Richardson A. PLoS ONE. 2014;9:e83988. doi: 10.1371/journal.pone.0083988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kolosova NG, Vitovtov AO, Muraleva NA, Akulov AE, Stefanova NA, Blagosklonny MV. Aging (Albany, NY) 2013;5:474–484. doi: 10.18632/aging.100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK. Cell Metab. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li JW, Vederas JC. Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 86.Newman DJ, Cragg GM. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bouslimani A, Sanchez LM, Garg N, Dorrestein PC. Nat. Prod. Rep. 2014;31:718–729. doi: 10.1039/c4np00044g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D, Polichuk DR, Teoh KH, Reed DW, Treynor T, Lenihan J, Fleck M, Bajad S, Dang G, Dengrove D, Diola D, Dorin G, Ellens KW, Fickes S, Galazzo J, Gaucher SP, Geistlinger T, Henry R, Hepp M, Horning T, Iqbal T, Jiang H, Kizer L, Lieu B, Melis D, Moss N, Regentin R, Secrest S, Tsuruta H, Vazquez R, Westblade LF, Xu L, Yu M, Zhang Y, Zhao L, Lievense J, Covello PS, Keasling JD, Reiling KK, Renninger NS, Newman JD. Nature. 2013;496:528–532. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- 89.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu C, Zhang R, Sun C, Zhang H, Xu C, Liu W, Gao W, Huang S, Chen L. J. Neurochem. 2015;135:466–478. doi: 10.1111/jnc.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen S, Zhou N, Zhang Z, Li W, Zhu W. Biochem. Biophys. Res. Commun. 2015;457:608–613. doi: 10.1016/j.bbrc.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 92.Quoc Trung L, Espinoza JL, Takami A. S. Nakao. PLoS ONE. 2013;8:e55183. doi: 10.1371/journal.pone.0055183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Madrigal-Perez LA, Nava GM, Gonzalez-Hernandez JC, Ramos-Gomez M. J. Bioenerg. Biomembr. 2015;47:331–336. doi: 10.1007/s10863-015-9615-y. [DOI] [PubMed] [Google Scholar]
- 94.Escote X, Miranda M, Menoyo S, Rodriguez-Porrata B, Carmona-Gutierrez D, Jungwirth H, Madeo F, Cordero RR, Mas A, Tinahones F, Clotet J, Vendrell J. Yeast. 2012;29:251–263. doi: 10.1002/yea.2903. [DOI] [PubMed] [Google Scholar]
- 95.Liu T, Qi H, Ma L, Liu Z, Fu H, Zhu W, Song T, Yang B, Li G. Rejuvenation Res. 2015;18:225–233. doi: 10.1089/rej.2014.1618. [DOI] [PubMed] [Google Scholar]
- 96.Li YM, Chan HY, Huang Y, Chen ZY. Mol. Nutr. Food Res. 2007;51:546–554. doi: 10.1002/mnfr.200600238. [DOI] [PubMed] [Google Scholar]
- 97.Wang X, Perumalsamy H, Kwon HW, Na YE, Ahn YJ. Sci. Rep. 2015;5:16127. doi: 10.1038/srep16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Asthana J, Mishra BN, Pandey R. Free Radic. Res. 2016;50:861–874. doi: 10.1080/10715762.2016.1187268. [DOI] [PubMed] [Google Scholar]
- 99.Chuang MH, Chiou SH, Huang CH, Yang WB, Wong CH. Bioorg. Med. Chem. 2009;17:7831–7840. doi: 10.1016/j.bmc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 100.Burhans WC, Weinberger M. Cell Cycle. 2009;8:2300–2302. doi: 10.4161/cc.8.14.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.K.J. Senthil Kumar, M. Gokila Vani, J.L. Mau, C.C. Lin, F.H. Chu, C.C. Wei, V.H. Liao, S.Y. Wang. Oncotarget (2016) [DOI] [PMC free article] [PubMed]
- 102.Rushaidhi M, Zhang H, Liu P. Neuroscience. 2013;234:116–124. doi: 10.1016/j.neuroscience.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 103.Edwards C, Canfield J, Copes N, Brito A, Rehan M, Lipps D, Brunquell J, Westerheide SD, Bradshaw PC. BMC Genet. 2015;16:8. doi: 10.1186/s12863-015-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Niemiec T, Sikorska J, Harrison A, Szmidt M, Sawosz E, Wirth-Dzieciolowska E, Wilczak J, Pierzynowski S. J. Physiol. Pharmacol. 2011;62:37–43. [PubMed] [Google Scholar]
- 105.Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, Hu E, Whelan SA, Wang JX, Jung G, Solis GM, Fazlollahi F, Kaweeteerawat C, Quach A, Nili M, Krall AS, Godwin HA, Chang HR, Faull KF, Guo F, Jiang M, Trauger SA, Saghatelian A, Braas D, Christofk HR, Clarke CF, Teitell MA, Petrascheck M, Reue K, Jung ME, Frand AR, Huang J. Nature. 2014;510:397–401. doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Havermann S, Humpf HU, Watjen W. Fitoterapia. 2016;113:123–127. doi: 10.1016/j.fitote.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 107.Zhang S, Ye J, Dong G. J. Mol. Neurosci. 2010;40:311–320. doi: 10.1007/s12031-009-9285-5. [DOI] [PubMed] [Google Scholar]
- 108.Lee EK, Jang EJ, Jung KJ, Kim DH, Yu BP, Chung HY. Exp. Gerontol. 2013;48:517–524. doi: 10.1016/j.exger.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 109.Coban J, Bingul I, Yesil-Mizrak K, Dogru-Abbasoglu S, Oztezcan S, Uysal M. Curr. Aging Sci. 2013;6:199–205. doi: 10.2174/18746098112059990011. [DOI] [PubMed] [Google Scholar]
- 110.Esrefoglu M, Iraz M, Ates B, Gul M. Ultrastruct. Pathol. 2012;36:244–251. doi: 10.3109/01913123.2012.679351. [DOI] [PubMed] [Google Scholar]
- 111.Deshmukh R, Kaundal M, Bansal V. Samardeep. Biomed. Pharmacother. 2016;81:56–62. doi: 10.1016/j.biopha.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 112.Pietsch K, Saul N, Chakrabarti S, Sturzenbaum SR, Menzel R, Steinberg CE. Biogerontology. 2011;12:329–347. doi: 10.1007/s10522-011-9334-7. [DOI] [PubMed] [Google Scholar]
- 113.Aydin AF, Coban J, Dogan-Ekici I, Betul-Kalaz E, Dogru-Abbasoglu S, Uysal M. Metab. Brain Dis. 2016;31:337–345. doi: 10.1007/s11011-015-9755-0. [DOI] [PubMed] [Google Scholar]
- 114.Aydin AF, Coban J, Dogan-Ekici I, Dogru-Abbasoglu S, Uysal M, Kocak-Toker N. Andrologia. 2015;47:1131–1138. doi: 10.1111/and.12392. [DOI] [PubMed] [Google Scholar]
- 115.Yuneva AO, Kramarenko GG, Vetreshchak TV, Gallant S, Boldyrev AA. Bull. Exp. Biol. Med. 2002;133:559–561. doi: 10.1023/A:1020273506970. [DOI] [PubMed] [Google Scholar]
- 116.Stvolinsky S, Antipin M, Meguro K, Sato T, Abe H, Boldyrev A. Rejuvenation Res. 2010;13:453–457. doi: 10.1089/rej.2009.1010. [DOI] [PubMed] [Google Scholar]
- 117.Zhang X, Jin C, Li Y, Guan S, Han F, Zhang S. Food Chem. Toxicol. 2013;58:50–55. doi: 10.1016/j.fct.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 118.Wei M, Lu Y, Liu D, Ru W. Biol. Pharm. Bull. 2014;37:1444–1449. doi: 10.1248/bpb.b14-00064. [DOI] [PubMed] [Google Scholar]
- 119.Seo HW, Cheon SM, Lee MH, Kim HJ, Jeon H, Cha DS. Evid. Based Complement Altern. Med. 2015;2015:524878. doi: 10.1155/2015/524878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feng Y, Yu YH, Wang ST, Ren J, Camer D, Hua YZ, Zhang Q, Huang J, Xue DL, Zhang XF, Huang XF, Liu Y. Pharm. Biol. 2016;54:1027–1034. doi: 10.3109/13880209.2015.1093510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.S.Q. Zheng, X.B. Huang, T.K. Xing, A.J. Ding, G.S. Wu, H.R. Luo. J. Gerontol. A (2016)
- 122.Shetty RA, Ikonne US, Forster MJ, Sumien N. Exp. Gerontol. 2014;58:208–218. doi: 10.1016/j.exger.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ishii N, Senoo-Matsuda N, Miyake K, Yasuda K, Ishii T, Hartman PS, Furukawa S. Mech. Ageing Dev. 2004;125:41–46. doi: 10.1016/j.mad.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 124.Weimer S, Priebs J, Kuhlow D, Groth M, Priebe S, Mansfeld J, Merry TL, Dubuis S, Laube B, Pfeiffer AF, Schulz TJ, Guthke R, Platzer M, Zamboni N, Zarse K, Ristow M. Nat. Commun. 2014;5:3563. doi: 10.1038/ncomms4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen J, Li Y, Zhu Q, Li T, Lu H, Wei N, Huang Y, Shi R, Ma X, Wang X, Sheng J. Mech. Ageing Dev. 2017;164:1–7. doi: 10.1016/j.mad.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 126.Abbas S, Wink M. Planta Med. 2009;75:216–221. doi: 10.1055/s-0028-1088378. [DOI] [PubMed] [Google Scholar]
- 127.Li L, Ng TB, Gao W, Li W, Fu M, Niu SM, Zhao L, Chen RR, Liu F. Life Sci. 2005;77:230–240. doi: 10.1016/j.lfs.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 128.Massie HR, Williams TR. Exp. Gerontol. 1979;14:109–115. doi: 10.1016/0531-5565(79)90025-1. [DOI] [PubMed] [Google Scholar]
- 129.Snell TW, Johnston RK. Exp. Gerontol. 2014;57:47–56. doi: 10.1016/j.exger.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rotimi SO, Bankole GE, Adelani IB, Rotimi OA. Immunopharmacol. Immunotoxicol. 2016;38:364–371. doi: 10.1080/08923973.2016.1214142. [DOI] [PubMed] [Google Scholar]
- 131.Sun K, Xiang L, Ishihara S, Matsuura A, Sakagami Y, Qi J. Biosci. Biotechnol. Biochem. 2012;76:640–645. doi: 10.1271/bbb.110535. [DOI] [PubMed] [Google Scholar]
- 132.Zhang SQ, Cai WJ, Huang JH, Wu B, Xia SJ, Chen XL, Zhang XM, Shen ZY. Exp. Gerontol. 2015;69:226–235. doi: 10.1016/j.exger.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 133.Cai WJ, Huang JH, Zhang SQ, Wu B, Kapahi P, Zhang XM, Shen ZY. PLoS ONE. 2011;6:e28835. doi: 10.1371/journal.pone.0028835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.H. Ma, Y. Ma, Z. Zhang, Z. Zhao, R. Lin, J. Zhu, Y. Guo, L. Xu. Int. J. Environ. Res. Public Health 13 (2016) [DOI] [PMC free article] [PubMed]
- 135.Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Harrison DE. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Buu-Hoi NP, Ratsimamanga AR. C. R. Seances Soc. Biol. Fil. 1959;153:1180–1182. [PubMed] [Google Scholar]
- 137.Richie JP, Jr, Mills BJ, Lang CA. Proc. Soc. Exp. Biol. Med. 1986;183:81–85. doi: 10.3181/00379727-183-42389. [DOI] [PubMed] [Google Scholar]
- 138.Spindler SR, Mote PL, Lublin AL, Flegal JM, Dhahbi JM, Li R. J. Gerontol. A. 2015;70:1479–1489. doi: 10.1093/gerona/glu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tsai SJ, Yin MC. Eur. J. Pharmacol. 2012;689:81–88. doi: 10.1016/j.ejphar.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 140.Zhang J, Lu L, Zhou L. Biochem. Biophys. Res. Commun. 2015;468:843–849. doi: 10.1016/j.bbrc.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 141.Park SK, Seong RK, Kim JA, Son SJ, Kim Y, Yokozawa T, Shin OS. Nutr. Res. Pract. 2016;10:3–10. doi: 10.4162/nrp.2016.10.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi YY, Maeda T, Fujii H, Yokozawa T, Kim HY, Cho EJ, Shibamoto T. Nutr Res. 2014;34:595–603. doi: 10.1016/j.nutres.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 143.Xu LQ, Xie YL, Gui SH, Zhang X, Mo ZZ, Sun CY, Li CL, Luo DD, Zhang ZB, Su ZR, Xie JH. Food Funct. 2016;7:4545–4555. doi: 10.1039/C6FO01057A. [DOI] [PubMed] [Google Scholar]
- 144.Wen H, Gao X, Qin J. Integr. Biol. (Camp) 2014;6:35–43. doi: 10.1039/C3IB40191J. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Y, Chen X, Yang L, Zu Y, Lu Q. Food Funct. 2015;6:927–931. doi: 10.1039/C4FO01051E. [DOI] [PubMed] [Google Scholar]
- 146.Zuo Y, Peng C, Liang Y, Ma KY, Chan HY, Huang Y, Chen ZY. Biogerontology. 2013;14:107–119. doi: 10.1007/s10522-012-9413-4. [DOI] [PubMed] [Google Scholar]
- 147.Yaguchi Y, Komura T, Kashima N, Tamura M, Kage-Nakadai E, Saeki S, Terao K, Nishikawa Y. Eur. J. Nutr. 2014;53:1659–1668. doi: 10.1007/s00394-014-0671-6. [DOI] [PubMed] [Google Scholar]
- 148.Kitani K, Yokozawa T, Osawa T. Ann. N. Y. Acad. Sci. 2004;1019:424–426. doi: 10.1196/annals.1297.075. [DOI] [PubMed] [Google Scholar]
- 149.Sangartit W, Pakdeechote P, Kukongviriyapan V, Donpunha W, Shibahara S, Kukongviriyapan U. Vascul. Pharmacol. 2016;87:199–208. doi: 10.1016/j.vph.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 150.Okada K, Wangpoengtrakul C, Tanaka T, Toyokuni S, Uchida K, Osawa T. J. Nutr. 2001;131:2090–2095. doi: 10.1093/jn/131.8.2090. [DOI] [PubMed] [Google Scholar]
- 151.Kitani K, Osawa T, Yokozawa T. Biogerontology. 2007;8:567–573. doi: 10.1007/s10522-007-9100-z. [DOI] [PubMed] [Google Scholar]
- 152.Xiang L, Nakamura Y, Lim YM, Yamasaki Y, Kurokawa-Nose Y, Maruyama W, Osawa T, Matsuura A, Motoyama N, Tsuda L. Aging (Albany, NY) 2011;3:1098–1109. doi: 10.18632/aging.100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Felix AA, Williams EG, Jha P, Lo Sasso G, Huzard D, Aebischer P, Sandi C, Rinsch C. J. Auwerx. Nat. Med. 2016;22:879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 154.An F, Yang G, Tian J, Wang S. Neural Regen. Res. 2012;7:2565–2575. doi: 10.3969/j.issn.1673-5374.2012.33.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee EB, Kim JH, Cha YS, Kim M, Song SB, Cha DS, Jeon H, Eun JS, Han S, Kim DK. Biomol. Ther. (Seoul) 2015;23:582–589. doi: 10.4062/biomolther.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.D.G. Souza, B. Bellaver, L.D. Bobermin, D.O. Souza, A. Quincozes-Santos. Purinergic Signal (2016) [DOI] [PMC free article] [PubMed]
- 157.de la Coba F, Aguilera J, de Galvez MV, Alvarez M, Gallego E, Figueroa FL, Herrera E. J. Dermatol. Sci. 2009;55:161–169. doi: 10.1016/j.jdermsci.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 158.Hur S, Lee H, Kim Y, Lee BH, Shin J, Kim TY. Eur. J. Pharmacol. 2008;582:1–11. doi: 10.1016/j.ejphar.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 159.Kawamura T, Mori N, Shibata K. J. Nutr. Sci. Vitaminol. (Tokyo) 2016;62:272–276. doi: 10.3177/jnsv.62.272. [DOI] [PubMed] [Google Scholar]
- 160.Bernardes de Jesus B, Schneeberger K, Vera E, Tejera A, Harley CB, Blasco MA. Aging Cell. 2011;10:604–621. doi: 10.1111/j.1474-9726.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Neurodegener. Dis. 2005;2:246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- 162.Bender A, Beckers J, Schneider I, Holter SM, Haack T, Ruthsatz T, Vogt-Weisenhorn DM, Becker L, Genius J, Rujescu D, Irmler M, Mijalski T, Mader M, Quintanilla-Martinez L, Fuchs H, Gailus-Durner V, de Angelis MH, Wurst W, Schmidt J, Klopstock T. Neurobiol. Aging. 2008;29:1404–1411. doi: 10.1016/j.neurobiolaging.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 163.Bakshi HA, Sam S, Feroz A, Ravesh Z, Shah GA, Sharma M. Asian Pac. J. Cancer Prev. 2009;10:887–890. [PubMed] [Google Scholar]
- 164.Peng XM, Gao L, Huo SX, Liu XM, Yan M. Phytother. Res. 2015;29:1137–1144. doi: 10.1002/ptr.5358. [DOI] [PubMed] [Google Scholar]
- 165.Wu H, Zhao J, Chen M, Wang H, Yao Q, Fan J, Zhang M. J. Mol. Neurosci. 2017;61:449–458. doi: 10.1007/s12031-017-0885-1. [DOI] [PubMed] [Google Scholar]
- 166.Li Y, Zhang Z. Int. J. Clin. Exp. Pathol. 2015;8:14099–14109. [PMC free article] [PubMed] [Google Scholar]
- 167.Zhu J, Mu X, Zeng J, Xu C, Liu J, Zhang M, Li C, Chen J, Li T, Wang Y. PLoS ONE. 2014;9:e101291. doi: 10.1371/journal.pone.0101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.J. Li, D. Cai, X. Yao, Y. Zhang, L. Chen, P. Jing, L. Wang, Y. Wang. Int. J. Mol. Sci. 17 (2016) [DOI] [PMC free article] [PubMed]
- 169.Hada B, Yoo MR, Seong KM, Jin YW, Myeong HK, Min KJ. J. Gerontol. A. 2013;68:226–234. doi: 10.1093/gerona/gls156. [DOI] [PubMed] [Google Scholar]
- 170.Pan W, Jiang S, Luo P, Wu J, Gao P. Nat. Prod. Res. 2008;22:719–725. doi: 10.1080/14786410802102246. [DOI] [PubMed] [Google Scholar]
- 171.Zhang Z, Han S, Wang H, Wang T. Arch. Gerontol. Geriatr. 2014;58:153–159. doi: 10.1016/j.archger.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 172.Peng C, Chan HY, Li YM, Huang Y, Chen ZY. Exp. Gerontol. 2009;44:773–783. doi: 10.1016/j.exger.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 173.Yang S, Long LH, Li D, Zhang JK, Jin S, Wang F, Chen JG. Aging Cell. 2015;14:1024–1033. doi: 10.1111/acel.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Danilov A, Shaposhnikov M, Plyusnina E, Kogan V, Fedichev P, Moskalev A. Oncotarget. 2013;4:1507–1526. doi: 10.18632/oncotarget.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nakagawa-Yagi Y, Sato Y, Matsumoto E, Nakatsuka S, Sakaki T, Muramatsu Y, Hara T, Aigaki T. BMC Complement Altern. Med. 2012;12:101. doi: 10.1186/1472-6882-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ye X, Linton JM, Schork NJ, Buck LB, Petrascheck M. Aging Cell. 2014;13:206–215. doi: 10.1111/acel.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang E, Wink M. PeerJ. 2016;4:e1879. doi: 10.7717/peerj.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang Z, Zhao Y, Wang X, Lin R, Zhang Y, Ma H, Guo Y, Xu L, Zhao B. Food Funct. 2016;7:1975–1984. doi: 10.1039/C5FO01302J. [DOI] [PubMed] [Google Scholar]
- 179.Sayed AA. J. Pharm. Pharmacol. 2011;63:423–428. doi: 10.1111/j.2042-7158.2010.01222.x. [DOI] [PubMed] [Google Scholar]
- 180.Lee EB, Ahn D, Kim BJ, Lee SY, Seo HW, Cha YS, Jeon H, Eun JS, Cha DS, Kim DK. Biomol. Ther. (Seoul) 2015;23:77–83. doi: 10.4062/biomolther.2014.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fischer M, Regitz C, Kahl M, Werthebach M, Boll M, Wenzel U. Mol. Nutr. Food Res. 2012;56:957–965. doi: 10.1002/mnfr.201200006. [DOI] [PubMed] [Google Scholar]
- 182.Xue YL, Ahiko T, Miyakawa T, Amino H, Hu F, Furihata K, Kita K, Shirasawa T, Sawano Y, Tanokura M. J. Agric. Food Chem. 2011;59:5927–5934. doi: 10.1021/jf104798n. [DOI] [PubMed] [Google Scholar]
- 183.Ayyadevara S, Bharill P, Dandapat A, Hu C, Khaidakov M, Mitra S, Shmookler Reis RJ, Mehta JL. Antioxid. Redox Signal. 2013;18:481–490. doi: 10.1089/ars.2011.4151. [DOI] [PubMed] [Google Scholar]
- 184.Asthana J, Yadav AK, Pant A, Pandey S, Gupta MM, Pandey R. Comp. Biochem. Physiol. C. 2015;169:25–34. doi: 10.1016/j.cbpc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 185.Benedetti MG, Foster AL, Vantipalli MC, White MP, Sampayo JN, Gill MS, Olsen A, Lithgow GJ. Exp. Gerontol. 2008;43:882–891. doi: 10.1016/j.exger.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zarse K, Jabin S, Ristow M. Eur. J. Nutr. 2012;51:765–768. doi: 10.1007/s00394-012-0341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Adachi H, Ishii N. J. Gerontol. A. 2000;55:B280–B285. doi: 10.1093/gerona/55.6.B280. [DOI] [PubMed] [Google Scholar]
- 188.Kampkotter A, Gombitang Nkwonkam C, Zurawski RF, Timpel C, Chovolou Y, Watjen W, Kahl R. Arch. Toxicol. 2007;81:849–858. doi: 10.1007/s00204-007-0215-4. [DOI] [PubMed] [Google Scholar]
- 189.Shukla V, Yadav D, Phulara SC, Gupta MM, Saikia SK, Pandey R. Free Radic. Biol. Med. 2012;53:1848–1856. doi: 10.1016/j.freeradbiomed.2012.08.594. [DOI] [PubMed] [Google Scholar]
- 190.Buchter C, Havermann S, Koch K, Watjen W. Eur. J. Nutr. 2016;55:257–265. doi: 10.1007/s00394-015-0843-z. [DOI] [PubMed] [Google Scholar]
- 191.Kampkotter A, Timpel C, Zurawski RF, Ruhl S, Chovolou Y, Proksch P, Watjen W. Comp. Biochem. Physiol. B. 2008;149:314–323. doi: 10.1016/j.cbpb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 192.Pant A, Asthana J, Yadav AK, Rathor L, Srivastava S, Gupta MM, Pandey R. Free Radic. Res. 2015;49:1384–1392. doi: 10.3109/10715762.2015.1075017. [DOI] [PubMed] [Google Scholar]
- 193.Honda Y, Tanaka M, Honda S. Aging Cell. 2010;9:558–569. doi: 10.1111/j.1474-9726.2010.00582.x. [DOI] [PubMed] [Google Scholar]
- 194.Akhoon BA, Pandey S, Tiwari S, Pandey R. Exp. Gerontol. 2016;78:47–56. doi: 10.1016/j.exger.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 195.Canuelo A, Esteban FJ, Peragon J. Eur. J. Nutr. 2016;55:639–650. doi: 10.1007/s00394-015-0884-3. [DOI] [PubMed] [Google Scholar]
- 196.Canuelo A, Peragon J. Proteomics. 2013;13:3064–3075. doi: 10.1002/pmic.201200579. [DOI] [PubMed] [Google Scholar]
- 197.Canuelo A, Gilbert-Lopez B, Pacheco-Linan P, Martinez-Lara E, Siles E, Miranda-Vizuete A. Mech. Ageing Dev. 2012;133:563–574. doi: 10.1016/j.mad.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 198.P. Shen, Y. Yue, Q. Sun, N. Kasireddy, K.H. Kim,Y. Park. Biofactors (2017) [DOI] [PubMed]
- 199.Schlernitzauer A, Oiry C, Hamad R, Galas S, Cortade F, Chabi B, Casas F, Pessemesse L, Fouret G, Feillet-Coudray C, Cros G, Cabello G, Magous R, Wrutniak-Cabello C. PLoS ONE. 2013;8:e78788. doi: 10.1371/journal.pone.0078788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Williams DS, Cash A, Hamadani L, Diemer T. Aging Cell. 2009;8:765–768. doi: 10.1111/j.1474-9726.2009.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee J, Kwon G, Park J, Kim JK, Lim YH. Exp. Biol. Med. (Maywood) 2016;241:1757–1763. doi: 10.1177/1535370216650054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gao L, Zhang R, Lan J, Ning R, Wu D, Chen D, Zhao W. J. Nat. Prod. 2016;79:3039–3046. doi: 10.1021/acs.jnatprod.6b00648. [DOI] [PubMed] [Google Scholar]
- 203.Fu X, Chin RM, Vergnes L, Hwang H, Deng G, Xing Y, Pai MY, Li S, Ta L, Fazlollahi F, Chen C, Prins RM, Teitell MA, Nathanson DA, Lai A, Faull KF, Jiang M, Clarke SG, Cloughesy TF, Graeber TG, Braas D, Christofk HR, Jung ME, Reue K, Huang J. Cell Metab. 2015;22:508–515. doi: 10.1016/j.cmet.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Honda Y, Araki Y, Hata T, Ichihara K, Ito M, Tanaka M, Honda S. J. Aging Res. 2015;2015:425261. doi: 10.1155/2015/425261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ludewig AH, Izrayelit Y, Park D, Malik RU, Zimmermann A, Mahanti P, Fox BW, Bethke A, Doering F, Riddle DL, Schroeder FC. Proc. Natl. Acad. Sci. USA. 2013;110:5522–5527. doi: 10.1073/pnas.1214467110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kim J, Kang YG, Lee JY, Choi DH, Cho YU, Shin JM, Park JS, Lee JH, Kim WG, Seo DB, Lee TR, Miyamoto Y, No KT. Mol. Cell. Endocrinol. 2015;412:216–225. doi: 10.1016/j.mce.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 207.Miller DL, Roth MB. Proc. Natl. Acad. Sci. USA. 2007;104:20618–20622. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Qabazard B, Li L, Gruber J, Peh MT, Ng LF, Kumar SD, Rose P, Tan CH, Dymock BW, Wei F, Swain SC, Halliwell B, Sturzenbaum SR, Moore PK. Antioxid. Redox Signal. 2014;20:2621–2630. doi: 10.1089/ars.2013.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nguyen TT, Caito SW, Zackert WE, West JD, Zhu S, Aschner M, Fessel JP, Roberts LJ., 2nd Aging (Albany, NY) 2016;8:1759–1780. doi: 10.18632/aging.101011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Heidler T, Hartwig K, Daniel H, Wenzel U. Biogerontology. 2010;11:183–195. doi: 10.1007/s10522-009-9239-x. [DOI] [PubMed] [Google Scholar]
- 211.Huang L, Li P, Wang G, Guan S, Sun X, Wang L. Free Radic. Res. 2013;47:316–324. doi: 10.3109/10715762.2013.773588. [DOI] [PubMed] [Google Scholar]
- 212.Hashimoto T, Horikawa M, Nomura T, Sakamoto K. Biogerontology. 2010;11:31–43. doi: 10.1007/s10522-009-9225-3. [DOI] [PubMed] [Google Scholar]
- 213.Edwards CB, Copes N, Brito AG, Canfield J, Bradshaw PC. PLoS ONE. 2013;8:e58345. doi: 10.1371/journal.pone.0058345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Powolny AA, Singh SV, Melov S, Hubbard A, Fisher AL. Exp. Gerontol. 2011;46:441–452. doi: 10.1016/j.exger.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hunt PR, Son TG, Wilson MA, Yu QS, Wood WH, Zhang Y, Becker KG, Greig NH, Mattson MP, Camandola S, Wolkow CA. PLoS ONE. 2011;6:e21922. doi: 10.1371/journal.pone.0021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fang EF, Waltz TB, Kassahun H, Lu Q, Kerr JS, Morevati M, Fivenson EM, Wollman BN, Marosi K, Wilson MA, Iser WB, Eckley DM, Zhang Y, Lehrmann E, Goldberg IG, Scheibye-Knudsen M, Mattson MP, Nilsen H, Bohr VA, Becker KG. Sci. Rep. 2017;7:46208. doi: 10.1038/srep46208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ogawa T, Kodera Y, Hirata D, Blackwell TK, Mizunuma M. Sci. Rep. 2016;6:21611. doi: 10.1038/srep21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Su S, Wink M. Phytochemistry. 2015;117:340–350. doi: 10.1016/j.phytochem.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 219.Pant A, Saikia SK, Shukla V, Asthana J, Akhoon BA, Pandey R. Exp. Gerontol. 2014;57:81–95. doi: 10.1016/j.exger.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 220.Asthana J, Yadav D, Pant A, Yadav AK, Gupta MM, Pandey R. J. Gerontol. A. 2016;71:1160–1168. doi: 10.1093/gerona/glv173. [DOI] [PubMed] [Google Scholar]
- 221.Schmeisser S, Schmeisser K, Weimer S, Groth M, Priebe S, Fazius E, Kuhlow D, Pick D, Einax JW, Guthke R, Platzer M, Zarse K, Ristow M. Aging Cell. 2013;12:508–517. doi: 10.1111/acel.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yu CW, How CM, Liao VH. Chemosphere. 2016;150:632–638. doi: 10.1016/j.chemosphere.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 223.Edwards C, Canfield J, Copes N, Rehan M, Lipps D, Bradshaw PC. Aging (Albany, NY) 2014;6:621–644. doi: 10.18632/aging.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lublin A, Isoda F, Patel H, Yen K, Nguyen L, Hajje D, Schwartz M, Mobbs C. PLoS ONE. 2011;6:e27762. doi: 10.1371/journal.pone.0027762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Baberschke N, Steinberg CE, Saul N. Chemosphere. 2015;124:122–128. doi: 10.1016/j.chemosphere.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 226.Filippopoulou K, Papaevgeniou N, Lefaki M, Paraskevopoulou A, Biedermann D, Kren V, Chondrogianni N. Free Radic. Biol. Med. 2017;103:256–267. doi: 10.1016/j.freeradbiomed.2016.12.042. [DOI] [PubMed] [Google Scholar]
- 227.Zarse K, Bossecker A, Muller-Kuhrt L, Siems K, Hernandez MA, Berendsohn WG, Birringer M, Ristow M. Horm. Metab. Res. 2011;43:241–243. doi: 10.1055/s-0030-1270524. [DOI] [PubMed] [Google Scholar]
- 228.Buchter C, Ackermann D, Havermann S, Honnen S, Chovolou Y, Fritz G, Kampkotter A, Watjen W. Int. J. Mol. Sci. 2013;14:11895–11914. doi: 10.3390/ijms140611895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yang J, Wan QL, Mu QZ, Wu CF, Ding AJ, Yang ZL, Qiu MH, Luo HR. Nat. Prod. Bioprospect. 2015;5:177–183. doi: 10.1007/s13659-015-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen Y, Onken B, Chen H, Xiao S, Liu X, Driscoll M, Cao Y, Huang Q. J. Agric. Food Chem. 2014;62:3422–3431. doi: 10.1021/jf500210p. [DOI] [PubMed] [Google Scholar]
- 231.Denzel MS, Storm NJ, Gutschmidt A, Baddi R, Hinze Y, Jarosch E, Sommer T, Hoppe T, Antebi A. Cell. 2014;156:1167–1178. doi: 10.1016/j.cell.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 232.Chandler-Brown D, Choi H, Park S, Ocampo BR, Chen S, Le A, Sutphin GL, Shamieh LS, Smith ED, Kaeberlein M. Front. Genet. 2015;6:316. doi: 10.3389/fgene.2015.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pietsch K, Saul N, Swain SC, Menzel R, Steinberg CE, Sturzenbaum SR. Front. Genet. 2012;3:48. doi: 10.3389/fgene.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Katiki LM, Ferreira JF, Gonzalez JM, Zajac AM, Lindsay DS, Chagas AC, Amarante AF. Vet. Parasitol. 2013;192:218–227. doi: 10.1016/j.vetpar.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 235.S.J. Kim, S.M. Beak,S.K. Park. J. Food Sci. (2017) [DOI] [PubMed]
- 236.Mark KA, Dumas KJ, Bhaumik D, Schilling B, Davis S, Oron TR, Sorensen DJ, Lucanic M, Brem RB, Melov S, Ramanathan A, Gibson BW, Lithgow GJ. Cell Rep. 2016;17:1227–1237. doi: 10.1016/j.celrep.2016.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ihara A, Uno M, Miyatake K, Honjoh S, Nishida E. Exp. Gerontol. 2016;87:40–47. doi: 10.1016/j.exger.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 238.Gerisch B, Rottiers V, Li D, Motola DL, Cummins CL, Lehrach H, Mangelsdorf DJ, Antebi A. Proc. Natl. Acad. Sci. USA. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Broue F, Liere P, Kenyon C, Baulieu EE. Aging Cell. 2007;6:87–94. doi: 10.1111/j.1474-9726.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- 240.Detienne G, De Haes W, Ernst UR, Schoofs L, Temmerman L. Exp. Gerontol. 2014;60:129–135. doi: 10.1016/j.exger.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 241.Havermann S, Chovolou Y, Humpf HU, Watjen W. PLoS ONE. 2014;9:e100256. doi: 10.1371/journal.pone.0100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Castro PV, Khare S, Young BD, Clarke SG. PLoS ONE. 2012;7:e29984. doi: 10.1371/journal.pone.0029984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.C.H. Chang, C.T. Ho,V.H. Liao. Mol. Nutr. Food Res. (2017)
- 244.Katsiki M, Chondrogianni N, Chinou I, Rivett AJ, Gonos ES. Rejuvenation Res. 2007;10:157–172. doi: 10.1089/rej.2006.0513. [DOI] [PubMed] [Google Scholar]
- 245.Ip FC, Ng YP, An HJ, Dai Y, Pang HH, Hu YQ, Chin AC, Harley CB, Wong YH, Ip NY. Neurosignals. 2014;22:52–63. doi: 10.1159/000365290. [DOI] [PubMed] [Google Scholar]
- 246.Choi MJ, Kim BK, Park KY, Yokozawa T, Song YO, Cho EJ. Biol. Pharm. Bull. 2010;33:421–426. doi: 10.1248/bpb.33.421. [DOI] [PubMed] [Google Scholar]
- 247.Heo SJ, Ko SC, Cha SH, Kang DH, Park HS, Choi YU, Kim D, Jung WK, Jeon YJ. Toxicol. In Vitro. 2009;23:1123–1130. doi: 10.1016/j.tiv.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 248.Wang P, Zhang Z, Ma X, Huang Y, Liu X, Tu P, Tong T. Mech. Ageing Dev. 2003;124:1025–1034. doi: 10.1016/j.mad.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 249.Wang P, Zhang Z, Sun Y, Liu X, Tong T. DNA Cell Biol. 2010;29:33–39. doi: 10.1089/dna.2009.0932. [DOI] [PubMed] [Google Scholar]
- 250.Chondrogianni N, Kapeta S, Chinou I, Vassilatou K, Papassideri I, Gonos ES. Exp. Gerontol. 2010;45:763–771. doi: 10.1016/j.exger.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 251.Torres A, Hochberg M, Pergament I, Smoum R, Niddam V, Dembitsky VM, Temina M, Dor I, Lev O, Srebnik M, Enk CD. Eur. J. Biochem. 2004;271:780–784. doi: 10.1111/j.1432-1033.2004.03981.x. [DOI] [PubMed] [Google Scholar]
- 252.Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 253.Lu SP, Kato M, Lin SJ. J. Biol. Chem. 2009;284:17110–17119. doi: 10.1074/jbc.M109.004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Liu Y, Wu Z, Feng S, Yang X, Huang D. Molecules. 2014;19:568–580. doi: 10.3390/molecules19010568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 256.Goldberg AA, Richard VR, Kyryakov P, Bourque SD, Beach A, Burstein MT, Glebov A, Koupaki O, Boukh-Viner T, Gregg C, Juneau M, English AM, Thomas DY, Titorenko VI. Aging (Albany, NY) 2010;2:393–414. doi: 10.18632/aging.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.A. Leonov, A. Arlia-Ciommo, S.D. Bourque, O. Koupaki, P. Kyryakov, P. Dakik, M. McAuley, Y. Medkour, K. Mohammad, T. Di Maulo, V.I. Titorenko. Oncotarget (2017) [DOI] [PMC free article] [PubMed]
- 258.Beauvais JE, Enesco HE. Exp. Gerontol. 1985;20:359–366. doi: 10.1016/0531-5565(85)90016-6. [DOI] [PubMed] [Google Scholar]
- 259.Lee SH, An HS, Jung YW, Lee EJ, Lee HY, Choi ES, An SW, Son H, Lee SJ, Kim JB, Min KJ. Biogerontology. 2014;15:153–164. doi: 10.1007/s10522-013-9487-7. [DOI] [PubMed] [Google Scholar]
- 260.Jiao J, Wei Y, Chen J, Chen X, Zhang Y. J. Funct. Foods. 2017;30:63–73. doi: 10.1016/j.jff.2016.12.039. [DOI] [Google Scholar]
- 261.Ma XK, Guo DD, Peterson EC, Dun Y, Li DY. Food Funct. 2016;7:3468–3479. doi: 10.1039/C6FO00422A. [DOI] [PubMed] [Google Scholar]
- 262.Xu Y, Gao Y, Zhong M, Li J, Cao H, Huang S, Wei R, Zhang K. Int. J. Biol. Macromol. 2017;101:603–611. doi: 10.1016/j.ijbiomac.2017.03.112. [DOI] [PubMed] [Google Scholar]
- 263.Jing B, Lv C, Li SX, Fu ML, Yin ZQ. Zhong Yao Cai. 2015;38:2563–2567. [PubMed] [Google Scholar]
- 264.Bisson JF, Nejdi A, Rozan P, Hidalgo S, Lalonde R, Messaoudi M. Br. J. Nutr. 2008;100:94–101. doi: 10.1017/S0007114507886375. [DOI] [PubMed] [Google Scholar]
- 265.Shahroudi MJ, Mehri S, Hosseinzadeh H. J. Pharmacopuncture. 2017;20:29–35. doi: 10.3831/KPI.2017.20.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhao H, Li J, Zhang J, Wang X, Hao L, Jia L. Int. J. Biol. Macromol. 2017;102:351–357. doi: 10.1016/j.ijbiomac.2017.04.039. [DOI] [PubMed] [Google Scholar]
- 267.Ngo HT, Hwang E, Seo SA, Park B, Sun ZW, Zhang M, Shin YK, Yi TH. J. Photochem. Photobiol. B. 2017;169:161–170. doi: 10.1016/j.jphotobiol.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 268.Peng C, Chan HY, Huang Y, Yu H, Chen ZY. J. Agric. Food Chem. 2011;59:2097–2106. doi: 10.1021/jf1046267. [DOI] [PubMed] [Google Scholar]
- 269.Bahadorani S, Hilliker AJ. Nutr. Res. 2008;28:377–382. doi: 10.1016/j.nutres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 270.Zou Y, Liu Y, Ruan M, Feng X, Wang J, Chu Z, Zhang Z. Int. J. Mol. Med. 2015;36:939–946. doi: 10.3892/ijmm.2015.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Liu HP, Chang RF, Wu YS, Lin WY, Tsai FJ. Evid. Based Complement Altern. Med. 2012;2012:735481. doi: 10.1155/2012/735481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Rawal S, Singh P, Gupta A, Mohanty S. Biomed. Res. Int. 2014;2014:910290. doi: 10.1155/2014/910290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gospodaryov DV, Yurkevych IS, Jafari M, Lushchak VI, Lushchak OV. Longev. Healthspan. 2013;2:5. doi: 10.1186/2046-2395-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zou S, Carey JR, Liedo P, Ingram DK, Yu B. Age (Dordr.) 2012;34:269–279. doi: 10.1007/s11357-011-9230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Schriner SE, Kuramada S, Lopez TE, Truong S, Pham A, Jafari M. Exp. Gerontol. 2014;60:220–230. doi: 10.1016/j.exger.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lin WS, Chen JY, Wang JC, Chen LY, Lin CH, Hsieh TR, Wang MF, Fu TF, Wang PY. Age (Dordr.) 2014;36:689–703. doi: 10.1007/s11357-013-9606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ghimire S, Kim MS. Biogerontology. 2017;18:263–273. doi: 10.1007/s10522-017-9686-8. [DOI] [PubMed] [Google Scholar]
- 278.Massie HR, Aiello VR, Williams TR. Mech. Ageing Dev. 1993;67:227–237. doi: 10.1016/0047-6374(93)90001-8. [DOI] [PubMed] [Google Scholar]
- 279.Wang L, Li YM, Lei L, Liu Y, Wang X, Ma KY, Chen ZY. Exp. Gerontol. 2015;69:189–195. doi: 10.1016/j.exger.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 280.Schriner SE, Katoozi NS, Pham KQ, Gazarian M, Zarban A, Jafari M. Biogerontology. 2012;13:105–117. doi: 10.1007/s10522-011-9357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Park JK, Kim CK, Gong SK, Yu AR, Lee MY, Park SK. Nutr. Res. Pract. 2014;8:526–532. doi: 10.4162/nrp.2014.8.5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wang Q, Huang Y, Qin C, Liang M, Mao X, Li S, Zou Y, Jia W, Li H, Ma CW, Huang Z. Oxid. Med. Cell Longev. 2016;2016:8956981. doi: 10.1155/2016/8956981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Sunagawa T, Shimizu T, Kanda T, Tagashira M, Sami M, Shirasawa T. Planta Med. 2011;77:122–127. doi: 10.1055/s-0030-1250204. [DOI] [PubMed] [Google Scholar]
- 284.Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Liu H, Liang F, Su W, Wang N, Lv M, Li P, Pei Z, Zhang Y, Xie XQ, Wang L, Wang Y. J. Ethnopharmacol. 2013;147:366–372. doi: 10.1016/j.jep.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 286.Wu Z, Smith JV, Paramasivam V, Butko P, Khan I, Cypser JR, Luo Y. Cell Mol. Biol. (Noisy-le-grand) 2002;48:725–731. [PubMed] [Google Scholar]
- 287.Chen J, Zhang J, Xiang Y, Xiang L, Liu Y, He X, Zhou X, Liu X, Huang Z. Food Funct. 2016;7:943–952. doi: 10.1039/C5FO01241D. [DOI] [PubMed] [Google Scholar]
- 288.Moriwaki T, Kato S, Kato Y, Hosoki A, Zhang-Akiyama QM. J. Clin. Biochem. Nutr. 2013;53:81–88. doi: 10.3164/jcbn.13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Feng S, Cheng H, Xu Z, Shen S, Yuan M, Liu J, Ding C. Int. J. Biol. Macromol. 2015;81:188–194. doi: 10.1016/j.ijbiomac.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 290.S.M. Won, H.U. Cha, S.S. Yi, S.J. Kim, S.K. Park. J. Med. Food (2016)
- 291.Wan F, Zhi D, Liu D, Xian J, Li M, AbuLizi A, Ju W, Li H. Biogerontology. 2014;15:377–387. doi: 10.1007/s10522-014-9508-1. [DOI] [PubMed] [Google Scholar]
- 292.Zhang Y, Lv T, Li M, Xue T, Liu H, Zhang W, Ding X, Zhuang Z. Pharmacogn. Mag. 2015;11:449–454. doi: 10.4103/0973-1296.160447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Jeon H, Cha DS. Chin J. Nat. Med. 2016;14:335–342. doi: 10.3724/SP.J.1009.2016.00335. [DOI] [PubMed] [Google Scholar]
- 294.Wang Z, Ma X, Li J, Cui X. Exp. Gerontol. 2016;82:139–149. doi: 10.1016/j.exger.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 295.Zhang H, Pan N, Xiong S, Zou S, Li H, Xiao L, Cao Z, Tunnacliffe A, Huang Z. Biochem. J. 2012;441:417–424. doi: 10.1042/BJ20110621. [DOI] [PubMed] [Google Scholar]
- 296.Huang CH, Hsu FY, Wu YH, Zhong L, Tseng MY, Kuo CJ, Hsu AL, Liang SS, Chiou SH. J. Nutr. Biochem. 2015;26:808–817. doi: 10.1016/j.jnutbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 297.Honda Y, Fujita Y, Maruyama H, Araki Y, Ichihara K, Sato A, Kojima T, Tanaka M, Nozawa Y, Ito M, Honda S. PLoS ONE. 2011;6:e23527. doi: 10.1371/journal.pone.0023527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Parikh I, Guo J, Chuang KH, Zhong Y, Rempe RG, Hoffman JD, Armstrong R, Bauer B, Hartz AM, Lin AL. Aging (Albany, NY) 2016;8:2814–2826. doi: 10.18632/aging.101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Rathor L, Pant A, Awasthi H, Mani D, Pandey R. Biogerontology. 2017;18:131–147. doi: 10.1007/s10522-016-9668-2. [DOI] [PubMed] [Google Scholar]
- 300.Yang X, Zhang P, Wu J, Xiong S, Jin N, Huang Z. J. Ethnopharmacol. 2012;141:41–47. doi: 10.1016/j.jep.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 301.Guan S, Li P, Luo J, Li Y, Huang L, Wang G, Zhu L, Fan H, Li W, Wang L. Free Radic. Res. 2010;44:813–820. doi: 10.3109/10715762.2010.485991. [DOI] [PubMed] [Google Scholar]
- 302.Wiegant FA, Surinova S, Ytsma E, Langelaar-Makkinje M, Wikman G, Post JA. Biogerontology. 2009;10:27–42. doi: 10.1007/s10522-008-9151-9. [DOI] [PubMed] [Google Scholar]
- 303.Scerbak C, Vayndorf EM, Hernandez A, McGill C, Taylor BE. Front. Aging Neurosci. 2016;8:173. doi: 10.3389/fnagi.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zheng S, Liao S, Zou Y, Qu Z, Shen W, Shi Y. Age (Dordr.) 2014;36:9719. doi: 10.1007/s11357-014-9719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kawano T, Kataoka N, Abe S, Ohtani M, Honda Y, Honda S, Kimura Y. Biosci. Biotechnol. Biochem. 2005;69:2479–2481. doi: 10.1271/bbb.69.2479. [DOI] [PubMed] [Google Scholar]
- 306.Stirpe M, Palermo V, Bianchi MM, Silvestri R, Falcone C, Tenore G, Novellino E, Mazzoni C. BMC Complement Altern. Med. 2017;17:200. doi: 10.1186/s12906-017-1666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Snare DJ, Fields AM, Snell TW, Kubanek J. Exp. Gerontol. 2013;48:1420–1427. doi: 10.1016/j.exger.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wan QL, Zheng SQ, Wu GS, Luo HR. Exp. Gerontol. 2013;48:499–506. doi: 10.1016/j.exger.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 309.Zhang Z, Li X, Li F, An L. Int. Immunopharmacol. 2016;38:426–433. doi: 10.1016/j.intimp.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 310.Wang J, Guo T, Peng QS, Yue SW, Wang SX. J. Cell Mol. Med. 2015;19:2607–2616. doi: 10.1111/jcmm.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Navrotskaya V, Oxenkrug G, Vorobyova L, Summergrad P. Am. J. Plant Sci. 2014;5:275–278. doi: 10.4236/ajps.2014.53037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ruan Q, Liu F, Gao Z, Kong D, Hu X, Shi D, Bao Z, Yu Z. Mech. Ageing Dev. 2013;134:89–97. doi: 10.1016/j.mad.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 313.Mora M, Medina-Leendertz SJ, Bonilla E, Teran RE, Paz MC, Arcaya JL. Invest. Clin. 2013;54:161–170. [PubMed] [Google Scholar]
- 314.Anisimov VN, Semenchenko AV, Yashin AI. Biogerontology. 2003;4:297–307. doi: 10.1023/A:1026299318315. [DOI] [PubMed] [Google Scholar]
- 315.Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kucukatay V, Bor-Kucukatay M, Gundogdu G, Erken G, Ozcan TO, Miloglu FD, Kadioglu Y. Folia Biol. (Praha) 2012;58:157–165. [PubMed] [Google Scholar]
- 317.Ryan MJ, Dudash HJ, Docherty M, Geronilla KB, Baker BA, Haff GG, Cutlip RG, Alway SE. Exp. Gerontol. 2010;45:882–895. doi: 10.1016/j.exger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Harrington LA, Harley CB. Mech. Ageing Dev. 1988;43:71–78. doi: 10.1016/0047-6374(88)90098-X. [DOI] [PubMed] [Google Scholar]
- 319.C. Vinod, A. Jagota. Biogerontology (2017)
- 320.Gent S, Kleinbongard P, Dammann P, Neuhauser M, Heusch G. Basic Res. Cardiol. 2015;110:2. doi: 10.1007/s00395-014-0460-7. [DOI] [PubMed] [Google Scholar]
- 321.Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Tong JJ, Chen GH, Wang F, Li XW, Cao L, Sui X, Tao F, Yan WW, Wei ZJ. Behav. Brain Res. 2015;284:138–152. doi: 10.1016/j.bbr.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 323.Spindler SR, Mote PL, Li R, Dhahbi JM, Yamakawa A, Flegal JM, Jeske DR, Li R, Lublin AL. Age (Dordr.) 2013;35:2099–2109. doi: 10.1007/s11357-012-9498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zou YX, Ruan MH, Luan J, Feng X, Chen S, Chu ZY. J. Nutr. Health Aging. 2017;21:314–319. doi: 10.1007/s12603-016-0752-8. [DOI] [PubMed] [Google Scholar]
- 325.Avanesian A, Khodayari B, Felgner JS, Jafari M. Biogerontology. 2010;11:45–52. doi: 10.1007/s10522-009-9227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kang HL, Benzer S, Min KT. Proc. Natl. Acad. Sci. USA. 2002;99:838–843. doi: 10.1073/pnas.022631999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Bergwitz C. Nephrol. Dial. Transplant. 2012;27:3399–3406. doi: 10.1093/ndt/gfs362. [DOI] [PubMed] [Google Scholar]
- 328.Spindler SR, Li R, Dhahbi JM, Yamakawa A, Mote P, Bodmer R, Ocorr K, Williams RT, Wang Y, Ablao KP. PLoS ONE. 2012;7:e39581. doi: 10.1371/journal.pone.0039581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Slack C, Alic N, Foley A, Cabecinha M, Hoddinott MP, Partridge L. Cell. 2015;162:72–83. doi: 10.1016/j.cell.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Tao D, Lu J, Sun H, Zhao YM, Yuan ZG, Li XX, Huang BQ. Acta Biochim. Biophys. Sin. (Shanghai) 2004;36:618–622. doi: 10.1093/abbs/36.9.618. [DOI] [PubMed] [Google Scholar]
- 331.Dubiley TA, Rushkevich YE, Koshel NM, Voitenko VP, Vaiserman AM. Biogerontology. 2011;12:179–184. doi: 10.1007/s10522-010-9308-1. [DOI] [PubMed] [Google Scholar]
- 332.Calvert S, Tacutu R, Sharifi S, Teixeira R, Ghosh P, de Magalhaes JP. Aging Cell. 2016;15:256–266. doi: 10.1111/acel.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ching TT, Chiang WC, Chen CS, Hsu AL. Aging Cell. 2011;10:506–519. doi: 10.1111/j.1474-9726.2011.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Evason K, Collins JJ, Huang C, Hughes S, Kornfeld K. Aging Cell. 2008;7:305–317. doi: 10.1111/j.1474-9726.2008.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Brandstadt S, Schmeisser K, Zarse K, Ristow M. Aging (Albany, NY) 2013;5:270–275. doi: 10.18632/aging.100548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Evason K, Huang C, Yamben I, Covey DF, Kornfeld K. Science. 2005;307:258–262. doi: 10.1126/science.1105299. [DOI] [PubMed] [Google Scholar]
- 337.Rathor L, Akhoon BA, Pandey S, Srivastava S, Pandey R. Age (Dordr.) 2015;37:113. doi: 10.1007/s11357-015-9850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Collins JJ, Evason K, Pickett CL, Schneider DL, Kornfeld K. PLoS Genet. 2008;4:e1000230. doi: 10.1371/journal.pgen.1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Chen X, McCue HV, Wong SQ, Kashyap SS, Kraemer BC, Barclay JW, Burgoyne RD, Morgan A. Mol. Neurodegener. 2015;10:51. doi: 10.1186/s13024-015-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Schmeisser S, Zarse K, Ristow M. Horm. Metab. Res. 2011;43:687–692. doi: 10.1055/s-0031-1286308. [DOI] [PubMed] [Google Scholar]
- 341.Srivastava D, Arya U, SoundaraRajan T, Dwivedi H, Kumar S, Subramaniam JR. Biogerontology. 2008;9:309–316. doi: 10.1007/s10522-008-9139-5. [DOI] [PubMed] [Google Scholar]
- 342.Saharia K, Arya U, Kumar R, Sahu R, Das CK, Gupta K, Dwivedi H, Subramaniam JR. Exp. Gerontol. 2012;47:188–197. doi: 10.1016/j.exger.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 343.Golegaonkar S, Tabrez SS, Pandit A, Sethurathinam S, Jagadeeshaprasad MG, Bansode S, Sampathkumar SG, Kulkarni MJ, Mukhopadhyay A. Aging Cell. 2015;14:463–473. doi: 10.1111/acel.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Liu S, Saul N, Pan B, Menzel R, Steinberg CE. Chemosphere. 2013;93:2373–2380. doi: 10.1016/j.chemosphere.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 345.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J. Nature. 2013;497:451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Bozovic V, Enesco HE. Arch. Gerontol. Geriatr. 1989;9:45–51. doi: 10.1016/0167-4943(89)90023-X. [DOI] [PubMed] [Google Scholar]
- 347.Liu J, Huang X, Withers BR, Blalock E, Liu K, Dickson RC. Aging Cell. 2013;12:833–841. doi: 10.1111/acel.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Aging Cell. 2008;7:552–560. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 349.Padalko VI. Biochemistry (Mosc.) 2005;70:986–989. doi: 10.1007/s10541-005-0213-1. [DOI] [PubMed] [Google Scholar]
- 350.Comfort A, Youhotsky-Gore I, Pathmanathan K. Nature. 1971;229:254–255. doi: 10.1038/229254a0. [DOI] [PubMed] [Google Scholar]
- 351.Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M, de Cabo R. Cell Rep. 2014;6:836–843. doi: 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Khavinson VK, Izmaylov DM, Obukhova LK, Malinin VV. Mech. Ageing Dev. 2000;120:141–149. doi: 10.1016/S0047-6374(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 353.Miquel J, Fleming J, Economos AC. Arch. Gerontol. Geriatr. 1982;1:159–165. doi: 10.1016/0167-4943(82)90016-4. [DOI] [PubMed] [Google Scholar]
- 354.Spindler SR, Li R, Dhahbi JM, Yamakawa A, Sauer F. PLoS ONE. 2012;7:e29782. doi: 10.1371/journal.pone.0029782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sagi O, Wolfson M, Utko N, Muradian K, Fraifeld V. Mech. Ageing Dev. 2005;126:249–254. doi: 10.1016/j.mad.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 356.Moskalev A, Shaposhnikov M. Aging (Albany, NY) 2011;3:391–394. doi: 10.18632/aging.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Sampayo JN, Olsen A, Lithgow GJ. Aging Cell. 2003;2:319–326. doi: 10.1046/j.1474-9728.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 358.Fischer N, Buchter C, Koch K, Albert S, Csuk R, Watjen W. J. Pharm. Pharmacol. 2016;69:73–8. doi: 10.1111/jphp.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Mekheimer RA, Sayed AA, Ahmed EA. J. Med. Chem. 2012;55:4169–4177. doi: 10.1021/jm2014315. [DOI] [PubMed] [Google Scholar]
- 360.Alavez S, Vantipalli MC, Zucker DJ, Klang IM, Lithgow GJ. Nature. 2011;472:226–229. doi: 10.1038/nature09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zhu J, Wu CF, Li X, Wu GS, Xie S, Hu QN, Deng Z, Zhu MX, Luo HR, Hong X. Bioorg. Med. Chem. 2013;21:4218–4224. doi: 10.1016/j.bmc.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 362.Zhu Y, Lu Y, Qu C, Miller M, Tian J, Thakur DP, Zhu J, Deng Z, Hu X, Wu M, McManus OB, Li M, Hong X, Zhu MX, Luo HR. Br. J. Pharmacol. 2015;172:3495–3509. doi: 10.1111/bph.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yang LP, Jiang FJ, Wu GS, Deng K, Wen M, Zhou X, Hong X, Zhu MX, Luo HR. PLoS ONE. 2015;10:e0136255. doi: 10.1371/journal.pone.0136255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Ding AJ, Wu GS, Tang B, Hong X, Zhu MX, Luo HR. Mol. Cell Biochem. 2016;426:101–109. doi: 10.1007/s11010-016-2884-x. [DOI] [PubMed] [Google Scholar]
- 365.Sawada M, Carlson JC, Enesco HE. Arch. Gerontol. Geriatr. 1990;10:27–36. doi: 10.1016/0167-4943(90)90041-4. [DOI] [PubMed] [Google Scholar]