Abstract
Abstract
Three new lycodine-type Lycopodium alkaloids, namely 1-methyllycodine (1), 8α-hydroxy-15,16-dehydro-des-N-methyl-α-obscurine (2), N-methyl-16-hydroxyhuperzine B (3), and one new natural lycodine-type Lycopodium alkaloid, N-methylhuperzine A (4), along with 11 known analogues (5–15), were isolated from the whole plants of club moss Huperzia serrata. The structures of 1–4 were elucidated on the basis of NMR spectroscopic and mass spectrometry data. Among them, compound 1 was the first lycodine-type alkaloid possessing a methyl group at C-1. In addition, the structure of 5 was confirmed by the single-crystal X-ray crystallography data and its 13C NMR was reported for the first time in current study. Compounds 1–5 were tested their BACE1 inhibitory activity.
Graphical Abstract
Electronic supplementary material
The online version of this article (doi:10.1007/s13659-017-0140-z) contains supplementary material, which is available to authorized users.
Keywords: Lycopodium alkaloids, Lycodine-type, Huperzia serrata, BACE1 inhibitory activity
Introduction
The Lycopodium alkaloids, which only found from the plants of the families Lycopodiaceae and Huperziaceae, have attracted broad interest from chemists and pharmacologists worldwide due to their intriguing carbon skeletons and biological activities [1–6]. Such alkaloids were divided into four major classes (lycopodine, lycodine, fawcettimine, and phlegmarine) by Canadian famous chemists Ayer et al. [7]. Particularly, lycodine-type alkaloids, which generally characterized by four connected six-membered rings, including a pyridone or pyridine ring, a piperidine ring, and a bicyclo[3.3.1]nonane core [8], are a unique class of compounds and have attracted great interest for their biological activities especially the extraordinary acetylcholinesterase (AChE) inhibition by huperzine A that has a potential of becoming a therapeutic agent for the treatment of Alzheimer’s disease [2, 9].
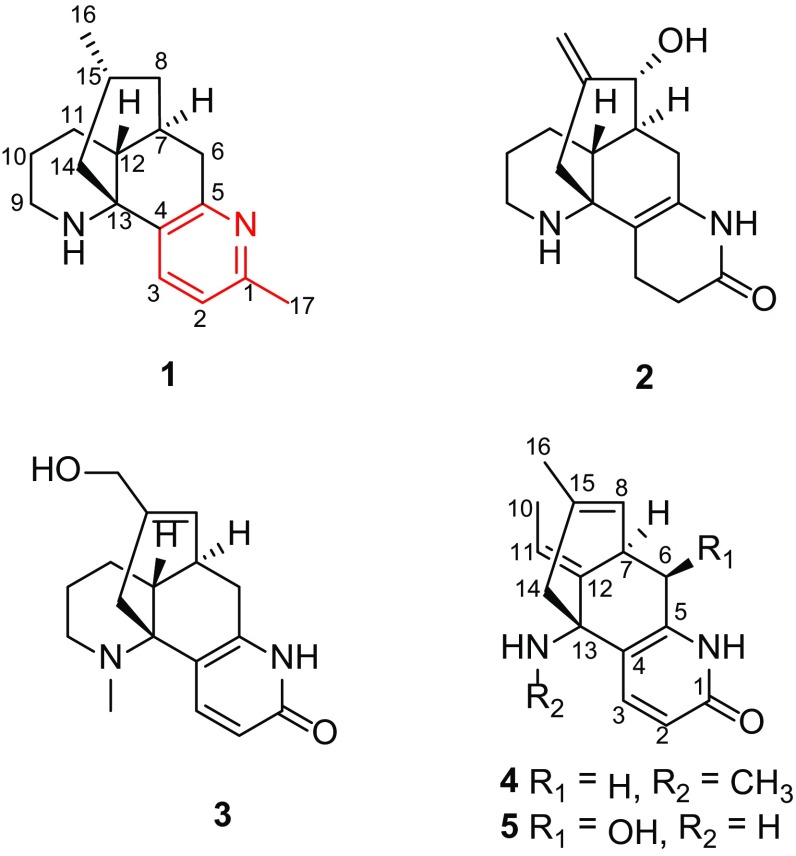
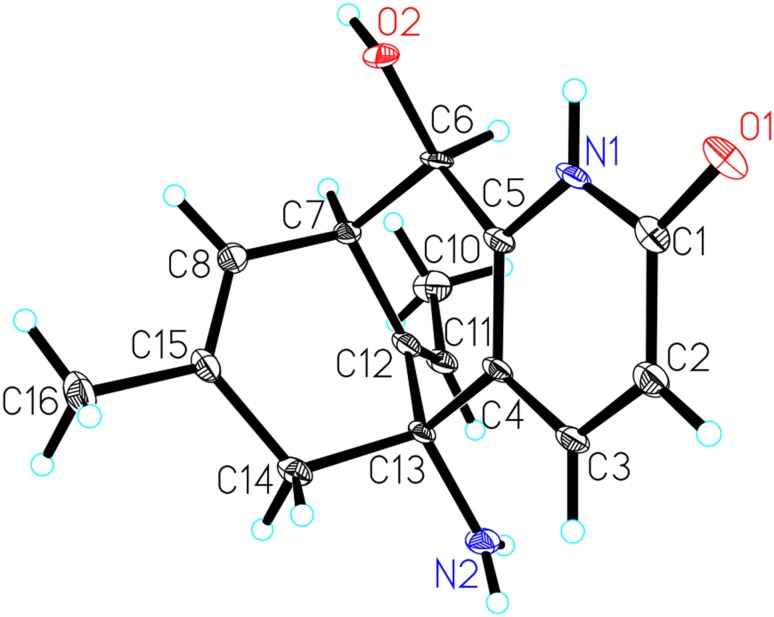
Chinese traditional medicinal plant Huperzia serrata, chiefly growing in rock crevice or somewhere dank in the forests, shrubs, or roadsides at the elevation of 300–2700 m in Southwestern and Southeastern China, belonged to the Huperziaceae family and was known for its therapeutic effect on contusions, pains, swellings, schizophrenia, and organophosphate poisoning in ancient China [10, 11]. Previously, phytochemical studies on H. serrata have led to a series of components [12, 13]. Among them, lycodine-type Lycopodium alkaloids were its main chemical and bioactive ingredients [14, 15], in particular, a type of alkaloids that possessed extraordinary AChE inhibition, such as the well-known huperzine A [2, 9]. As part of an ongoing research program aimed at exploring more Lycopodium alkaloids with fascinating structures and bioactivities serving as lead compounds for drug discovery, three new lycodine-type alkaloids, 1-methyllycodine (1), 8α-hydroxy-15,16-dehydro-des-N-methyl-α-obscurine (2), N-methyl-16-hydroxyhuperzine B (3), and one new natural Lycopodium alkaloid N-methylhuperzine A (4) were isolated from H. serrata (Fig. 1), together with 11 known analogues. The known compounds were identified as 6β-hydroxyhuperzine A (5) [16], huperzine A (6) [2, 9], huperzine B (7) [2, 9], casuarine B (8) [17], huperzinine (9) [18], lycodine (10) [19], N-methyllycodine (11) [20, 21], carinatumine B (12) [22], des-N-methyl-β-obscurine (13) [23], N-demethylhuperzinine (14) [24], and 16-hydroxyhuperzine B (15) [25] by comparison of their spectroscopic data with those reported in the literature. Their structures were determined by extensive spectroscopic analysis. Among them, the structure of 5 was confirmed by the single-crystal X-ray crystallography data (Fig. 2) and its 13C NMR data (Table 2) was reported for the first time in current study. In addition, these findings might provide more information for the biological activities study and synthesis of lycodine-type alkaloids. Reported herein are the isolation, structure elucidation and bioactivity investigation results of compounds 1–5.
Fig. 1.
Structures of compounds 1–5
Fig. 2.
The X-ray structure of compound 5
Table 2.
13C NMR spectroscopic data of 1–5 (δ in ppm)
| No. | 1 a | 2 b | 3 b | 4 c | 5 c |
|---|---|---|---|---|---|
| 1 | 157.8 s | 173.6 s | 165.8 s | 165.6 s | 165.3 s |
| 2 | 122.0 d | 31.6 t | 118.3 d | 118.1 d | 119.1 d |
| 3 | 133.5 d | 19.8 t | 143.1 d | 142.3 d | 141.3 d |
| 4 | 126.6 s | 111.6 s | 121.9 s | 120.7 s | 123.7 s |
| 5 | 157.7 s | 131.3 s | 144.1 s | 145.9 s | 145.9 s |
| 6 | 35.0 t | 28.0 t | 30.0 t | 35.2 t | 72.1 d |
| 7 | 33.1 d | 41.6 d | 35.3 d | 34.3 d | 42.2 d |
| 8 | 42.7 t | 78.8 d | 126.3 d | 125.8 d | 121.3 d |
| 9 | 40.4 t | 43.3 t | 51.8 t | ||
| 10 | 23.3 t | 27.3 t | 21.1 t | 12.5 q | 12.5 q |
| 11 | 24.3 t | 26.7 t | 26.9 t | 114.3 d | 115.0 d |
| 12 | 41.6 d | 38.1 d | 34.3 d | 137.3 s | 140.3 s |
| 13 | 61.2 s | 58.2 s | 58.3 s | 61.1 s | 55.7 s |
| 14 | 47.4 t | 42.4 t | 39.9 t | 50.7 t | 50.2 t |
| 15 | 26.0 d | 147.4 s | 137.8 s | 135.4 s | 136.6 s |
| 16 | 21.5 q | 115.1 t | 66.4 t | 22.7 q | 22.9 q |
| 17 | 24.2 q | ||||
| N-CH3 | 38.0 q | 29.7 q |
aRecorded at 125 MHz in C5D5N
bRecorded at 125 MHz in CD3OD
cRecorded at 150 MHz in CD3OD
Results and Discussion
The air-dried and powdered whole plants of H. serrata were extracted with 60% EtOH for three times. The extract was partitioned between EtOAc and 1.0% HCl/H2O. Water-soluble materials, which were adjusted to pH 9 with 17% ammonia solution, were then extracted with CHCl3 to afford an alkaloidal extract. Further column chromatography (CC) over MCI gel, normal-phase silica gel, and Sephadex LH-20 led to the isolation of three new lycodine-type alkaloids (1–3), one new natural lycodine-type Lycopodium alkaloid (4), together with 11 known ones (5–15).
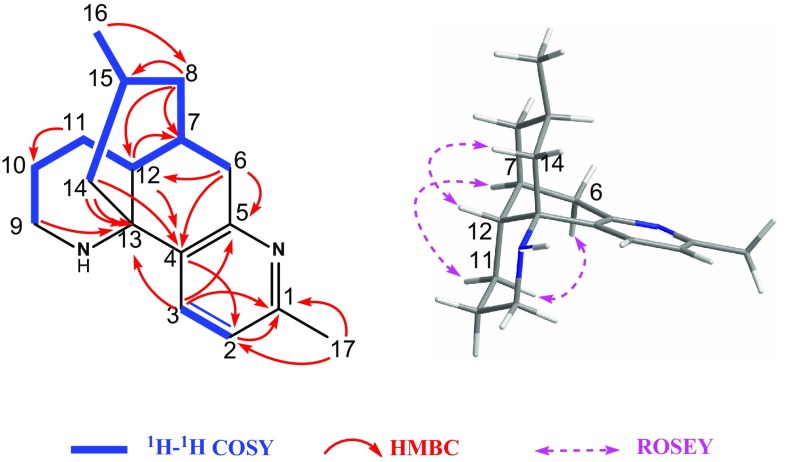
Compound 1 possessed a molecular formula of C17H24N2 as deduced from the HR–ESI–MS analysis ([M+H]+ m/z 257.2014, Calcd 257.2018), corresponding to seven degrees of unsaturation. Its IR spectrum showed strong absorption at 3433 and 1677 cm−1, indicated the NH and double bond groups, respectively. The existence of a pyridine moiety was revealed by the absorption bands at 209 and 273 nm in its UV spectrum and two characteristic proton signals [δ H 8.31 and 7.07 (each 1H, d, J = 7.9 Hz)] in the downfield region of the 1H NMR spectrum. The 13C NMR and DEPT spectra exhibited 17 carbon resonances (Table 2), including three sp 2 quaternary carbons (δ C 126.6, 157.7, and 157.8), one sp 3 quaternary carbon (δ C 61.2), three sp 3 methines (δ C 26.0, 33.1, and 41.6), two sp 2 methines (δ C 122.0 and 133.5), six sp 3 methylenes (δ C 23.3, 24.3, 35.0, 40.4, 42.7 and 47.4), and two methyls (δ C 21.5 and 24.2) with corresponding protons as one double signal at δ H 0.56 and one singlet signal at δ H 2.55 (Table 1), respectively, in the 1H NMR spectrum. The above information suggested that 1 should be a lycodine-related Lycopodium alkaloid. Comparison of the 1H and 13C NMR spectroscopic data of 1 with those of the known alkaloid lycodine (10) [19] revealed that they were structural analogues. A major difference was the presence of one more methyl group [δ H 2.55 (3H, s), δ C 24.2] at C-1 in 1, which was confirmed by the significant HMBC correlations from H-17 (δ H 2.55) to C-1 (δ C 157.8) and C-2 (δ C 122.0) (Fig. 3), as well as the obvious triplet signal of H-1 in 10 was disappeared in 1. It is worth noting that 1 was the first lycodine-type Lycopodium alkaloid possessing a methyl group at C-1.
Table 1.
1H NMR spectroscopic data of 1–5 (δ in ppm, J in Hz)
| No. | 1 a | 2 b | 3 b | 4 c | 5 c |
|---|---|---|---|---|---|
| 2a | 7.07 (d, 7.9) | 2.37 (m) | 6.39 (d, 9.5) | 6.41 (d, 9.4) | 6.43 (d, 9.4) |
| 2b | 2.31 (m) | ||||
| 3a | 8.31 (d, 7.9) | 2.16 (2H, m) | 7.94 (d, 9.5) | 7.68 (d, 9.4) | 7.90 (d, 9.4) |
| 3b | |||||
| 6a | 3.17 (dd, 19.0, 7.2) | 2.33 (dd, 17.8, 7.5) | 2.89 (dd, 17.9, 5.4) | 2.77 (dd, 17.0, 4.8) | 4.60 (d, 5.2) |
| 6b | 2.76 (d, 19.0) | 1.56 (d, 17.8) | 2.30 (d, 17.9) | 2.57 (d, 17.0) | |
| 7 | 1.98 (overlapped) | 1.94 (m) | 2.48 (m) | 3.63 (t, 4.8) | 3.74 (dd, 5.2, 3.5) |
| 8a | 1.57 (overlapped) | 3.93 (d, 2.6) | 5.71 (br d, 4.3) | 5.42 (d, 4.8) | 5.55 (br d, 3.5) |
| 8b | 1.21 (overlapped) | ||||
| 9a | 3.54 (br d, 12.7) | 2.76 (br d, 12.3) | 2.61 (2H, overlapped) | ||
| 9b | 2.84 (td, 12.7, 2.5) | 2.42 (td, 12.3, 3.0) | |||
| 10a | 1.97 (overlapped) | 1.62 (2H, overlapped) | 1.87 (dt, 12.6, 4.1) | 1.71 (d, 6.8) | 1.72 (d, 6.7) |
| 10b | 1.55 (overlapped) | 1.27 (overlapped) | |||
| 11a | 1.38 (br d, 16.7) | 1.43 (2H, overlapped) | 1.62 (m) | 5.46 (q, 6.8) | 5.63 (q, 6.7) |
| 11b | 1.11 (ddd, 16.7, 13.4, 3.9) | 1.34 (ddd, 17.6, 12.6, 4.4) | |||
| 12 | 2.17 (d, 13.4) | 2.14 (m) | 2.09 (dt, 12.6, 4.1) | ||
| 14a | 2.11 (br d, 11.9) | 2.39 (d, 12.9) | 2.65 (d, 16.7) | 2.24 (d, 16.5) | 2.29 (d, 16.8) |
| 14b | 1.87 (t, 11.9) | 1.97 (d, 12.9) | 1.94 (d, 16.7) | 2.05 (d, 16.5) | 2.16 (d, 16.8) |
| 15 | 1.20 (overlapped) | ||||
| 16a | 0.56 (d, 7.8) | 4.91 (br s) | 3.79, 3.83 (ABq, 13.4) | 1.53 (s) | 1.58 (s) |
| 16b | 4.71 (br s) | ||||
| 17 | 2.55 (s) | ||||
| N-CH3 | 2.69 (s) | 2.09 (s) |
aRecorded at 500 MHz in C5D5N
bRecorded at 500 MHz in CD3OD
cRecorded at 600 MHz in CD3OD
Fig. 3.
Key 2D NMR correlations of compound 1
The relative configuration of 1 was deduced by the ROESY experiment (Fig. 3) and the coupling constant. Biogenetially, H-12 and H-7 of the lycodine-type Lycopodium alkaloids were β- and α-orientated, respectively, which were also supported by the diagnostic ROESY correlations of H-6a with H-11b and H-7 with H-11a. Irradiation of H-12 enhanced the signal of H-14a, indicating that these protons were on the same facial plane. Furthermore, the methyl group at C-15 was located at an equatorial position by the large coupling constant (11.9 Hz) between H-14a and H-15. Thus, the structure of 1 was determined and named as 1-methyllycodine.
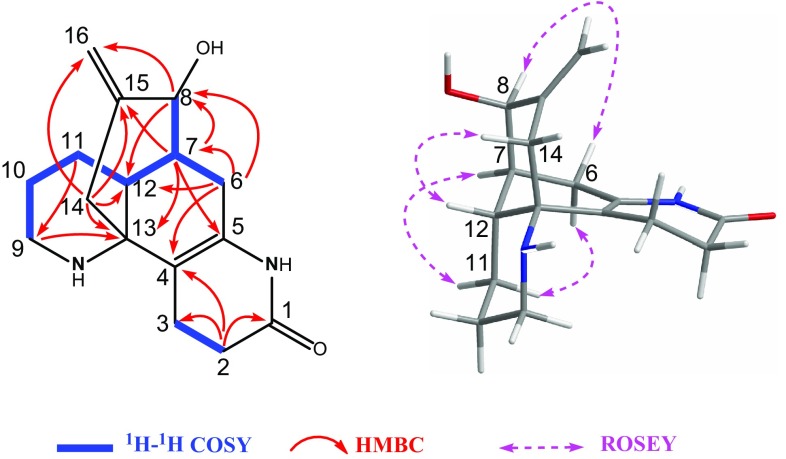
Compound 2 was assigned a molecular formula of C16H22N2O2 as determined by HR-EI-MS ([M]+ m/z 274.1674, Calcd 274.1681), requiring seven degrees of unsaturation. The IR spectrum was indicative of the presence of a hydroxy (3426 cm−1) and an amide carbonyl (1660 cm−1) groups. Its 1D NMR spectroscopic data (Tables 1, 2) showed general features analogous to those of des-N-methyl-α-obscurine [23, 27]. Differing from the latter, compound 2 has one more hydroxy group and a ∆15 double bond in its structure. The carbon resonance at δ C 78.8 was assigned to C-8 bearing a hydroxy group, based on the HMBC correlations of H-8 (δ H 3.93) with C-12 (δ C 38.1), C-14 (δ C 42.4), and C-16 (δ C 115.1), and of H2-6 (δ H 1.56 and 2.33) and H-7 (δ H 1.94) with C-8, together with the 1H-1H COSY correlations of H-7/H-8 (Fig. 4). Moreover, the downfield shifts of C-15 (δ C 147.4) and C-16 (δ C 115.1) and the downfield shift of H-16 [δ H 4.91 and 4.71 (each 1H, br s)] for 2 indicated the double bond was located between C-15 and C-16 at the terminal, which was also elucidated by the HMBC correlations of H-14b and H-8 with C-16, and of H-7 and H-14a (δ H 2.39) with C-15.
Fig. 4.
Key 2D NMR correlations of compound 2
In the ROESY spectrum (Fig. 4), the β - and α-orientation of H-12 and H-7, respectively, were revealed by the observed correlations of H-6a/H-11b, H-7/H-11a, and H-12/H-14b. Furthermore, the obvious ROESY correlation of H-8/H-6b allowed the assignment of OH-8 as α -orientated. On the basis of the above evidence, the structure of 2 was assigned as 8α-hydroxy-15,16-dehydro-des-N-methyl-α-obscurine.
Compound 3 showed the pseudo-molecular ion peak at m/z 287.1759 [M+H]+ (Calcd 287.1760) in the HR–ESI–MS, which established a molecular formula of C17H22N2O2, indicating eight degrees of unsaturation. IR absorptions implied the presence of amide carbonyl (1657 cm−1) and hydroxy (3421 cm−1) functionalities. The absorption bands at 231 and 310 nm in its UV spectrum and two characteristic proton signals [δ H 7.94 and 6.39 (each 1H, d, J = 9.5 Hz)] in the low field region of the 1H NMR spectrum revealed the existence of an α-pyridone moiety. Additionally, an N-methyl group [δ H 2.69 (3H, s), δ C 38.0] was also displayed in the 1H, 13C NMR, and DEPT spectra (Tables 1, 2). Analysis of the 1D NMR spectra of 3 revealed that its spectroscopic data closely resembled those of 16-hydroxyhuperzine B (15) [25], a known lycodine-type alkaloid previously isolated from Lycopodium casuarinoides. The only difference between those two compounds was that 3 possessed an additional N-methyl group. Consequently, compound 3 was assumed to be the N-methylated derivative of 16-hydroxyhuperzine B, which was confirmed by observed key HMBC correlations from N-methyl (H-17) at δ H 2.69 to C-9 (δ C 51.8) and C-13 (δ C 58.3). The observed ROESY correlations of H-6a/H-11b, H-7/H-11a, and H-12/H-14b indicated that H-12 and H-7 were β - and α-orientated, respectively. Hence, the structure of 3 was elucidated as N-methyl-16-hydroxyhuperzine B.
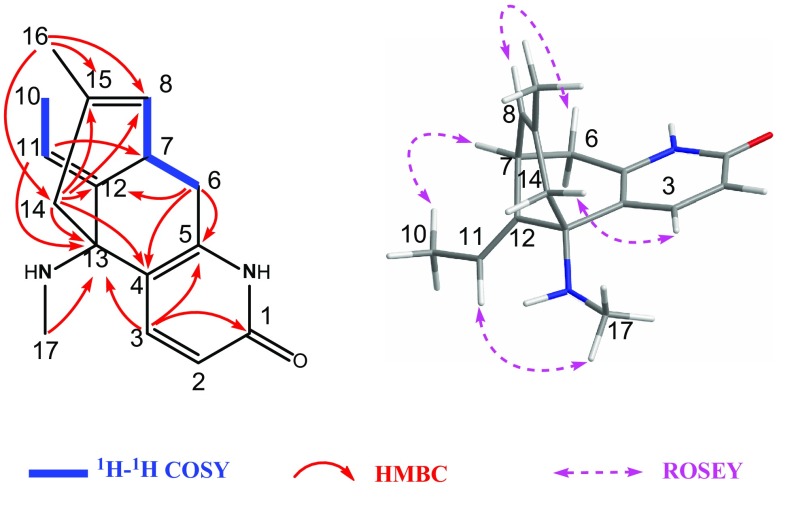
Compound 4 was assigned with a molecular ion peak at m/z 257.1653 [M+H]+ (Calcd 257.1654) in the HR–ESI–MS, coincided with the molecular formula of C16H20N2O, which required eight degrees of unsaturation. IR spectrum revealed the presence of NH (3431 cm−1) and amide carbonyl (1657 cm−1) groups. The 13C NMR and DEPT spectra displayed 16 carbon signals (Table 2), including five sp 2 quaternary carbons [one carbonyl (δ C 165.6) and four olefinic (δ C 120.7, 135.4, 137.3, and 145.9)], one sp 3 quaternary carbon (δ C 61.1), one sp 3 methines (δ C 34.3), four sp 2 methines (δ C 114.3, 118.1, 125.8, and 142.3), two sp 3 methylenes (δ C 35.2 and 50.7), and three methyls (δ C 12.5, 22.7, and 29.7) with corresponding protons as double signal at δ H 1.71 and singlet signals at δ H 1.53 and 2.09, respectively, in the 1H NMR spectrum (Table 1). The above data allowed 4 to be a N-methyl derivative of huperzine A, which was confirmed by the observed key HMBC correlation between the proton at δ H 2.09 with the sp 3 quaternary carbon C-13 at δ C 61.1 (Fig. 5). The observed ROESY correlations (Fig. 5) of H-6b/H-8 and of H-7/H-10 (δ H 1.71) indicated that H-7 was α-orientated. The R * configuration of C-13 was assigned by the clear ROESY correlations of H-3 with H-14a and H-11 with H-17. Accordingly, the structure of 4 was characterized as N-methylhuperzine A, which reported previously as a synthetic product from the methylation of huperzine A [2, 9]. To our knowledge, compound 4 was isolated for the first time from natural resources.
Fig. 5.
Key 2D NMR correlations of compound 4
Compounds 1-5 were evaluated for their β-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) inhibitory activity. Unfortunately, the results showed that all the compounds were inactive (IC50 values >100 μM).
Experimental Section
Gernal Experimental Procedures
UV spectra were recorded with a Shimadzu UV-2401A spectrophotometer. IR spectra were recorded on Bruker Tensor 27 spectrometer with KBr pellets. 1D and 2D NMR spectra were carried out on Bruker AM-400, DRX-500, or AVANCE III-600 spectrometers. Chemical shifts were reported using TMS as the internal standard. ESI–MS were run on Shimadzu UPLC–IT–TOF–MS instrument. HR–ESI–MS spectra were measured using Agilent G 6230 TOF MS (Agilent). EI–MS and HR–EI–MS spectra were measured with a Waters AutoSpec Premier P776 mass spectrometer (Waters, Milford, MA, USA).Crystal analysis were performed on a Bruker APEX DUO diffractometer equipped with an APEX II CCD, using Cu Kα radiation (λ = 1.54178 Å). Cell refinement and data reduction were performed with Bruker SAINT. Column chromatography (CC) was performed on silica gel (100–200 or 200–300 mesh; Qingdao Marine Chemical Co. Ltd., Qingdao, China), Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Sala, Sweden) and MPLC was performed on a Lisui EZ Purify III System packed with MCI gel (CHP20P, 75–150 mm; Mitsubishi Chemical Corporation, Tokyo, Japan). Precoated silica gel GF254 plates (Qingdao Haiyang Chemical Co. Ltd.) were used for thin-layer chromatography (TLC). Fractions were monitored by TLC and spots were visualized by Dragendorff’s reagent.
Plant Material
The club moss H. serrata was collected from Taijiang County, Guizhou Province, People’s Republic of China in July, 2012. The plant was identified by one of the authors, Prof. Lu-Tai Pan (Guiyang College of Traditional Chinese Medicine). And the voucher specimen (No. 20120312h) was deposited at the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences.
Extraction and Isolation
The aerial parts of club moss H. serrata (40 kg) were chopped into sections and extracted with 60% EtOH/H2O under reflux for three times (24 h × 3). The resultant extract was partitioned between EtOAc and 1% HCl/H2O solution to afford ethyl acetate and water soluble fractions, respectively. The water-soluble fractions were adjusted to pH 9 with 17% ammonia solution, and then extracted with CHCl3 to give an alkaloidal extract (106 g). The alkaloidal extract was subjected to a MCI gel CC (MeOH/H2O, 5 to 100%) to afford fractions I–V. Fraction I (18.0 g) was further chromatographed over a silica gel CC (CHCl3/MeOH, 50:1 → 5:1) to give subfractions (Fr. I–I to Fr. I–III). Fr. I–II (3.4 g) was subjected to a silica gel CC (EtOAc/MeOH, 25:1 → 5:1) to afford compounds 8 (5.3 mg) and 9 (35.0 mg). From Fr. I–III (18.6 g), compounds 7 (12.5 mg) and 10 (10.8 mg) were obtained after purified by a silica gel CC (CHCl3/MeOH, 30:1 → 10:1). Fraction II (15.0 g) was subjected to a silica gel CC eluted with petroleum ether (PE)/EtOAc/Et2NH, 100:2:1 → 50:50:1, to give subfractions (Fr. II–I to Fr. II–III). Fr. II–I (2.4 g) was separated over a silica gel CC (EtOAc/MeOH, 15:1 → 5:1) to yield compounds 2 (9.5 mg), 4 (5.3 mg), and 6 (18.7 mg). Fr. II–II (1.6 g) was purified by CC over a silica gel (CHCl3/MeOH, 40:1 → 1:1) to give compound 3 (30.1 mg). Fraction III (12.9 g) was subjected to a Sephadex LH-20 CC (MeOH) to yield subfractions (Fr. III–I to Fr. III–III). Fr. III–II (3.5 g) was chromatographed on a silica gel CC (CHCl3/EtOAc/MeOH, 20:5:1 → 5:5:1) to afford compounds 12 (16.0 mg) and 13 (3.7 mg). Fraction IV (11.4 g) was performed on repeated silica gel CC (PE/acetone/Et2NH, 80:1:1 → 50:5:1 and then EtOAc/MeOH, 35:1 → 10:1) to provide subfractions (Fr. IV–I to Fr. IV–III). Fraction IV–I (2.5 g) was chromatographed over a silica gel CC (CHCl3/MeOH, 20:1 → 5:1) to furnish compound 14 (16.4 mg). Fraction IV–II (3.9 g) was submitted to a Sephadex LH-20 CC (MeOH) and further purified via a silica gel CC (PE/acetone/Et2NH, 50:1:1 → 50:10:1) to produce compounds 1 (8.7 mg) and 15 (8.7 mg). The last Fraction V (9.3 g) was applied to repeated silica gel CC (CHCl3/acetone, 30:1 → 1:1 and then PE/EtOAc, 10:1 → 1:1) and purified via a Sephadex LH-20 CC (MeOH) to yield compounds 5 (18.5 mg) and 11 (6.6 mg).
1-Methyllycodine (1)
Colorless oil; – 8.8 (c = 0.12, MeOH); UV (MeOH) λ max (log ε) 209 (3.56), 273 (3.40), 378 (1.91), 452 (1.56), 570 (0.91) nm; IR (KBr) ν max 3433, 2929, 1677, 1429, 1204, 1134, 837, and 722 cm−1; 1H NMR and 13C NMR data see Tables 1 and 2; ESIMS (positive) m/z 257 [M+H]+; HRESIMS (positive) m/z 257.2014 [M+H]+ (calcd for C17H24N2, 257.2018).
8α-Hydroxy-15,16-dehydro-des-N-methyl-α-obscurine (2)
Colorless solid; mp 264–265 °C; – 40.4 (c = 0.11, MeOH); UV (MeOH) λ max (log ε) 202 (3.64), 254 (3.73) nm; IR (KBr) ν max 3426, 2926, 1660, 1440, 1383, 1220, 1026, 905, and 651 cm−1; 1H NMR and 13C NMR data see Tables 1 and 2; EIMS m/z 274 [M]+ (10), 260 (8), 203 (100), 175 (41), 91 (10); HREIMS (positive) m/z 274.1674 [M]+ (calcd for C16H22N2O2, 274.1681).
N-Methyl-16-hydroxyhuperzine B (3)
Colorless solid; mp 200–201 °C; – 44.8 (c = 0.12, MeOH); UV (MeOH) λ max (log ε) 203 (4.00), 231 (3.95), 310 (3.82) nm; IR (KBr) ν max 3421, 2929, 1657, 1453, 1304, 1105, 837, and 515 cm−1; 1H NMR and 13C NMR data see Tables 1 and 2; ESIMS (positive) m/z 287 [M+H]+; HRESIMS (positive) m/z 287.1759 [M+H]+ (calcd for C17H22N2O2, 287.1760).
N-Methylhuperzine A (4)
Colorless solid; mp 235–236 °C; – 97.6 (c = 0.11, MeOH); UV (MeOH) λ max (log ε) 202 (3.95), 232 (3.97), 312 (3.86) nm; IR (KBr) ν max 3431, 2927, 1657, 1610, 1441, 1121, 839, and 652 cm−1; 1H NMR and 13C NMR data see Tables 1 and 2; ESIMS (positive) m/z 257 [M+H]+; HRESIMS (positive) m/z 257.1653 [M+H]+ (calcd for C16H20N2O, 257.1654).
6β-Hydroxyhuperzine A (5)
Colorless crystals; mp 207–210 °C; – 145.2 (c = 0.11, MeOH); UV (MeOH) λ max (log ε) 203 (3.93), 232 (3.80), 310 (3.65) nm; IR (KBr) ν max 3415, 2926, 1655, 1598, 1441, 1383, 1090, 841, and 621 cm−1; 1H NMR and 13C NMR data see Tables 1 and 2; ESIMS (positive) m/z 259 [M+H]+; HRESIMS (positive) m/z 259.1443 [M+H]+ (calcd for C15H18N2O2, 259.1447).
X-ray Crystal Structure Analysis
Crystal analysis were performed on a Bruker APEX DUO diffractometer equipped with an APEX II CCD, using Cu Kα radiation (λ = 1.54178 Å). Cell refinement and data reduction were performed with Bruker SAINT.
The structure of 5 was solved by direct methods using SHELXS-97. Refinements were performed with SHELXL-97 using full-matrix least-squares, with anisotropic displacement parameters for all the non-hydrogen atoms. The H-atoms were placed in calculated positions and refined using a riding model. Crystallographic data for 5 in this paper have been deposited with the Cambridge Crystallographic Data Centre (CCDC 1518517). Copies of the data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk.
X-ray Crystal Data for 6β-Hydroxyhuperzine A (5)
C15H18N2O2·H2O, M = 276.33, a = 14.227(7) Å, b = 12.474(6) Å, c = 8.948(5) Å, α = 90°, β = 113.406(7)°, γ = 90°, V = 1457.4(13) Å3, T = 100(2) K, space group C2, Z = 4, μ(MoKα) = 0.088 mm−1, 6582 reflections measured, 3687 independent reflections (R int = 0.0400). The final R 1 values were 0.0840 (I > 2σ(I)). The final wR(F 2) values were 0.2254 (I > 2σ(I)). The final R 1 values were 0.0914 (all data). The final wR(F 2) values were 0.2326 (all data). The goodness of fit on F 2 was 1.168. Flack parameter = 0.3(7).
BACE1 Inhibitory Activity Assay
Compounds 1–5 were assessed for β-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) inhibitory activity. BACE1 inhibitory evaluation was tested using a fluorescence resonance energy transfer (FRET) assay kit supplied by PanVera (Kit P2985, Madison, WI, USA). The kit was using purified baculovirus expression BACE1 and substrates of a new red FRET peptide substrates, which were based on the “Swedish” mutation. The BACE1 FRET assay was carried out according to the principle described in Ref. [26]. The first orally available non-peptidic β-secretase inhibitor LY2811376, [28] which had an IC50 value of 401.21 nM, was using as a positive control.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by the NSFC-Joint Foundation of Yunnan Province (No. U1502223), the National Natural Science Foundation of China (No. 21402212), the Science and Technology Program of Yunnan province (No 2015FB173), and the CAS “Light of West China” Program and Youth Innovation Promotion Association CAS (X.D. Wu).
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13659-017-0140-z) contains supplementary material, which is available to authorized users.
Contributor Information
Xing-De Wu, Email: wuxingde@mail.kib.ac.cn.
Qin-Shi Zhao, Email: qinshizhao@mail.kib.ac.cn.
References
- 1.Siengalewicz P, Mulzer J, Rinner U. Alkaloids. 2013;72:1–151. doi: 10.1016/b978-0-12-407774-4.00001-7. [DOI] [PubMed] [Google Scholar]
- 2.Liu JS, Zhu YL, Yu CM, Zhou YZ, Han YY, Wu FW, Qi BF. Can. J. Chem. 1986;64:837–839. doi: 10.1139/v86-137. [DOI] [Google Scholar]
- 3.Hirasawa Y, Tanaka T, Koyama K, Morita H. Tetrahedron Lett. 2009;50:4816–4819. doi: 10.1016/j.tetlet.2009.05.072. [DOI] [Google Scholar]
- 4.Hirasawa Y, Kobayashi JI, Morita H. Heterocycles. 2009;77:679–729. doi: 10.3987/REV-08-SR(F)6. [DOI] [Google Scholar]
- 5.Ma X, Gang R. David. Nat. Prod. Rep. 2004;21:752–772. doi: 10.1039/b409720n. [DOI] [PubMed] [Google Scholar]
- 6.Kitajima M, Takayama H. Top. Curr. Chem. 2012;309:1–32. doi: 10.1007/128_2011_126. [DOI] [PubMed] [Google Scholar]
- 7.Ayer WA. Nat. Prod. Rep. 1991;8:455–463. doi: 10.1039/np9910800455. [DOI] [PubMed] [Google Scholar]
- 8.Tang Y, Fu Y, Xiong J, Li M, Ma GL, Yang GX, Wei BG, Zhao Y, Zhang HY, Hu JF. J. Nat. Prod. 2013;76:1475–1484. doi: 10.1021/np4003355. [DOI] [PubMed] [Google Scholar]
- 9.Liu JS, Yu CM, Zhou YZ, Han YY, Qi BF, Zhu YL. Acta Chem. Sin. 1986;44:1035–1040. [Google Scholar]
- 10.Shu S, Zhao X, Wang W, Zhang G, Cosoveanu A, Ahn Y, Wang M. World J. Microbiol. Biotechnol. 2014;30:3101–3109. doi: 10.1007/s11274-014-1737-6. [DOI] [PubMed] [Google Scholar]
- 11.Wang HB, Tan CH, Tan JJ, Qu SJ, Chen YL, Li YM, Jiang SH, Zhu DY. Nat. Prod. Res. 2009;23:1363–1366. doi: 10.1080/14786410802253239. [DOI] [PubMed] [Google Scholar]
- 12.Tan CH, Wang BD, Jiang SH, Zhu DY. Planta Med. 2002;68:188–190. doi: 10.1055/s-2002-20243. [DOI] [PubMed] [Google Scholar]
- 13.Tan CH, Jiang SH, Zhu DY, Huperzine P. Tetrahedron Lett. 2000;41:5733–5736. doi: 10.1016/S0040-4039(00)00893-5. [DOI] [Google Scholar]
- 14.Jiang WW, Liu F, Gao X, He J, Cheng X, Peng LY, Wu XD, Zhao QS. Fitoterapia. 2014;99:72–77. doi: 10.1016/j.fitote.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Ying YM, Liu XS, Tong CP, Wang JW, Zhan ZJ, Shan WG. Helv. Chim. Acta. 2014;97:1433–1439. doi: 10.1002/hlca.201400015. [DOI] [Google Scholar]
- 16.Ayer WA, Browne LM, Orszanska H, Valenta Z, Liu J. Can. J. Chem. 1989;67:1538–1540. doi: 10.1139/v89-234. [DOI] [Google Scholar]
- 17.Liu F, Wu XD, He J, Deng X, Peng LY, Luo HR, Zhao QS. Tetrahedron Lett. 2013;54:4555–4557. doi: 10.1016/j.tetlet.2013.06.083. [DOI] [Google Scholar]
- 18.Yuan SQ, Wei TT. Yaoxue Xuebao. 1988;23:516–520. [PubMed] [Google Scholar]
- 19.Anet FAL, Eves CR. Can. J. Chem. 1958;36:902–909. doi: 10.1139/v58-130. [DOI] [Google Scholar]
- 20.Ayer WA, Iverach GG. Can. J. Chem. 1960;38:1823–1826. doi: 10.1139/v60-247. [DOI] [Google Scholar]
- 21.Nakashima TTS, Peter P, Browne LM, Ayer WA. Can. J. Chem. 1975;53:1936–1942. doi: 10.1139/v75-270. [DOI] [Google Scholar]
- 22.Choo CY, Hirasawa Y, Karimata C, Koyama K, Sekiguchi M, Kobayashi JI, Morita H. Bioorg. Med. Chem. 2007;15:1703–1707. doi: 10.1016/j.bmc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Ayer WA, Kasitu GC. Can. J. Chem. 1989;67:1077–1086. doi: 10.1139/v89-163. [DOI] [Google Scholar]
- 24.Shen YC, Chen CH. J. Nat. Prod. 1994;57:824–826. doi: 10.1021/np50108a021. [DOI] [PubMed] [Google Scholar]
- 25.Zhang DB, Chen JJ, Song QY, Zhang L, Gao K. Molecules. 2014;19:9999–10010. doi: 10.3390/molecules19079999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu Y, Gao H, Xu F, Wang C, Liu P, Yang G, Sun Q, Xu P. Chem. Biol. Drug Des. 2012;80:775–780. doi: 10.1111/cbdd.12016. [DOI] [PubMed] [Google Scholar]
- 27.Alam SN, Adams KAH, MacLean DB. Can. J. Chem. 1964;42:2456–2466. doi: 10.1139/v64-361. [DOI] [Google Scholar]
- 28.May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, Monk SA, Mathes BM, Mergott DJ, Watson BM, Stout SL, Timm DE, Smith Labell E, Gonzales CR, Nakano M, Jhee SS, Yen M, Ereshefsky L, Lindstrom TD, Calligaro DO, Cocke PJ, Greg Hall D, Friedrich S, Citron M, Audia JE. J. Neurosci. 2011;31:16507–16516. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.