Abstract
The pathological progression of osteoarthritis (OA) involves degradation of articular cartilage matrix. Type II collagen is the main component of cartilage matrix, which is degraded by pro-inflammatory cytokines such as IL-1β mediated by MMP-13. Nebivolol, a licensed drug used for the treatment of hypertension in clinics, displays its anti-inflammatory capacity in various conditions. However, whether Nebivolol has a protective effect on cartilage matrix degradation has not been reported before. In this study, we investigated the effects of Nebivolol on regulating the expression of MMP-13 and degradation of type II collagen. Our results indicate that Nebivolol alleviated the increase in gene expression, protein expression, and activity of MMP-13 induced by IL-1β. Importantly, IL-1β strikingly reduced the levels of type II collagen in cell culture supernatants, which was reversed by treatment with Nebivolol in a dose-dependent manner. Mechanistically, Nebivolol was found to alleviate the increased levels of phosphorylated IκBα and reduced levels of total IκBα induced by IL-1β, which subsequently mitigated p65 nuclear translocation and the transcriptional activity of NF-κB. Furthermore, our results indicated that IL-1β treatment resulted in a significant increase in expression of the transcriptional factor interferon regulatory factor-1 (IRF-1) at both the mRNA and protein levels, which was significantly ameliorated by treatment with Nebivolol. The combination of these findings suggests that Nebivolol can potentially be applied in human OA treatment.
Keywords: Osteoarthritis (OA), Nebivolol, Il-1β, MMP-13, NF-κB, Collagen
Introduction
Osteoarthritis (OA) is a group of diseases and mechanical abnormalities affecting millions of people worldwide. The pathological progression involves degradation of articular cartilage matrix and the sub-chondral bone (Patra and Sandell 2011). Previous studies suggest that the inhibition of cartilage loss and the promotion of cartilage repair are important issues to address. However, there is currently no cure for OA (Loeser et al. 2012). Cartilage matrix degradation is mainly mediated by matrix metalloproteinases (MMPs) Goldring and Marcu 2009). Type II collagen is the main component of cartilage matrix. In particular, MMP-13 is the most potent enzyme in cleaving type II collagen. Pro-inflammatory cytokines such as interleukin-1β (IL-1β) play pivotal roles in degradation of type II collagen. It is well known that IL-1β induces the activation of pro-inflammatory transcriptional factor nuclear factor-κB (NF-κB) through phosphorylation of IκB kinase (IKK) and IκB. Activation of NF-κB induces inflammation-related gene expression, such as matrix metalloproteinase-13 (MMP-13) (Mengshol et al. 2000). Transcriptional activation of MMP-13 induced by IL-1β is also mediated by the transcriptional factor interferon regulatory factor-1 (IRF-1), the expression of which in OA chondrocytes is significantly higher compared to those in normal chondrocytes (Lu et al. 2014). It is suggested that inhibition of MMP-13 expression may show some beneficial effects of chondro-protection on the pathological conditions such as OA (Johnson et al. 2007).
Nebivolol, the third-generation β-blocker, has been approved by the Food and Drug Administration (FDA) for the treatment of hypertension in clinics. Nebivolol has been shown to reduce systemic vascular resistance and improve diastolic function (Mercanoglu et al. 2010). In addition, clinical investigations also demonstrated that Nebivolol is capable of reducing mortality and morbidity in elderly patients with heart failure (Dobre et al. 2007). Notably, a recent study demonstrated that Nebivolol significantly attenuated vascular remodeling by attenuating hypertension-induced increases in aortic NAD(P)H oxidase activity, superoxide production, TBARS concentrations, nitrotyrosine levels, TGF-β upregulation, and MMP-2 and MMP-9 expression/activity (Ceron et al. 2013). Another study also displayed the anti-pro-inflammatory property of Nebivolol by showing that Nebivolol downregulates Ox-LDL-induced elevation of adhesion molecules such as ICAM-1, ICAM-2, ICAM-3, E-selectin, and P-selectin (Garbin et al. 2008). However, it is unknown whether Nebivolol regulates the expression of MMP-13 and degradation of type II collagen. In this study, we report that Nebivolol ameliorated the induction of MMP-13 and reduction of type II collagen through blocking both the IκBα/NF-κB and STAT-1/IRF-1 pathways.
Materials and methods
Isolation and culture of chondrocytes from human articular cartilage
Healthy femoral head articular cartilage specimens were obtained from 12 generally healthy patients aged 50–71 years (mean age 61.2 ± 2.7 years) undergoing joint replacement surgery and isolated for human articular chondrocytes. The samples were collected with written approvals from the Institutional Ethics Committee at Dalian University and also from the patients. Cartilage pieces were minced finely and digested at 37 °C with 0.2% collagenase (type II; Sigma-Aldrich) for 4 h in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA). Chondrocytes released from matrix were filtered through a nylon mesh filter to isolate single cells. Isolated cells were cultured in DMEM medium containing 20 mM HEPES and 10% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 mg/ml) (pH = 7.4). Cells were treated with10 ng/ml IL-1β in the presence or absence of various concentrations of Dl-Nebivolol for 24 h. Stat1 small interfering RNA (siRNA) (Cell Signaling Technology) was transfected into cells by DharmaFECT reagent according to the manufacturer’s instructions for 24 h before treatment with IL-1β (10 ng/ml). Human chondrocytes were infected with an Iκ-Bα-dominant negative mutant (Iκ-Bα-S32A/S36A; DNM IκBα) adenovirus (Vector Biolabs, USA) or control. Twenty-four hours later, cells were treated with IL-1β (10 ng/ml) in the presence or absence of Nebivolol (10 μM) for another 24 h.
Real-time PCR
Intracellular RNA was extracted from chondrocytes by using Qiazol (Qiagen, France). NanoDrop was used to measure the quantity of RNA. Reverse transcription was performed on 1 μg RNA by use of the iScript cDNA Synthesis Kit (Bio-Rad, USA) to synthesize cDNA. Gene expression at messenger RNA (mRNA) levels was determined by real-time PCR with GoTaq qPCR Master Mix (Promega, Charbonnières les Bains, France) in the LC480 LightCycler Real-Time PCR system (Roche Applied Science, Meylan, France). Target gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Following primers were used in this study: humanIRF-1 forward 5′-ctggagagggtctcgctgt-3′ and reverse 5′-ttctggggtcactggtctgt-3′, human MMP-13 forward 5′-ccagtctccgaggagaaaca-3′ and reverse 5′-aaaaacagctccgcatcaac-3′, and human GAPDH forward 5′-ccacatcgctcagacaccat-3′ and reverse 5′-ccaggcgcccaatacg-3′.
MMP-13 enzymatic activity assays
Upon completion of indicated treatment, MMP-13 activity in the culture supernatants was described previously (Francin et al. 2014). The synthetic fluorogenic substrate Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 was used to determine MMP-13 activity in continuous assays. Results were normalized to concentration of proteins in the culture supernatant.
Western blot analysis
Human chondrocytes were washed with PBS. The cellular lysates were prepared by using the cell lysis buffer. Twenty micrograms of total intracellular proteins was resolved on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to immobilon polyvinyl difluoride (PVDF) membranes (Bio-Rad, USA). The transferred membranes were blocked with 4% BSA for 1 h at room temperature, followed by incubation with the primary antibodies against IκB kinase (IKK)α/β, p-IKKα/β, IkBα, p-IkBα, p65, p-p65, and collagen II (1:1000, Santa Cruz) overnight at 4 °C. Then, cells were washed for three times and the blots were subsequently incubated with the secondary goat anti-rabbit or anti-mouse antibodies conjugated with horseradish peroxidase (1:1000) for 1 h at room temperature. The blots were developed with enhanced chemiluminescence reagent. For MMP-13 and type II collagen expressions in cell culture supernatants, media was collected after indicated treatment, followed by concentrated with TCA and acetone as described previously (Liacini et al. 2005). MMP-13 and type II collagen expressions at protein levels were evaluated by western blot analysis.
Luciferase reporter assays
Human chondrocytes were transfected with pNF-κB-Luc using Lipofectamine 2000 (Invitrogen). Transfected cells were treated with 10 ng/ml IL-1β in the presence or absence of various concentrations of Dl-nebivolol for 24 h. Then, cells were lysed and luciferase activity was determined by use of a Dual-Luciferase Kit (Promega, USA) and TD-20/20 luminometer (TurnerDesigns, USA) according to the manufacturer’s instructions.
Immunofluorescence
The expressions of IRF-1 at protein levels were determined by the immunofluorescence assay. Briefly, cells were washed for three times with PBS and fixed with 4% paraformaldehyde for 10 min at room temperature (RT), followed by permeabilization with 0.1% Triton X-100 on ice. Then, cells were blocked with 5% normal goat serum in phosphate-buffered saline (PBS) for 1 h at RT and incubated with primary antibodies diluted in TBS (1:500). Two hours later, cells were incubated with Alexa-594-conjugated secondary antibodies diluted in TBS (1:200) for 1 h at RT. Nuclear was stained with 4′,6-diamidino-2-phenylindole (DAPI) (Vector labs, USA).
Statistical analysis
Experimental data are shown as arithmetic mean ± SD. Statistical analysis was evaluated using unpaired Student’s t test and one-way ANOVA followed by Dunnett’s analysis. P < 0.05 was considered significantly different.
Results
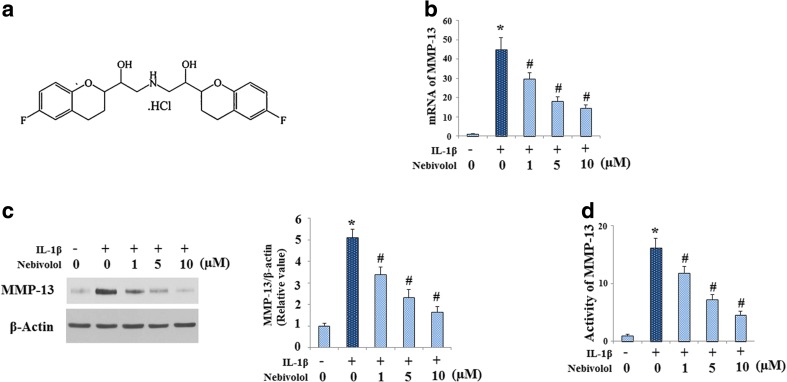
Nebivolol is one of the most important β1-adrenergic receptor blockers; the molecular structure of which is shown in Fig. 1a. Firstly, we investigated whether Nebivolol regulated IL-1β-induced gene expression of MMP-13 by quantitative real-time PCR. As shown in Fig. 1b, IL-1β treatment at the concentration of 10 ng/ml significantly increased the expression of MMP-13 at mRNA levels, which was attenuated by Nebivolol in a dose-dependent manner from 1 to 10 μM. Consistently, western blot analysis results revealed that Nebivolol could ameliorate the effect of IL-1β on inducing the protein expression of MMP-13 in cell culture supernatants (Fig. 1c). In addition, Nebivolol alleviated the increase in MMP-13 activity induced by IL-1β (Fig. 1d).
Fig. 1.
Effects of Nebivolol on IL-1β (10 ng/ml)-mediated gene expression and activation of matrix metalloproteinase (MMP)-13. Cells were treated with IL-1β (10 ng/ml) in the absence or presence of Nebivolol from 1, 5, and 10 μM for 24 h. a Molecular structure of Nebivolol. b mRNA levels of MMP-13 determined by real-time PCR. c Protein levels of MMP-13 in cell culture supernatants were determined by western blot analysis. d Activity of MMP-13 in cell culture supernatants was determined (*P < 0.01 vs. untreated group, # P < 0.01 vs. IL-1β-treated group)
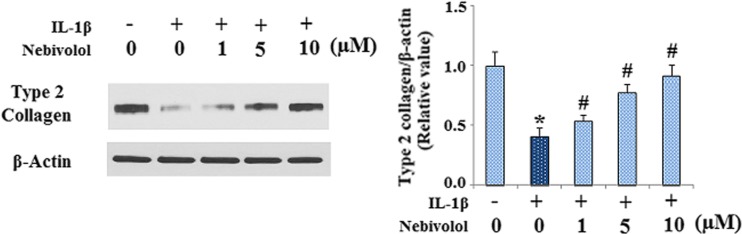
MMP-13 cleaves fibrillar collagens with preference to type II collagen. Therefore, we examined the effects of Nebivolol on the levels of type II collagen by western blot analysis. The results in Fig. 2 showed that IL-1β strikingly reduced the levels of type II collagen in cell culture supernatants, which was abolished by treatment with Nebivolol in a dose-dependent manner.
Fig. 2.
Nebivolol mitigates the degradation of type II collagen induced by IL-1β in human chondrocytes. Cells were treated with IL-1β (10 ng/ml) in the absence or presence of Nebivolol from 1, 5, and 10 μM for 24 h. The levels of collagen type II in cell culture supernatants were measured by western blot analysis (P < 0.01 vs. control group, # P < 0.01 vs. IL-1β-treated group)
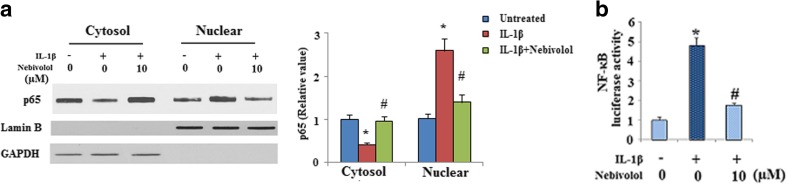
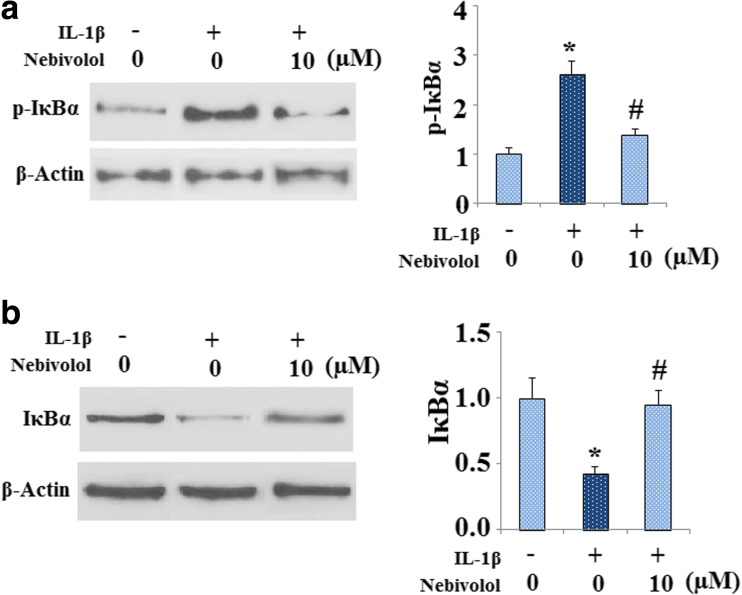
It is well known that the transcriptional factor NF-κB and its upstream signals are involved in the induction of MMPs by IL-1β in chondrocytes. We then investigated whether IL-1β-induced activation of NF-κB was affected by Nebivolol. Activation of the NF-κB transcription factor is associated with nuclear translocation of the p65 component of the complex. Nuclear proteins were extracted from chondrocytes and used for western blot analysis. As shown in Fig. 3a, IL-1β treatment promoted p65 nuclear translocation. However, treatment with Nebivolol mitigated p65 nuclear translocation induced by IL-1β. Consistently, the NF-κB-Luc reporter assay demonstrated that IL-1β drastically induced NF-κB luciferase activity, which was markedly alleviated by Nebivolol treatment (Fig. 3b). The activity of NF-κB is negatively regulated by IκB binding. IκB phosphorylation and subsequent degradation plays a pivotal role in regulating NF-κB activation. Our results indicate that IL-1β treatment resulted in a significant increase in the level of phosphorylated IκBα, which was significantly alleviated by treatment with Nebivolol (Fig. 4a). In addition, the effects of Nebivolol on IκBα degradation were evaluated by western blot analysis. Treatment with IL-1β induced a significant reduction of IκB-α. However, Nebivolol treatment attenuated the degradation of IκB-α (Fig. 4b). In order to confirm the role of the IκBα/NF-κB pathway in MMP-13 expression, cells were infected with either a recombinant adenovirus encoding a dominant negative mutant form of IκBα (IκBα-S32A/S36A; DNM IκBα) or control, followed by treatment with IL-1β in the presence or absence of Nebivolol. Overexpression of IκBα was verified by western blot analysis in Fig. 5a. Results in Fig. 5b indicate that expression of the dominant negative mutant IκB (DNM IκBα) blocked the effects of IL-1β and Nebivolol on the expression of extracellular MMP-13.
Fig. 3.
Nebivolol mitigates the activation of NF-κB. Cells were treated with IL-1β (10 ng/ml) in the absence or presence of 10 μM Nebivolol for 24 h. a Effects of Nebivolol on p65 nuclear translocation, GAPDH, and Lamin B served as controls in the cytosol and nucleus, respectively. b NF-κB luciferase reporter assays. Human chondrocytes transfected with pNF-κB-Luc reporter were treated with IL-1β (10 ng/ml) in the presence or absence of Nebivolol as indicated for 24 h before measuring luciferase activity (*P < 0.01 vs. non-treated control, # P < 0.01 vs. IL-1β-treated group)
Fig. 4.
Nebivolol mitigates the phosphorylation and degradation of IκBα in human chondrocytes induced by IL-1β. Cells were treated with IL-1β (10 ng/ml) in the absence or presence of 10 μM Nebivolol for 24 h. a Western blot and quantification analysis revealed that the phosphorylation of IκBα as induced by the administration of IL-1β was mitigated by Nebivolol. b Western blot and quantification analysis revealed that the reduction of IκBα as induced by the administration of IL-1β was attenuated by Nebivolol (*P < 0.01 vs. non-treated control, # P < 0.01 vs. IL-1β-treated group)
Fig. 5.
Human chondrocytes were infected with an IκBα-dominant negative mutant (IκBα-S32A/S36A; DNM IκBα) adenovirus (Vector Biolabs, USA) or control. Twenty-four hours later, cells were treated with IL-1β (10 ng/ml) in the presence or absence of Nebivolol (10 μM) for another 24 h. EV empty virus, DNM IκBα an IκBα-dominant negative mutant (IκBα-S32A/S36A; DNM IκBα) adenovirus. a Western blot analysis revealed the successful overexpression of DNM IκBα. b Expression of MMP-13 in the media was identified by western blot analysis (*P < 0.01 vs. non-treated EV control, # P < 0.01 vs. EV + IL-1β-treated group)
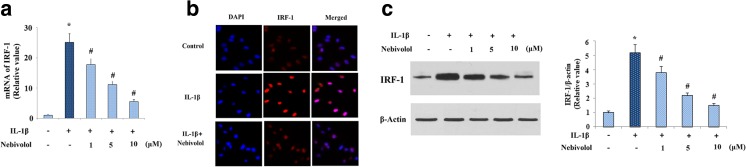
Previous studies also showed that transcriptional activation of MMP-13 induced by IL-1β is mediated by the transcriptional factor IRF-1. We further investigated whether IRF-1 participated in the inhibitory effects of Nebivolol on MMP-13 expression. Real-time PCR results displayed that treatment with Nebivolol attenuated the elevation of IRF-1 induced by IL-1β in a dose-dependent manner (Fig. 6a). Consistently, immunofluorescence study revealed that the expression of IRF-1 at protein levels was ameliorated by Nebivolol (Fig. 6b). In addition, the inhibitory effects of Nebivolol on IRF-1 expression at protein levels were confirmed by the western blot analysis (Fig. 6c).
Fig. 6.
Nebivolol mitigates the expression of IRF-1 at both mRNA and protein levels. a Cells were treated with IL-1β (10 ng/ml) in the absence or presence of Nebivolol from 1, 5, and 10 μM for 24 h. mRNA levels of IRF-1 were determined by real-time PCR. b Cells were treated with IL-1β (10 ng/ml) in the absence or presence of 10 μM Nebivolol for 24 h. Protein expression of IRF-1 was measured by an immunofluorescence assay. c Cells were treated with IL-1β (10 ng/ml) in the absence or presence of Nebivolol for 24 h. Protein expression of IRF-1 was measured by the western blot analysis (*P < 0.01 vs. non-treated control, # P < 0.01 vs. IL-1β-treated group)
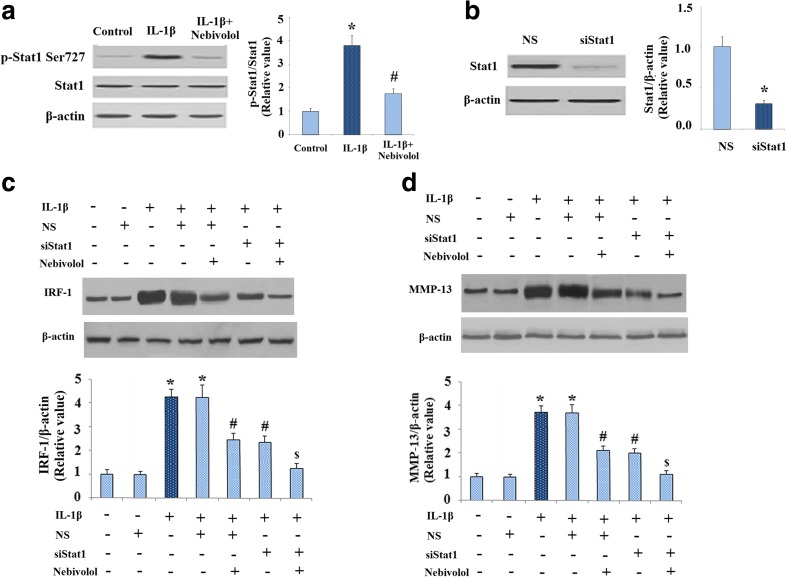
Expression of IRF-1 is regulated by signal transducers and activator of transcription 1 (STAT1). Therefore, we investigated whether Stat1 is involved in the regulatory roles of Nebivolol in IRF-1 expression. Activation of Stat1 is mediated by serine phosphorylation at amino acid 727 (Ser727). As shown in Fig. 7, IL-1β obviously increased phosphorylated levels of Stat1 at Ser727, which was ameliorated by treatment with Nebivolol. However, levels of total Stat1 maintain consistent. We next used siRNA transfection to confirm the role of STAT1 in IRF-1 and MMP-13 expressions. Successful knockdown of STAT1 was shown in Fig. 7b. As shown in Fig. 7c, d, knockdown of STAT1 reduced both intracellular IRF-1 and extracellular MMP-13, indicating the principal involvement of STAT1. In addition, silence of STAT1 by STAT1 siRNA promoted the inhibitory effects of Nevibolol on IRF-1 and MMP-13 expressions. These findings suggest that Nebivolol might alleviate the expressions of IRF-1 and MMP-13 through abolishing the activation of Stat1.
Fig. 7.
Nebivolol ameliorates phosphorylation of Stat1 in human chondrocytes induced by IL-1β. a Western blot and quantification analysis revealed that the phosphorylation of Stat1 as induced by the administration of IL-1β (10 ng/ml) was mitigated by 10 μM Nebivolol. b Cells were transfected with 20 nM siRNAs targeting STAT1 for 24 h and incubated further with IL-1β (10 ng/ml) in the presence or absence of various concentration of Dl-nebivolol for 24 h. The level of STAT1 was determined by western blot analysis. c Expression of IRF in intracellular extracts was identified by western blot. d Expression of MMP-13 in the media was identified by western blot (*P < 0.01 vs. non-treated control, # P < 0.01 vs. IL-1β-treated group, $ P < 0.01 vs. IL-1β + siSTAT1-treated group)
Discussion
The progressive degeneration of articular cartilage including chondrocyte loss and degradation of the extracellular matrix (ECM) is one of the main symptoms in OA (Vignon et al. 2000). It is well known that pro-inflammatory cytokines secreted by chondrocytes, such as IL-1β, contributes to the progression of OA (Goldring 2000). Chondrocyte apoptosis is an important hallmark of OA (Heraud et al. 2000). IL-1β treatment triggers large-scale chondrocyte apoptosis (Yasuhara et al. 2005). Specifically, administration of IL-1β facilitated the generation of reactive oxygen species (ROS), activated mitogen-activated protein kinase (MAPK) pathways, which led to chondrocyte apoptosis (Zhou et al. 2008). Thus, chondrocytes treated with IL-1β provided a useful model of OA chondrocytes (Sanchez et al. 2005a, 2005b). Anti-apoptotic capacity of Nebivolol has been reported in previous studies (Tsoporis et al. 2014). The effect of Nebivolol on IL-1β-induced apoptosis remains unknown and needs to be elucidated in future studies. Notably, administration of IL-1β has been used to mimic the status of chondrocytes in OA (Sanchez et al. 2005a, 2005b). In this study, we found that Nebivolol promoted type II collagen matrix production in IL-1β-stimulated articular chondrocytes, one type of model OA chondrocytes. Specifically, Nebivolol could ameliorate the expression and activity of MMP-13. The underlying mechanism of Nebivolol is associated with both NF-κB and IRF-1 pathways. These findings demonstrated that Nebivolol promoted matrix production in IL-1β-stimulated chondrocytes, suggesting that Nebivolol might represent a therapeutic potential for the treatment of cartilage damage in OA.
The degradation of collagen starts at the articular surface (Wu et al. 2002). As one of principal collagenases degrading cartilage matrix under pathological conditions such as OA, MMP-13 is overexpressed in the synovial space in many cases of human OA (Senolt et al. 2006). It is well known that MMP-13 is largely responsible to degrade cartilage collagen matrix in articular joints, especially type II collagen (Takaishi et al. 2008). An in vivo study with mice expressing an active human collagenase (MMP-13) transgene have shown that extensive damage to collagen and aggrecan occurs, leading to cartilage thinning, before overt fibrillation is seen. Consistently, Wen and colleagues reported that IL-1β treatment induced MMP-13 expression in the SW1353 chondrocyte cell line by activation of the transcription factor NF-κB (Wen et al. 2006). NF-κB is a protein complex that controls transcription of DNA in regulating pro-inflammatory signaling pathway, including MMP and pro-inflammatory cytokine secretion. In this study, we found that Nebivolol mitigated the expression of MMP-13. Consistently, Nebivolol has been reported to mitigate the expressions/activities of MMP-2 and MMP-9 (Ceron et al. 2013). In this study, Nevibolol was used at the concentrations of 1, 5, and 10 μM, which are consistent with previous studies showing that Nebivolol exerts various pharmacological actions in a range of micromolar concentrations and are comparable to the concentrations used for treatment of patients (Dogan et al. 2014). Consistently, it was shown that increasing amounts of the Nevibolol (from 1 to 25 μM) increased the production of nitric oxide and reduced the generation of ROS in endothelial cells (Cominacini et al. 2003). Pretreatment with 10 μM Nebivolol suppressed autophagy, restored mitochondrial biogenesis, leading to decreased mitochondrial reactive oxygen species (mtROS) generation in H9C2 cells (Xie et al. 2016). In addition, Nevibolol in a range of micromolar concentrations had a protective effect in isolated canine coronary arteries (Gao et al. 1991).
The transcription factor IRF-1 is also induced by IL-1β to regulate inflammation. MMP-13 is selectively regulated by IRF-1, the level of which is elevated in several chronic inflammatory diseases including OA (Lu et al. 2014). Our finding that Nebivolol could attenuate IL-1β-induced expression of IRF-1 might suggest its significance in IL-1β-mediated damage of cartilage. Activation of STAT1 through phosphorylation at Ser727 has been reported to be involved in the expression of IRF-1. The existence of JAK/STAT pathway plays an essential role in the process of inflammation in chondrocytes. Our results display that treatment with Nebivolol is able to mitigate the induction of IRF-1 by attenuating the activation of Stat1, exploring the underlying mechanisms. The relationship between the JAK/STAT/IRF-1 pathway and the NF-κB pathway is complex. Interestingly, NF-κB has been reported to regulate IL-1β-induced expression of IRF-1. Particularly, IL-1β activated IKK complex, the phosphorylation and degradation of IκBα, thereby liberated NF-κB (Shultz et al. 2009). Activation of the phosphorylation of p65 then interacts with IRF1 directly or indirectly to affect its binding ability. IL-1β stimulates p65 interaction with IRF-1 to regulate the platelet-derived growth factor (PDGF)-D promoter activity in smooth muscle cells (Liu and Khachigian 2009). Activation of STAT1 can cooperate with NF-κB to promote enhanced transcription of target genes, including IRF-1 (Ohmori et al. 1997). It is possible that there is a synergistic action between NF-кB and IRF-1 on regulating the expression of MMP-13 and degradation of type II collagen. Indeed, it has been reported that the activation of NF-κB and IRF-1 synergistically activated the RANTES promoter elements in response to TNF-α and IFN-γ and treatment (Lee et al. 2000). Logical next steps are to explore the possibility of a similar relationship between IRF1 and NF-кB in NF-κB and/or IRF1-deficient cells and to delineate the mechanism by which Nevibolol ameliorates IL-1β-induced MMP-13 expression and type II collagen degradation. Targeted strategies that interfere with IκBα/NF-κB and STAT-1/IRF-1 pathways could offer novel potential therapeutic options for OA treatment. There is currently no therapeutic with a clearly demonstrated ability to modify the course of OA (Cheng and Visco 2012). The inhibitory effects of Nebivolol against IL-1β-induced type II collagen degradation found in this study suggest its possible use in OA.
Footnotes
Zhigang Li and Baoyi Liu are the co-first authors.
References
- Ceron CS, Rizzi E, Guimarães DA, Martins-Oliveira A, Gerlach RF, Tanus-Santos JE. Nebivolol attenuates prooxidant and profibrotic effects of nebivolol on endothelial gene expression during oxidative stress in human umbilical vein endothelial cells mechanisms involving TGF-β and MMPs, and decreases vascular remodeling in renovascular hypertension. Free Radic Biol Med. 2013;65:47–56. doi: 10.1016/j.freeradbiomed.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Cheng DS, Visco CJ (2012) Pharmaceutical therapy for osteoarthritis. PM R 4:S82–S88 [DOI] [PubMed]
- Cominacini L, Fratta Pasini A, Garbin U, Nava C, Davoli A, Criscuoli M, Crea A, Sawamura T, Lo Cascio V. Nebivolol and its 4-keto derivative increase nitric oxide in endothelial cells by reducing its oxidative inactivation. J Am Coll Cardiol. 2003;42(10):1838–1844. doi: 10.1016/j.jacc.2003.06.011. [DOI] [PubMed] [Google Scholar]
- Dobre D, van Veldhuisen DJ, Mordenti G, Vintila M, Haaijer-Ruskamp FM, et al. Tolerability and dose-related effects of nebivolol in elderly patients with heart failure: data from the study of the effects of Nebivolol intervention on outcomes and Rehospitalisation in Seniors with Heart Failure (SENIORS) trial. Am Heart J. 2007;154:109–115. doi: 10.1016/j.ahj.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Dogan A, Karabacak M, Tayyar S, Erdogan D, Ozaydin M. Comparison of the effects of carvedilol and nebivolol on diastolic functions of the left ventricle in patients with non-ischemic heart failure. Cardiol J. 2014;21(1):76–82. doi: 10.5603/CJ.a2013.0062. [DOI] [PubMed] [Google Scholar]
- Francin J, Abot A, Guillaume C, et al. Association between adiponectin and cartilage degradation in human osteoarthritis. Osteoarthr Cartil. 2014;22:519–526. doi: 10.1016/j.joca.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Gao Y, Nagao T, Bond RA, Janssens WJ, Vanhoutte PM. Nebivolol induces endothelium-dependent relaxations of canine coronary arteries. J Cardiovasc Pharmacol. 1991;17:964–969. doi: 10.1097/00005344-199106000-00016. [DOI] [PubMed] [Google Scholar]
- Garbin U, Fratta Pasini A, Stranieri C, Manfro S, Mozzini C, Boccioletti V, Pasini A, Cominacini M, Evangelista S, Cominacini L. Effects of nebivolol on endothelial gene expression during oxidative stress in human umbilical vein endothelial cells. Mediat Inflamm. 2008;2008:567–590. doi: 10.1155/2008/367590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:59–65. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heraud F, Heraud A, Harmand MF. Apoptosis in normal and osteoarthritic human articular cartilage. Ann Rheum Dis. 2000;59:959–965. doi: 10.1136/ard.59.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AR, Pavlovsky AG, Ortwine DF, Prior F, Man CF, Bornemeier DA, Banotai CA, Mueller WT, McConnell P, Yan C, Baragi V, Lesch C, Roark WH, Wilson M, Datta K, Guzman R, Han HK, Dyer RD. Discovery and characterization of a novel inhibitor of matrix metalloprotease-13 that reduces cartilage damage in vivo without joint fibroplasia side effects. J Biol Chem. 2007;282:27781–27791. doi: 10.1074/jbc.M703286200. [DOI] [PubMed] [Google Scholar]
- Lee AH, Hong JH, Seo YS. Tumour necrosis factor-a and interferon-c synergistically activate the RANTES promoter through nuclear factor κB and interferon regulatory factor 1 (IRF-1) transcription factors. Biochem J. 2000;350:131–138. doi: 10.1042/bj3500131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liacini A, Sylvester J, Li WQ, Zafarullah M. Mithramycin downregulates proinflammatory cytokine induced matrix metalloproteinase gene expression in articular chondrocytes. Arthritis Res Ther. 2005;7:R777–R783. doi: 10.1186/ar1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MY, Khachigian LM. Histone deacetylase-1 is enriched at the platelet-derived growth factor-D promoter in response to interleukin-1beta and forms a cytokine-inducible gene-silencing complex with NF-kappab p65 and interferon regulatory factor-1. J Biol Chem. 2009;284:35101–35112. doi: 10.1074/jbc.M109.061903. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zeng C, Zhao H, Lian L, Dai Y. Glatiramer acetate inhibits degradation of collagen II by suppressing the activity of interferon regulatory factor-1. Biochem Biophys Res Commun. 2014;448(3):323–328. doi: 10.1016/j.bbrc.2014.03.041. [DOI] [PubMed] [Google Scholar]
- Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor κB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Mercanoglu GO, Pamukcu B, Safran N, Mercanoglu F, Fici F, et al. Nebivolol prevents remodeling in a rat myocardial infarction model: an echocardiographic study. Anadolu Kardiyol Derg. 2010;10:18–27. doi: 10.5152/akd.2010.006. [DOI] [PubMed] [Google Scholar]
- Ohmori Y, Schreiber RD, Hamilton TA. Synergy between interferon-γ and tumor necrosis factor-α in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor κB. J Bio Chem. 1997;1(272(23)):14899–14907. doi: 10.1074/jbc.272.23.14899. [DOI] [PubMed] [Google Scholar]
- Patra D, Sandell LJ. Evolving biomarkers in osteoarthritis. J Knee Surg. 2011;24:241–249. doi: 10.1055/s-0031-1286192. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE. Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes. This effect is mimicked by interleukin-6, −1beta and oncostatin M pre-treated nonsclerotic osteoblasts. Osteoarthr Cartil. 2005;13:979–987. doi: 10.1016/j.joca.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE. Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes. This effect is mimicked by interleukin-6, −1beta and oncostatin pre-treated non-sclerotic osteoblasts. Osteoarthr Cartil. 2005;13:979–987. doi: 10.1016/j.joca.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Senolt L, Grigorian M, Lukanidin E, Simmen B, Michel BA, Pavelka K, Gay RE, Gay S, Neidhart M. S100A4 is expressed at site of invasion in rheumatoid arthritis synovium and modulates production of matrix metalloproteinases. Ann Rheum Dis. 2006;65:1645–1648. doi: 10.1136/ard.2005.047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz DB, Rani MR, Fuller JD, et al. Roles of IKK-β, IRF1, and p65 in the activation of chemokine genes by interferon-γ. J Interf Cytokine Res. 2009;29:817–824. doi: 10.1089/jir.2009.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi H, Kimura T, Dalal S, Okada Y, D’Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr Pharm Biotechnol. 2008;9:47–54. doi: 10.2174/138920108783497659. [DOI] [PubMed] [Google Scholar]
- Tsoporis J, Rizos IK, Toumpoulis IK, Salpeas V, Izhar S. Parker TG (2014) Nebivolol suppresses hypoxic-induced rat thoracic aortic smooth muscle cell apoptosis by a mechanism involving nitric oxide production, HSP70 upregulation and inhibition of P53 phosphorylation. Arterioscler Thromb Vasc Biol. 2014;34:A119. [Google Scholar]
- Vignon E, Arlot M, Meunier P, Vignon G. Quantitative histological changes in osteoarthritic hip cartilage. Morphometric analysis of osteoarthritic and 26 normal human femoral heads. Clin Orthop Relat Res. 2000;103(1974):269–278. [PubMed] [Google Scholar]
- Wen D, Nong Y, Morgan JG, Gangurde P, Bielecki A, Dasilva J, Keaveney M, Cheng H, Fraser C, Schopf L, Hepperle M, Harriman G, Jaffee BD, Ocain TD, Xu Y. A selective small molecule IκB kinase β inhibitor blocks nuclear factor κB-mediated inflammatory responses in human fibroblast-like synoviocytes, chondrocytes and mast cells. J Pharmacol Exp Ther. 2006;317:989–1001. doi: 10.1124/jpet.105.097584. [DOI] [PubMed] [Google Scholar]
- Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, Poole AR. Sites of collagenase cleavage and denaturation of of type II collagen in articular cartilage in ageing and osteoarthritis and their relationship to the distribution of the collagenases MMP-1 and MMP-13. Arthritis Rheum. 2002;46:2087–2094. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- Xie Q, Wei T, Huang C, Liu P, Sun M, Shen W, Gao P. Nebivolol ameliorates cardiac NLRP3 inflammasome activation in a juvenile-adolescent animal model of diet-induced obesity. Sci Rep. 2016;6:34326. doi: 10.1038/srep34326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara R, Miyamoto Y, Akaike T, Akuta T, Nakamura M, Takami M, Morimura N, Yasu K, Kamijo R. Interleukin-1b induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species dependent manner. Biochem J. 2005;389:315–323. doi: 10.1042/BJ20041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1β-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop research. 2008;26:1643–1648. doi: 10.1002/jor.20683. [DOI] [PubMed] [Google Scholar]