Abstract
Phytochemicals extracted from flowers, roots and bark, leaves, and other plant sources have been used extensively throughout human history with varying levels of efficacy in prevention and treatment of disease. Recently, advanced methods for characterization and clinical use of these materials have allowed modern understanding of their properties to be used as immunomodulatory agents that act by enhancement of endogenous cytoprotective mechanisms, avoiding interference with normal physiologic signaling and highly effective medical treatment with minimal adverse side effects. Simple methods have been identified for improving their biological effects, such as thermal conditioning by heating or freezing—prominent example being heat treatment of lycopene and tetrahydrocannabinol. The present investigation shows improvement of the ability of heat to augment splenocyte proliferation, natural killer (NK) cell activities, and antioxidant capacity of the flavonoid luteolin-7-O-β-glucoside (L7G) in comparison with the native (non heat-treated) molecule, while further demonstrating that both the native and the heat-treated variants exhibit comparable antioxidant properties, as evidenced by their effects in macrophages by inhibition of nitric oxide production and lysosomal enzyme activity in experiments that strengthen lysosomal membrane integrity. Outcomes of these studies suggest that heat-treated L7G shows promise for use in immunotherapy, including anti-cancer regimens, as shown by its improvement of NK cell cytotoxicity.
Keywords: Luteolin-7-O-β-glucoside, Heated luteolin-7-O-β-glucoside, Immunomodulatory potential, Cellular antioxidant activity
Introduction
Since the middle of the last century, it has been evidenced that the immune system can recognize and reject tumors. Immunotherapy, which involves the cancer patient’s immune system by improving its ability to recognize the tumor or providing a missing immune effector functions, is one treatment approach that holds promise of a life-long cure (Finn 2012). Besides, the immunotherapy is a potential application as a treatment for inflammatory bowel disease (Geem et al. 2015). Therefore, it is important to discover items that help to modulate the immune system, to protect our body from bacterial infections, inflammatory diseases, and tumor development such as plants or molecules that are widespread in plants. Some epidemiological studies have shown that consuming fruits and vegetables rich in phenolic compounds leads to a general well-being of consumers (Gil et al. 2000). The antioxidant capacity of phenolic compounds plays a role in health-promoting capacity (Mellor and Naumovski 2016). In fact, during periods of stress such as drought or sun exposure, many polyphenols produced by plants contribute to stress tolerance for animals that consume them. This phenomenon of xenohormesis is a more conjectural theory, in general suggesting that animals and fungi are able to sense chemical signals produced by plants and other autotrophs in response to stress. Thus, secondary metabolites such as resveratrol and quercetin, produced by plants in response to stressors, act as physiologic signals within animals that consume them, which have evolved to activate endogenous cytoprotective processes in the animals, thus conferring survival advantages. Moreover, this model predicts that such “xenohormetic” molecules interact with conserved domains in cellular proteins critical for regulation of stress responses, as agonists, antagonists, and other means by which cellular activities are affected in ways that promote whole organism survival. This might explain the variety of underlying action mechanisms of molecules, which all serve to protect the animal consumer (Baur and Sinclair 2008; Hooper et al. 2010; Howitz and Sinclair 2008). During times of stress, plants synthesize many polyphenolic molecules that stimulate sirtuins. In fact, several polyphenols are able to interact with the mammalian sirtuin genes that regulate a number of transcription factors (p53, NF-κB, PPAR) which play key roles in stress responses, cell metabolism, and differentiation (Greiss and Gartner 2009). In this context, survival pathways in animals have retained the ability to respond to plant stress signaling molecules, as they provide useful predictions about the state of the environment and/or the food supply (Kennedy 2014; Leonov et al. 2015; Zhang and Tsao 2016). This ability would permit organisms to get ready and survive adversity. These benefits have been attributed to phenolic compounds which enhance the host stress responses (Allard et al. 2009; Lamming et al. 2004), particularly flavonoids which are relatively abundant in human diet (Batra and Sharma 2013) such us luteolin-7-O-β-glucoside (L7G) also known as cynaroside. This flavone possesses a wide range of biological activities including cytotoxic, anti-cancerous (Ahmed and Kamel 2014; Baskar et al. 2011), and anti-inflammatory potentials (Francisco et al. 2014). Furthermore, cynaroside presents antioxidant (Olennikov et al. 2013) and antimicrobial activities (Žemlička et al. 2014). The 7-O-glucoside luteolin is present in aqueous artichoke extracts (Wittemer et al. 2005), flower buds of Lonicera japonica (Hu et al. 2015) and Bidens parviflora Willd (Li et al. 2008), and apple fruits (De Paepe et al. 2013). Previous studies dealing with thermal stability of several plant molecules reported that heat treatment of some flavonoids as rutin, naringin, eriodictyol, and mesquitol (Chaaban et al. 2017) and pigments as lycopene (Vallverdu-Queralt et al. 2015) improved their antioxidant activity. Thus, we undertook in this study to investigate the effect of thermal processing, such as that undergone during transformation steps of raw material to finished products, of L7G, and to evaluate the involvement of its heat treatment in improving their potential cancerous preventing capacity by enhancing its cellular antioxidant activity, as well as its immunomodulatory potential.
Materials and methods
Reagents
Native and thermally treated luteolin-7-O-β-glucoside was provided by the Laboratory of Biomolecular Engineering, ENSAIA-INPL, University of Lorraine, Vandoeuvre-lès-Nancy.
Thermal treatment
The treatment of luteolin-7-glucoside was conducted at 130 °C in an oil-based bath for 2 h within Biomolecular Engineering Laboratory at the University of Lorraine, France (Chaaban et al. 2017). Flavonoids were diluted in dimethylsulfoxide (DMSO) stock solutions prior to addition to cell culture medium. The final concentration of dimethylsulfoxide (DMSO) never exceeded 0.1% (v/v).
Animals
Specific pathogen-free male BALB/c mice (20–22 g) were obtained from Pasteur Institute (Tunis, Tunisia). The mice were housed under standard conditions of temperature, humidity, and light (12 h light/dark) in an accredited pathogen-free facility. They were fed a commercial pellet diet and water ad libitum throughout the experiment period. All experiments were performed in accordance with the guidelines for the care and use of laboratory animals as published by the National Institute of Health. All experiments received the explicit approval of the Ethics Animal Committee in Tunisia.
Preparation of primary splenocytes
Spleen mice lymphocytes were obtained as previously reported (Limem et al. 2011). After washing with phosphate-buffered saline (PBS, pH 7.4), cells were resuspended in complete RPMI medium (Gibco BRL) containing 10% fetal bovine serum (FBS; Gibco) and 100 mg/ml gentamicin (Gibco BRL, Paisley, UK). Other mice were used to provide peritoneal macrophages as previously reported. Cell viability was assessed using the trypan blue exclusion technique.
Cell treatment
Splenocytes (5 × 106 cells per ml) were treated with various concentrations of molecules, then supplemented separately with optimal concentration of lectin (5 μg/ml) or lipopolysaccharide from Escherichia coli 0127:B8 (LPS) (5 μg/ml), for priming T cells and B cells, respectively. Macrophages (3 × 105 cells per well) were incubated with various concentrations of flavones with or without a unique concentration of LPS (5 μg/ml) solubilized in RPMI 1640 medium supplemented with 10% FBS and 100 mg/ml gentamicin (Manosroi et al. 2003). Cells were maintained at 37 °C in a 5% CO2-humidified incubator.
Lymphocyte T and B proliferation assay
Lymphocyte proliferation assay was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Mosmann 1983). Splenocyte suspension, in RPMI 1640 medium (5 × 106 cells/ml; 100 μl aliquot/well), was pre-incubated in a 96-well plate for 24 h, before the adding of mitogens (LPS or lectin, each at 5 μg/ml) and tested compounds solubilized in RPMI. Cells were then incubated at 37 °C in a humidified 5% CO2 atmosphere for an additional 48 h. Thereafter, 40 μl of MTT (5 mg/ml) was added to RPMI solution and incubated for 2 h at 37 °C. Plates were then centrifuged again, and the MTT removed from each well was dissolved in 100 μl of dimethylsulfoxide (98% DMSO). After incubation at 37 °C for 15 min, absorbance of formazan, formed in each well, was measured at 570 nm in a microplate reader (Thermo Scientific, Vantaa, Finland). The percentage of proliferation was finally calculated using the following equation:
Natural killer cell activity
NK cell activity was established as previously described by Sarangi et al. (2006), with minor modifications. Briefly, spleens prepared as described above were exploited as the source of effector cells; isolated splenocytes were seeded into 96-well microtiter plates at 5 × 106 cells/ml. The cells were then stimulated by different concentrations of the tested samples and incubated at 37 °C in 5% CO2 atmosphere for 24 h. To eliminate direct effects of samples on target cells, spleens were washed once with RPMI 1640, then 100-μl aliquots of target K562 cells (5 × 104 cells/ml) were added to each well. The plates were then incubated for 4 h, at 37 °C in 5% CO2 atmosphere. Three kinds of controls were achieved: target cell control (K562 cells), blank control (splenocytes incubated with K562 cells), and effector cell control (splenocyte cells). NK cell activity was calculated as follows:
Cytotoxic T lymphocyte assay
Cell-mediated cytotoxicity assay was achieved using MTT assay. Cytotoxicity of T lymphocytes was assessed as previously described for NK cell activity, with modification of target cells. In fact, B16F10 melanoma cells (5 × 104 cells/ml; yielding a 100:1 expected effector-target ratio) were added to each well, as target cells, in 50-μl aliquots. The plates were then incubated for 24 h at 37 °C in 5% CO2 atmosphere. Cytotoxic T lymphocyte (CTL) activity was calculated as follows: CTL activity (%) = 100 × (Abs T − (Abs S − Abs E)) / Abs T; where Abs T = absorbance value of target cells (B16F10), Abs S = absorbance value of test samples incubated with splenocytes and B16F10 cells, and Abs E = absorbance value of untreated splenocytes.
Assessment of lysosomal enzyme activity
Lysosomal enzyme activity (reflected by acid phosphatase activity in macrophages) was determined as previously described by Manosroi et al. (2005), with some modifications. This activity was measured at 405 nm in a microplate reader (Thermo Scientific, Vantaa, Finland). The absorbance values obtained were compared with control cells.
Measurement of nitrite production
The amount of NO released by macrophages was measured by determining the amounts of accumulated nitrite (NO− 2) in cell-free supernatants, via the Griess reaction (Green et al. 1982). Cells were incubated for 48 h in the presence of increasing concentrations of the tested samples, and NO production was determined by measuring absorbance of 100 μl of harvested culture supernatant incubated for 15 min with 100 μl of Griess reagent at 570 nm. The absorbance values obtained were compared with positive control cells treated with LPS.
Assessment of lysosomal membrane permeabilization
Acridine orange (AO) staining was performed to label lysosomes as described previously with some modification (Persson and Vainikka 2013). Briefly, macrophages were seeded at a density of 5 × 104 cells per well. Triplicate wells were then treated with 10 μl of each sample with 1 μl of acridine orange (0.1 mg/ml). After 15 min incubation, fluorescence of each well was determined using a fluorescence microplate reader (BioTek, Winooski, USA) with 538 nm emission and at 485-nm excitation filters. Plates include triplicate control wells with macrophages treated with AO; blank wells contained cells with PBS and sample wells contained macrophages incubated with native or heated L7G and AO. The percentage of lysosome permeability (LP) was calculated as follows:
Macrophage-mediated cytotoxicity assay
Macrophage-mediated cytotoxicity (MMC) was performed as previously reported by Ferrari et al. (1990) with slight modifications. Peritoneal macrophages (1.5 × 105 cells per well) were pre-incubated for 16 h at 37 °C in a 5% CO2-humidified atmosphere, then treated with tested concentrations during 24 h. Afterward, target B16-F10 melanoma cells (104 cells per well; yielding a 15:1 expected effector-target ratio) were added to each well. Cells were then incubated at 37 °C for an additional 24 h. Controls are macrophages incubated with target cells. Macrophage-mediated cytotoxicity activity was then measured using an MTT assay. MMC activity was calculated as follows:
Cellular anti-oxidant activity assay
Cellular anti-oxidant activity (CAA) assay, developed by Wolfe and Liu (2007), was employed to measure the antioxidant potential of the tested samples. Briefly, splenocytes and macrophages were seeded at a density of 5 × 105 and 6 × 104 cells per well, respectively (in 100 μl PBS). Triplicate wells were then treated with 10 μl of each sample and 5 μl of a 25-μM solution of 2′,7′-dichlorofluorescin diacetate (DCFH-DA; Fluka, Steinheim, Germany). After 1 h incubation, a 100-μl aliquot of 600 μM solution of 2,2′-azobis(2-amidinopropane) dihydrochloride (ABAP) (Sigma-Aldrich, Steinheim, Germany) in PBS was applied to the cells. In this method, DCFH-DA is taken up by cells and deacetylated to DCFH (2′,7′-dichlorofluorescin). Peroxyl radicals generated from ABAP lead to the oxidation of DCFH to fluorescent dichlorofluorescin (DCF). Accordingly, cells treated with natural compounds that have any anti-oxidant activity should have lower fluorescence compared to untreated cells. Fluorescence of each well was followed every 5 min during 1 h using a fluorescence microplate reader (BioTek, Winooski, USA) with 538 nm emission and at 485-nm excitation filters. Each plate included triplicate control and blank wells: control wells contained cells treated with DCFH-DA and the oxidant ABAP; blank wells contained cells with PBS but without oxidant ABAP. Fluorescence values for the blank samples and initial fluorescence values were subtracted from the sample fluorescence values. The area under the fluorescence versus time curve was integrated at each time point to calculate the CAA units using the following equation:
where ∫SA is the integrated area under the sample fluorescence versus time curve, and ∫CA is the integrated area under the control fluorescence versus time curve.
Statistical analysis
Data are expressed as the arithmetic means ± SD of three independent experiments. The statistical significance was evaluated by the two-way ANOVA test, using GraphPad Prism software version 6.01. P values less than 0.05 were deemed as significant.
Results
hL7G modulates proliferation of T and B lymphocytes
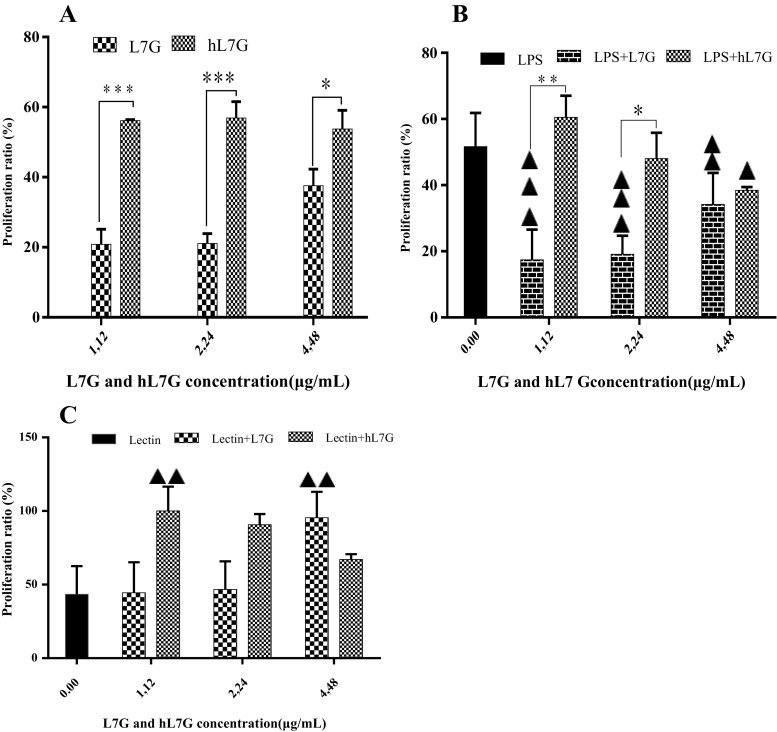
The first set of analysis examines the impact of luteolin-7-O-glucoside and its derivatives issued from thermal treatment on activation of murine splenocytes. It appears that splenocyte proliferation increases significantly in the presence of hL7G compared to L7G (Fig. 1a). In fact, hL7G increases both B and T lymphocyte proliferation compared to L7G, except at the highest tested concentration, where we observed a comparable proliferation of the B lymphocytes, whereas a better proliferation of T lymphocytes was obtained with L7G. Native molecule decreases significantly B lymphocyte proliferation compared to the control cells incubated with LPS (Fig. 1b, c), whereas hL7G induces significantly B cell proliferation only at the lowest tested concentration (1.12 μg/ml), compared to control cells incubated with LPS (Fig. 1b). The proliferation of T lymphocytes increases significantly, only when exposed to the highest tested concentration of L7G (4.48 μg/ml), as compared to cells incubated only with lectin. While, when exposed to hL7G, T cells proliferate in an inverse dose-dependent manner compared to control cells incubated with lectin.
Fig. 1.
Effect of native luteolin 7-O-glucoside (L7G) and heated luteolin 7-O-glucoside (hL7G) on splenocyte proliferation (5 × 105 cells/well). Cells were incubated for 48 h at 37 °C and 5% CO2with a increasing concentrations of samples without mitogen; b LPS (lipopolysaccharide from Escherichia coli) (5 μg/ml) in the absence or presence of L7G and hL7G; or c lectin (5 μg/ml) in the absence or presence of L7G and hL7G. Control cells were incubated with RPMI-1640 only. Data shown are mean percentage proliferation (±SD) from three independent experiments. The statistical significance of results was evaluated by Sidak’s multiple comparisons test and the Tukey’s multiple comparisons test respectively. Filled triangle, p < 0.031, value significantly different compared with control cells. Double filled triangles, p < 0.003, value significantly different compared with control cells. *p < 0.023, value significantly different from molecule-treated cells. ***p < 0.0001, value significantly different from molecule-treated cells
hL7G enhances the cytotoxicity of T cells
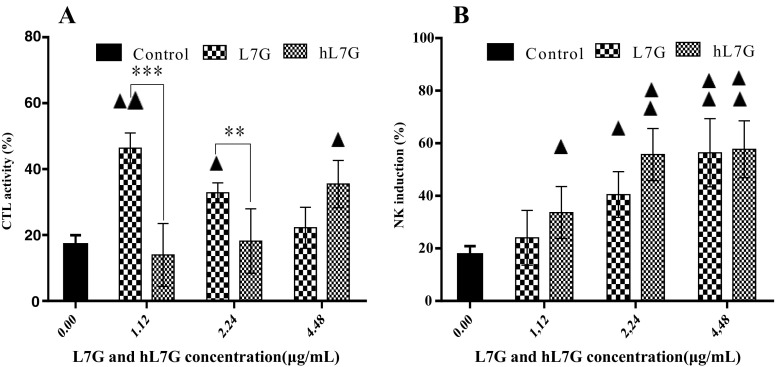
To further compare the immunomodulatory effects of native and heated L7G, we evaluated the cytotoxic effect of CTLs on target cells by co-culture of splenocytes with B16F10 tumor cells, as CTL-sensitive cells. It appears that cells treated with 4.48 μg/ml hL7G as well as cells incubated with decreasing doses of native L7G exhibited a significantly higher cytotoxicity than those incubated with B16F10 target cells only (Fig. 2a). Thus, native molecule seems to be more significantly efficient than the heated one, in inducing T cell cytotoxicity. It is very important to note that both L7G and hL7G provoked no direct cytotoxicity against B16F10 tumor cells, indicating that T cells are responsible for B16F10 lysis.
Fig. 2.
In vitro effect of various concentrations of native luteolin 7-O-glucoside (L7G) and heated luteolin 7-O-glucoside (hL7G) on mouse cytotoxic T cell activity (CTL) (a) and on mouse natural killer (NK) cell acitvity (b). Splenocytes were cultured with different concentrations of L7G and hL7G for 24 h at 37 °C and 5% CO2 before target cells K652 or B16F10 were added (at expected 100:1 E/T ratio). NK induction and CTL activity were then measured in the presence of different concentrations of the tested molecules using the MTT test .Values shown are mean (±SD) percentage of cytotoxicity from six different observations. The statistical significance of results was evaluated by Tukey’s multiple comparisons test. Filled triangle, p < 0.025, value significantly different compared with control cells. Double filled triangles, p < 0.002, value significantly different compared with control cells. **p < 0.002, value significantly different from molecule-treated cells. ***p < 0.0002, value significantly different from molecule-treated cells
hL7G decreases natural killer cell activity
We evaluated NK cell activity and specially its cytotoxic potential against cancerous target cells by co-culture with K562 cells, which are NK-sensitive cells. We firstly revealed that splenocytes displayed a weak cytotoxicity, about 14%, against K562 tumor cells. NK cell activity was significantly enhanced by both L7G and hL7G in a dose-dependent manner as compared to the control cells. Whereas, hL7G was more efficient in enhancing NK cell activity than L7G except at the highest tested concentration (4.48 μg/ml). It is very important to note that both molecules provoked no direct cytotoxicity against K562 tumor cells, indicating that NK cells are responsible of K562 lysis.
hL7G reduces nitric oxide production
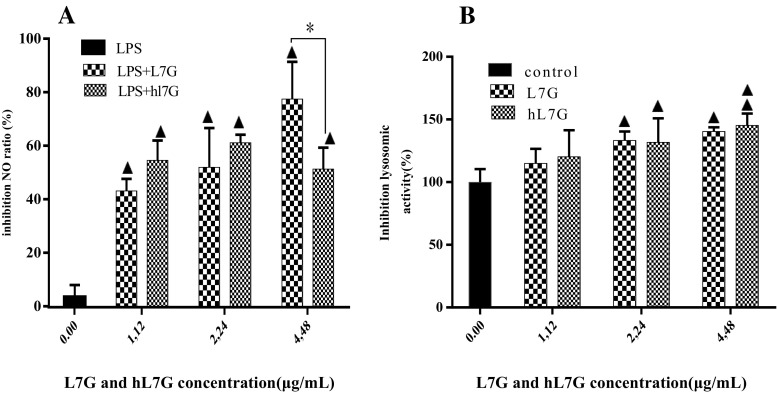
The ability of native and heated L7G to inhibit the release of nitric oxide (NO) was measured through nitrite formation, which is a stable breakdown product of NO. It appears from our results (Fig. 3a) that the different tested concentrations of L7G and hL7G reduced significantly in a dose-dependent manner the nitrite accumulation in peritoneal macrophages cultured in the presence of LPS.
Fig. 3.
Effect of native luteolin 7-O-glucoside (L7G) and heated luteolin 7-O-glucoside (hL7G) in inhibition of production of the nitrite by mouse peritoneal macrophages stimulated by lipopolysaccharide (LPS) (5 μg/ml) (a) and lysosomal enzyme activity (b). a Macrophages (2 × 105 cells/well) were incubated in the presence of LPS and increasing concentrations of L7G and hL7G for 48 h at 37 °C and 5% CO2. Cells treated with 5 μg/ml of LPS alone were used as positive control. b Macrophages (2 × 105 cells/well) were incubated in the presence of increasing concentrations of molecules for 48 h at 37 °C and 5% CO2. Control (untreated cells) included cells incubated with RPMI 1640 medium. Data shown are mean (±SD) percentages of NO production and lysosomal enzyme activity from three independent experiments. The statistical significance of results was evaluated by Tukey’s multiple comparison test and Sidak’s multiple comparison test, respectively. Filled triangle, p < 0.030, value significantly different compared with control cells. Double filled triangles, p < 0.003, value significantly different compared with control cells. *p < 0.030, value significantly different from molecule-treated cells
hL7G modulates cellular lysosomal enzyme activity
Native and heated L7G decreased markedly, in a dose-dependent manner, macrophage lysosomal activity (Fig. 3b). A significant inhibition was recorded at 2.24 and 4.48 μg/ml in comparison with untreated cells. No remarkable difference was detected between native and heated L7G.
hL7G ensures the integrity of lysosomal membrane
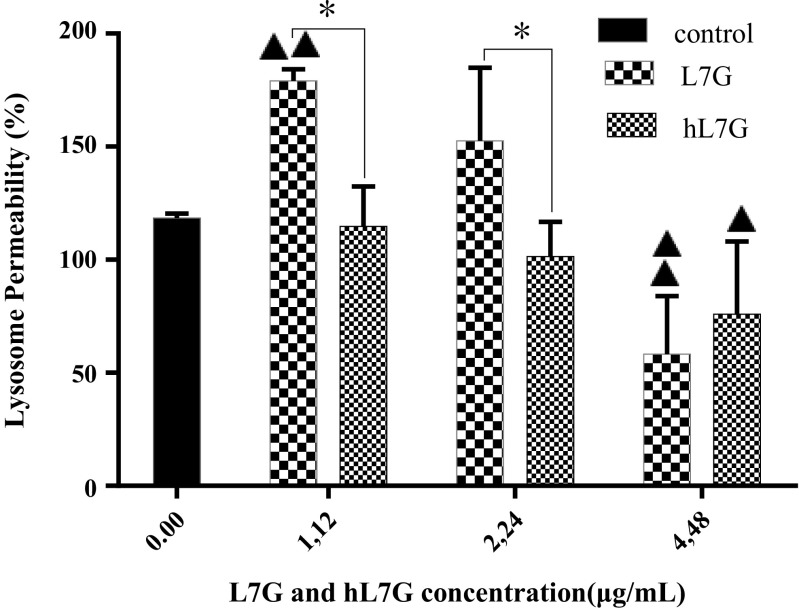
The permeabilization of lysosomes was analyzed by measuring the emission of green fluorescence, which is indicative of leakage of AO from the acidic compartment into the cytosol. Both L7G and hL7G at the concentration of 4.48 μg/ml induce remarkable decrease in green fluorescence in comparison with untreated cells (Fig. 4), revealing a reduction of lysosome content leakage. On the other hand, hL7G was more efficient in preserving lysosomal membrane integrity, as evidenced by the decrease of green fluorescence obtained with 1.12 and 2.24 μg/ml of hL7G compared to the native molecule.
Fig. 4.
Effect of native luteolin 7-O-glucoside (L7G) and heated luteolin 7-O-glucoside (hL7G) at various tested concentrations, on macrophage lysosome permeability (LP). Peritoneal macrophages (5 × 104 cells/well) were incubated with different doses of molecules at 37 °C and 5% CO2. Control cells correspond to macrophages incubated only with acridine orange (AO). Values are mean (±SD) percentage of LP from three different measurements. The statistical significance of results was evaluated by Tukey’s multiple comparison test. Filled triangle, p < 0.027, value significantly different compared with control cells. Double filled triangles, p < 0.006, value significantly different compared with control cells. *p < 0.027, value significantly different from molecule-treated cells
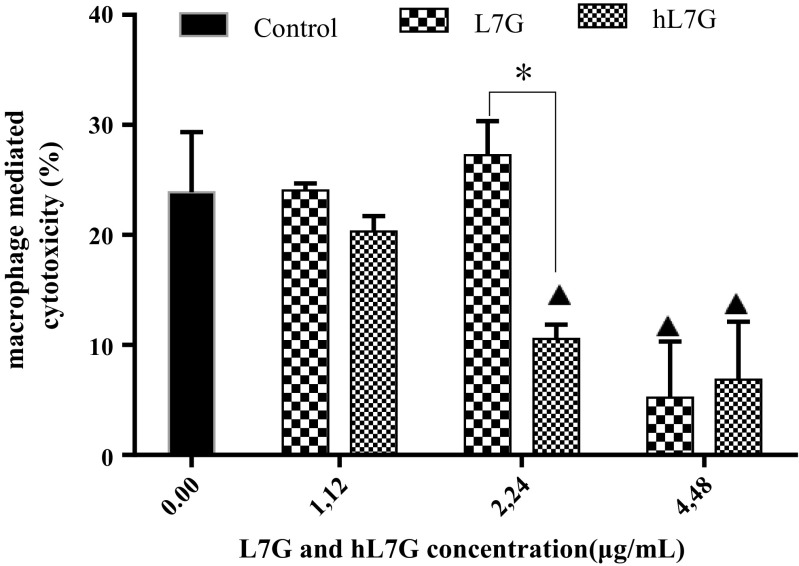
hL7G reduces macrophage-mediated cytotoxicity
Macrophage-mediated cytotoxicity was assessed by co-culturing peritoneal macrophages with B16F10 tumor cells. By comparing with the control cells (macrophages incubated with B16F10 target cells), a significant reduction of macrophage cytotoxicity was detected at 4.48 μg/ml of native L7G and 2.24 and 4.48 μg/ml of hL7G (Fig. 5). Whereas L7G exhibited more important cytotoxicity than hL7G.
Fig. 5.
Effect of native luteolin 7-O-glucoside (L7G) and heated luteolin 7-O-glucoside (hL7G) at various tested concentrations, on cytotoxicity mediated by macrophages in vitro. Peritoneal macrophages (1.5 × 105 cells/well) were cultured with different doses of molecules during 24 h before B16F10 target cells were added (at expected 15:1 E/T ratio). Control consists of macrophages incubated with target cells. Macrophage-mediated cytotoxicity activity was then measured using an MTT assay. Values shown are mean (±SD) percentage of cytotoxicity from six different observations. The statistical significance of results was evaluated by Tukey’s multiple comparison test. Filled triangle, p < 0.030, value significantly different compared with control cells. *p < 0.040, value significantly different from molecule-treated cells
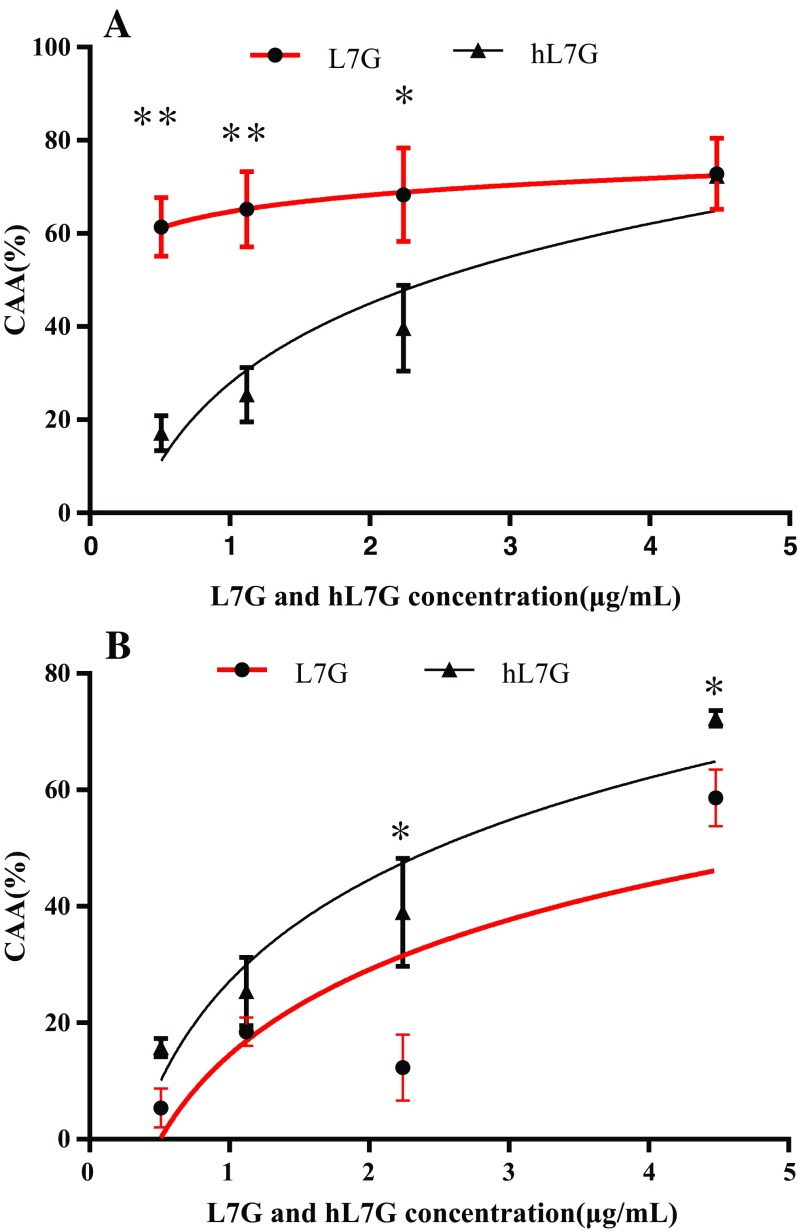
hL7G expands the cellular antioxidant activity of splenocytes and macrophages
Native and heated luteolin 7-O-glucoside was able to inhibit the oxidation of DCFH in macrophages, by 72.81 and 72.32%, respectively, at 4.48 μg/ml. Native L7G exhibited stronger CCA compared to the heated molecule (Fig. 6a). The mean effective concentrations (EC50) for heated and natural L7G were 2.31 and 0.07 μg/ml, respectively. On the other hand, treated cynaroside was more efficient in protecting splenocytes against peroxyl radical-induced oxidation compared to native molecules. The EC50 obtained with both treated and untreated L7G were 2.61 and 4.03 μg/ml, respectively.
Fig. 6.
Dose-response curve for oxidation of DCFH to DCF in macrophages (6 × 104 cells per well) (a) and splenocytes (5 × 105 cells per well) (b) using a cellular antioxidant activity assay in the presence of native luteolin 7-O-glucoside (L7G) and heated luteolin 7-O-glucoside (hL7G). The curves shown in each graph are from a single experiment (mean ± SD, n = 3). The statistical significance of results was evaluated by Sidak’s multiple comparisons test. *p < 0.010, value significantly different from molecule-treated cells. **p < 0.0001, value significantly different from molecule-treated cells
Discussion
The immune system is the ultimate defense against infectious diseases and tumor and cancer growth. Modulation of immune response through stimulation or suppression may help in maintaining a disease-free state. For the above reasons, we focused on how L7G can modulate lymphocyte and macrophage responses as well as other inflammatory mediators. Nevertheless, numerous studies reported that thermal conditioning of molecules by heating, a necessary step in transformation of human diet from raw materials to finished products, should improve their biological effects (Boileau et al. 2002; Chaaban et al. 2017; Vallverdu-Queralt et al. 2015). In this study, we investigate the effect of heat processing on biological activities of L7G. We compared the immunopotential of both native and heated L7G by assessing their effect on murine splenocyte proliferation. It appears from our results that heating ameliorates splenocyte proliferation induced by L7G, and specially T cells, making them interesting candidates to be used in immunotherapy approach. This is a new approach which consists of inducing anti-tumor immunity by enhancing CD4+ and CD8+ T cells and thus inhibiting tumor expansion as described by Leavy (2012). It has been recorded that immunological methods that remove and/or activate anti-tumor immunity can be very effective in the treatment of cancer (Zhou 2014).
Natural killer cells are a component of the innate immune system with the capacity to kill cells and produce cytokines through the interaction of several cell receptors with their cognate ligands (Caligiuri 2008; Warren and Smyth 1999). NK cells recognize and destroy cells lacking self-MHC molecules (Ljunggren and Karre 1990). Many studies have shown that flavonoids can stimulate NK cytotoxic activity (Mokdad-Bzeouich et al. 2016). CTL cells are also necessary for defense against viral infections and cancer processes. They constitute the major anti-tumor effector population and are recognized for their involvement in host resistance against tumor growth and dissemination (Whiteside and Herberman 1995). It is well known that CTLs are involved in the FAS-FASL pathway to eliminate self-reactive lymphoid cells, whereas NK cells are involved in the granulocyte-exocytosis pathway using perforin and granzymes with various substrate specificities secreted by exocytosis to induce the apoptosis of target cells (Trapani and Smyth 2002). In this report, we have demonstrated that heated L7G promotes more efficiently natural killer cytotoxicity, against erythromyeloblastoid leukemia K562 cells, than T lymphocyte cytotoxicity against murine melanoma B16F10. These results suggested potential synergistic effect of hL7G upon cytolytic proteins (granzyme, perforin) to kill the cell targeted. In this study, we noticed that the cytotoxicity of lymphocyte is obtained only at the highest tested concentration of hL7G, while NK cell activity occurred at all the tested doses. This may be due to an early activation of NK cell activity; however, CTL activity will be targeted later. In fact, Herberman et al. (1979) have shown that T cell immunity may come into play only as a relatively late event and may be more important in further resistance to progressive tumor growth. This result is of great importance as far as NK cells are currently used in anti-tumor therapies by suppressing tumor growth (Pietra et al. 2015).
Besides, macrophages are known to represent a main recognition system for foreign cells, including tumor cells. Their anti-tumor potential is often more efficient than other kinds of immunocompetent cells (Dumont et al. 1988). The enhancement of tumoricidal activity of macrophages by plants and their polyphenolic compounds has been previously reported. In fact, Oršolić and Bašić (2005) have demonstrated that some polyphenols (caffeic acid, quercetin, chrysin, and naringenin) increase the tumoricidal activity of macrophages against Ehrlich ascite tumor cells. It appears from our study that only the low tested concentrations of both hL7G (1.12 μg/ml) and L7G (1.12 μg/ml, 2.24 μg/ml), induced macrophage cytotoxicity against target B16F10 cells. These data suggest that both hL7G and L7G might interfere moderately with the growth of B16F10 tumor cells, during the early phase of treatment, leading to a considerable elimination of melanoma cells. A possible explanation given by Bucana et al. (1976) suggests that the tested samples induce the expression of an endosome-localized cytotoxin, which is released onto the tumor cell surface only during cell-cell contact. Another similar proposed mechanism of the macrophage-mediated cytotoxicity is that a soluble factor released by macrophages might alter the integrity of the tumor cell membrane and/or pericellular environment of these cells (Cleveland et al. 1974).
The low cytotoxicity exhibited by high doses of both L7G and hL7G against melanoma cells should be ascribed to the anti-inflammatory-inducing potential of these molecules, as revealed by their capacity to downregulate both nitric oxide (NO) release by LPS-treated peritoneal macrophages and lysosomal activity of macrophages. It is admitted that infected macrophages release pro-inflammatory mediators such as NO, by the action of inducible NO synthase (iNOS), to exert key functions during immune response (Jakhar et al. 2014). Compared with LPS, a potent inducer of macrophage NO production (MacMicking et al. 1997), we demonstrated that both native and heated cynaroside inhibited significantly the release of NO by peritoneal macrophages. This is in accordance with data reported by Ha et al. (2006) and Francisco et al. (2014). They found that L7G induced weak NO release by LPS-stimulated macrophages. Our findings are similar to those reported by Hu and Kitts (2004), who showed the inhibitory effect of L7G on nitric oxide production, in LPS-activated RAW264.7 cells.
In recent years, production of a large quantity of NO has been associated with various diseases such as arthritis, autoimmune diseases, and chronic inflammation (Arteel et al. 1999; Moncada et al. 1991). In fact, excessive amounts of NO can directly inhibit mitochondrial complexes I and IV, activate the enzyme poly-ADP ribose polymerase (PARP), resulting in depletion of cellular energy stores (Liu and Huang 2008), and damage a wide array of molecules including DNA and proteins (Mokdad-Bzeouich et al. 2016). Therefore, inhibition of high-output NO production by native or heated L7G could be a potential therapeutic approach for the treatment of various inflammatory diseases.
Destruction of tumor cells by macrophages represents a multistep process involving the activation of macrophages, the recognition and binding to tumor cells, and the production of lysis factors, as lysosomal phosphatase enzymes, which ultimately destroy neoplastic cells (Hamilton and Adams 1987). The present study showed that both L7G and hL7G decreased lysosomal phosphatase activity and ensured the integrity of the lysosomal membrane, confirming their capacity to treat inflammatory disorders and to reduce the tissue injury induced by lysosomal enzymes.
Previous studies have reported that production of NO induced by LPS is mediated through the induction of iNOS and cyclooxygenase (COX-2) expression, which is in turn regulated by the transcription factors nuclear factor (NF-κB) and activator protein (AP)-1. These two factors are regulated by a cascade of events that leads to the activation of mitogen-activated protein kinases (MAPKs) and protein kinase B (Akt) (Park and Song 2013). For our part, we believe that L7G and hL7G inhibited Akt phosphorylation, contributing thus to the suppression of both NF-κB and (AP)-1 activities, which results in reduced expression of inflammatory mediators in macrophage cells. Ha et al. (2006) reported that L7G acts as anti-inflammatory element, by inhibiting cytokine release from both LPS-stimulated macrophages and phytohemagglutinin-activated lymphocyte T helper (Th1) cells.
Reactive oxygen species (ROS) are free radicals that contain an oxygen atom. They are formed as natural by-products of the normal metabolism of oxygen and have important roles in cell signaling (Rada and Leto 2008). Otherwise, as ROS are known to be involved in many diseases and tumor angiogenesis, through the release of vascular endothelial growth factor and angiopoietin (Fiaschi and Chiarugi 2012), we investigated the antioxidant potential of both native and heated L7G. We obtained an important dose depending on cellular antioxidant activity of L7G in murine macrophages. The present finding corroborates the ideas of Qiusheng et al. (2004) who suggested that L7G exhibits a protective effect against hepatic oxidative injury damage induced by chemicals. Likewise, other studies reported antioxidative effect of L7G (Žemlička et al. 2014). Surprisingly, heated L7G has significantly greater antioxidant properties than inherent L7G towards peroxyl radical-induced DCFH2 oxidation in splenocytes. However, Murakami et al. (2004) reported that luteolin-7-glucoside, heated at 100 °C for 360 min, has retained 85% of its radical scavenging activity. This weak loss of L7G antioxidant activity should be due to its stability during the heating process.
Our result was confirmed by Dewanto et al. (2002) who reported that heat processing of tomatoes at 88 °C induces an increase of its total antioxidant activity. This activity was correlated to increasing lycopene amounts, suggesting that thermal processing enhances the nutritional value of tomatoes by increasing the bioaccessible lycopene content. Similarly, Vallverdu-Queralt et al. (2015) reported that the antioxidant activity and the total carotenoid, α-carotene, β-carotene, and (Z)-lycopene contents in tomatoes increase during their thermal treatment. The above studies highlighted the advantages of eating heated plants, as far as this process increases levels of lycopene isomers which should be responsible for the antioxidant potential of tomatoes, knowing that isomeric forms of lycopene are found in the blood and tissues of humans and animals consuming a lycopene-containing diet (Boileau et al. 2002). Ettlinger and Yegles (2016) reported a strong increase of cannabinol and benzoylecgonine content in hair after its thermal treatments. This change was due to the conversion of tetrahydrocannabinol into cannabinol and cocaine into benzoylecgonine. On the other hand, Chaaban et al. (2017) reported that degradation of some flavonoids such as rutin, naringin, mesquitol, and eriodictyol, by heat processing, strengthens their antioxidant capacities. In fact, the antioxidant activity of flavonoids is linked to some structural elements, such as the presence of hydroxyl groups at positions 3 and 5, enone structure conjugated with carbonyl group in C4, and ortho-dihydroxy (catechol) structure in the B ring (Chaaban et al. 2017; Procházková et al. 2011).
Conclusion
In conclusion, native and thermally treated luteolin-7-glucoside exhibited important immunomodulatory activity. HL7G activates B and T cells more efficiently than native molecules. It enhances the cytotoxicity of NK cells. Besides, both native and heated L7G exhibited comparable anti-inflammatory activity. Whereas heated L7G presents higher cellular antioxidant activity than native molecules in splenocytes. Therefore, we suggest that such molecules should be useful in chemotherapeutic treatments of some types of cancer, as adjuvant or in a preventive strategy.
Acknowledgements
The authors acknowledge the “Ministère Tunisien de l’Enseignement Supérieur et de la Recherche Scientifique” for its support.
Compliance with ethical standards
All experiments were performed in accordance with the guidelines for the care and use of laboratory animals as published by the National Institute of Health. All experiments received the explicit approval of the Ethics Animal Committee in Tunisia.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ahmed SA, Kamel EM. Cytotoxic activities of flavonoids from Centaurea scoparia. ScientificWorldJournal. 2014;2014:274207. doi: 10.1155/2014/274207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard JS, Perez E, Zou S, De Cabo R. Dietary activators of Sirt1. Mol Cell Endocrinol. 2009;299:58–63. doi: 10.1016/j.mce.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteel GE, Kadiiska MB, Rusyn I, Bradford BU, Mason RP, Raleigh JA, Thurman RG. Oxidative stress occurs in perfused rat liver at low oxygen tension by mechanisms involving peroxynitrite. Mol Pharmacol. 1999;55:708–715. [PubMed] [Google Scholar]
- Baskar AA, Ignacimuthu S, Michael GP, Al Numair KS. Cancer chemopreventive potential of luteolin-7-O-glucoside isolated from Ophiorrhiza mungos. Linn Nutr Cancer. 2011;63:130–138. doi: 10.1080/01635581.2010.516869. [DOI] [PubMed] [Google Scholar]
- Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives 3. Biotech. 2013;3:439–459. doi: 10.1007/s13205-013-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. What is xenohormesis? Am J Pharmacol Toxicol. 2008;3:152–159. doi: 10.3844/ajptsp.2008.152.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau TW, Boileau AC, Erdman JW., Jr Bioavailability of all-trans and cis-isomers of lycopene. Exp Biol Med (Maywood) 2002;227:914–919. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- Bucana C, Hoyer LC, Hobbs B, Breesman S, McDaniel M, Hanna MG., Jr Morphological evidence for the translocation of lysosomal organelles from cytotoxic macrophages into the cytoplasm of tumor target cells. Cancer Res. 1976;36:4444–4458. [PubMed] [Google Scholar]
- Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaban H et al. (2017) Effect of heat processing on thermal stability and antioxidant activity of six flavonoids Journal of Food Processing and Preservation
- Cleveland RP, Meltzer MS, Zbar B. Tumor cytotoxicity in vitro by macrophages from mice infected with mycobacterium bovis strain. BCG J Natl Cancer Inst. 1974;52:1887–1895. doi: 10.1093/jnci/52.6.1887. [DOI] [PubMed] [Google Scholar]
- De Paepe D, Servaes K, Noten B, Diels L, De Loose M, Van Droogenbroeck B, Voorspoels S. An improved mass spectrometric method for identification and quantification of phenolic compounds in apple fruits. Food Chem. 2013;136:368–375. doi: 10.1016/j.foodchem.2012.08.062. [DOI] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Dumont S, Hartmann D, Poindron P, Oberling F, Faradji A, Bartholeyns J. Control of the antitumoral activity of human macrophages produced in large amounts in view of adoptive transfer. Eur J Cancer Clin Oncol. 1988;24:1691–1698. doi: 10.1016/0277-5379(88)90069-7. [DOI] [PubMed] [Google Scholar]
- Ettlinger J, Yegles M. Influence of thermal hair straightening on cannabis and cocaine content in hair. Forensic Sci Int. 2016;265:13–16. doi: 10.1016/j.forsciint.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Fornasiero MC, Isetta AM (1990) MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods 131(2):165–172 [DOI] [PubMed]
- Fiaschi T, Chiarugi P (2012, 2012) Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol:762825. doi:10.1155/2012/762825 [DOI] [PMC free article] [PubMed]
- Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23(Suppl 8):viii6–viii9. doi: 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco V, et al. Chemical characterization and anti-inflammatory activity of luteolin glycosides isolated from lemongrass. J Funct Foods. 2014;10:436–443. doi: 10.1016/j.jff.2014.07.003. [DOI] [Google Scholar]
- Geem D, Harusato A, Flannigan K, Denning TL. Harnessing regulatory T cells for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1409–1418. doi: 10.1097/MIB.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Greiss S, Gartner A. Sirtuin/Sir2 phylogeny, evolutionary considerations and structural conservation. Mol Cells. 2009;28:407. doi: 10.1007/s10059-009-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CL, Weng CY, Wang L, Lian TW, Wu MJ. Immunomodulatory effect of Glossogyne tenuifolia in murine peritoneal macrophages and splenocytes. J Ethnopharmacol. 2006;107:116–125. doi: 10.1016/j.jep.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Hamilton TA, Adams DO. Molecular mechanisms of signal transduction in macrophages. Immunol Today. 1987;8:151–158. doi: 10.1016/0167-5699(87)90145-9. [DOI] [PubMed] [Google Scholar]
- Herberman RB, et al. Natural killer cells: characteristics and regulation of activity. Immunol Rev. 1979;44:43–70. doi: 10.1111/j.1600-065X.1979.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL, Tytell M, Vigh L. Xenohormesis: health benefits from an eon of plant stress response evolution. Cell Stress Chaperones. 2010;15:761–770. doi: 10.1007/s12192-010-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008;133:387–391. doi: 10.1016/j.cell.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Kitts DD. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol Cell Biochem. 2004;265:107–113. doi: 10.1023/B:MCBI.0000044364.73144.fe. [DOI] [PubMed] [Google Scholar]
- Hu W, Guo T, Jiang WJ, Dong GL, Chen DW, Yang SL, Li HR. Effects of ultrahigh pressure extraction on yield and antioxidant activity of chlorogenic acid and cynaroside extracted from flower buds of Lonicera japonica. Chin J Nat Med. 2015;13:445–453. doi: 10.1016/S1875-5364(15)30038-8. [DOI] [PubMed] [Google Scholar]
- Jakhar R, Paul S, Chauhan AK, Kang SC. Morin hydrate augments phagocytosis mechanism and inhibits LPS induced autophagic signaling in murine macrophage. Int Immunopharmacol. 2014;22:356–365. doi: 10.1016/j.intimp.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Kennedy DO. Polyphenols and the human brain: plant “secondary metabolite” ecologic roles and endogenous signaling functions drive benefits. Adv Nutr. 2014;5:515–533. doi: 10.3945/an.114.006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Wood JG, Sinclair DA. Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol. 2004;53:1003–1009. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- Leavy O. Tumour immunology: a close-range dual hit for tumour immunity. Nat Rev Immunol. 2012;12:227. doi: 10.1038/nri3189. [DOI] [PubMed] [Google Scholar]
- Leonov A, Arlia-Ciommo A, Piano A, Svistkova V, Lutchman V, Medkour Y, Titorenko VI. Longevity extension by phytochemicals. Molecules. 2015;20:6544–6572. doi: 10.3390/molecules20046544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YL, Li J, Wang NL, Yao XS. Flavonoids and a new polyacetylene from Bidens parviflora Willd. Molecules. 2008;13:1931–1941. doi: 10.3390/molecules13081931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limem I, Harizi H, Ghedira K, Chekir-Ghedira L. Leaf extracts from Phlomis crinita Cav. subs. mauritanica Munby affect immune cell functions in vitro. Immunopharmacol Immunotoxicol. 2011;33:309–314. doi: 10.3109/08923973.2010.504926. [DOI] [PubMed] [Google Scholar]
- Liu VW, Huang PL. Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc Res. 2008;77:19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-S. [DOI] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Manosroi A, Saraphanchotiwitthaya A, Manosroi J. Immunomodulatory activities of Clausena excavata Burm. f. wood extracts. J Ethnopharmacol. 2003;89:155–160. doi: 10.1016/S0378-8741(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Manosroi A, Saraphanchotiwitthaya A, Manosroi J. In vitro immunomodulatory effect of Pouteria cambodiana (Pierre ex Dubard) Baehni extract. J Ethnopharmacol. 2005;101:90–94. doi: 10.1016/j.jep.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Mellor DD, Naumovski N. Effect of cocoa in diabetes: the potential of the pancreas and liver as key target organs, more than an antioxidant effect? Int J Food Sci Technol. 2016;51(4):829–841. doi: 10.1111/ijfs.13075. [DOI] [Google Scholar]
- Mokdad-Bzeouich I, Mustapha N, Sassi A, Bedoui A, Ghoul M, Ghedira K, Chekir Ghedira L. Investigation of immunomodulatory and anti-inflammatory effects of eriodictyol through its cellular anti-oxidant activity. Cell Stress Chaperones. 2016;21:773–781. doi: 10.1007/s12192-016-0702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murakami M, Yamaguchi T, Takamura H, Atoba T. Effects of thermal treatment on radical-scavenging activity of single and mixed polyphenolic compounds. J Food Sci. 2004;69:FCT7–FCT10. doi: 10.1111/j.1365-2621.2004.tb17848.x. [DOI] [Google Scholar]
- Olennikov DN, Chirikova NK, Okhlopkova ZM, Zulfugarov IS. Chemical composition and antioxidant activity of Tanara Oto (Dracocephalum palmatum Stephan), a medicinal plant used by the North-Yakutian nomads. Molecules. 2013;18:14105–14121. doi: 10.3390/molecules181114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oršolić N, Bašić I. Water-soluble derivative of propolis and its polyphenolic compounds enhance tumoricidal activity of macrophages. J Ethnopharmacol. 2005;102:37–45. doi: 10.1016/j.jep.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Park CM, Song YS. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-kappaB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr Res Pract. 2013;7:423–429. doi: 10.4162/nrp.2013.7.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson HL, Vainikka LK. Increased lysosomal membrane permeabilization in oxidant-exposed macrophages of human fibrotic lungs. J Cell Death. 2013;6:69–74. doi: 10.4137/JCD.S13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietra G et al (2015) Human natural killer cells: news in the therapy of solid tumors and high-risk leukemias. Cancer Immunol Immunother. doi:10.1007/s00262-015-1744-y [DOI] [PMC free article] [PubMed]
- Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Qiusheng Z, Xiling S, Xubo MS, Changhai W. Protective effects of luteolin-7-glucoside against liver injury caused by carbon tetrachloride in rats. Pharmazie. 2004;59:286–289. [PubMed] [Google Scholar]
- Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangi I, Ghosh D, Bhutia SK, Mallick SK, Maiti TK. Anti-tumor and immunomodulating effects of Pleurotus ostreatus mycelia-derived proteoglycans. Int Immunopharmacol. 2006;6:1287–1297. doi: 10.1016/j.intimp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- Vallverdu-Queralt A, Regueiro J, de Alvarenga JF, Torrado X, Lamuela-Raventos RM. Carotenoid profile of tomato sauces: effect of cooking time and content of extra virgin olive oil. Int J Mol Sci. 2015;16:9588–9599. doi: 10.3390/ijms16059588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren HS, Smyth MJ. NK cells and apoptosis. Immunol Cell Biol. 1999;77:64–75. doi: 10.1046/j.1440-1711.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- Whiteside TL, Herberman RB. The role of natural killer cells in immune surveillance of cancer. Curr Opin Immunol. 1995;7:704–710. doi: 10.1016/0952-7915(95)80080-8. [DOI] [PubMed] [Google Scholar]
- Wittemer SM, Ploch M, Windeck T, Muller SC, Drewelow B, Derendorf H, Veit M. Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of artichoke leaf extracts in humans. Phytomedicine. 2005;12:28–38. doi: 10.1016/j.phymed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Wolfe KL, Liu RH (2007) Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem 55(22):8896–8907 [DOI] [PubMed]
- Žemlička L, et al. Physicochemical and biological properties of luteolin-7-O-β-d-glucoside (cynaroside) isolated from Anthriscus sylvestris (L.) Hoffm Monatsh Chem. 2014;145:1307–1318. doi: 10.1007/s00706-014-1228-3. [DOI] [Google Scholar]
- Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- Zhou J (2014) Advances and prospects in cancer immunotherapy. New J Sci:2014