Abstract
Neoadjuvant (or induction) chemotherapy can be used for cervical cancer patients with locally advanced disease; this treatment is followed by radical surgery and/or radiation therapy. Cisplatin is considered to be the most active platinum agent drug for this cancer, with a response rate of 20%. In order to understand how the cisplatin treatment affects the stress response, in this work, we performed an exploratory study to analyze a number of stress proteins before and after cisplatin neoadjuvant chemotherapy. The study involved 14 patients; the pre- and post-chemotherapy paired biopsies were examined by hematoxylin and eosin staining and by immunohistochemistry. The proteins evaluated were p53, P16/INK4A, MSH2, nuclear protein transcriptional regulator 1 (NUPR1), and HSPB1 (total: HSPB1/t and phosphorylated: HSPB1/p). These proteins were selected because there is previous evidence of their relationship with drug resistance. The formation of platinum-DNA adducts was also studied. There was a great variation in the expression levels of the mentioned proteins in the pre-chemotherapy biopsies. After chemotherapy, p53 was not significantly affected by cisplatin, as well as P16/INK4A and MSH2 while nuclear NUPR1 content tended to decrease (p = 0.056). Cytoplasmic HSPB1/t expression levels decreased significantly following cisplatin therapy while nuclear HSPB1/t and HSPB1/p tended to increase. Since the most significant changes following chemotherapy appeared in the HSPB1 expression levels, the changes were confirmed by Western blot. The platinum-DNA adducts were observed in HeLa cell in apoptosis; however, in the tumor samples, the platinum-DNA adducts were observed in morphologically healthy tumor cells; these cells displayed nuclear HSPB1/p. Further mechanistic studies should be performed to reveal how HSPB1/p is related with drug resistance. When the correlations of the markers with the response to neoadjuvant chemotherapy were examined, only high pre-chemotherapy levels of cytoplasmic HSPB1/p correlated with a poor clinical and pathological response to neoadjuvant cisplatin chemotherapy (p = 0.056) suggesting that this marker could be useful opening its study in a larger number of cases.
Keywords: Cervical cancer, Neoadjuvant chemotherapy, Cisplatin, DNA damage, Heat shock proteins, Molecular markers, HSPB1
Introduction
In Argentina, there are approximately 3000 new cases of cervical cancer every year, whereas in Latin America and other low-income countries, this disease is among the most frequent cancers affecting mainly socially vulnerable women (Arrossi 2008; Ferlay et al. 2012; Tadessse 2015). Cervical cancer is a human papillomavirus HPV-related disease; practically all cases of cervical cancer are preceded by persistent infections with oncogenic subtypes of HPV (WHO 2016). In Argentina as well as in other countries, a vaccine against HPV has been included in the immunization program expecting to decrease the incidence of cervical cancer (Arrossi et al. 2012). Unfortunately, more years are needed to see the effect of immunization and many patients with cervical cancer are still diagnosed at late stages of the disease, for example when the cancer is locally advanced. Radiotherapy with concomitant platinum-based chemotherapy is the standard treatment for locally advanced disease but the response rate is still poor; these patients frequently show incomplete local control, disease recurrence, and distant metastasis particularly in patients with a large tumor burden, with lymph node metastasis, and/or with parametrial infiltration (Pearcey et al. 2002; Al-Mansour and Verschraegen 2010). In order to improve the patient’s outcome, an alternative treatment for these patients is the use of neoadjuvant chemotherapy followed by radical surgery and treatment completion with radiochemotherapy (Al-Mansour and Verschraegen 2010; Luvero et al. 2016). The aim of the neoadjuvant treatment is to reduce the tumor volume rendering radical surgery feasible and at the same time kill the possible micrometastasis. The advantages and disadvantages of the addition of neoadjuvant chemotherapy to standard treatment have been analyzed recently by Narayan et al. (2016). These authors found a better clinical response when neoadjuvant chemotherapy was applied; however, about 40% of the patients still showed a poor outcome.
In cervical cancer patients, the main drug used in neoadjuvant and adjuvant treatments is cisplatin. This drug is effective against various cancer types producing cross-links of purine bases on the DNA [90% are 1,2-intrastrand d(GpG) adducts] with important consequences: DNA damage, activation of DNA repair mechanisms, blocking cell division, and inducing apoptosis (Dasari and Tchounwou 2014). Cisplatin toxicity is also due to oxidative stress since the compound induces reactive oxygen species that can result in both apoptosis and necrosis; this and other mechanisms of cisplatin action have been reviewed elsewhere (Dasari and Tchounwou 2014; Fong 2016). Among the mechanism of cisplatin resistance, we can mention reduced uptake or retention of the drug by the tumor with decreased platinum-DNA adduct formation. Cisplatin exposure induces a stress response in the cells and therefore can awake a heat shock protein (HSP) and stress protein response. The participation of HSPs in DNA repair pathways and their implications in cancer therapy and drug sensitivity have been reviewed (Nadin and Ciocca 2010). In order to expand our understanding of the stress response following cisplatin exposure, in the present report, we have performed an exploratory study to analyze particular stress proteins in biopsies from patients with locally advanced cervical cancer receiving cisplatin neoadjuvant chemotherapy. The study involved 14 patients, the pre- and post-chemotherapy paired biopsies were examined by hematoxylin and eosin staining (H&E) and by immunohistochemistry (IHC); in 5 cases, Western blot was also used. In addition, the formation of cisplatin-DNA adducts was evaluated in the pre- and post-chemotherapy biopsies. The proteins evaluated were HSPB1 (also known as HSP27) (total: HSPB1/t and phosphorylated form: HSPB1/p), MSH2, nuclear protein 1 transcriptional regulator (NUPR1), p53, and P16/INK4A; these proteins were selected because there is evidence that they are related to drug resistance. For instance, in a recent study, we have reported interactions between HSPB1 and the MSH2 (DNA repair protein) and the potential role of these proteins on temozolomide resistance mechanism in human gliomas (Castro et al. 2015). Mismatch repair proteins are important in the processing of cisplatin-DNA cross-links (Dasari and Tchounwou 2014; Sawant et al. 2015). NUPR1 is a multifunctional stress-induced protein (Cano et al. 2011) implicated in pancreatic oncogenesis and in protecting cells from stress by inhibiting apoptosis (Hamidi et al. 2012). Chemoresistance has been described as enhanced in breast cancer cells through the NUPR1-PI3K/Akt-phospho-p21 axis in p53-negative cells (Vincent et al. 2012), and this protein has been implicated with gemcitabine resistance in pancreatic ductal adenocarcinoma cells (Palam et al. 2015). During stressful situations, p53 modulates particular subsets of genes driving the cells to growth arrest followed by survival or toward apoptosis, and has been implicated in platinum resistance (Thakur and Ray 2016) and in DNA double-strand break (DSB) repair pathway choice (Moureau et al. 2016). P16/INK4A has been implicated in chemotherapy resistance in osteosarcoma patients (Robl et al. 2015); in contrast, in malignant pleural mesothelioma patients, high P16/INK4A levels correlated with a better survival after chemotherapy (cisplatin is among the drugs of choice to treat mesothelioma patients) (Jennings et al. 2015). Moreover, P16/INK4A has been described as a requirement for cisplatin resistance in human cervical carcinoma SiHa cells (Li et al. 2015). It is therefore important to study the effects of cisplatin in cancer patients to know the response mounted by the cells with the aim to find biomarkers to predict resistance to the drug avoiding the administration of chemotherapy with lack of benefits.
Patients and methods
Patients
From May 2011 to September 2016, 14 patients with locally advanced cervical cancer (stages 1B2–3B) were considered eligible and thus included for this study. Patients were required to have histological proof of invasive carcinoma, to be at least 18 years of age, have a performance status of 90% by the Karnofsky scale, serum biochemical markers within the normal range, and a normal cardiac and renal function. The patients were assessed to be metastasis free at the time of diagnosis by careful clinical evaluation, and by abdominal and pelvic computed axial tomography. None of the patients had previously received any treatment for the disease. The main clinical and pathological characteristics of the patients are shown in Table 1. All indications were discussed in a multidisciplinary oncology meeting and the informed signed consent was obtained from the patients according to our research ethics requirements approved by the Ethic Committee of the Argentine Foundation for Cancer Research of Mendoza in accordance with the precepts established by the Helsinki Declaration.
Table 1.
Main characteristics of the patients entered into the study
| Patient | Age | Diagnosis | Neoadjuvant chemotherapy | Clinical response | Pathological response | Response to NCa |
|---|---|---|---|---|---|---|
| 1 | 45 | Squamous carcinoma | Cisplatin | 2B → 3A | Poor | Resistant |
| 2 | 36 | Squamous carcinoma | Cisplatin | 1B2 → 1B1 | Good | Sensitive |
| 3 | 47 | Adenocarcinoma | Cisplatin | 1B2 → 1B1 | Poor | Resistant |
| 4 | 40 | Squamous carcinoma | Cisplatin | 2B → 2B | Poor | Resistant |
| 5 | 66 | Squamous carcinoma | Cisplatin | 2B → 2A | Good (NTa) | Sensitive |
| 6 | 47 | Squamous carcinoma | Cisplatin | 2B → 4 | Poor | Resistant |
| 7 | 39 | Squamous carcinoma | Cisplatin | 1B2 → 1B2 | Good | Sensitive |
| 8 | 39 | Squamous carcinoma | Cisplatin | 2A → 1B1 | Good | Sensitive |
| 9 | 39 | Squamous carcinoma | Cisplatin | 2B → 2A1 | Good (NT) | Sensitive |
| 10 | 67 | Squamous carcinoma | Cisplatin | 3B → 3A | Good | Sensitive |
| 11 | 39 | Squamous carcinoma | Cisplatin | 2B → 2A1 | Good | Sensitive |
| 12 | 47 | Squamous carcinoma | Cisplatin | 2B → 4 | Poor | Resistant |
| 13 | 40 | Squamous carcinoma | Cisplatin | 3B → 4 | Poor | Resistant |
| 14 | 46 | Squamous carcinoma | Cisplatin | 2A → 1B | Good (NT) | Sensitive |
aAbbreviation used: NC neoadjuvant chemotherapy, NT no tumor after cisplatin neoadjuvant chemotherapy
Neoadjuvant chemotherapy
Initial diagnosis was made by a small surgical biopsy under colposcopic examination (pre-chemotherapy biopsy). Once the presence of the tumor was confirmed, the patients received two cycles of cisplatin neoadjuvant chemotherapy (100 mg/m2 administered in 4 days); the treatment was repeated at 21 days. The post-chemotherapy biopsies were taken at day 5 after the second neoadjuvant treatment, the samples were paraffin-included after formalin fixation, and in five cases an additional sample was taken and stored frozen for Western blot. Although the experimental conditions ended after neoadjuvant chemotherapy, we are briefly mentioning the treatments to complete the therapy after neoadjuvant chemotherapy. The patients received complete removal of the tumors (Wertheim-Meigs operation) and/or radiotherapy (50–60 Gy). After these treatments, patients were evaluated by positron emission tomography; those without evidence of disease were periodically controlled while those with evidence of disease received chemotherapy (taxanes and/or carboplatin according to the performance status).
Immunohistochemistry
Pre- and post-chemotherapy samples were immediately fixed in 10% buffered formalin and embedded in paraffin. Serial 5-μm-thick sections were mounted onto 3-aminopropyltrietoxysilane (Sigma, St. Louis, MO, USA) coated slides for subsequent analysis. The primary antibodies and their dilutions are presented in Table 2. The rabbit polyclonal antibody against HSPB1 (HSP25/27) was kindly provided by Dr. M. Gaestel (Max-Delbrück Center for Molecular Medicine, Berlin, Germany). The rabbit polyclonal antibody against specific intrastrand platinum-DNA adducts was kindly provided by Dr. M.C. Poirier (NIH, USA). The platinum-DNA adducts are persistent for many months in human tissues subsequent to cisplatin treatment (Poirier et al. 1992). The specificity of the antibody was increased by absorption of the antibody (diluted 1:100 in dilution buffer: 0.02 M NaPO4H2, 0.15 M NaC1, 0.04% sodium azide, 1% BSA, pH 7.6) with DNA, adding 20 μl of Salmon Testes DNA (phenol-chloroform extract, 10 mg/mL, Sigma) to 1000 μl of the diluted antibody. After 48 h of incubation at 4 °C, the antibody was centrifuged at 10,000 rpm for 20 min, and the supernatant used at a final dilution of 1:2000. For this antibody, the slides were pre-incubated with 2 M HCl followed by heat for antigen retrieval (30 min each step); this protocol gave the most successful immunostainings. The evaluation was done by subtractive comparative immunohistochemistry (pre- and post-chemotherapy). In addition to the antibodies presented in Table 2, antibodies against low and high molecular weight cytokeratins 35H11 and 34BE12 and against CD45 (from Dako) were applied only to those samples with difficulty to identify the tumor cells in the post-chemotherapy biopsies. Before adding the antibodies, the tissue sections were treated with heat for antigen retrieval (pH 6). Tissue sections were incubated with the primary antibodies overnight at 4 °C in humidity chambers. We used, as second antibody, biotinylated anti-rabbit and anti-mouse IgG (whole molecule) followed by avidin and biotinylated horseradish peroxidase complex (Vector Laboratories, Inc., Burlingame, CA, USA) at 1:200 dilutions (45 min). Diaminobenzidine (DAB, Vector Lab.) was used as chromogen substrate according to the manufacturer’s instructions. Slides were lightly counterstained with hematoxylin and observed with a Nikon Eclipse E200 microscope. Sections from the serial biopsies (pre- and post-chemotherapy) were always processed together. Nonspecific mouse IgG1 antibody and purified rabbit pre-immune serum (Dako, Kingsgrove, NSW, Australia) were used as isotype-negative controls. All of the slides were reviewed and scored separately by two observers who were blinded to the clinical outcome of the patients; discordant cases were re-evaluated and resolved by consensus.
Table 2.
Antibodies used for immunohistochemistry
| Antibody to Source | Dilution | |
|---|---|---|
| p53 | Mouse MAba (Dako, Glostrup, Denmark) | 1:100 |
| P16/INK4A | Mouse MAb (Abcam, USA) | 1:500 |
| NUPR1 | Rabbit PAb (Antibodies-online Inc., Atlanta, USA) | 1:500 |
| MSH2 | Mouse MAb (Merck Millipore, USA) | 1:500 |
| HSPB1/t | Rabbit PAb to hybrid Hsp25/27 (Germany) | 1:500 (1:1000 for wb) |
| HSPB1/p | Rabbit MAb [p Ser78] (Novus Littleton, CO, USA) | 1:50 |
| Cis-DNA A | Rabbit PAb (Dr. Poirier, NIH, USA) | 1:2000 |
aAbbreviations used: MAb monoclonal antibody, PAb polyclonal antibody, wb Western blot, t total meaning the non-phosphorylated and phosphorylated form of the protein, p phosphorylated form of the protein using the antibody Y175, Cis-DNA A cisplatin-DNA adducts
The samples were evaluated in intensity and proportion of cells with positive immunoreactions using a scoring system reported previously (Elledge et al. 1993). Briefly, the intensity score used was no staining = 0, weak staining = 1, moderate staining = 2, and strong staining = 3; the proportion score used was <1% = 0, 1–10% = 1, 11–30% = 2, 31–66% = 3, and >66% = 4.
Western blot analysis
Western blot analysis was performed on pre- and post-chemotherapy biopsies stored at −70 °C. Tissue samples were homogenized with lysis buffer (25 mM Tris–HCl, pH 7.5, 5 mM ethylenediamine tetraacetic acid, pH 7.5, 250 mM NaCl, 1% Triton X-100) and centrifuged 30 min to 13,000 rpm at 4 °C. The protein lysate (40–60 μg per lane) from the supernatant was subjected to electrophoresis on 12.5% SDS-polyacrylamide gels (w/v) and transferred to nitrocellulose filters, as previously described (Fanelli et al. 1998). One lane was loaded with a positive control (cytosol containing HSPB1). Molecular weight markers (Rainbow Protein Markers, Amersham) were myosin (200 kDa), phosphorylase b (97.4 kDa), BSA (69 kDa), ovalbumin (46 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (21.5 kDa), and lysozyme (14.3 kDa). The nitrocellulose filters were blocked with 5% BSA in PBS and 0.05% Tween 20 (v/v) and incubated with the primary antibody described in Table 2. After incubation overnight at 4 °C, the filters were incubated with biotinylated rabbit antibody to mouse immunoglobulins. The secondary antibody was purchased from Dako (Glostrup) and was used at a dilution of 1:2500 for 90 min. Then the filters were incubated with peroxidase-labeled streptavidin–biotin complex at a dilution of 1:5000 for 45 min to detect the proteins immunoenzymatically. The bands were developed using chemiluminescence reagents according to the manufacturer’s instructions (Dupont NEN, Boston, MA).
Treatment of HeLa cells
Human cervical cancer HeLa cells obtained from ATCC were cultured in DMEM media with the addition of 10% fetal bovine serum (FBS) (Internegocios S.A., Buenos Aires). When they reached 85% of confluence, the cells were subjected to serum-free media containing the following treatments: 0, 25, 50, and 100 μM cisplatinum (Filaxis Laboratories, Argentina) during 4 h. The selected doses were previously described by Sottile et al. (2015). The cells were fixed in 4% buffered formalin and treated for immunohistochemistry.
Statistical analyses
The Wilcoxon signed-rank nonparametric test was used to determine whether differences found in the pre- and post-chemotherapy biopsies were significant. Fisher’s exact test and Welch t test were used to determine whether the expression of the markers studied correlate with the clinical and pathological response of the patients. Statistical analyses were performed using R version 3.3.1, and a p = 0.05 was considered statistically significant.
Results
The clinical response to neoadjuvant chemotherapy was evaluated under colposcopic examination re-evaluating the clinical stage of the disease at day 5 after the second cycle of cisplatin therapy. The pathological response to neoadjuvant chemotherapy was evaluated in the paired biopsies assessing in the H&E stained sections the effects of chemotherapy: presence of massive apoptosis, necrosis, and cytological changes in the tumor cells, mitotic and apoptotic indexes. Both the clinical and pathological responses are presented in Table 1. After neoadjuvant chemotherapy, the clinical response (tumor stage) and the pathological response correlated significantly (13/14, 93%). We mention here that in a large study performed on cervical cancer patients, the clinical response to neoadjuvant chemotherapy appeared as an independent predictor of disease-free survival (Li et al. 2016).
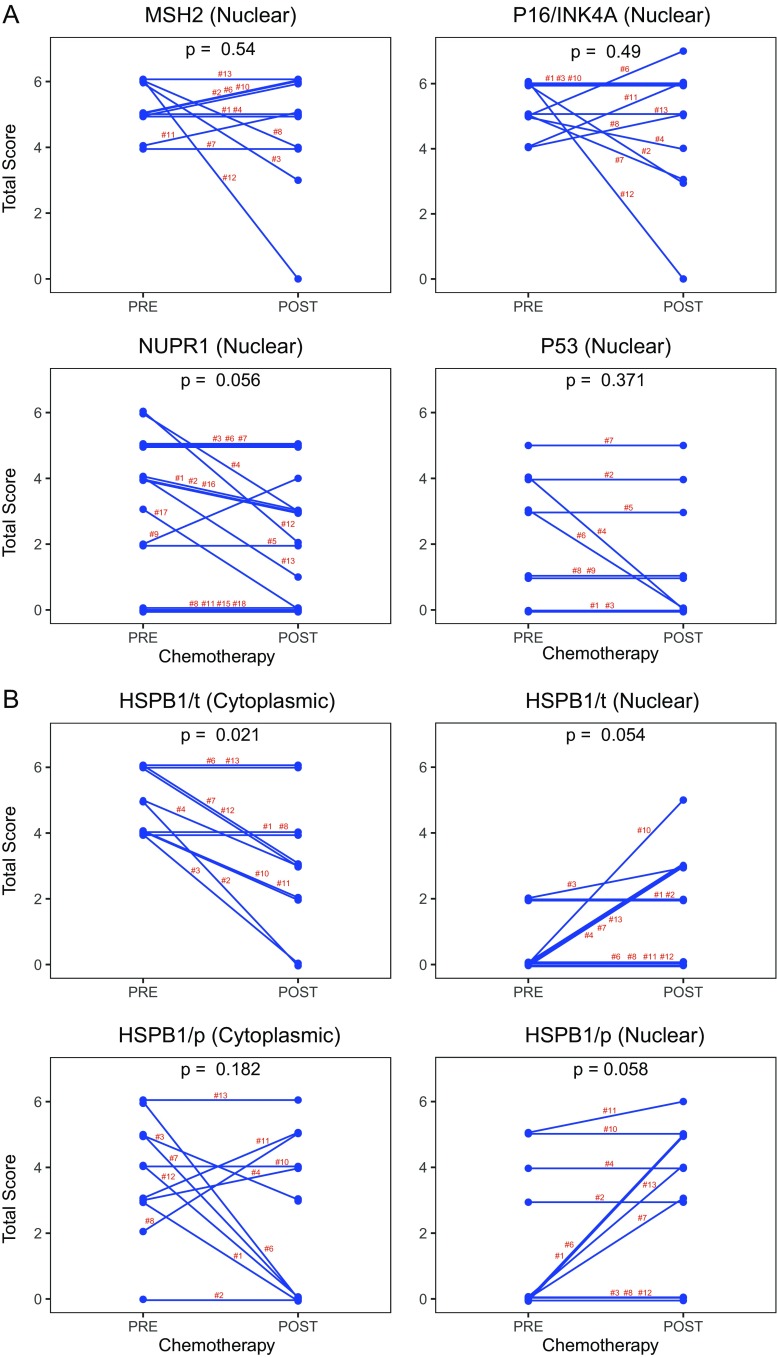
We then evaluated the immunostainings in the paired pre- and post-chemotherapy biopsies. Examples of the findings in case #1 corresponding to a patient with poor clinical and pathological response (Table 1) are shown in Fig. 1. The significant changes appeared in the expression of HSPB1, the total content of this protein decreased after chemotherapy at the cytoplasmic level while its expression increased at the nuclear level which corresponded to the phosphorylated form of the protein (Fig. 2).
Fig. 1.
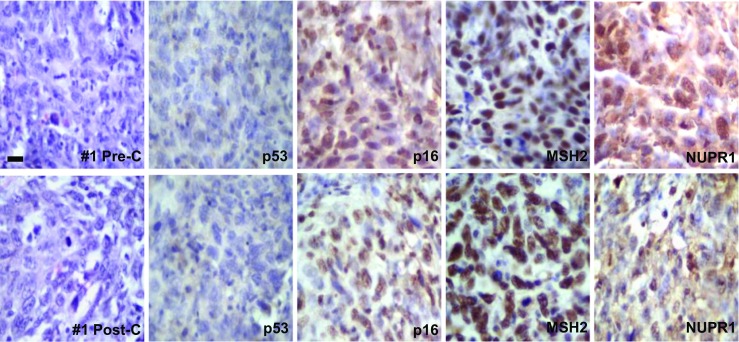
Representative photographs of case #1 showing the pre- and post-chemotherapy biopsies. No significant changes were observed in the H&E sections. p53 protein was not mutated/inactivated. Most tumor cells expressed p16, MSH2, and NUPR1 in the nucleus and no significant changes were noted in the post-chemotherapy biopsies. For comparative purposes, all photographs are shown with the same magnification, bar = 25 μm
Fig. 2.
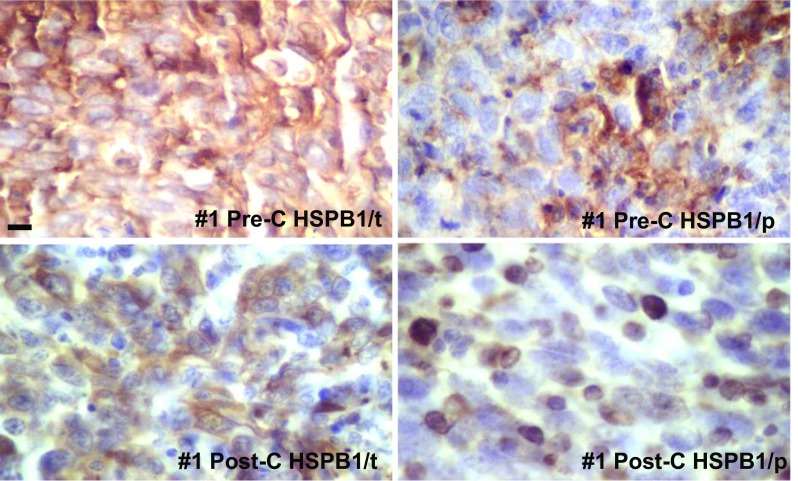
Representative images of the levels of HSPB1/t and HSPB1/p (case #1) as revealed by IHC in the pre- and post-chemotherapy biopsies. Note a modest decrease in HSPB1/t content after chemotherapy, and a strong immunostaining in the nuclei of several cells in the HSPB1/p following chemotherapy. For comparative purposes, all photographs are shown with the same magnification, bar = 35 μm
A similar situation was noted in a patient with good clinical and pathological response (case #2, Table 1) where again the total content of HSPB1 decreased after chemotherapy while the expression of HSPB1/p increased in the nuclei of the tumor cells (Fig. 3). The other molecular markers did not change significantly in this patient.
Fig. 3.

Representative photographs of case #2 showing the pre- and post-chemotherapy biopsies. Following chemotherapy, the tumor cells showed increased apoptosis and large nuclei (H&E sections). p53 protein appeared mutated/inactivated and no significant changes were noted in the post-chemotherapy biopsy. HSPB1 total and HSPB1 phosphorylated levels decreased after chemotherapy with an increase at the nuclear level (in same cells). Tumor cell heterogeneity is evident, and some areas showed cell regeneration after chemotherapy (HSPB1/t and HSPB1/p). The arrows point to nuclear HSPB1/p. The enlarged tissue section shows large nuclei following chemotherapy (some showing intense HSPB1/p). For comparative purposes, all photographs are shown with the same magnification, bar = 45 μm, except in the last were bar = 15 μm
A summary of the comparative changes in the proteins analyzed in all of the studied patients is shown in Fig. 4. In those cases where it was difficult to identify the presence of tumor cells in the post-chemotherapy biopsies, we performed immunohistochemistry with antibodies against high and low molecular weight cytokeratins and against CD45; no tumors cells were found in cases # 5, 9, and 14 (they are not shown in Fig. 4). Our finding revealed that cisplatin treatment induced a significant decrease in the content of cytoplasmic HSPB1/t (p = 0.021). Other changes showed marginal significance and were (a) a decrease in the nuclear NUPR1 levels and (b) an increase in nuclear HSPB1/t and HSPB1/p levels. No significant changed were noted in the other markers.
Fig. 4.
Expression levels of the molecular markers in the paired pre- and post-chemotherapy biopsies. a MSH2 (nuclear), P16/INK4A (nuclear), NUPR1 (nuclear), and p53 (nuclear). Note that in most of the samples, the levels of these markers suffered little modifications following chemotherapy. b HSPB1/t (cytoplasmic and nuclear) and HSPB1/p (cytoplasmic and nuclear). Note that the cytoplasmic levels of HSPB1/t decreased after chemotherapy, while the HSPB1/t and HSPB1/p nuclear levels increased (Wilcoxon test)
Since the most significant changes following chemotherapy appeared in the HSPB1 expression levels, the changes were confirmed by Western blot using the antibody that detected both the non-phosphorylated and the phosphorylated forms of the protein (Fig. 5).
Fig. 5.

Western blot and IHC of selected cases comparing HSPB1 levels in the paired pre- and post-chemotherapy biopsies. Note in the Western blot the absence of HSPB1/t in the post-chemotherapy biopsies in cases # 3 and # 2. For comparative purposes, case # 3 was selected to compare in the IHC the levels of the HSPB1/t and HSPB1/p in the pre- and post-chemotherapy biopsies. Note that HSPB1 practically disappeared after chemotherapy. In contrast, in case # 11, the protein remained in the Western blot and the IHC shows the increase of HSPB1/p in the nuclei of the tumor cells. E empty lane, Pre-C pre-chemotherapy, Post-C post-chemotherapy. Bar = 20 μm in case # 12, and bar = 15 μm in case #11
HeLa cells were treated with cisplatin to confirm that we were able to detect the platinum-DNA adducts (Fig. 6). In the clinical samples, the visualization of the platinum-DNA adducts was clear in some samples but not in others (Fig. 6). We were expecting to observe the platinum-DNA adducts in all the tumor tissues following chemotherapy but this was not the case; at present, we do not have a clear explanation for this inconsistency. In this figure, we can also observe relatively high nuclear HSPB1/p expression levels in the pre-chemotherapy as well as in the post-chemotherapy tumor cells.
Fig. 6.

Cisplatin-DNA adducts in HeLa cells and in a tissue sample (showing also HSPB1/p). HeLa cells were used as a positive control to show the formation of cisplatin-DNA adducts. Note the absence of a positive reaction in absence of cisplatin; these cells show mitosis and apoptosis. When cisplatin was added, the positive reaction appeared in the nuclei in the form of dots (cisplatin 50 μM) or in all the nuclei in apoptotic cells (cisplatin 100 μM). In contrast, in the tumor tissue, a light background appeared in the nuclei before cisplatin therapy. After cisplatin treatment, the positive reaction for adducts increased considerably; however, the tumor cells did not show apoptosis and the positive reaction appeared even in mitotic cells. In the same tissue sample, HSPB1/p appeared in tumor cells in the pre-chemotherapy biopsy and the HSPB1/p nuclear immunostaining increased in the post-chemotherapy biopsy. Pre-C pre-chemotherapy, Post-C post-chemotherapy. Bar = 15 μm in HeLa cells, and bar = 10 μm in case #4
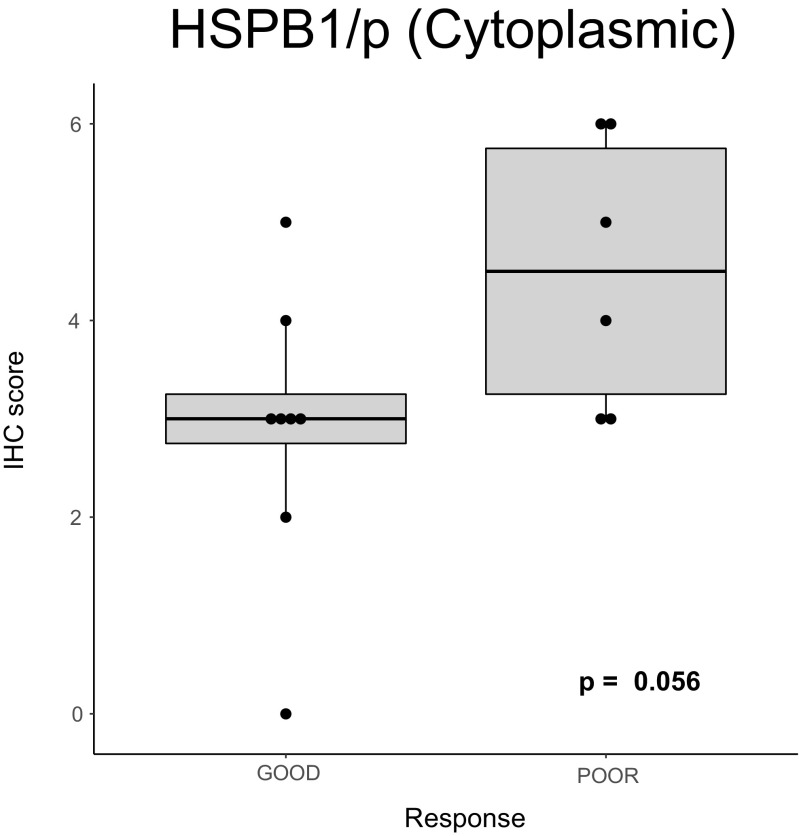
Regarding the correlations of the molecular markers with the clinical and pathological response of the tumors to neoadjuvant chemotherapy, we first analyzed the pre-chemotherapy levels of markers: none of these markers showed significant correlations except for high HSPB1/p cytoplasmic levels which correlated with a poor response to chemotherapy (Fig. 7). We then analyzed the changes after chemotherapy finding no correlations with the response to chemotherapy.
Fig. 7.
Correlation of the HSPB1/p cytoplasmic levels in the pre-chemotherapy biopsies with the response to cisplatin neoadjuvant chemotherapy
Discussion
Our study revealed that in patients with advanced cervical cancer, cisplatin neoadjuvant chemotherapy can induce significant changes in HSPB1 expression levels. The total content of the protein tended to decrease, its phosphorylation increased, and the protein was relocated from the cytoplasm to the nucleus. All of the patients received cisplatin chemotherapy, so we can state that this anticancer drug is responsible for these changes. In previous works, the expression of HSPB1 has been reported as changed by several anticancer drugs like doxorubicin or epirubicin in breast cancer (Vargas-Roig et al. 1998; Nadin et al. 2014), temozolomide in glioma cells (Castro et al. 2015), and gemcitabine in pancreatic cancer cells (Taba et al. 2010; Kang et al. 2015). The effect of cisplatin on HSPB1 has also been reported, for example in ovarian cancer cells HSPB1 (and HSPA) have been found up-regulated in cisplatin resistant cells, and the cells were more sensitive to the anticancer drug when HSPB1 levels were decreased (Yamamoto et al. 2001). Cisplatin induced the expression of HSPB1 (total) in HSC-2 (oral squamous carcinoma) and A549 (lung cancer) cells but not in 16HBE14o- (normal bronchial epithelial cells), IFN-γ administration suppressed the induction of HSPB1 by cisplatin facilitating tumor cell death (Oba et al. 2008). Cisplatin induced HSPB1 (total) expression in human hepatocellular carcinoma cells and the protein has been involved in cytoprotection by the unfolded protein response to cisplatin-induced apoptosis (Chen et al. 2011). Cisplatin induced HSPB1 phosphorylation via p38 MAPK in drug-resistant oral squamous cancer cells, and when quercetin was administered (suppressing HSPB1/p), apoptosis was increased (Chen et al. 2012). More recently, a modest increase of HSPB1 protein levels has been reported after cisplatin treatment in mesenchymal stem cells harvested from bone marrow samples of healthy donors, the changes in HSPB1 and other HSPs have been linked to cisplatin resistance (Nicolay et al. 2016). These authors have not evaluated the HSPB1 protein localization and/or its phosphorylation status. In contrast to the previous studies, Stope et al. (2016) have recently reported a significant reduction of HSPB1 caused by both paclitaxel and carboplatin in SK-OV-3 ovarian cancer cells. The same authors also reported no changes in intracellular HSPB1 levels in another two ovarian cancer cell lines (OVCAR-3 and TOV-21G); interestingly, increased extracellular HSPB1 levels were noted after chemotherapy administration in SK-OV-3 cells.
Therefore, from our results and from those mentioned above, it seems clear that (a) in cultured tumor cells, the HSPB1 protein levels (including protein phosphorylation) are modulated by cisplatin administration; (b) the modulation of HSPB1 by cisplatin differs according to the different cancer cell types; and (c) the HSPB1 appears implicated in cisplatin resistance. Mechanistically, we are tempted to speculate that when HSPB1 is decreased in the cytoplasm by phosphorylation and hence translocated to the nucleus, the HSPB1 releases client proteins, loses its antiapoptotic properties, and the tumors cells are more prone to dying. However, this scenario does not fit well with our findings; we clearly observed tumor cells with very low or no cytoplasmic HSPB1 content and with high nuclear HSPB1 levels and these cells survived 2 cycles of cisplatin as shown in Fig. 6. It is still not clear how the HSPB1 is related to drug resistance. Ovarian cancer cells transfected with HSPB1 siRNA have shown a significantly lower ability to form colonies after exposure to paclitaxel compared with cells treated with paclitaxel alone (Song et al. 2009). The authors related this effect with increasing production of reactive oxygen species. HSPB1 interacts with several client proteins; in a previous study, we reported that HSPB1 down-regulation induces PTEN up-regulation in MCF-7 cells and PTEN phosphatase is a negative regulator of the PI3 kinase/Akt signaling pathway (Cayado-Gutiérrez et al. 2013).
Our results stress that both, the total content of HSPB1 at cytoplasmic level and the HSPB1 phosphorylation status implicated in HSPB1 localization into the nucleus, are important to regulate the behavior of the cancer cells. Our results are in agreement to those of Kang et al. (2015) who have reported that the ratio of phosphorylated/nonphosphorylated HSPB1 (rather than the cellular level of HSPB1 itself) acts biphasically as a cell signal for survival or death depending on the stress intensities in gemcitabine-resistant pancreatic cancer cells. The consequences of HSPB1 phosphorylation in cancer have been reviewed elsewhere (Katsogiannou et al. 2014).
In contrast to in vitro studies, we found very few reports relating HSPB1 with cisplatin in cancer patients. One of them was performed in locally advanced esophageal adenocarcinoma patients that received neoadjuvant chemotherapy with 5-fluorouracil/cisplatin; the authors reported that a negative and weak staining for HSPB1 was associated with non-response to neoadjuvant chemotherapy; however, a survival disadvantage for patients with negative/weak HSPB1 staining could not be shown (Langer et al. 2008). The other study was performed in patients with retinoblastoma; HSPB1 cytoplasmic immunoreactivity increased significantly in the tumor cells after chemotherapy with carboplatin, etoposide, and vincristine, suggesting that these cells were protected against the cytotoxic drugs (Kase et al. 2009). In these previous reports, the authors did not study the phosphorylation status of HSPB1 and there are important differences in the tumor types; in esophageal adenocarcinomas and in retinoblastomas, the initial HSPB1 levels (before chemotherapy) are lower than in squamous cervical carcinomas (Puy et al. 1989; Doak et al. 2014, and the present study). Of interest here is also that in multivariate analysis performed in patients with esophageal squamous cell carcinomas, negative HSPB1 levels (immunostaining in <20% of the cells) predicted a good effect of radiotherapy and chemo-radiotherapy (with cisplatin/nedaplatin/5-fluorouracil) (Miyazaki et al. 2005). They found that other molecular markers (p53, p21, bax, bcl2, and HSP70) were not as reliable as HSPB1. A previous study performed on peripheral blood lymphocytes reported that in platinum-treated patients, the HSPB1 nuclear/cytoplasmic ratio was related to disease-free survival and overall survival (Nadin et al. 2007).
At this point, we do not want to be over speculative since the number of patients entered into our study was low; however, the results on the value of HSPB1 as a predictive factor to cisplatin chemotherapy in patients with advanced cervical cancer are encouraging. In a recent article, other molecular marker (aldehyde dehydrogenase 1, converting retinol to retinoic acid) has been found as a predictive marker of chemoresistance in cervical cancer patients treated with neoadjuvant chemotherapy (Xie et al. 2016). In addition, Zhu et al. (2016) have reported that in cervical cancer patients treated with cisplatin-based neoadjuvant chemotherapy, high levels of mTOR, HIF-1α, c-Myc, and PKM2 were found associated with a positive chemotherapy response. We are hoping that in the future these molecular markers can be used to predict the response of cancer patients to cisplatin-based chemotherapy.
Our study revealed that in most cases, the cisplatin neoadjuvant chemotherapy was not significantly affecting the expression levels of MSH2, p16, and p53. Only nuclear NUPR1 levels tended to decrease. We did not find a significant correlation between high p16 levels and poor response to cisplatin treatment in vivo; nevertheless, the results show a tendency that might confirm the findings of Li et al. (2016) performed in SiHa cells, and are therefore interesting to be studied further. MSH2 was reported to be highly expressed during cell stress when the phosphorylated HSPB1 translocates to the nucleus, both taking part in the mismatch repair system (Nadin et al. 2007; Castro et al. 2015); furthermore, the protein in the heterocomplex with MSH6 was found to be required for cisplatin sensitivity (Sawant et al. 2015). We therefore hypothesized that up-regulated MSH2 could be a possible predictor for good response to chemotherapy. However, in the present study, we observed a slight tendency toward poor response of the patients when the protein was highly expressed in the pre-chemotherapy biopsies. Finally, the expression of the other two molecular markers examined, p53 and NUPR1, were not associated with the response to cisplatin neoadjuvant chemotherapy.
In conclusion, our study supports the importance of HSPB1 in relationship to chemo resistance to cisplatin induction chemotherapy in patients with cervical cancer. Further studies should be directed to increase the number of patients to validate the results and to explore the possible molecular pathways implicated using cervical cancer cells.
Acknowledgments
This work was partially supported by CONICET grant (PIP 11220110100836 DAS 30844). The authors confirm that the founder had no influence over the study design, content of the article, or selection of this journal.
Footnotes
N. Real, G. N. Castro, and F. D. Cuello-Carrión have contributed equally to this work.
References
- Al-Mansour Z, Verschraegen C. Locally advanced cervical cancer: what is the standard of care? Curr Opin Oncol. 2010;22:503–512. doi: 10.1097/CCO.0b013e32833af426. [DOI] [PubMed] [Google Scholar]
- Arrossi S (2008) Proyecto para el mejoramiento del Programa Nacional de Prevención del Cáncer de Cuello Uterino en Argentina. 1ra. Edicion. Buenos Aires. Organizacion Panamericana de la Salud, 163 p. ISBN: 978–950–710-114-4
- Arrossi S, Maceira V, Paolino M, Sankaranarayanan R. Acceptability and uptake of HPV vaccine in Argentina before its inclusion in the immunization program: a population-based survey. Vaccine. 2012;30:2467–2474. doi: 10.1016/j.vaccine.2012.01.032. [DOI] [PubMed] [Google Scholar]
- Cano CE, Hamidi T, Sandi MJ, Iovanna JL. Nupr1: the Swiss-knife of cancer. J Cell Physiol. 2011;226:1439–1443. doi: 10.1002/jcp.22324. [DOI] [PubMed] [Google Scholar]
- Castro GN, Cayado-Gutiérrez N, Zoppino FC, Fanelli MA, Cuello-Carrión FD, Sottile M, Nadin SB, Ciocca DR. Effects of temozolomide (TMZ) on the expression and interaction of heat shock proteins (HSPs) and DNA repair proteins in human malignant glioma cells. Cell Stress Chaperones. 2015;20:253–265. doi: 10.1007/s12192-014-0537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayado-Gutiérrez N, Moncalero VL, Rosales EM, Berón W, Salvatierra EE, Alvarez-Olmedo D, Radrizzani M, Ciocca DR. Downregulation of Hsp27 (HSPB1) in MCF-7 human breast cancer cells induces upregulation of PTEN. Cell Stress Chaperones. 2013;18:243–249. doi: 10.1007/s12192-012-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Dai RY, Duan CY, Liu YP, Chen SK, Yan DM, Chen CN, Wei M, Li H. Unfolded protein response suppresses cisplatin-induced apoptosis via autophagy regulation in human hepatocellular carcinoma cells. Folia Biol (Praha) 2011;57:87–95. [PubMed] [Google Scholar]
- Chen SF, Nieh S, Jao SW, Liu CL, Wu CH, Chang YC, Yang CY, Lin YS. Quercetin suppresses drug-resistant spheres via the p38 MAPK-Hsp27 apoptotic pathway in oral cancer cells. PLoS One. 2012;7(11):e49275. doi: 10.1371/journal.pone.0049275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;0:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doak SH, Jenkins GJ, Parry EM, Griffiths AP, Baxter JN, Parry JM. Differential expression of the MAD2, BUB1 and HSP27 genes in Barrett's oesophagus-their association with aneuploidy and neoplastic progression. Mutat Res. 2014;547:133–144. doi: 10.1016/j.mrfmmm.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Elledge RM, Ciocca DR, Langone G, McGuire WL. Estrogen receptor, progesterone receptor, and HER-2/neu protein in breast cancers from pregnant patients. Cancer. 1993;71:2499–2506. doi: 10.1002/1097-0142(19930415)71:8<2499::AID-CNCR2820710812>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Fanelli MA, Cuello Carrión FD, Dekker J, Schoemaker J, Ciocca DR (1998) Serological detection of heat shock protein hsp27 in normal and breast cancer patients. Cancer Epidemiol Biomarkers Prev 7:791–795 [PubMed]
- Ferlay J, Soerjomataram I, Ervik M, et al. (2012) GLOBOCAN 2012 v1. 0, Cancer incidence and mortality worldwide: IARC cancer Base No. 11. Lyon, France: International Agency for Research [on-line]. http://globocan.iarc.fr
- Fong CW. Platinum anti-cancer drugs: free radical mechanism of Pt-DNA adduct formation and anti-neoplastic effect. Free Radic Biol Med. 2016;95:216–229. doi: 10.1016/j.freeradbiomed.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Hamidi T, Algül H, Cano CE, Sandi MJ, Molejon MI, Riemann M, Calvo EL, Lomberk G, Dagorn J-C, Weih F, Urrutia R, Schmid RM, Iovanna JL. Nuclear protein 1 promotes pancreatic cancer development and protects cells from stress by inhibiting apoptosis. J Clin Invest. 2012;122:2092–2103. doi: 10.1172/JCI60144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JC, Murer B, O'Grady A, Hearn LM, Harvey BJ, Kay EW, Thomas W. Differential p16/INK4A cyclin-dependent kinase inhibitor expression correlates with chemotherapy efficacy in a cohort of 88 malignant pleural mesothelioma patients. Br J Cancer. 2015;113:69–75. doi: 10.1038/bjc.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Choi HJ, Kang S, Kim SY, Hwang YS, Je S, Han Z, Kim JH, Song JJ. Ratio of phosphorylated HSP27 to nonphosphorylated HSP27 biphasically acts as a determinant of cellular fate in gemcitabine-resistant pancreatic cancer cells. Cell Signal. 2015;27:807–817. doi: 10.1016/j.cellsig.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Kase S, Parikh JG, Rao NA. Expression of heat shock protein 27 and alpha-crystallins in human retinoblastoma after chemoreduction. Br J Ophthalmol. 2009;93:541–544. doi: 10.1136/bjo.2008.145508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsogiannou M, Andrieu C, Rocchi P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front Genet. 2014;5:346. doi: 10.3389/fgene.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R, Ott K, Specht K, Becker K, Lordick F, Burian M, Herrmann K, Schrattenholz A, Cahill MA, Schwaiger M, Hofler H, Wester HJ. Protein expression profiling in esophageal adenocarcinoma patients indicates association of heat-shock protein 27 expression and chemotherapy response. Clin Cancer Res. 2008;14:8279–8287. doi: 10.1158/1078-0432.CCR-08-0679. [DOI] [PubMed] [Google Scholar]
- Li Y, Xiao S, Dan L, Xue M. P16INK4A is required for cisplatin resistance in cervical carcinoma SiHa cells. Oncol Lett. 2015;9:1104–1108. doi: 10.3892/ol.2014.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Huang K, Zhang Q, Shen J, Zhou H, Yang R, Wang L, Liu J, Zhang J, Sun H, Jia Y, Du X, Wang H, Deng S, Ding T, Jiang J, Lu Y, Li S, Wang S, Ma D (2016) Early response to neoadjuvant chemotherapy can help predict long-term survival in patients with cervical cancer. Oncotarget. doi: 10.18632/oncotarget.11460 [DOI] [PMC free article] [PubMed]
- Luvero D, Plotti F, Aloisi A, Capriglione S, Ricciardi R, Miranda A, Lopez S, Scaletta G, De Luca G, Benedetti-Panici P, Angioli R. Patients treated with neoadjuvant chemotherapy + radical surgery + adjuvant chemotherapy in locally advanced cervical cancer: long-term outcomes, survival and prognostic factors in a single-center 10-year follow-up. Med Oncol. 2016;33:110. doi: 10.1007/s12032-016-0830-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kato H, Faried A, Sohda M, Nakajima M, Fukai Y, Masuda N, Manda R, Fukuchi M, Ojima H, Tsukada K, Kuwano H. Predictors of response to chemo-radiotherapy and radiotherapy for esophageal squamous cell carcinoma. Anticancer Res. 2005;25:2749–2755. [PubMed] [Google Scholar]
- Moureau S, Luessing J, Harte EC, Voisin M, Lowndes NF (2016) A role for the p53 tumour suppressor in regulating the balance between homologous recombination and non-homologous end joining. Open Biol. 6(9) [DOI] [PMC free article] [PubMed]
- Nadin S, Ciocca DR (2010) Participation of heat shock proteins in DNA repair mechanisms in cancer. In: DNA Damage Repair, Repair Mechanisms and Aging. Editor: Allison E. Thomas, pp. 165–186. ISBN 978–1–61668-914-8
- Nadin SB, Vargas-Roig LM, Drago G, Ibarra J, Ciocca DR. Hsp27, Hsp70 and mismatch repair proteins hMLH1 and hMSH2 expression in peripheral blood lymphocytes from healthy subjects and cancer patients. Cancer Lett. 2007;252:131–146. doi: 10.1016/j.canlet.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Nadin SB, Sottile ML, Montt-Guevara MM, Gauna GV, Daguerre P, Leuzzi M, Gago FE, Ibarra J, Cuello-Carrión FD, Ciocca DR, Vargas-Roig LM. Prognostic implication of HSPA (HSP70) in breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy. Cell Stress Chaperones. 2014;19:493–505. doi: 10.1007/s12192-013-0475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, Sharma N, Kapoor A, Sharma R, Kumar N, Singhal M, Purohit R, Jakhar SL, Beniwal S, Kumar HS, Sharma A. Pros and cons of adding of neoadjuvant chemotherapy to standard concurrent chemoradiotherapy in cervical cancer: a regional cancer center experience. J Obstet Gynaecol India. 2016;66:385–390. doi: 10.1007/s13224-015-0698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay NH, Lopez Perez R, Rühle A, Trinh T, Sisombath S, Weber K-J, Ho AD, Debus J, Saffrich R, Huber PE. Mesenchymal stem cells maintain their defining stem cell characteristics after treatment with cisplatin. Sci Rep. 2016;6:20035. doi: 10.1038/srep20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba M, Yano S, Shuto T, Suico MA, Eguma A, Kai H. IFN-gamma down-regulates Hsp27 and enhances hyperthermia-induced tumor cell death in vitro and tumor suppression in vivo. Int J Oncol. 2008;32:1317–1324. doi: 10.3892/ijo_32_6_1317. [DOI] [PubMed] [Google Scholar]
- Palam LR, Gore J, Craven KE, Wilson JL, Korc M. Integrated stress response is critical for gemcitabine resistance in pancreatic ductal adenocarcinoma. Cell Death Dis. 2015;6:e1913. doi: 10.1038/cddis.2015.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcey R, Brundage M, Drouin P, Jeffrey J, Johnston D, Lukka H, MacLean G, Souhami L, Stuart G, Tu D. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20:966–972. doi: 10.1200/JCO.2002.20.4.966. [DOI] [PubMed] [Google Scholar]
- Poirier MC, Reed E, Litterst CL, Katz D, Gupta-Burt S. Persistence of platinum-ammine-DNA adducts in gonads and kidneys of rats and multiple tissues from cancer patients. Cancer Res. 1992;52:149–153. [PubMed] [Google Scholar]
- Puy LA, Lo Castro G, Olcese JE, Lotfi HO, Brandi HR, Ciocca DR. Analysis of a 24-kilodalton (KD) protein in the human uterine cervix during abnormal growth. Cancer. 1989;64:1067–1073. doi: 10.1002/1097-0142(19890901)64:5<1067::AID-CNCR2820640518>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Robl B, Pauli C, Botter SM, Bode-Lesniewska B, Fuchs B. Prognostic value of tumor suppressors in osteosarcoma before and after neoadjuvant chemotherapy. BMC Cancer. 2015;15:379. doi: 10.1186/s12885-015-1397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant A, Kothandapani A, Zhitkovich A, Sobol RW, Patrick SM. Role of mismatch repair proteins in the processing of cisplatin interstrand cross-links. DNA Repair (Amst) 2015;35:126–136. doi: 10.1016/j.dnarep.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song TF, Zhang ZF, Liu L, Yang T, Jiang J, Li P. Small interfering RNA-mediated silencing of heat shock protein 27 (HSP27) increases chemosensitivity to paclitaxel by increasing production of reactive oxygen species in human ovarian cancer cells (HO8910) J Int Med Res. 2009;37:1375–1388. doi: 10.1177/147323000903700512. [DOI] [PubMed] [Google Scholar]
- Sottile ML, Losinno AD, Fanelli MA, Cuello-Carrión FD, Montt-Guevara MM, Vargas-Roig LM, Nadin SB. Hyperthermia effects on Hsp27 and Hsp72 associations with mismatch repair (MMR) proteins and cisplatin toxicity in MMR-deficient/proficient colon cancer cell lines. Int J Hyperth. 2015;31:464–475. doi: 10.3109/02656736.2015.1026848. [DOI] [PubMed] [Google Scholar]
- Stope MB, Wiegank L, Weiss M, Diesing K, Koensgen D, Burchardt M, Zygmunt M, Mustea A. Drug-induced modulation of heat shock protein HSPB1 in an ovarian cancer cell model. Anticancer Res. 2016;36:3321–3327. [PubMed] [Google Scholar]
- Taba K, Kuramitsu Y, Ryozawa S, Yoshida K, Tanaka T, Maehara S, Maehara Y, Sakaida I, Nakamura K. Heat-shock protein 27 is phosphorylated in gemcitabine-resistant pancreatic cancer cells. Anticancer Res. 2010;30:2539–2543. [PubMed] [Google Scholar]
- Tadessse SK. Socio-economic and cultural vulnerabilities to cervical cancer and challenges faced by patients attending care at Tikur Anbessa hospital: a cross sectional and qualitative study. BMC Womens Health. 2015;15:75. doi: 10.1186/s12905-015-0231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur B, Ray O. p53 loses grip on PIK3CA expression leading to enhanced cell survival during platinum resistance. Mol Oncol. 2016;10:1283–1295. doi: 10.1016/j.molonc.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR (1998) Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer 79:468–475 [DOI] [PubMed]
- Vincent AJ, Ren S, Harris LG, Devine DJ, Samant RS, Fodstad O, Shevde LA. Cytoplasmic translocation of p21 mediates NUPR1-induced chemoresistance NUPR1 and p21 in chemoresistance. FEBS Lett. 2012;586:3429–3434. doi: 10.1016/j.febslet.2012.07.063. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2016) Human papillomavirus (HPV) and cervical cancer. On line http://www.who.int/mediacentre/factsheets/fs380/en/
- Xie Q, Liang J, Rao Q, Xie X, Li R, Liu Y, Zhou H, Han J, Yao T, Lin Z. Aldehyde dehydrogenase 1 expression predicts chemoresistance and poor clinical outcomes in patients with locally advanced cervical cancer treated with neoadjuvant chemotherapy prior to radical hysterectomy. Ann Surg Oncol. 2016;23:163–170. doi: 10.1245/s10434-015-4555-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Okamoto A, Isonishi S, Ochiai K, Ohtake Y. Heat shock protein 27 was up-regulated in cisplatin resistant human ovarian tumor cell line and associated with the cisplatin resistance. Cancer Lett. 2001;168:173–181. doi: 10.1016/S0304-3835(01)00532-8. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wu J, Zhang W, Luo H, Shen Z, Cheng H, Zhu X. PKM2 enhances chemosensitivity to cisplatin through interaction with the mTOR pathway in cervical cancer. Sci Rep. 2016;6:30788. doi: 10.1038/srep30788. [DOI] [PMC free article] [PubMed] [Google Scholar]