Abstract
Heatstroke is associated with systemic inflammatory response syndrome, leading to multiple organ dysfunction and death. Currently, there is no specific treatment decreasing hyperthermia-induced inflammatory/hemostatic derangements. Emerging studies indicate that histones leaking from damaged cells into the extracellular space are toxic, pro-inflammatory, and pro-thrombotic. We therefore hypothesize that serum histones (sHs) are elevated during heatstroke and are associated with the severity of the disease. Sixteen dogs with heatstroke and seven healthy controls were included in the study. Median serum histones (sHs) upon admission in dogs with heatstroke were significantly higher (P = 0.043) compared to that in seven controls (13.2 vs. 7.3 ng/mL, respectively). sHs level was significantly higher among non-survivors and among dogs with severe hemostatic derangement (P = 0.049, median 21.4 ng/mL vs. median 8.16 ng/mL and P = 0.038, 19.0 vs. 7.0 ng/mL, respectively). There were significant positive correlation between sHs and urea (r = 0.8, P = 0.02); total CO2 (r = 0.661, P = 0.05); CK (r = 0.678, P = 0.04); and prothrombin time (PT) 12 h post presentation (r = 0.888, P = 0.04). The significant positive correlation between sHs and other heatstroke severity biomarkers, and significant increase among severely affected dogs, implies its role in inflammation/oxidation/coagulation during heatstroke. sHs, unlike other prognostic and severity biomarkers in heatstroke, can be pharmacologically manipulated, offering a potential therapeutic target.
Keywords: Inflammation, Canine, Hemostatic, Heparin, Protein C
Introduction
Heatstroke occurs with exposure to a hot environment (classical heatstroke) or due to strenuous physical exercise (exertional heatstroke) and inability to dissipate the accumulated body heat (Epstein and Roberts 2011; Leon and Bouchama 2015; Shibolet and Farfel 1975). In dogs, it is characterized by core temperatures higher than <41 °C, acute collapse, and neurological abnormalities (Bruchim et al. 2006). Hyperthermia leads to inflammatory cytokine production, reactive oxygen and nitrogen species generation, and endotoxemia and endothelial injury, thus contributing to the increased vascular permeability, and in turn edema formation, similarly to sepsis (Bouchama and Knochel 2002; Leon and Helwig 2010a, b). Our previous studies in dogs with naturally occurring heatstroke pinpointed several prognostic clinical and laboratory abnormalities (Aroch et al. 2009; Bruchim et al. 2006, 2009, 2016; Segev et al. 2015b). Severe heatstroke complications in dogs commonly include rhabdomyolysis, acute kidney injury (AKI), acute respiratory distress syndrome (ARDS), and disseminated intravascular coagulation (DIC) (Bruchim et al. 2006, 2009). Despite early appropriate body cooling and intensive supportive treatment, mortality rates of heatstroke in humans and dogs remain around 50% (Aroch et al. 2009; Bouchama and Knochel 2002; Bruchim et al. 2006, 2016). Currently, there is no specific treatment to slow down or significantly decrease the life-threatening inflammatory and hemostatic derangements in canine heatstroke.
Histones are highly conserved, positively charged nuclear proteins, serving as the basic structure block unit of the chromatin. Emerging studies indicate that histones leak from damaged and activated immune system cells (e.g., neutrophils and mast cells) and by neutrophil extracellular traps (NETs) into the extracellular space, exhibiting toxic, pro-inflammatory, and pro-thrombotic properties (Ginsburg and Kohen 1995; Ginsburg et al. 2016; Hoeksema et al. 2016; Xu et al. 2009, 2015). Extracellular serum histones (sHs) have been hypothesized to act as potential mediators of lethal systemic infectious (e.g., sepsis and peritonitis) and non-infectious (e.g., ischemia-reperfusion injury, pancreatitis, drug-induced tissue toxicity, and stroke) inflammatory diseases (Chen et al. 2014; Xu et al. 2009, 2015). Their serum concentration increases in experimental E. coli infusion, inducing endotoxemia and septic shock in rats (Xu et al. 2009) as well as in humans with sepsis (Ekaney et al. 2014).
Studies in animals and in humans suggest that sHs concentration may serve as a biomarker of the severity of stress and the outcome, as well as a potential, novel therapeutic target in several inflammatory diseases. Since heatstroke is a severe systemic inflammatory response syndrome (SIRS), resembling sepsis in many ways, (Bouchama and Knochel 2002), it is likely that sHs concentrations will show similar changes in dogs with heatstroke. To test sHs in dogs, we included seven healthy young dogs as controls, and 16 dogs with naturally occurring heatstroke. We show in this investigation that sHs are markedly elevated in detrimental heatstroke. This elevation accompanies severe hemostatic derangement as well indicating sHs role in inflammation/coagulation in heatstroke in dogs. sHs concentration in canine heatstroke will potentially provide useful diagnostic and prognostic information, which may become an attractive therapeutic target.
Material and methods
Selection of dogs and data collection
Seven healthy young (median 3.5 years, range; 2–5 years old), large breed dogs (median body weight 27 kg, range; 20–35 kg), four males, and three females were selected as controls for measurement of sHs concentration (Table 1). Dogs were deemed healthy based on normal medical history, physical examination, and complete blood count (CBC) findings. Dogs with naturally occurring environmental or exertional heatstroke were prospectively and consecutively enrolled, with their owners’ signed consent and with approval of the Hebrew University Veterinary Teaching Hospital (HUVTH) Ethics Committee. Heatstroke was diagnosed based on a history of strenuous exercise, exposure to a hot environment, or both, and acute onset of appropriate clinical signs (i.e., acute collapse and CNS dysfunction, increased body temperature, tachypnea, or tachycardia) in dogs that were otherwise healthy prior to the insult (Bruchim et al. 2016). Dogs with pre-existing diseases, based on history and physical examination, were excluded. The dogs underwent physical examination at presentation and were monitored throughout hospitalization. Data were collected from their medical records, including anamnesis and demographic data, physical examination and laboratory findings, the duration of hospitalization, occurrence of secondary complications (i.e., severe coagulation derangements), and the outcome (died, euthanized, or discharged alive).
Table 1.
Breed, gender, and body weight in 16 dogs with naturally occurring heatstroke and seven healthy controls
| Dogs with heatstroke (n = 16) | Healthy control dogs (n = 7) | ||||||
|---|---|---|---|---|---|---|---|
| Dog breed | Sex | Body weight | Age | Dog breed | Sex | Body weight (kg) | Age (years) |
| Dog de Bordeaux | M | 60 | 2.5 | Australian Shepherd | F | 23 | 4 |
| Mixed breed | M | 23 | 3 | Boxer | F | 26 | 3 |
| German Shepherd | M | 32 | 3.5 | Mixed breed | F | 28 | 2 |
| Mixed breed | M | 28 | 3.0 | Border Collie | M | 20 | 3 |
| Mixed breed | F | 16 | 8.0 | Mixed breed | M | 35 | 5 |
| Dog de Bordeaux | F | 48 | 5.0 | Mixed breed | M | 27 | 3 |
| Belgian Malinios | F | 25 | 6.5 | German Shepherd | M | 28 | 5 |
| Border Collie | M | 24 | 9.0 | ||||
| Caucasian Shepherd | M | 70 | 4.5 | ||||
| Mixed breed | M | 18 | 2.0 | ||||
| Standard Poodle | F | 7 | 2.0 | ||||
| Weimaraner | F | 25 | 4.0 | ||||
| Australian Shepherd | M | 30 | 1.5 | ||||
| Mixed breed | M | 50 | 4.0 | ||||
| Mixed breed | M | 23 | 3.5 | ||||
| Siberian Husky | M | 23 | 3 | ||||
| Median (range) | 25 (7–70) | 3.5 (2–9) | 27 (20–35) | 3.5 (2–5) | |||
F female, M male
Markers of organ damage
To evaluate organ damage, the potential origin of serum histones, and association of consensus biomarkers of the severity of disease with sHs level, several laboratory analytes were measured and monitored. Serum activities of alanine amino transferase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) were used to assess hepatobiliary damage. Muscle damage was assessed by serum activities of AST and creatine kinase (CK). Kidney injury was assessed by serum creatinine and urea concentrations. AKI was diagnosed based on the International Renal Interest Society (IRIS) guidelines, (Segev et al. 2015a). Serum lactate and total CO2 and venous blood gas analysis were done to assess acid-base balance and hypoxia. Severe hemostatic derangement (SHD) was diagnosed if prothrombin time (PT) and activated thromboplastin time (aPTT) were prolonged >150% their upper reference limit (URL), presence of thrombocytopenia (platelets <150 × 109/L) along with clinical signs of spontaneous bleeding (e.g., petechiae, ecchymosis, melena, hematochezia hematuria, or hematemesis).
Survival
Dogs discharged alive from the hospital were considered survivors, while those died or euthanized due to deterioration during hospitalization were defined as non-survivors.
Serum histones
Total sHs concentration was measured using a commercially available EIA (Assay Design, NY), according to manufacturers’ instructions. Briefly, analyses were done in duplicates. Samples were placed into the wells, biotinylated anti-histone antibodies were added, and the sealed plate was incubated for 2 h at room temperature. The plate was then washed with Tris buffered solution, leaving only plate-bound histones. Histones, then, were stained with a horseradish peroxide catalyzed reaction for 30 min. The dyed solution was read by a spectrophotometer (Bio-Rad, i-MARK microplate reader, USA) at 450 nm. The optical density (OD) is directly proportional to total histone concentration.
Hematological and biochemical tests
Whole blood samples for CBC were collected at presentation in potassium-EDTA tubes and analyzed within 30 min of collection. Blood smears, stained with modified Wright’s staining solution, were used to evaluate blood cell morphology, to manually count the platelet number and to exclude platelet clumping as a cause of spurious thrombocytopenia. The manual platelet count was performed by averaging platelet numbers in ten randomly selected oil (×100) fields and multiplying the average by 20,000 cells/μL (Aroch et al. 2009).
Whole blood samples for PT and aPTT were collected in 3.2% trisodium-citrate tubes at presentation and at 4 and 12 h post presentation (PP). Blood samples were collected from a peripheral IV catheter upon its placement at presentation, and from dedicated sampling peripheral IV catheters thereafter, using a standard method. Samples were centrifuged within 30 min of collection, and harvested plasmas were either analyzed immediately, at the attending clinician’s decision, or stored at −80 °C pending analysis, which was performed within 12 months, in two single runs, using a coagulometric auto analyzer (ACL-9000, Instrumentation Laboratory, Milano, Italy). Whole blood samples for serum biochemistry were collected in plain tubes with gel separators, allowed to clot, and centrifuged within 30 min of collection, and harvested sera were either analyzed immediately at the attending clinician’s decision, or refrigerated (at 4 °C) and analyzed within 12 h of collection or stored at −80 °C pending analysis, done within 12 months of collection, using an autoanalyzer (Cobas Integra 400 Plus, Roche, Mannheim, Germany; at 37 °C).
Treatment protocol
All dogs were treated according to a current standardized HUVTH protocol for heatstroke. At presentation, two intravenous catheters were placed, one for IV fluid therapy and another for blood sampling. Dogs presented with hyperthermia were treated by whole body irrigation with tap water and fanning with a ventilator until a core temperature of 39.5 °C was reached. All dogs received IV fluids (lactated Ringer’s solution; bolus of 50 mL/kg, followed by constant infusion rate (CRI) of 7.5 mL/kg/h), IV broad spectrum antibiotics (amoxycillin/clavulanic acid; 15 mg/kg q12h, metronidazole; 15 mg/kg q12h, famotidine; 0.5–1 mg/kg IV q24h, and metoclopramide; 0.02 mg/kg/h at CRI). Arterial blood pressure (ABP) was measured every 4 h (Cardell, Midmark, Tampa, FL).
Statistical methods
The distribution pattern of continuous variables was assessed using Q-Q plot. Because of the limited cohort size and because most of the continuous variables did not have normal distribution, non-parametric tests were used and data are presented as median and range. Comparison of all continuous variables between the survivors and the non-survivors was performed using the Mann-Whitney U test. Due to abnormal distribution of histones concentration, logarithmic transformation was performed, distribution was assessed by Q-Q plot. Since the transformed results showed normal distribution, Student’s t test was used to compare sHs concentration between survivors and non-survivors, and between dogs with heatstroke and controls. Spearman correlation test was used to assess the correlation between two quantitative variables. Receiver operator curve (ROC) analysis was applied for finding an optimal cutoff point of sHs concentration (with the best balance between sensitivity and specificity) between survivors and non- survivors, and between dogs with sHs and severe hemostatic derangement. All tests were two-tailed and P ≤ 0.05 was considered significant. Statistical analyses were performed using a statistical software package (SPSS 22.0 for Windows, SPSS Inc., Chicago, IL, USA).
Results
Signalment and physical parameters
The study included 16 dogs with naturally occurring heatstroke (environmental nine dogs, 57% and exertional seven dogs, 43%), 11 male, and 5 females, with a median body weight of 25 kg (range 7.5–70 kg) and median age of 3.5 years (range 2–9 years) (Table 1). The median lag of time from the heat-induced insult to presentation to the HUVTH was 3.8 h (range 2–17 h). There were no significant age and body weight differences between dogs with heatstroke and the controls (P > 0.05).
The most common clinical signs at presentation included signs consisted with shock and collapse (14/16, 87%); petechiae (10/16, 62%); and seizures or coma (6/16, 37%). All six dogs presented to referral clinics prior to presentation to the HUVTH had rectal temperature (Tre) > 40 °C. Only 5/16 (37%) had a Tre > 40 °C at presentation to the HUVTH. At presentation, the median arterial systolic and diastolic blood pressures were 128 mmHg (range 78–185) and 72 mmHg (range 33–132), respectively.
Serum biochemistry, hematology, and complications
The medians of the leukocyte count, ALP, and GGT activities and calculated bicarbonate concentration were significantly higher in the non-survivors compared to the survivors (P < 0.05, Table 2). Severe hemostatic derangement was recorded in 9/16 dogs (50%), and AKI in 5/16 (31%).
Table 2.
Total serum histones, leukocyte count, platelets, serum enzyme activities, and lactate and total CO2 concentrations, pH, and clotting in ten non-surviving and six surviving dogs with naturally occurring heatstroke
| Analyte (reference interval; units) | Survivors (n = 6) median (range) | Non-survivors (n = 10) median (range) | P value1 |
|---|---|---|---|
| Serum histones (ng/mL) | 8.16 (1.0–19) | 21.4 (4.2–40) | 0.0492 |
| White blood cell count (6–10 × 103/μL) | 10.5 (6.8–19.1) | 18.5 (6.7–35.0) | 0.05 |
| Hematocrit (35–50%) | 62 (50–72) | 57 (23–65) | 0.28 |
| Platelets (>143 × 103) | 93 (57–170) | 115 (61–431) | 0.36 |
| Lactate (0–1.8 mmol/L) | 2.2 (0.9–9.9) | 2.9 (1.6–8.9) | 0.54 |
| Aspartate transaminase (14–80 U/L) | 416 (2223–1623) | 3293 (104–6153) | 0.62 |
| Alanine aminotransferase (19–67; U/L) | 130 (86–182) | 522 (44–1929) | 0.92 |
| Creatine kinase (20–160 U/L) | 9333 (461–40,000) | 40,000 (684–54,558) | 0.69 |
| γ-glutamyl transpeptidase (0–6 U/L) | 1.5 (1.7–6.5) | 12.7 (6.1–26.3) | 0.039 |
| Alkaline phosphatase (13–190 U/L) | 77 (28–100) | 202 (15–254) | 0.049 |
| Creatinine (0.5–1.2 mg/dL) | 1.5 (1.1–1.8) | 1.6 (0.6–2.8) | 0.71 |
| Urea (10–45 mg/dL) | 47 (31–68) | 59 (6–83) | 0.6 |
| Prothrombin time (6.0–8.4 s) | 12.6 (6.9–30.0) | 15.8 (7–27) | 0.5 |
| aPTT (11–17.4 s)3 | 30.6 (12–100) | 43.0 (21–100) | 0.6 |
| pH (7.35–7.45) | 7.38 (7.22—7.4) | 7.26 (7.08–7.38) | 0.07 |
| Bicarbonate (18–22 mmol/L) | 16.5 (15.1–21.1) | 13.2 (9.9–17.2) | 0.04 |
| Total CO2 (22–26 mmol/L) | 9.2 (9.0–13.7) | 10.7 (5.2–16.6) | 0.7 |
aMedian values compared using the Mann-Whitney non-parametric test
bLogarithmic transformation was performed, distribution was assessed by Q-Q plot, and Student’s t test was used to compare sHs concentration between survivors and non-survivors
cActivated partial thromboplastin time
Serum histones
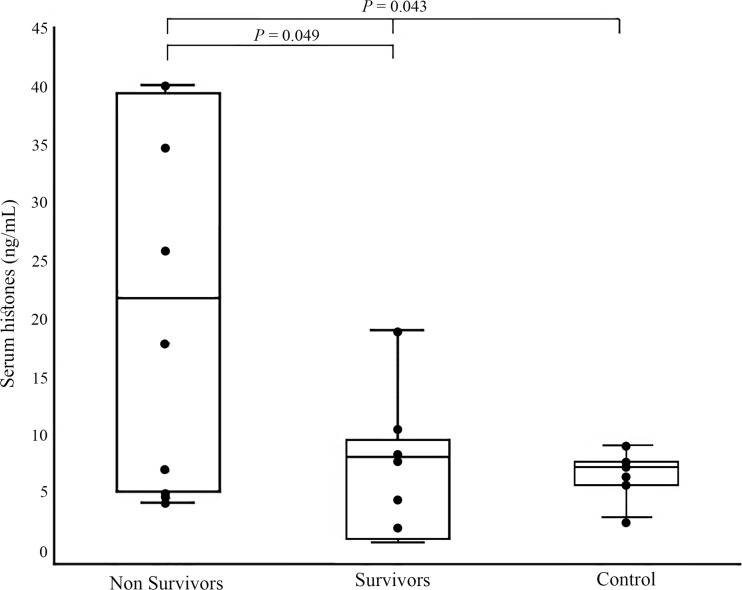
sHs concentration in the control and heatstroke groups were 7.1 ng/mL (range 3.0–9.1) and 13.2 ng/mL (range 2.9–40), respectively, and was significantly higher (P = 0.043) in the latter. Median sHs concentration was significantly higher (P = 0.049) in the non-survivors compared to that in the survivors, (median 21.3 ng/mL, range 4.2–40.0 vs. median 8.16 ng/mL, range 1.0–19.0, respectively) (Fig.1). sHs was also significantly higher (P = 0.038) among dogs with severe hemostatic derangement compared to that in other dogs (19.0 ng/mL, range 4.6–40 vs. 7.0 ng/mL, range 4.6–34.6, respectively) (Fig. 2). There was significant correlation between sHs concentration at presentation and serum urea (r = 0.8, P = 0.028) and total CO2 (r = 0.66, P = 0.026) concentrations; CK activity (r = 0.68, P = 0.045); and PT at 12 h PP (r = 0.89, P = 0.044). The area under the ROC curve of sHs concentration as marker of survival was 0.72, with cutoff point of 13.7 ng/mL corresponded to a sensitivity of 60% and specificity of 88%. There was no significant difference in sHs concentrations between genders, both within the heatstroke group and within the control group. No significant correlations were noted between sHs concentration and the body weight of the dogs or their age.
Fig. 1.
Median total serum histones concentration at presentation to the hospital in ten non- survivor, six survivors, and seven healthy controls dogs with naturally occurring heatstroke. Median total serum histones are significantly increased (P = 0.043) in all dogs with naturally occurring heatstroke compared to their level in seven healthy dogs. Median total serum histones in non-survivors was significantly (P = 0.049) higher among non-survivors compared to survivors
Fig. 2.
Median total serum histones concentration in 16 dogs with naturally occurring heatstroke. Median total serum histones concentration was significantly higher among nine dogs with sustained severe hemostatic derangement compared to seven dogs without severe hemostatic derangement
Discussion
This study demonstrates for the first time that sHs concentration in dogs is measurable and it is increased during naturally occurring heatstroke. Histone levels positively correlate with the severity of the disease as reflected by its significantly higher level in the non-survivors and among dogs with severe hemostatic derangement, and with other organ failure biomarkers. This scenario fits with a condition of sHs leakage into circulation in response to inflammatory challenge, contributing to endothelial dysfunction, organ failure, and death during sepsis (Xu et al. 2009). As these proteins can be targeted pharmacologically and be neutralized by heparin, protein C, and specific antibodies it may serve as a potential therapeutic target in the future, in canine heatstroke in particular and in other inflammatory/infective diseases in general.
In the present study, seven healthy young dogs were served as controls and provided histone baseline in the peripheral circulation of normal dogs with no evidence of inflammation. We have found that all the seven dogs had sHs level relatively uniform compared to critically ill dogs with naturally occurring heatstroke, demonstrating significantly elevated sHs levels, but with large variability (ranging between 2.9–40 and 3.0–9.1 ng/mL in heatstroke and controls, respectively) (Fig. 1). This variability may be attributed to the time elapsed from the initiation of the heat insult and admission to medical treatment, as well as potentially the threshold severity for histones release into the blood. This may explain the similarity in sHs levels between controls and survivors seen in the present study. sHs release threshold during heatstroke may be affected by hypoxia as implicated by the observed decrease in the total CO2, HCO3-, and pH, under these conditions. The change in sHs was significantly correlated with changes total CO2, while decrease in PH and HCO3- were linked to higher mortality in the heatstroke dogs in the present study and previously (Bruchim et al. 2016). Interestingly, five dogs (three survivors and two non-survivors) had lower sHs than the median of the controls (<7.3 ng/mL), which may be attributed to possible clearance of small quantities of histones by macrophage, or by enzymatic cleavage by protease in plasma such as activated protein C (Ekaney et al. 2014). Similarly, Ekaney et al. (2014) have shown that sHs were not significantly increased in non-septic and in minor trauma patients but were increased only in septic and major trauma patients, suggesting a severity threshold of cellular damage and organ failure for histones release into extracellular space (Ekaney et al. 2014).
The proposed source of sHs is both activated immune cells (e.g., neutrophils and mast cells) by extracellular traps in a process called netosis (Chen et al. 2014; Zawrotniak and Rapala-Kozik 2013), and by necrotic and dying cells which release their content (Xu et al. 2015). In the present study, we did not find a correlation between leukocyte count and sHs level, although both were significantly higher among non-survivors. We did find a correlation between sHs level and CK released due to severe rhabdomyolysis reflected by the dramatic increase in muscle enzymes CK, AST, and ALT (Table 2). Median CK increased 400 folds than the normal range, and significantly positively correlated with histones levels upon admission. This correlation may indicate the severity of heatstroke and perhaps, the source of histones leakage into circulation from damaged and necrotic cells.
One of the most devastating consequences of heatstroke is formation of hemostatic derangement and DIC, which play a major role in the morbidity of this condition (Bruchim et al. 2006, 2009, 2016; Segev et al. 2015b). In a study of 30 dogs with naturally occurrence heatstroke, 80% sustained DIC and both PT and aPTT were above their reference interval (RI), while PC activity was <RI (Brucim et al. 2017). In that study, upon admission, there was no significant difference between outcome groups in these analytes. However, 12 h PP there was a remarkable and significant difference between outcome groups in coagulation analytes (Bruchim et al. 2017). Similarly, in the present study 50% of the dogs with heatstroke sustained marked hemostatic derangement, sHs levels were significantly elevated and were positively correlated with PT 12 h PP, suggesting that sHs might play a major role in the formation hemostatic abnormalities (Allen et al. 2015; Zawrotniak and Rapala-Kozik 2013) which may complicate dogs with heatstroke, and contribute to their death.
Histone administration to rats leads to neutrophil migration, endothelial injury and dysfunction, and hemorrhages resulting in animal death (Xu et al. 2009). The mechanism of the toxic effect of sHs is only partially understood. Xu’s et al. hypothesized that histones play a role in sepsis, such that the positively charged histones bind to negatively charged phospholipids in the cell plasma membrane leading to increase transmembrane conductance, membrane disruption, and finally calcium influx (Xu et al. 2009, 2015). This toxicity is significantly reduced by blocking sHs by activated protein C (APC) (Xu et al. 2009), heparin (Iba et al. 2015), and non-anticoagulant heparin (Wildhagen et al. 2014). It has been recently suggested that histone is a unique alarming which if inhibited can stop its effect; however, it is highly possible that histones does not damage cell by its own, but always through a “cross talk”: (i.e., synergism) with other pro-inflammatory agonists components, released from activated neutrophils (e.g., oxidant proteases, phospholipases) (Ginsburg and Kohen 1995; Ginsburg et al. 2016). Currently, there is no specific treatment to stop, slow down, or decrease the life-threatening inflammatory and hemostatic derangements in heatstroke. In many dogs presented to our emergency services (Bruchim et al. 2017) due to heatstroke, the hemostatic profile at presentation is unremarkable; however, with time, many develop severe hemostatic derangement and DIC. Despite intensive clotting factors and natural anticoagulant replacement therapy (e.g., anthirombin and PC), the mortality rate is relatively high (50%).
Xu et al. found that a mixture of histones was cytotoxic, and this toxicity was attributed mainly to histones 3 and 4 (H3 and H4, respectively) (Xu et al. 2009). In the present study, we have evaluated total serum histones level which includes all 5 types of histones. Possibly, specifically measuring the serum concentration of H3 and H4 will increase the specificity of histone concentration in heatstroke and SIRS, although this might decrease the sensitivity. A recent clinical study in human ICU patients has shown a significant increase of serum H4 in septic patients compared to other non-septic ICU patients (Ekaney et al. 2014). Presently, we have chosen to measure general total sHs levels, rather than specific ones, as the literature lack data on canine histone concentration in healthy and in critically ill patients. Therefore, by using more sensitive ELISA kit that measures all 5 subtypes of histones, we believe that it will increase the probability of having positive results, as shown in our preliminary data in which have high specificity but relatively low sensitivity (89 and 60%, respectively). Future studies of dogs with heatstroke and SIRS, in which H3 or H4 are to be specifically measured, are warranted.
This study has two major limitations. First, the size of the cohort is limited, thereby decreasing the power of the statistical analyses. However, this is to be regarded as a preliminary investigation of sHs in dogs in general, and specifically in heatstroke. Moreover, as their concentration was higher in non-surviving dogs compared to the survivors, sHs concentration has prognostic usefulness, warranting future studies. The second limitation is the inevitable variable clinical severity of naturally occurring heatstroke vs. experimental conditions. This variability results from differences in severity of the initial heat insult, lag of times from onset of heatstroke to presentation, and in previous treatment administered by referring clinics.
In conclusion, sHs are detectable in healthy control dogs, their concentration are increased in dogs with heatstroke, and are significantly higher among non-survivors and among dogs with severe hemostatic disorders. Their level correlates with severity of disease and other organ failure parameters, and potentially may contribute to cellular damage, inflammation and coagulation activation, organ failure, and death in dogs with naturally occurring heatstroke. As sHs can be neutralized pharmacologically, they may serve as potential promising therapeutic target in the future.
References
- Allen KS, Sawheny E, Kinasewitz GT. Anticoagulant modulation of inflammation in severe sepsis. World J Crit Care Med. 2015;4(2):105–115. doi: 10.5492/wjccm.v4.i2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroch I, Segev G, Loeb E, Bruchim Y. Peripheral nucleated red blood cells as a prognostic indicator in heatstroke in dogs. J Vet Intern Med. 2009;23(3):544–551. doi: 10.1111/j.1939-1676.2009.0305.x. [DOI] [PubMed] [Google Scholar]
- Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Bruchim Y, Klement E, Saragusty J, Finkeilstein E, Kass P, Aroch I. Heat stroke in dogs: a retrospective study of 54 cases (1999-2004) and analysis of risk factors for death. J Vet Intern Med. 2006;20(1):38–46. doi: 10.1892/0891-6640(2006)20[38:hsidar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bruchim Y, Loeb E, Saragusty J, Aroch I. Pathological findings in dogs with fatal heatstroke. J Comp Pathol. 2009;140(2–3):97–104. doi: 10.1016/j.jcpa.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Bruchim Y, Segev G, Kelmer E, Codner C, Marisat A, Horowitz M. Hospitalized dogs recovery from naturally occurring heatstroke; does serum heat shock protein 72 can provide prognostic biomarker? Cell Stress Chaperones. 2016;21(1):123–130. doi: 10.1007/s12192-015-0645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchim Y, Kelmer E, Segev G, Aroch I (2017) Hemostatic abnormalities in dogs with naturally-occurring heatstroke. J Vet Emerg Crit Care 27(3):315–324. doi:10.1111/vec.12590 [DOI] [PubMed]
- Chen R, Kang R, Fan XG, Tang D. Release and activity of histone in diseases. Cell Death Dis. 2014;5:e1370. doi: 10.1038/cddis.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekaney ML, Otto GP, Sossdorf M, Sponholz C, Boehringer M, Loesche W, Claus RA. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit Care. 2014;18(5):543. doi: 10.1186/s13054-014-0543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein Y, Roberts WO. The pathopysiology of heat stroke: an integrative view of the final common pathway. Scand J Med Sci Sports. 2011;21(6):742–748. doi: 10.1111/j.1600-0838.2011.01333.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg I, Kohen R. Cell damage in inflammatory and infectious sites might involve a coordinated "cross-talk" among oxidants, microbial haemolysins and ampiphiles, cationic proteins, phospholipases, fatty acids, proteinases and cytokines (an overview) Free Radic Res. 1995;22(6):489–517. doi: 10.3109/10715769509150323. [DOI] [PubMed] [Google Scholar]
- Ginsburg I, Koren E, Varani J, Kohen R. Nuclear histones: major virulence factors or just additional early sepsis markers? A comment. Inflammopharmacology. 2016;24(5):287–289. doi: 10.1007/s10787-016-0279-y. [DOI] [PubMed] [Google Scholar]
- Hoeksema M, van Eijk M, Haagsman HP, Hartshorn KL. Histones as mediators of host defense, inflammation and thrombosis. Future Microbiol. 2016;11(3):441–453. doi: 10.2217/fmb.15.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba T, Hashiguchi N, Nagaoka I, Tabe Y, Kadota K, Sato K. Heparins attenuated histone-mediated cytotoxicity in vitro and improved the survival in a rat model of histone-induced organ dysfunction. Intensive Care Med Exp. 2015;3(1):36. doi: 10.1186/s40635-015-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LR, Bouchama A. Heat stroke. Compr Physiol. 2015;5(2):611–647. doi: 10.1002/cphy.c140017. [DOI] [PubMed] [Google Scholar]
- Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J Appl Physiol. 2010;109(6):1980–1988. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Leon LR, Helwig BG. Role of endotoxin and cytokines in the systemic inflammatory response to heat injury. Front Biosci (Schol Ed) 2010;2:916–938. doi: 10.2741/s111. [DOI] [PubMed] [Google Scholar]
- Segev G, Daminet S, Meyer E, De Loor J, Cohen A, Aroch I, Bruchim Y. Characterization of kidney damage using several renal biomarkers in dogs with naturally occurring heatstroke. Vet J. 2015;206(2):231–235. doi: 10.1016/j.tvjl.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Segev G, Aroch I, Savoray M, Kass PH, Bruchim Y. A novel severity scoring system for dogs with heatstroke. J Vet Emerg Crit Care (San Antonio) 2015;25(2):240–247. doi: 10.1111/vec.12284. [DOI] [PubMed] [Google Scholar]
- Shibolet S, Farfel Z. Letter: heparin therapy for heatstroke. Ann Intern Med. 1975;82(6):857–858. doi: 10.7326/0003-4819-82-6-857_2. [DOI] [PubMed] [Google Scholar]
- Wildhagen KC, Garcia de Frutos P, Reutelingsperger CP, Schrijver R, Areste C, Ortega-Gomez A, Nicolaes GA. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. 2014;123(7):1098–1101. doi: 10.1182/blood-2013-07-514984. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Huang Y, Mao P, Zhang J, Li Y. Sepsis and ARDS: the dark side of histones. Mediat Inflamm. 2015;2015:205054. doi: 10.1155/2015/205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawrotniak M, Rapala-Kozik M. Neutrophil extracellular traps (NETs)—formation and implications. Acta Biochim Pol. 2013;60(3):277–284. [PubMed] [Google Scholar]