Abstract
Serine proteinases play important roles in innate immunity and insect development. We isolated a serine proteinase gene, designated AccSp10, from the Chinese honeybees (Apis cerana cerana). RT-qPCR and a Western blot analysis at different pupal development stages indicated that AccSp10 might be involved in melanin formation in pupae and promote pupal development. In adult workers, the expression of AccSp10 was upregulated by treatments mimicking harmful environments such as the presence of Bacillus bombysepticus, different temperatures (4, 24 and 42 °C), HgCl2, H2O2 and paraquat; the exception was treatment with VC (vitamin C), which did not upregulate AccSp10 expression. Western blot confirmed the results. A disc diffusion assay indicated that recombinant AccSp10 accelerated E. coli cell death during stimulation with harmful substances (HgCl2, paraquat and cumene hydroperoxide). These findings suggest that AccSp10 may be involved in the pupal development of Chinese honeybees and protection against microorganisms and abiotic harms.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-017-0818-5) contains supplementary material, which is available to authorized users.
Keywords: Apis cerana cerana, Serine proteinase, Microorganism and abiotic stresses, Expression analysis, Antibacterial activity
Introduction
Insects are the most widely distributed species in the world, often living in environments with many pathogens (for example, fungi and Gram-negative bacteria) and various unfavourable environmental stressors (extreme temperature, heavy metals, pesticides), which can cause serious oxidative damage (Halliwell and Gutterridge 1989; Yao et al. 2013). All of these factors pose a threat to the lives of insects, including the Chinese honeybees (Apis cerana cerana), which is an important species in maintaining the balance of regional ecologies in China.
When pathogens enter insects, insects can initiate a type of humoral immunity, which is an important innate immune response involving serine proteinases that includes coagulation, melanisation and Toll pathway signalling (Muta and Iwanaga 1996; Iwanaga et al. 1998; Jiang et al. 2010). These serine proteinase cascade pathways can rapidly amplify responses to infection and stimulate pathogen killing (An et al. 2009). The coagulation mechanism is well known based on studies in horseshoe crab; plasmozyme is activated by serine proteinase cascade reactions, leading to the coagulation of haemolymph for pathogen clearance (Muta and Iwanaga 1996; Iwanaga et al. 1998). In the melanisation pathway, after pathogen-associated molecular patterns such as fungal β-1, 3-glucan or bacterial peptidoglycan are recognized by pattern recognition proteins in insects (Gupta et al. 2005; Hughes 2012), a series of serine proteinases in haemolymph are activated, which leads to proPO-activating proteinase (PAP) activation and the transformation of proPO (prophenoloxidase) into PO (phenoloxidase) (Gorman et al. 2007; Wang and Jiang 2007). PO is the key proteinase that catalyses the formation of melanin. This pathway plays an important role in microbe killing and wound healing (Mavrouli et al. 2005; Jiang et al. 2010). The Toll molecule was originally shown to be a type 1 transmembrane receptor that controls dorsal-ventral patterning of the Drosophila embryo (Anderson et al. 1985a, b; Hashimoto et al. 1988) and later was identified to be involved in host resistance against pathogens (Lemaitre et al. 1996; Ligoxygakis et al. 2002; Michel et al. 2001). Many serine proteinases with clip-domains have been found in Drosophila, Manduca sexta and Bombyx mori, which participate in Toll pathway activation and stimulate the synthesis of antimicrobial peptides (metchnikowins, thanatin, magainin) (Kim et al. 2008; An et al. 2009; Roh et al. 2009; Ligoxygakis et al. 2002; Kambris et al. 2006; Bulet et al. 1999; Lehrer and Ganz 1999).
Additional studies have found that extracellular serine proteases are required for dorsal-ventral growth of the Drosophila embryo (Belvin and Anderson 1996; Trapasso and Simpson 1998). Ahola et al. (2015) reported that the serine protease family was associated with larval growth rate, development time and pupal weight in the Glanville fritillary butterfly. In mammalian preimplantation embryos, a serine protease (hepsin) was also found to be expressed (Vu et al. 1997). These imply that serine proteases might play a role in the development of various organisms.
These serine proteinases that participate in immunity and insect development are conserved proteinases in terms of their structure, which contains a conservative catalytic triad with the His, Asp and Ser residues (Carter and Wells 1988; Polgár 2005; Jiang and Kanost 2000). Additionally, many serine proteinases have clip-domains at their amino terminus, but little is known about the functions of clip-domains at present (Zou et al. 2006; Ross et al. 2003; Veillard et al. 2015). Serine proteinases exist in haemolymph in the form of zymogens, which are cut and activated at specific sites and then activate downstream proteins, when pathogens invade insects (James and Sielecki 1986; Jiang and Kanost 2000, 2010). At present, functional serine proteinases have been found in Bombyx mori, Drosophila melanogaster, Anopheles gambiae, Tenebrio molitor, Holotrichia diomphalia and Manduca sexta, particularly in Drosophila melanogaster and Manduca sexta (An et al. 2011; Gorman et al. 2007; An et al. 2009). However, the functions of most clip-domain proteases are unknown, even in well-studied insect species.
All of the previous data indicate that serine proteinases play a crucial role in innate immunity and insect development. However, the melanisation reaction pathway that these enzymes involved has not understood well (Tang et al. 2006; An et al. 2009), particularly in the Chinese honeybees (A. cerana cerana). Furthermore, little is known about the functions of serine proteinases when insects are subjected to abiotic stresses. Thus, the study of serine proteinases in the Chinese honeybees (A. cerana cerana) is necessary. Here, we identified a gene (AccSp10) from the Chinese honeybees (A. cerana cerana) for the first time and predicted that it is a serine proteinase gene that may be involved in Chinese honeybee development and protection against microorganisms and environmental harms.
Materials and methods
Specimens
Insects used in the following experiments were Chinese honeybees (A. cerana cerana) from Shandong Agricultural University (Taian, China). According to the criteria of Michelette and Soares (1993), the honeybees in the experiments were used at the following stages: eggs; larvae (L1–L6), pupae including prepupae (P0), white eyes (Pw), pink eyes (Pp), brown eyes (Pb) and dark eyes (Pd); and adults (A1, 1-day post-emergence; A15, 15-day post-emergence; and A30, 30-day post-emergence). The whole bodies of eggs, larvae, pupae and 1-day-old adult workers were collected from the hive. The A15 or A30-day-old adult workers were obtained by marking newly emerged bees with paint. These honeybees were breed at a constant humidity (70%) and temperature (34 °C) and under a 24-h dark regimen (Alaux et al. 2010). The whole bodies of bees were flash frozen in liquid nitrogen and stored at −80 °C.
Bacillus bombysepticus was preserved in our laboratory.
Treatments
The 1-day-old post-emergence adult workers were divided into groups (n = 40). Groups 1–3 were exposed to 4, 24 and 42 °C, respectively. Groups 4–6 were treated with Bacillus bombysepticus (OD600 ≈ 100), HgCl2 (3 mg/mL) and VC (0.02 mg/mL), which was added to food. Group 7 was injected with H2O2 (0.5 μL of a 2 mM dilution) between the first and the second abdominal segments. Paraquat (10 μM) was daubed on the thoracic notum of worker bees in Group 8. At specific times, specimens were collected and immediately frozen in liquid nitrogen and stored at −80 °C.
Preparation of RNA and cDNA
The extraction of total RNA and complementary DNA (cDNA) synthesis were performed as previously described (Yao et al. 2013).
PCR amplification conditions
Primers are shown in Table 1. The PCR amplification conditions were 10 min at 94 °C, 40 s at 94 °C, 40 s at 47 °C, 1 min 20 s at 72 °C for 35 cycles, 10 min at 72 °C (primers SC1 and SC2 (listed in Table 1)) and 10 min at 94 °C, 40 s at 94 °C, 40 s at 50 °C, 1 min 30 s at 72 °C for 35 cycles, 10 min at 72 °C (primers RP1 and RP2 (listed in Table 1)), respectively.
Table 1.
The primers used in this study
| Primers | Primer sequence (5′–3′) | Description |
|---|---|---|
| SC1 | TGAAGGCATGTCTTGATTATC | Synthesizing cDNA primer, forward |
| SC2 | CCTTTGTATTATTAATCTGGCC | Synthesizing cDNA primer, reverse |
| SQ1 | CAATGAATGGATAAGACCCAGTTG | Q-PCR primer, forward |
| SQ2 | GTCCACCACTATCTCCCTG | Q-PCR primer, reverse |
| β-s | GTTTTCCCATCTATCGTCGG | Standard control primer, forward |
| β-x | TTTTCTCCATATCATCCCAG | Standard control primer, reverse |
| RP1 | GGATCCTTAGATAAAGGCGAAGCATG BamHI | Primer of protein expression, forward |
| RP2 | TCCGAGATCTGGCCAAACTATATTCTC XhoI | Primer of protein expression, reverse |
Cloning of open reading frame of AccSp10
The internal fragment of AccSp10 cDNA was obtained using primers SC1 and SC2, which were designed based on the conserved sequence of Sp10 from Apis mellifera, Apis dorsata and Apis florea. Subsequently. The PCR product (open reading frame (ORF) without the 5′ and 3′ cDNA ends) was ligated into the pEASY-T3 vector and transformed into Escherichia coli strain DH5α for sequencing.
Bioinformatics analysis
The bioinformatics tools (http://blast.ncbi.nlm.nih.gov/Blast.cgi) at the National Center for Biotechnology Information (NCBI) were used to analyse conserved sequences of AccSp10. The homologous sequences of AccSp10 in other species from NCBI were aligned using the DNAMAN software. The theoretical isoelectric point and molecular mass of the predicted protein were predicted with the online tool PeptideMass (http://us.expasy.org/tools/peptide). SWISS-MODEL was used to predict the advanced structure of the AccSp10 protein. Antibacterial activity loci were predicted using the website http://aps.unmc.edu/AP/.
RT-qPCR analysis
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was used to identify the expression patterns of the AccSp10 gene. Total RNA of different samples was extracted separately, and the cDNA was synthesized as described previously (Yao et al. 2013). A 278-bp fragment of AccSp10 was obtained using primers SQ1 and SQ2 (listed in Table 1). The housekeeping gene β-actin from A. cerana cerana (XM640276) was used as a control gene, because it has been verified as the most stably expressed gene in honeybees (Scharlaken et al. 2008; Zhou et al. 2016). SYBR Premix Ex Taq (TaKaRa, Dalian, China) was used in a 25-μL volume on a CFX96™ Real-Time System (Bio-Rad, Hercules, CA, USA). We have first validated the primers of AccSp10. The melting curves had single peak. The amplification efficiency (E) and the correlation coefficients (R 2) were 95.1% and 0.998, respectively. The PCR cycling conditions were as follows: 95 °C for 30 s and then 40 cycles (95 °C for 5 s, 55 °C for 15 s and 72 °C for 15 s). Lastly, there was a melting cycle from 65 to 95 °C. Each sample was repeated in triplicate, and at least three samples were used for each primer pair. The linear relation, amplification efficiency and data analysis were performed using the CFX Manager Software version 1.1. The analysis of the significant differences was conducted using Duncan’s multiple range tests with the Statistical Analysis System (SAS) software version 9.1.
Protein expression and purification of recombinant AccSp10
The ORF fragment of AccSp10 cDNA (without signal sequence), amplified using a pair of primers containing BamHI and XhoI (primers RP1 and RP2), was inserted into the expression vector pET-21a (+) and was then transformed into E. coli BL21 cells. The cells were cultured in Luria-Bertani (LB) broth with kanamycin at 37 °C until the cell density reached 0.4–0.6 OD600. Then, the AccsSp10 expression was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 28 °C for 6 h. The bacterial cells were harvested by centrifugation at 5000×g for 10 min at 4 °C, resuspended in a loading buffer.
As described previously, the induced protein was collected and then purified using a HisTrap™ FF column (GE Healthcare, Uppsala, Sweden) according to the manufacturer’s instructions. Twelve percent sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to detect the target protein.
Recombinant AccSp10 protein identification
SDS-PAGE was performed to characterize the recombinant AccSp10 protein. A target band was picked up and further identified by matrix-assisted laser desorption/ionization-time-of-flight tandem mass spectrometry (MALDI-TOF/TOF, ultrafleXtreme™, Brucher, Germany). In brief, the protein sample was digested with trypsin (Promega, Madison, WI) after by DTT reduction and iodine generation acetamide alkylation process. The extracted peptide mixtures were analysed by MALDI-TOF/TOF MS. All peptide spectra were matched with the online Mascot program (http://www.matrixscience.com) against the NCBIprot databases. Carbamidomethyl of Cys was chosen as fixed modification; oxidation of Met was chosen as dynamic modification; and two missing tryptic cleavage were allowed.
Antibody preparation and Western blot analysis
The purified recombinant AccSp10 (200 μg) was mixed with an equal volume of Freund’s complete adjuvant (Sigma, USA) and was injected into the New Zealand rabbit. Second injection was the same with first after 2 weeks. Subsequently, three successive injections of 100 μg of protein mixed with an equal volume of Freund’s incomplete adjuvant were administered at 2-week intervals. Fourteen days after the final injection, blood was collected and centrifuged at 3000 rpm for 5 min. The supernatant antibodies were stored at −70 °C, for further application.
To analyse the expression of the AccSp10 gene at the protein level, total protein was extracted from A. cerana cerana at four different pupal developmental stages following treatment with microorganisms and at 24 °C. Anti-AccSp10 serum was used as the primary antibody at a 1:400 (v/v) dilution, and peroxidase-conjugated goat anti-rabbit IgG was used as the secondary antibody at a 1:2000 (v/v) dilution. Anti-β-actin (Sigma, USA) was used as a control. The proteins were detected using a SuperSignal® West Pico Trial Kit (Thermo Science Pierce, IL, USA).
Antibacterial assay of recombinant AccSp10 protein in vitro
A modified disc diffusion assay from Burmeister et al. (2008) was performed. After overexpressing AccSp10 in E. coli BL21, the protein was incubated on LB-kanamycin agar plates at 37 °C for 1 h, and filter discs (6-mm diameter) with different concentrations of HgCl2, paraquat and cumene hydroperoxide were placed on the agar surface for 12 h at 37 °C. Then, the inhibition zones were measured. E. coli BL21 cells with empty pET-21a (+) were used as the negative control.
ProPO activation assay
Recombinant proteins were mixed with tissue protein extract from prepupae, which had a low basal PO activity. The mixtures were incubated at room temperature for 10 min. Protein concentration was measured using the BCA Protein Assay Kit (Jiancheng, Nanjing, China). Forty-microliter tissue protein extract with recombinant proteins (30 μg) in 500 μL of phosphate-buffered saline containing protease inhibitors was mixed with 1500 μL of phosphate-buffered saline saturated with l-3,4-dihydroxiphenylalanine (Sigma, USA). After incubation at room temperature for 30 min, the absorbance at 470 nm of the samples was measured. One unit of PO activity was defined as the unit amount of enzyme producing an increase in absorbance (ΔA 470) of 0.001 μg/min. Each experiment was repeated at least three times.
Statistical analysis
Statistics were performed using the mean ± SD from triplicate experiments (n = 3). Statistical significance was determined by Duncan’s multiple range test using the SAS version 9.1 software program (SAS Institute, Cary, NC, USA). Significance was set at P < 0.05.
Results
Characterization of AccSp10
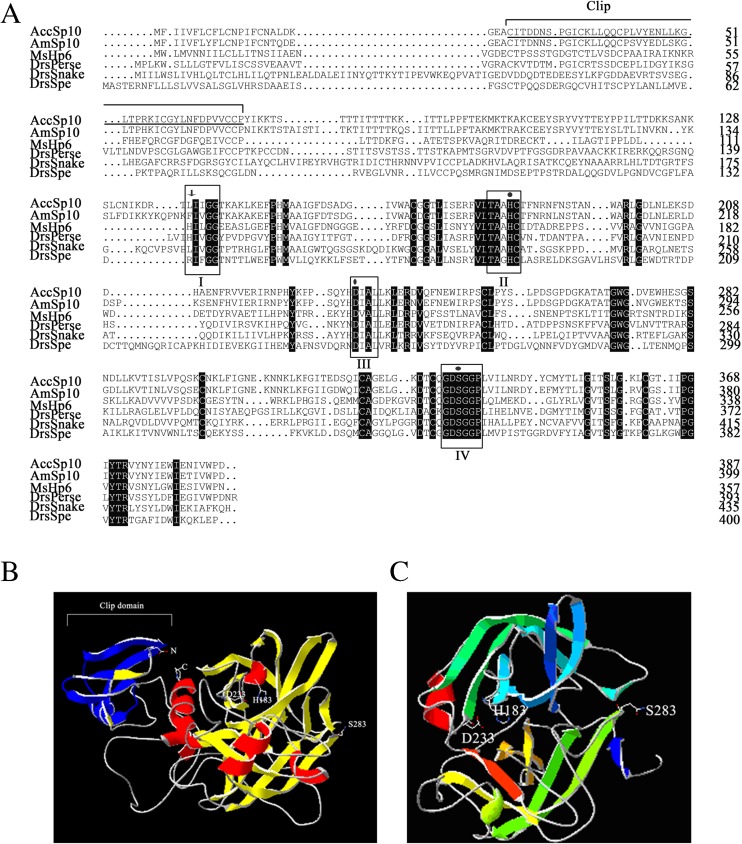
The ORF of AccSp10 (GenBank accession number: AKT73552) is 1164 bp and encodes a 387-amino-acid polypeptide with a predicted theoretical isoelectric point (pI) of 7.60 and a molecular mass of 43.39 kDa, as shown in Fig. 1a. Alignment of the AccSp10 protein was performed with homologous proteins from other species, specifically Apis mellifera Sp10 (XP_001120043.2), Manduca sexta HP6 (AAV91004.1), Drosophila melanogaster Persephone (NP_573297.1), Drosophila melanogaster snake, isoform A (NP_524338.2) and Drosophila melanogaster Spatzle-Processing enzyme (NP_651168.1). To understand the relationship between structure and function for the AccSp10 protein, the tertiary structures of AccSp10 and cleaved AccSp10 were predicted; the catalytic triad and clip-domain of AccSp10 are identified in blue, as shown in Fig. 1b, c.
Fig. 1.
a Multiple amino acid sequence alignment of AccSp10. The three conserved regions (II, III and IV) are marked with black boxes. The three amino acid residues (His183, Asp233 and Ser283) of the catalytic triad of AccSp10 are indicated with dots. The protein active site (Leu138 and Ile139) is indicated with arrows. The clip-domain is underlined. b The tertiary structure of AccSp10. The tertiary structure of AccSp10 was built using homology modelling in SWISS-MODEL. The catalytic triad of AccSp10 (His183, Asp233 and Ser283), N-terminus and C-terminus are shown. The structure of the clip-domain is indicated in blue. c The tertiary structure of cleaved AccSp10. The catalytic triad (His183, Asp233 and Ser283) is shown (colour figure online)
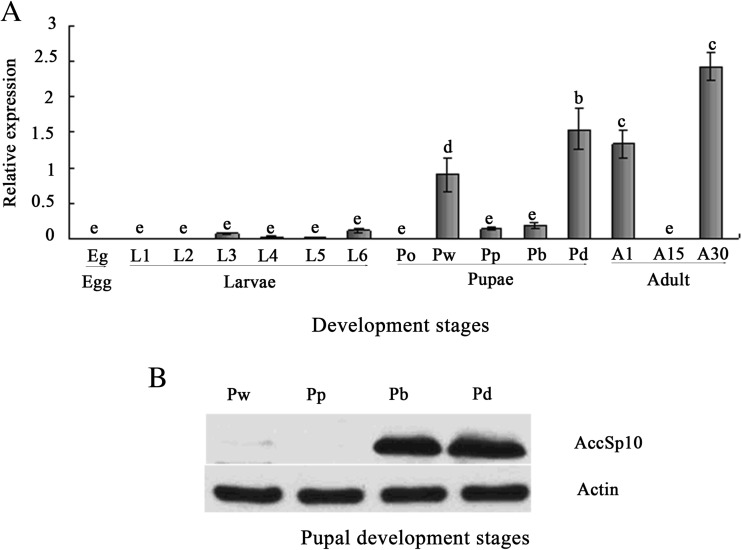
Transcription of AccSp10 and a Western blot analysis at pupal development
The RT-qPCR results showed that the expression of AccSp10 was low in larvae and highest in the 30-day-old adult workers. In the pupae, the transcription level of AccSp10 was highest in the dark eye stage compared to the other pupal stages (Fig. 2a). From white eyes (Pw) to dark eyes (Pd), pupal colour slowly become black, particularly the eyes, indicating that melanisation might be very rich; thus, we selected pupal stages to evaluate the expression of AccSp10 at the protein level. In the Western blot assay, anti-AccSp10 was used to detect AccSp10. The results indicated that the protein level of AccSp10 increases from white eyes (Pw) to dark eyes (Pd) in the pupae (Fig. 2b).
Fig. 2.
Expression profiles of AccSp10 and a Western blot analysis of AccSp10 at different developmental stages. a Transcriptional expression levels of AccSp10 at different developmental stages: egg, larvae from the first to sixth instars (L1–L6), pupae (Po prepupae, Pw white eyes, Pp pink eyes, Pb brown eyes, Pd dark eyes) and adults (A1 1-day post-emergence, A15 15-day post-emergence, A30 30-day post-emergence). The data represent the means ± SEM (n = 3). Letters above the columns indicate significant differences (P < 0.001). b A Western blot analysis of AccSp10 at different pupal developmental stages. Pw white eyes, Pp pink eyes, Pb brown eyes, Pd dark eyes. The β-actin protein was used as an internal control. All lanes were loaded with equivalent amounts of protein
Transcriptional expression of AccSp10 and a Western blot analysis under microorganism and abiotic conditions
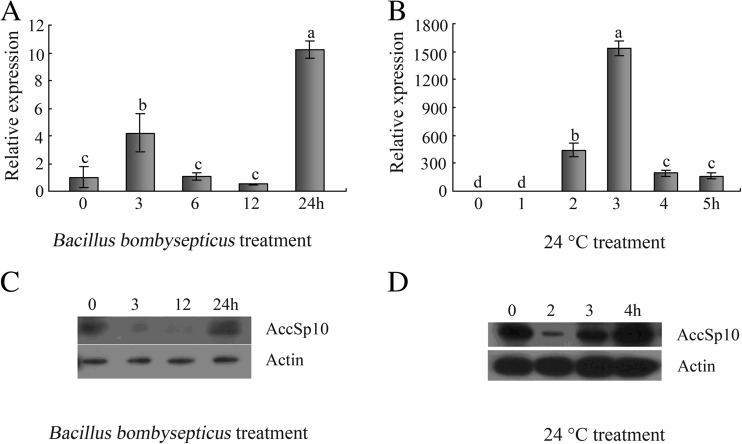
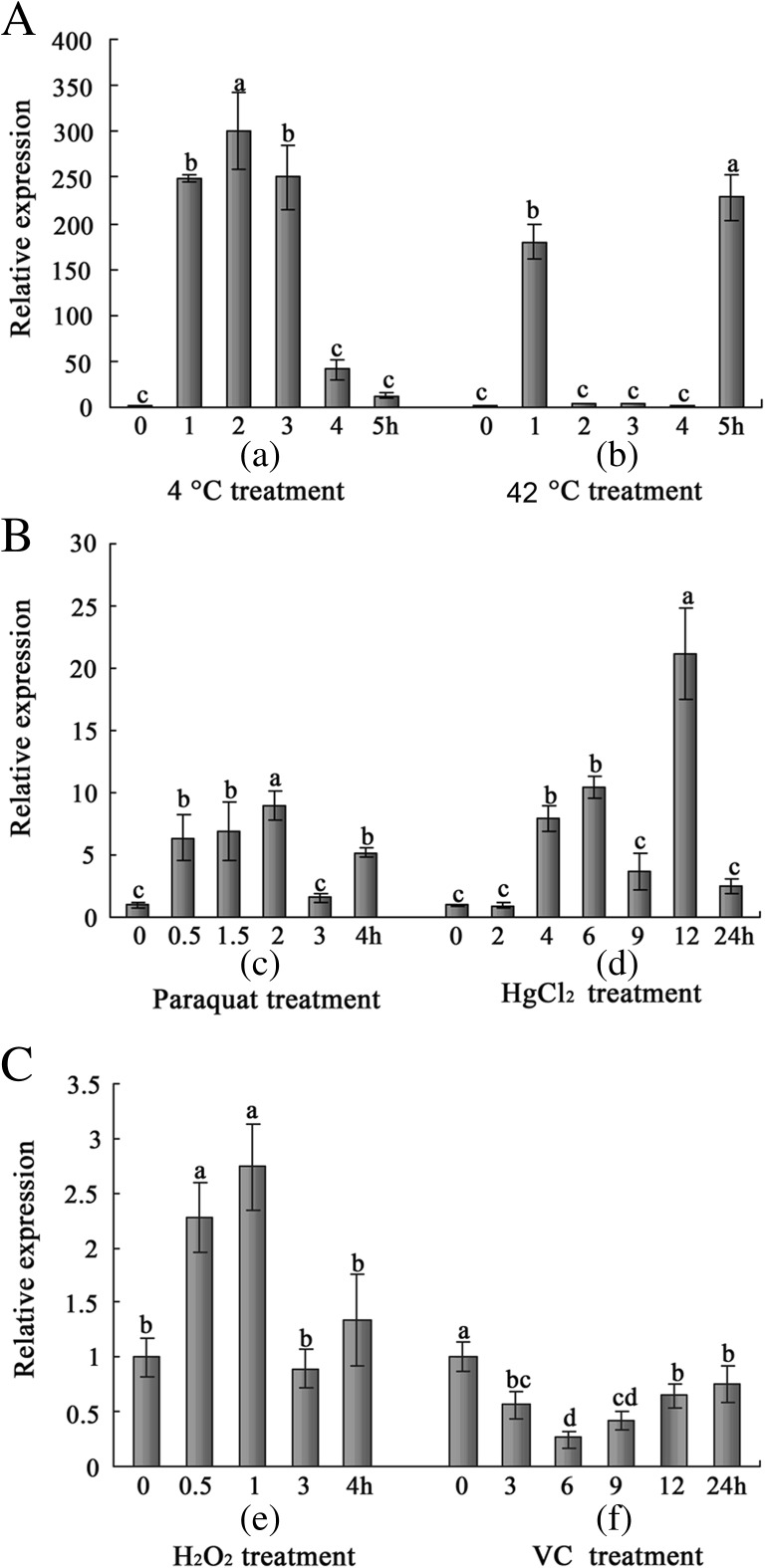
The RT-qPCR results indicated that the expression of AccSp10 was upregulated under Bacillus bombysepticus and abiotic conditions (4 °C, 24 °C, 42 °C, HgCl2, H2O2 and paraquat) but not under VC treatment (Figs. 3a, b and 4a–c). During the Western blot assay, anti-AccSp10 was used to detect AccSp10. Under microorganism treatment and at 24 °C, AccSp10 protein levels first declined and then rose at different treatment times (Fig. 3c, d).
Fig. 3.
The expression profiles of AccSp10 and the Western blot analysis of AccSp10 with Bacillus bombysepticus (OD600 ≈ 100) and 24 °C treatments. a, b Transcriptional expression levels of AccSp10 with Bacillus bombysepticus (OD600 ≈ 100) and 24 °C treatments. The data are the means ± SEM (n = 3). Letters above the columns indicate significant differences (P < 0.001). c, d The Western blot analysis of AccSp10 with Bacillus bombysepticus (OD600 ≈ 100) and 24 °C treatments. The β-actin protein was used as an internal control. All lanes (each condition) were loaded with equivalent amounts of protein
Fig. 4.
The expression profiles of AccSp10 under different abiotic environmental conditions. These conditions were temperature (a), paraquat (b(c)), HgCl2 (b(d)), H2O2 (c(e)) and VC (c(f)). The β-actin gene was used as an internal control. The data represent the means ± SEM (n = 3). Letters above the columns indicate significant differences (P < 0.001)
Overexpression and purification of AccSp10
The ORF fragment of AccSp10 cDNA (without the signal sequence) was expressed in E. coli BL21, and the recombinant AccSp10 protein was purified with HisTrap™ FF columns and is shown in lanes 6 and 7 (Fig. 5). The concentration of the soluble recombinant protein was approximately 1.8 μg/μL.
Fig. 5.
An SDS-PAGE analysis of the expression and purification of recombinant AccSp10 protein. Lanes 1 and 2, non-induced and induced overexpression of recombinant AccSp10 protein (10 μL, respectively); lane 3, protein molecular weight marker; lanes 4 and 5, suspension of sonicated recombinant AccSp10 protein (10 μL, respectively); lanes 6 and 7, purified recombinant AccSp10 protein (18 and 10.8 μg, respectively)
Recombinant AccSp10 protein identification
The target strip, as shown in Fig. S1A (provided as Supplemental data), was picked up and further identified by MALDI-TOF/TOF MS. The results showed that the protein score (130) was greater than 93 (P < 0.05) and significant; the matched protein was serine proteinase 10 (GenBank accession number: AKT73552.1, Apis cerana cerana), as shown in Fig. S1B (provided as Supplemental data). The matched peptides were shown in Fig. S1C (provided as Supplemental data). These results indicated that the recombinant protein was the same protein coding by the sequence.
Antibacterial assay with recombinant AccSp10 protein
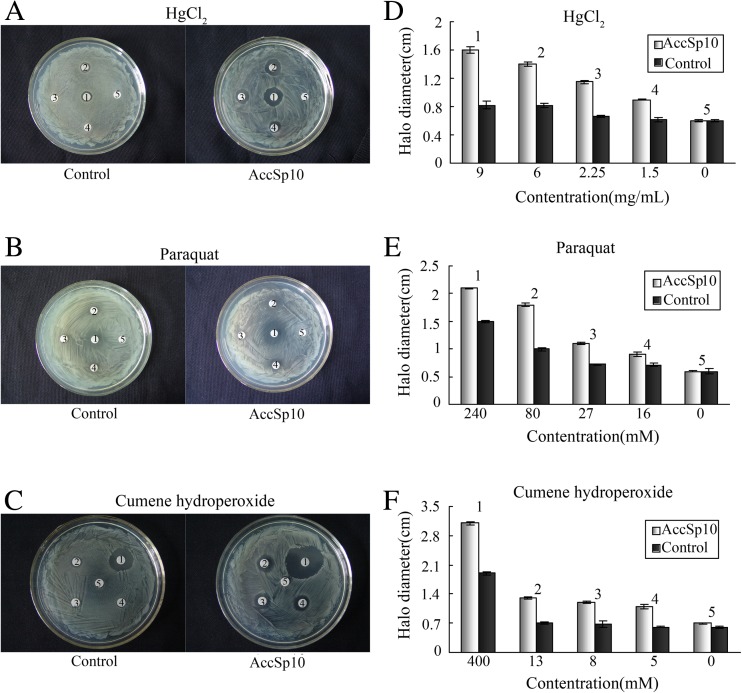
To evaluate the capacity of recombinant AccSp10 as a serine protease in vitro, a disc diffusion assay was performed. E. coli cells with overexpressing AccSp10 were exposed to HgCl2, paraquat and cumene hydroperoxide. The results showed that the death zones of E. coli with overexpressing AccSp10 were larger in diameter on experimental plates than control plates (Fig. 6).
Fig. 6.
Antibacterial activity assay with recombinant AccSp10 protein employing the disc diffusion method. LB agar plates were inoculated with 5 × 108 cells. E. coli BL21 cells transfected with empty pET-21a (+) vector were used as controls. The filter discs labelled 1 represent the negative controls. Filter discs labelled 2, 3, 4 and 5 represent different concentrations of HgCl2 (a, d), paraquat (b, e) and cumene hydroperoxide (c, f). The diameters of the death zones were measured. The data shown are the means ± SEM (n = 3)
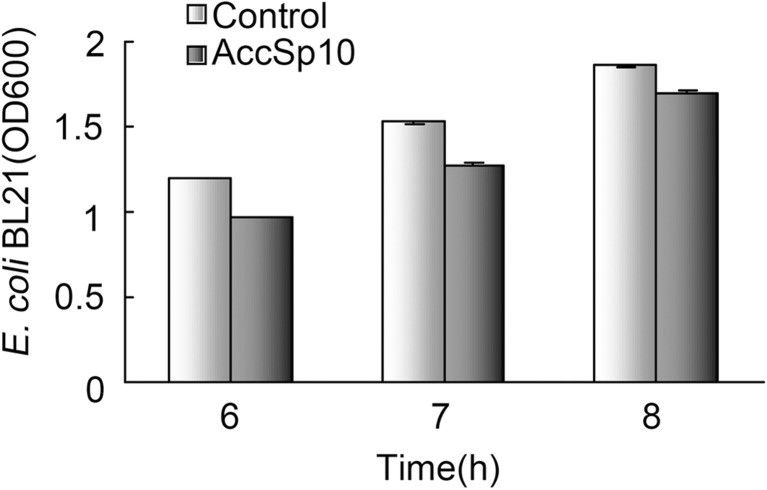
After culturing the bacteria with AccSp10 6, 7 and 8 h, the absorbance of bacteria was measured at 600 nm, respectively; results showed that the absorbance of bacteria with AccSp10 was less than the control (without AccSp10) (Fig. 7).
Fig. 7.
The AccSp10 inhibition for the growth of Escherichia coli BL21 cells. The inhibition for the growth of E. coli BL21 cells. The absorbance was measured after 6, 7 and 8 h, respectively. E. coli BL21 cells transfected with empty pET-21a (+) vector were used as controls
ProPO activation assay
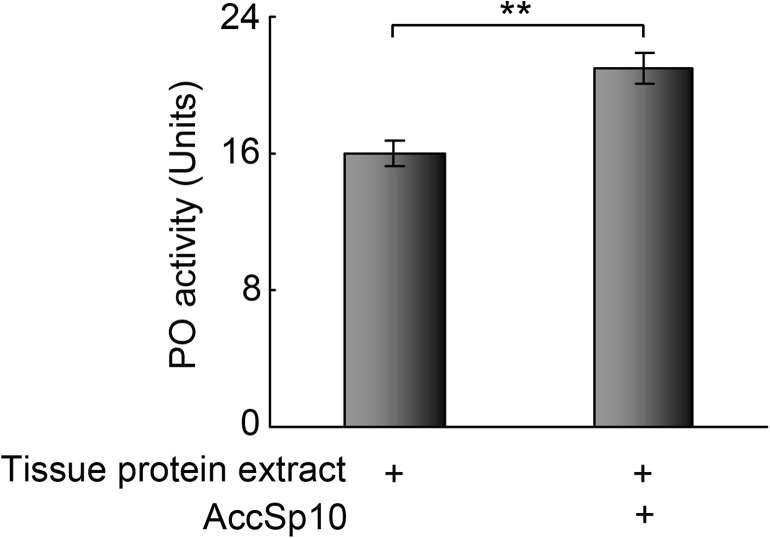
The PO activity of tissue protein extract with recombinant AccSp10 was higher than the control (Fig. 8).
Fig. 8.
The proPO activation assay. PO activity was measured using dopamine as a substrate. The tissue protein extract without recombinant AccSp10 was used as the control. The data represent the means ± SEM (n = 3). Asterisks indicate a significant difference from the control at P < 0.001
Discussion
In insects, serine proteinases with serine in their active centres are likely to be involved in development and innate immunity physiology (Krem and Di Cera 2002; Rawlings and Barrett 1993; Zou et al. 2006). According to reports, many serine proteinases with clip-domains were shown to participate in melanism reactions to help clear pathogens and initiate the Toll pathway, in which antimicrobial peptides are produced (An et al. 2009; Jiang et al. 2010; Aggarwal and Silverman 2008; Gabriella et al. 2011). These studies were also performed in Manduca sexta and Drosophila melanogaster. In this study, a serine proteinase gene from A. cerana cerana, designated AccSp10, was cloned and preliminarily analysed under simulated environments for the first time. The results suggest that AccSp10 takes part in stress responses and likely plays a part in honeybee development and protection against harmful damage.
A sequence analysis indicates that AccSp10 is a typical serine proteinase with three highly conserved sequences (TAAHC, DIAL and GDSGGP) in which the residues His183, Asp233 and Ser283 form a catalytic triad that is essential for a serine protease to maintain catalytic functions (Jiang and Kanost 2000). The protein active site (Leu138 and Ile139) was predicted from which AccSp10 was cleaved and activated with a surplus molecular mass of 27.78 kDa. After AccSp10 is activated, it may then activate downstream interacting proteins. These features of serine proteinases have been identified in many studies (Gupta et al. 2005; Jiang et al. 2010). In addition, a clip-domain (residues from Cys25 to Pro70) was predicted in the AccSp10 sequence. Studies have indicated that serine proteinases with clip-domains play a significant role in embryonic development and immune responses (An et al. 2009; Belvin and Anderson 1996; Iwanaga et al. 1998; Kanost et al. 2004; Ligoxygakis et al. 2002). Based on previous studies, it appears that AccSp10 is a typical serine protease with a clip-domain and might possess the same functions as other serine proteases such as MsHp6 or DrsPersephone (An et al. 2009; Ming et al. 2014).
Melanin synthesis and deposition in the cuticle occur at specific times during insect development. Phenoloxidase is an important enzyme in the biosynthesis of melanin and exists as an inactive precursor, prophenoloxidase. When phenoloxidase is activated by a cascade of serine proteases, it can catalyse reactions that lead to melanin and cuticle sclerotization (Andersen 1985; Hiruma and Riddiford 1988; Yoshida and Ashida 1986). Bitondi et al. (1998) reported that cuticular pigmentation of Apis mellifera correlated with phenoloxidase activation. The process of cuticular pigmentation begins at the pupal phase in Apis mellifera (Rembold 1987). This study indicated that serine proteases activate phenoloxidase and give rise to melanin during pupal development. To further probe the function of AccSp10 in development, the expression pattern of the AccSp10 gene was evaluated at different developmental stages. The transcription levels of the AccSp10 gene were measured using qPCR and exhibited large differences at all experimental stages, as shown in Fig. 2a. The transcription expression of AccSp10 was highest in the dark eyes (Pd) stage than the other pupal stages. The Western blot assay showed that the AccSp10 protein was upregulated gradually with pupal growth, and there was greater accumulation at the brown eyes (Pb) and dark eyes (Pd) stages. Pw (white eyed) is the junction from larva to pupae, which is a period during which cells become highly differentiated, and many organs tend to formation, for example, formation of the head, thorax, abdomen and legs; the result of AccSp10 for RT-qPCR is not in line with WB at Pw stage, as shown in Fig. 2, suggesting that the AccSp10 may quickly participate in other development processes besides melanisation reaction or the translator process of AccSp10 may be regulated after transcription. These results indicated that AccSp10 was likely involved in the production of melanin and promoted the development of pupae, especially changes in body surface colour, which might facilitate improved insect immunity.
Studies have shown that serine proteinases can initiate cascade reactions to resist pathogen invasion and promote wound healing (Muta and Iwanaga 1996; Lavine and Strand 2001; Jiang et al. 2010). To further understand the functions of AccSp10, external environment conditions were imitated. In this study, we found that the transcriptional expression of AccSp10 was upregulated when adult workers were treated with Bacillus bombysepticus, as shown in Fig. 3a. Western blot showed that AccSp10 protein levels first declined and then rose, as shown in Fig. 3c. This suggested that AccSp10 may take part in the elimination of Bacillus bombysepticus via lash-up and stress reactions. When accepting the external stimulus, organisms could occur lash-up and stress reactions (lash-up reactions showed quick reflexes, but stress reactions slow), AccSp10 expressed less in the early during lash-up reactions because of instinct, for example, during bacterial immune challenge at 3 h, at the same time AccSp10 protein was largely used to resist abiotic stresses; then, with the emergence of stress reactions, the expression of AccSp10 was greatly upregulated by hormone; so the transcription of AccSp10 was lower at 3 h and higher at 24 h, the result of Western blot was gradually higher from 3 to 24 h and the protein was less at 3 h than the control because of being consumed. This phenomenon could be observed under other stresses. The expression of AccSp10 was also upregulated at extreme temperatures (4, 24 and 42 °C) and under harmful abiotic conditions at different time points (treated with HgCl2, H2O2 and paraquat) (Fig. 4a–c(e)). An and Choi (2010) found that temperature is an abiotic environmental factor that induces physiological changes in organisms. When the temperature is higher than a certain range, it can induce damage in insects, including water imbalance, changes in the ion concentration in cells, membrane destruction and structural changes to DNA and proteins (Yoder and Denlinger 1991; Hallman and Denlinger 1998; Walter et al. 1990; Jaenicke 1991; Greenspan et al. 1980). Cold injury globally influenced biological structures and processes and was a primary cause of insect death (Hodkova and Hodek 2004; Koštál et al. 2004, 2007). The best survival temperature of Chinese honeybees was approximately 34 °C, so 4, 24 and 42 °C were extreme temperatures. When adult workers were exposed to extreme temperatures (4, 24 and 42 °C), AccSp10 mRNA accumulation was greater than controls and higher especially at 24 than 4 and 42 °C (Figs. 3b and 4a). The results indicated that Chinese honeybees had stronger ability to regulate expression of AccSp10 to pull through abiotic harms at 24 than 4 and 42 °C. Upregulation of the AccSp10 protein was similarly shown in the Western blot assay at 24 °C (Fig. 3d). These results might imply some other potential role of AccSp10 in response to extreme temperatures, suggesting that this gene may play a role in overwintering and oversummering similar with the Hsps (Yang et al. 2016). Li et al. (2005) reported that heavy metal could destroy the activity of the insect antioxidant enzyme system and cause cell damage. H2O2, a typical oxidant, can cause oxidative damage, resulting in cell death (Goldshmit et al. 2001). Paraquat, one of the most widely employed herbicides in the world, can produce super oxygen anion free radicals and damage the chloroplast membranes of plant. The upregulated AccSp10 at different times after exposure to extreme temperatures, HgCl2, H2O2 and paraquat suggested that this gene play a potential role in environmental adaptation. However, we also found that AccSp10 was downregulated when honeybees were treated with VC (Fig. 4c(f)). Etlik et al. (1997) reported that vitamins protected red blood cells from lipoperoxidation induced by sulphur dioxide. This suggested that the workers likely enhance immunity against oxidative damage under these conditions and maintain life through other processes rather than the AccSp10 pathway. All of the previous data indicate that AccSp10 might be involved in pathogen clearance, wound healing and protection against microorganisms and abiotic harm.
To further demonstrate the role of AccSp10 in pathogen elimination, an in vitro disc diffusion assay was performed. The killing zones for E. coli overexpressing AccSp10 were larger in diameter on the experimental plates than on control plates (Fig. 6), indicating that AccSp10 plays a role in killing E. coli cells after activation by different concentrations of HgCl2, paraquat and cumene hydroperoxide. In addition, while culturing the bacteria with AccSp10, we also found that it took longer for the bacteria with AccSp10 to reach the same OD600 (Fig. 7); above indicated that AccSp10 might inhibit bacterial growth. The MIC (minimum inhibitory concentration) assay could further measure the antibacterial specificity of recombinant AccSp10, but because the recombinant protein we got was easy to form precipitation, we felt regretted extremely that we could not obtain a MIC value of recombinant AccSp10. To further explore the antibacterial mechanism exerted by AccSp10, the antibacterial activity loci of AccSp10 were predicted using the website http://aps.unmc.edu/AP/. We found that there were two antimicrobial loci (showed in blue and red colours) present in activated AccSp10, as shown in Fig. S2a and S2b (provided as Supplemental data). This indicated that AccSp10 might play an antimicrobial role like other antimicrobial peptides (Bulet et al. 1999). All of the previous data suggest that AccSp10 is likely involved in the clearance of E. coli cells to protect honeybees against bacterial damage. To further verify this view, we performed the proPO activation assay; result showed that tissue protein extract had low basal PO activity that could be activated dramatically by addition of recombinant AccSp10 (Fig. 8). This powerfully indicated that AccSp10 could kill pathogen by melanisation pathway.
In conclusion, we identified a gene from A. cerana cerana, AccSp10; predicted its physiological course, including its participation during different developmental stages and then analysed its functions in the elimination of harmful microorganisms and resistance to abiotic stresses. Furthermore, new studies will test whether AccSp10 is really involved in the melanism response or Toll pathway signalling to protect honeybees against pathogen damage. Furthermore, this information will provide a foundation for the prevention and cure of honeybee diseases.
Electronic supplementary material
A MALDI-TOF/TOF MS of recombinant AccSp10 protein. a A SDS-PAGE of the recombinant AccSp10 protein. 1 and 2 lanes, suspension of sonicated recombinant AccSp10 protein (10 μL) and purified recombinant AccSp10 protein (5μg). The target strip is indicated with arrows. b Mascot Score Histogram and Concise Protein Summary Report. c Matched peptides shown in bold red. (DOCX 358 kb)
The antibacterial activity locus of the AccSp10 protein. The antibacterial activity loci of the AccSp10 protein were predicted using the APD database. Two antibacterial activity loci are shown in the tertiary structure of activated AccSp10 built using homology modelling in SWISS-MODEL. a The first antibacterial activity locus (from A145 to A182) is shown in blue. b The second antibacterial activity locus (from L326 to T371) is shown in red. (DOCX 322 kb)
Acknowledgments
This work was financially supported by the earmarked fund for China Agriculture Research System (no. CARS-45), the National Natural Science Foundation of China (no. 31572470), Shandong Province Modern Agricultural Technology System Innovation Team Special Fund (SDAIT-24-04) and Shandong Province Agricultural Fine Varieties Breeding Projects (2014–2016).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-017-0818-5) contains supplementary material, which is available to authorized users.
Contributor Information
Baohua Xu, Phone: +86-538-8245679, Email: bhxu@sdau.edu.cn.
Xingqi Guo, Phone: +86-538-8245679, Email: xqguo@sdau.edu.cn.
References
- Aggarwal K, Silverman N. Positive and negative regulation of the Drosophila immune response. BMB Rep. 2008;41:267–277. doi: 10.5483/BMBRep.2008.41.4.267. [DOI] [PubMed] [Google Scholar]
- Ahola V, Koskinen P, Wong SC, Kvist J, Paulin L, Auvinen P, Saastamoinen M, Frilander MJ, Lehtonen R, Hanski I. Temperature- and sex-related effects of serine protease alleles on larval development in the Glanville fritillary butterfly. J Evol Biol. 2015;28(12):2224–2235. doi: 10.1111/jeb.12745. [DOI] [PubMed] [Google Scholar]
- Alaux C, Ducloz F, Crauser D, Le Conte Y. Diet effects on honeybees immunocompetence. Biol Lett. 2010;6:562–565. doi: 10.1098/rsbl.2009.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An MI, Choi CY. Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: effects on hemolymph and biochemical parameters. Comparative Biochemistry & Physiology Part B Biochemistry & Molecular Biology. 2010;155:34–42. doi: 10.1016/j.cbpb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- An C, Ishibashi J, Ragan EJ, Jiang H, Kanost MR. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathway. J Biol Chem. 2009;284:19716–19726. doi: 10.1074/jbc.M109.007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Budd A, Kanost MR, Michel K. Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes. Cellular & Molecular Life Sciences Cmls. 2011;68:1929–1939. doi: 10.1007/s00018-010-0543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SO. 2—Sclerotization and tanning of the cuticle. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology biochemistry and pharmacology. Oxford: Pergamon Press; 1985. pp. 59–74. [Google Scholar]
- Anderson KV, Bokla L, Nüssleinvolhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985;42:791–798. doi: 10.1016/0092-8674(85)90275-2. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Jürgens G, Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985;42:779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- Belvin MP, Anderson KV. A conserved signalling pathway: the Drosophila toll-dorsal pathway. Annal Review of Cell and Developmental Biology. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- Bitondi MMG, Mora IM, Simões ZLP, Figueiredo VLC. The Apis mellifera, pupal melanization program is affected by treatment with a juvenile hormone analogue. J Insect Physiol. 1998;44:499–507. doi: 10.1016/S0022-1910(97)00113-3. [DOI] [PubMed] [Google Scholar]
- Bulet P, Hetru C, Dimarcq JL, Hoffmann D. Antimicrobial peptides in insects; structure and function. Developmental & Comparative Immunology. 1999;23:329–344. doi: 10.1016/S0145-305X(99)00015-4. [DOI] [PubMed] [Google Scholar]
- Burmeister C, Luërsen K, Heinick A, Hussein A, Domagalski M, Walter RD, Liebau E. Oxidative stress in Caenorhabditis elegans: protective effects of the Omega class glutathione transferase (GSTO-1) Faseb Jounal. 2008;22:343–354. doi: 10.1096/fj.06-7426com. [DOI] [PubMed] [Google Scholar]
- Carter P, Wells JA. Dissecting the catalytic triad of a serine protease. Nature. 1988;332:564–568. doi: 10.1038/332564a0. [DOI] [PubMed] [Google Scholar]
- Etlik O, Tomur A, Tuncer M, Ridvanağaoğlu AY, Andaç O. Protective effect of antioxidant vitamins on red blood cell lipoperoxidation induced by SO2 inhalation. Journal of Basic & Clinical Physiology & Pharmacology. 1997;8:31–43. doi: 10.1515/jbcpp.1997.8.1-2.31. [DOI] [PubMed] [Google Scholar]
- Gabriella F, Ioannis E, Ffrench-Constant RH, István V. A serine proteinase homologue, SPH-3, plays a central role in insect immunity. J Immunol. 2011;186:4828–4834. doi: 10.4049/jimmunol.1003246. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H2O2. Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:46379–46385. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- Gorman MJ, Wang Y, Jiang H, Kanost MR. Manduca sexta hemolymph proteinase 21 activates prophenoloxidase-activating proteinase 3 in an insect innate immune response proteinase cascade. J Biol Chem. 2007;282:11742–11749. doi: 10.1074/jbc.M611243200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan RJ, Finn JA, Hall JC. Acetylcholinesterase mutants in drosophila and their effects on the structure and function of the cental nervous system. J Comp Neurol. 1980;189:741–774. doi: 10.1002/cne.901890409. [DOI] [PubMed] [Google Scholar]
- Gupta S, Wang Y, Jiang H. Manduca sexta prophenoloxidase serine proteinase homologs (SPHs) simultaneously. Insect Biochem Mol Biol. 2005;35:241–248. doi: 10.1016/j.ibmb.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutterridge JMC. Production against radical damage: systems with problems. second. Oxford: Free Radical Biology and Medicine Clarendon Press; 1989. [Google Scholar]
- Hallman GJ, Denlinger DL. Temperature sensitivity in insects and application in integrated pest management. Oxford: Westview Press; 1998. p. 29. [Google Scholar]
- Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Hiruma K, Riddiford LM. Granular phenoloxidase involved in cuticular melanization in the tobacco hornworm: regulation of its synthesis in the epidermis by juvenile hormone. Dev Biol. 1988;130:87–97. doi: 10.1016/0012-1606(88)90416-2. [DOI] [PubMed] [Google Scholar]
- Hodkova M, Hodek I. Photoperiod, diapause and cold-hardiness. European Journal of Entomology. 2004;101:445–458. doi: 10.14411/eje.2004.064. [DOI] [Google Scholar]
- Hughes AL. Evolution of the betaGRP/GNBP/beta-1,3-glucanase family of insects. Immunogenetics. 2012;64:549–558. doi: 10.1007/s00251-012-0610-8. [DOI] [PubMed] [Google Scholar]
- Iwanaga S, Kawabata S, Muta T. New types of clotting factors and defense molecules found in horseshoe crab hemolymph: their structures and functions. J Biol Chem. 1998;123:1–15. doi: 10.1093/oxfordjournals.jbchem.a021894. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Protein stability and molecular adaptation to extreme conditions. Eur J Biochem. 1991;202:715–728. doi: 10.1111/j.1432-1033.1991.tb16426.x. [DOI] [PubMed] [Google Scholar]
- James MN, Sielecki AR. Molecular structure of an aspartic proteinase zymogen, porcine pepsinogen, at 1.8 A resolution. Nature. 1986;319:33–38. doi: 10.1038/319033a0. [DOI] [PubMed] [Google Scholar]
- Jiang H, Kanost MR. The clip-domain family of serine proteinases in arthropods. Insect Biochem Mol Biol. 2000;30:95–105. doi: 10.1016/S0965-1748(99)00113-7. [DOI] [PubMed] [Google Scholar]
- Jiang H, Vilcinskas A, Kanost MR. Immunity in lepidopteran insects. Adv Exp Med Biol. 2010;34:89–100. doi: 10.1007/978-1-4419-8059-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris Z, Brun S, Jang IH, Nam HJ, Romeo Y, Takahashi K, Lee WJ, Ueda R, Lemaitre B. Drosophila, immunity: a large-scale in vivo RNAi screen identifies five serine proteases required for Toll activation. Curr Biol. 2006;16:808–813. doi: 10.1016/j.cub.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Jiang H, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Kim CH, Kim SJ, Kan H, Kwon HM, Roh KB, Jiang R, Yang Y, Park JW, Lee HH, Ha NC, Kang HJ, Nonaka M, Söderhäll K, Lee BL. A three-step proteolytic cascade mediates the activation of the peptidoglycan-induced toll pathway in an insect. J Biol Chem. 2008;283:1799–7607. doi: 10.1074/jbc.M705747200. [DOI] [PubMed] [Google Scholar]
- Koštál V, Vambera J, Bastl J. On the nature of pre-freeze mortality in insects: water balance, ion homeostasis and energy charge in the adults of Pyrrhocoris apterus. Jounal of experimental biology. 2004;207:1509–1521. doi: 10.1242/jeb.00923. [DOI] [PubMed] [Google Scholar]
- Koštál V, Renault D, Mehrabianová A, Bastl J. Insect cold tolerance and repair of chill-injury at fluctuating thermal regimes: role of ion homeostasis. Comparative Biochemistry & Physiology Part A Molecular & Integrative Physiology. 2007;147:231–238. doi: 10.1016/j.cbpa.2006.12.033. [DOI] [PubMed] [Google Scholar]
- Krem MM, Di Cera E. Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem Sci. 2002;27:67–74. doi: 10.1016/S0968-0004(01)02007-2. [DOI] [PubMed] [Google Scholar]
- Lavine MD, Strand MR. Surface characteristics of foreign targets that elicit an encapsulation response by the moth Pseudoplusia includens. J Insect Physiol. 2001;47:965–974. doi: 10.1016/S0022-1910(01)00071-3. [DOI] [PubMed] [Google Scholar]
- Lehrer RI, Ganz T. Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol. 1999;11:23–27. doi: 10.1016/S0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Li L, Liu X, Guo Y, Ma E. Activity of the enzymes of the antioxidative system in cadmium-treated Oxya chinensis, (Orthoptera Acridoidae) Environmental Toxicology & Pharmacology. 2005;20:412–416. doi: 10.1016/j.etap.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science. 2002;297:114–116. doi: 10.1126/science.1072391. [DOI] [PubMed] [Google Scholar]
- Mavrouli MD, Tsakas S, Theodorou GL, Lampropoulou M, Marmaras VJ. Map kinases mediate phagocytosis and melanization via prophenoloxidase activation in medfly hemocytes. Biochimica Et Biophysica Acta-biomembranes. 2005;1774:145–156. doi: 10.1016/j.bbamcr.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Michel T, Reichhart JM, Hoffmann JA, Royet J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature. 2001;414:756–759. doi: 10.1038/414756a. [DOI] [PubMed] [Google Scholar]
- Michelette ERDF, Soares AEE. Characterization of preimaginal developmental stages in Africanized honey bee workers (Apis mellifera L) Apidologie. 1993;24:431–440. doi: 10.1051/apido:19930410. [DOI] [Google Scholar]
- Ming M, Obata F, Kuranaga E, Miura M. Persephone/Spatzle pathogen sensors mediate the activation of Toll receptor signalling in response to endogenous danger signals in apoptosis-deficient Drosophila. J Biol Chem. 2014;289:7558–7568. doi: 10.1074/jbc.M113.543884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muta T, Iwanaga S. The role of hemolymph coagulation in innate immunity. Curr Opin Immunol. 1996;8:41–47. doi: 10.1016/S0952-7915(96)80103-8. [DOI] [PubMed] [Google Scholar]
- Polgár L. The catalytic triad of serine peptidases. Cellular & Molecular Life Sciences Cmls. 2005;62:2161–2172. doi: 10.1007/s00018-005-5160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ. Evolutionary families of peptidases. J Cell Biochem. 1993;290:205–218. doi: 10.1042/bj2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold H. Caste specific modulation of juvenile hormone titers in Apis mellifera. Insect Biochemistry. 1987;17:1003–1006. doi: 10.1016/0020-1790(87)90110-7. [DOI] [Google Scholar]
- Roh KB, Kim CH, Lee H, Kwon HM, Park JW, Ryu JH, Kurokawa K, Ha NC, Lee WJ, Lemaitre B, Söderhäll K, Lee BL. Proteolytic cascade for the activation of the insect toll pathway induced by the fungal cell wall component. J Biol Chem. 2009;284:19474–19481. doi: 10.1074/jbc.M109.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Jiang H, Kanost MR, Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene. 2003;304:117–131. doi: 10.1016/S0378-1119(02)01187-3. [DOI] [PubMed] [Google Scholar]
- Scharlaken B, de Graaf DC, Goossens K, Brunain M, Peelman LJ, Jacobs FJ. Reference gene selection for insect expression studies using quantitative real-time PCR: the honeybees, Apis mellifera, head after a bacterial challenge. J Insect Sci. 2008;8:1–10. doi: 10.1673/031.008.3301. [DOI] [Google Scholar]
- Tang H, Kambris Z, Lemaitre B, Hashimoto C. Two proteases defining a melanization cascade in the immune system of Drosophila. J Biol Chem. 2006;281:28097–28104. doi: 10.1074/jbc.M601642200. [DOI] [PubMed] [Google Scholar]
- Trapasso LM, Simpson RM. Positive and negative regulation of Easter, a member of the serine protease family hat controls dorsal-ventral patterning in the Drosophila embryo. Development. 1998;125:1261–1267. doi: 10.1242/dev.125.7.1261. [DOI] [PubMed] [Google Scholar]
- Veillard F, Troxler L, Reichhart JM. Drosophila melanogaster clip-domain serine proteases: structure, function and regulation. Biochimie. 2015;122:255–269. doi: 10.1016/j.biochi.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Vu TK, Liu RW, Haaksma CJ, Tomasek JJ, Howard EW. Identification and cloning of the membrane-associated serine protease, hepsin, from mouse preimplantation embryos. J Biol Chem. 1997;272:31315–31320. doi: 10.1074/jbc.272.50.31315. [DOI] [PubMed] [Google Scholar]
- Walter MF, Biessmann H, Petersen NS. Heat shock causes the collapse of the intermediate filament cytoskeleton in Drosophila, embryos. Dev Genet. 1990;11:270–279. doi: 10.1002/dvg.1020110405. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiang H. Reconstitution of a branch of the Manduca sexta prophenoloxidase activation cascade in vitro: snake-like hemolymph proteinase 21 (HP21) cleaved by HP14 activates prophenoloxidase-activating proteinase-2 precursor. Insect Biochem Mol Biol. 2007;37:1015–1025. doi: 10.1016/j.ibmb.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XQ, Zhang YL, Wang XQ, Dong H, Gao P, Jia LY. Characterization of multiple heat-shock protein transcripts from Cydia pomonella: their response to extreme temperature and insecticide exposure. J Agric Food Chem. 2016;64:4288–4298. doi: 10.1021/acs.jafc.6b01914. [DOI] [PubMed] [Google Scholar]
- Yao P, Lu W, Meng F, Wang X, Xu B, Guo X. Molecular cloning, expression and oxidative stress response of a mitochondrial thioredoxin peroxidase gene (Acctpx-3) from Apis cerana cerana. J Insect Physiol. 2013;59:273–282. doi: 10.1016/j.jinsphys.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Yoder JA, Denlinger DL. Water balance in flesh fly pupae and water vapor absorption associated with diapause. J Exp Biol. 1991;157:273–286. [Google Scholar]
- Yoshida H, Ashida M. Microbial activation of two serine enzymes and prophenoloxidase in the plasma fraction of hemolymph of the silkworm, Bombyx mori. Insect Biochemistry. 1986;16:539–545. doi: 10.1016/0020-1790(86)90031-4. [DOI] [Google Scholar]
- Zhou Y, Wang F, Liu F, Wang C, Yan Y, Guo X, Xu B. Cloning and molecular identification of triosephosphate isomerase gene from Apis cerana cerana and its role in response to various stresses. Apidologie. 2016;1:1–13. [Google Scholar]
- Zou Z, Lopez DL, Kanost MR, Evans JD, Jiang H. Comparative analysis of serine protease-related genes in the honeybees genome: possible involvement in embryonic development and innate immunity. Insect Mol Biol. 2006;15:603–614. doi: 10.1111/j.1365-2583.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A MALDI-TOF/TOF MS of recombinant AccSp10 protein. a A SDS-PAGE of the recombinant AccSp10 protein. 1 and 2 lanes, suspension of sonicated recombinant AccSp10 protein (10 μL) and purified recombinant AccSp10 protein (5μg). The target strip is indicated with arrows. b Mascot Score Histogram and Concise Protein Summary Report. c Matched peptides shown in bold red. (DOCX 358 kb)
The antibacterial activity locus of the AccSp10 protein. The antibacterial activity loci of the AccSp10 protein were predicted using the APD database. Two antibacterial activity loci are shown in the tertiary structure of activated AccSp10 built using homology modelling in SWISS-MODEL. a The first antibacterial activity locus (from A145 to A182) is shown in blue. b The second antibacterial activity locus (from L326 to T371) is shown in red. (DOCX 322 kb)