Abstract
Polyhydroxyalkanoates (PHA) have been produced by some bacteria as bioplastics for many years. Yet their commercialization is still on the way. A few issues are related to the difficulty of PHA commercialization: namely, high cost and instabilities on molecular weights (Mw) and structures, thus instability on thermo-mechanical properties. The high cost is the result of complicated bioprocessing associated with sterilization, low conversion of carbon substrates to PHA products, and slow growth of microorganisms as well as difficulty of downstream separation. Future engineering on PHA producing microorganisms should be focused on contamination resistant bacteria especially extremophiles, developments of engineering approaches for the extremophiles, increase on carbon substrates to PHA conversion and controlling Mw of PHA. The concept proof studies could still be conducted on E. coli or Pseudomonas spp. that are easily used for molecular manipulations. In this review, we will use E. coli and halophiles as examples to show how to engineer bacteria for enhanced PHA biosynthesis and for increasing PHA competitiveness.
Keywords: PHB, Polyhydroxyalkanoates, Extremophiles, Halophiles, Next generation industrial biotechnology, NGIB, Metabolic engineering, Pathway engineering, Morphology engineering, Contents
1. Introduction
Polyhydroxyalkanoates (PHA) have been produced since the 1980s with limited market success [1], [2], [3]. Many challenges are related to the limited PHA commercialization (Table 1), especially the high production cost and instability on thermo-mechanical properties resulted from unstable molecular weights (Mw) and structures, that are also associated with unstable PHA synthase activity and monomer supplies [4], [5], [6], [7], [8], [9], [10]. Efforts have been made to meet these challenges [5], [11], [12], [13].
Table 1.
Challenges for producing cost competitive PHA.
| Problems | Reasons | Solutions | Reference |
|---|---|---|---|
| High energy demands | Sterilization and intensive aeration | Unsterile and micro-aerobic processes | [17] |
| Low substrates to PHA conversions | Substrates are consumed for other purposes | Deletion or weakening PHA unrelated pathways | [18], [19] |
| Unstable PHA structures | Multiple pathways consuming PHA precursors | Deletion or weakening PHA unrelated pathways | [19], [20] |
| Unstable batch Mw | Unstable PHA synthase activity | Controlling PHA synthase activity | [8], [10] |
| Slow growth | Binary fission et al. | Multiple fission et al. | [21] |
| Discontinuous processes | Avoid possible contamination | Use contamination resistant strains | [16], [17], [22] |
| Expensive downstream | Complexity to extract and purify products | Morphology engineering | [23], [24] |
The high cost is the result of high energy demand related to complicated sterilization and intensive aeration, low conversion of carbon substrates (S) to PHA products (P), slow growth of microorganisms, discontinuous processes and expensive downstream processing et al. (Table 1) [14], [15]. The use of extremophilic bacteria combined with metabolic engineering and synthetic biology could fully address these issues [16], [17].
Future engineering on PHA producing microorganisms should be focused on contamination resistant bacteria especially extremophiles, developments of engineering approaches for the extremophiles (which is called “Next Generation Industry Biotechnology” or “NGIB”, which will be discussed in section 6 in this paper), increase on carbon substrates to PHA conversion and controlling Mw of PHA (Table 1). The concept proof studies could still be conducted on E. coli or Pseudomonas spp. that are easily used for molecular manipulations. In this review, we will use E. coli, Pseudomonas spp., and halophiles as examples to show how to engineer bacteria for better PHA biosynthesis and for increased PHA application competitiveness.
2. Redirecting substrates to PHA conversion pathways
In many cases, substrates are the most important factor for high production cost. This is especially true for PHA production [11]. For example, the production of PHA containing non 3-hydroxubutyrate (3HB) monomers requires fatty acid(s) as substrate for formation of other non 3HB short-chain-length (scl) or medium-chain-length (mcl) monomers [25], [26], [27], [28], [29]. Since most fatty acids will be beta-oxidized to acetyl-CoA for the uses of many other biosynthesis pathways other than for PHA synthesis, it wastes a lot of expensive fatty acids for generating acetyl-CoA (Fig. 1) [20], which can be formed from low-cost glucose [18], [30]. Due to the beta-oxidation, fatty acid substrates conversion to PHA products are very low, resulting in high cost of PHA production.
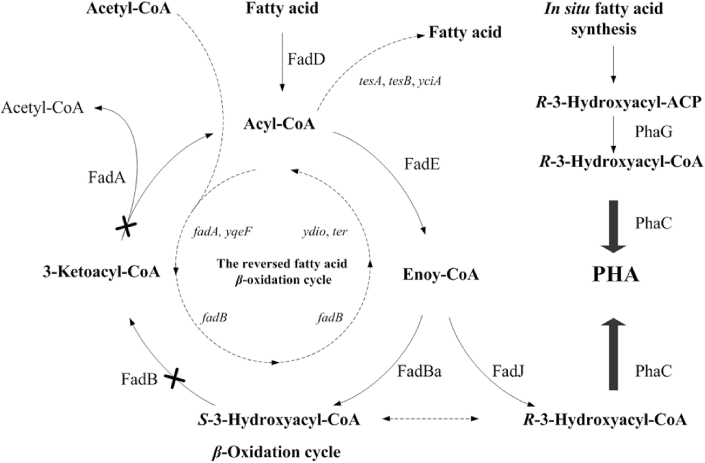
Fig. 1.
Biosynthesis of PHA from fatty acids via beta-oxidation pathway. Deletions on enzymes (FadA+FadB) in beta-oxidation pathways allow most fatty acid(s) to channel to PHA synthesis, thus significantly improve substrates to product PHA ratios.
The substrate to PHA conversion efficiency has been significantly improved with the deletions of enzymes FadA and FadB in the beta-oxidation pathway of Pseudomonas putida or P. entomophila (Fig. 1), as fatty acid substrates were mostly converted into 3-hydroxyacyl-CoA for PHA synthesis instead of being oxidized to become acetyl-CoA [20], [31], [32]. Beta-oxidation pathway deleted Pseudomonas spp. have been reported to produce PHA containing 3-hydroxyhexanoate (3HHx), 3-hydroxyoctanoate (3HO), 3-hydroxydecanoate (3HD) and 3-hydroxydodecanoate (3HDD) in the forms of homopolymers, block- or random copolymers [33].
A metabolically engineered Escherichia coli has been constructed by co-expressing genes involved in succinate degradation in Clostridium kluyveri and P(3HB) accumulation pathway of Ralstonia eutropha. This engineered E. coli can produce poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)] from glucose. Also, E. coli native succinate semialdehyde dehydrogenase genes sad and gabD were both deleted to enhance the carbon flux to 4HB biosynthesis [18]. Povolo et al. [34] reported that the production of P(3HB-co-3HV-co-4HB) terpolymer can be obtained directly by the use of lactose or waste raw materials such as cheese whey as carbon sources. Cerrone et al. [35] demonstrated the use of a mannitol rich ensiled grass press juice (EGPJ) as a renewable carbon substrate for polyhydroxyalkanoates (PHA) production. Fed-batch cultivations of Burkholderia sacchari IPT101 using EGPJ as sole carbon source produced 44.5 g/L CDW containing 33% poly-3-hydroxybutyrate (PHB) in 36 h. Park et al. [36] constructed a sucrose utilization pathway in Ralstonia eutropha NCIMB11599 and R. eutropha 437–540 by introducing the Mannheimia succiniciproducens MBEL55E sacC gene that encodes β-fructofuranosidase. β-fructofuranosidase excreted into the culture medium could hydrolyze sucrose to glucose and fructose, which efficiently used sucrose as carbon sources by recombinant R. eutropha strains. A high P(3HB) content of 73.2 wt% was obtained when R. eutropha NCIMB11599 was cultured in nitrogen-free chemically defined medium containing 20 g/L of sucrose. Qi group [37] synthesized mcl-PHAs in E. coli directly from glucose by engineering the reversed fatty acid β-oxidation cycle. After deletion of the major thioesterases and expression of a low-substrate-specificity PHA synthase from Pseudomonas stutzeri 1317, the engineered E. coli produced 12.10 wt% of cell dry weight scl-mcl PHA copolymers, of which 21.18 mol% was 3-hydroxybutyrate and 78.82 mol% was medium-chain-length monomers.
3. Stabilization of PHA monomer ratios and molecular weights (Mw)
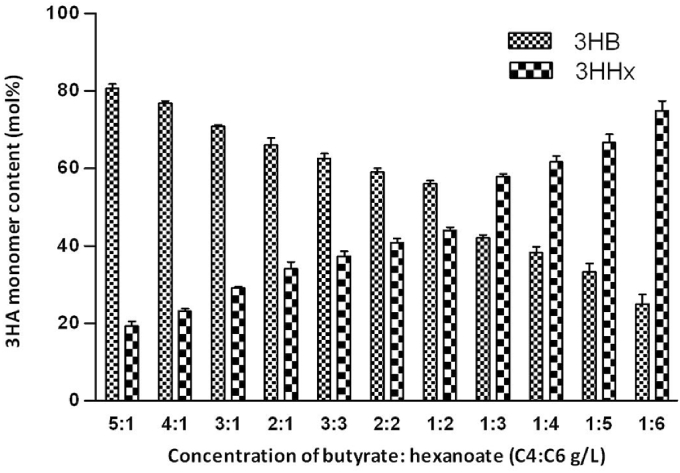
Due to the fluctuation of PHA synthase activity and monomer supplies, monomer ratios in copolymers and PHA Mw vary from batch to batch, this is not desirable by any consumer. Therefore, a lot of efforts have been made to stabilize the PHA monomer structures and Mw. Tripathi et al. [19] used the above beta-oxidation deleted Pseudomonas putida KT2442 as a platform for the biosynthesis of polyhydroxyalkanoates with adjustable monomer contents and compositions. The monomer ratios can be precisely controlled by feeding fatty acids with a predefined ratio (Fig. 2). They achieved to prepare random copolymers PHBHHx or block copolymers consisting of precisely adjustable 3-hydroxybutyrate (3HB) and 3-hydroxyhexanoate (3HHx). The materials thus showed stable properties if the monomer ratios were stable [19], [38].
Fig. 2.
A beta-oxidation deleted Pseudomonas putida KT2442 was successfully used as a platform for the biosynthesis of polyhydroxyalkanoates with adjustable 3HB/3HHx ratios depending on fatty acid mixture fed to the culture [19].
Similarly, Wang Ying et al. [39] succeeded in synthesizing homopolymers of C5 (3-hydroxyvalerate) to C14 (4-hydroxytetradecanoate) using beta-oxidation deleted P. entomophila LAC23 grown on different fatty acids as precursors, respectively. The examples clearly demonstrate that beta-oxidation deleted mutants can help control PHA monomer structures, as also evidenced by several other studies [40].
Some factors have a direct impact on the molecular weight of PHA. Such as the concentration (or activity) of PHA synthase, the occurrence of a chain transfer reaction, the catalytic activity of PHA synthase and the simultaneous degradation of PHA during biosynthesis [41]. PHA molecular weight needs to be controlled to tailor the physical properties of the polymer. PHA molecular weight can be reduced via the addition of chain transfer agents such as poly(ethylene glycol) (PEG), methanol, ethanol and isopropanol to the culture medium during production and mutations in the N-terminus of PHA synthase [42], [43]. Moreover, PHA molecular weights can be controlled by adjusting PHA synthase activity. CRISPRi (Clustered regularly interspaced short palindromic repeats interference) was able to control the phaC transcription and thus PhaC activity. Li Dan et al. [10] found that PHB content, molecular weight and polydispersity were approximately in direct and reverse proportion to the PhaC activity, respectively. Very importantly, a higher PhaC activity led to more intracellular PHB accumulation yet with less PHB molecular weights and wider polydispersity, PHB contents could be controlled in the ranges of 2.0–75% cell dry weights, molecular weights from 2 to 6 millions Dalton and a polydispersity of 1.2–1.43 in 48 h shake flask studies. This Mw control studies should be further confirmed in large scale fermentor studies.
4. Acceleration of cell growth
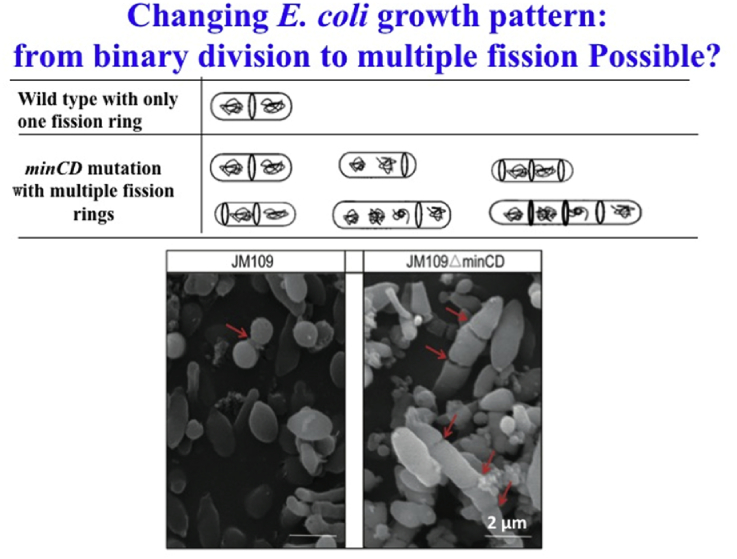
Most bacteria are grown in a common binary fission way (Fig. 3). Wu Hong et al. [21] changed the binary fission to multiple fission by deleting fission related genes minC and minD together, leading to the formation of multiple fission rings (Z-rings) in several positions of an elongated cell, achieving cell division into more than two daughter cells at same time (Fig. 3). In addition, some genes related to cell division process including ftsQ, ftsL, ftsW, ftsN and ftsZ, together with the cell shape control gene mreB, were all overexpressed in E. coli JM109 ΔminCD to further improve cell growth and PHA production. This resulted in more cell dry weights (CDW) and more than 80% polyhydroxybutyrate (PHB) accumulation increases compared to its binary fission control. This study demonstrates that changing the cell division pattern and cell morphology help accelerate cell growth and PHB accumulation. In another related study, Wu Hong et al. [44] further demonstrated that a combination of the multiple division pattern with elongated cell shape of E. coli improved PHB production.
Fig. 3.
The common bacterial binary fission can be changed to multiple fission by deleting fission related genes minC and minD together, leading to the formation of multiple fission rings (Z-rings) in several positions of an elongated cell, achieving cell division into more than two daughter cells.
In addition, Tyo group [45] developed a toggle switch that uses glucose sensing to decouple growth and production phase strategy. This industrially relevant auto-inducible genetic switch responds to glucose availability to precisely time the expression of burdensome pathway enzymes for enhanced bio-production, which improved growth by 2-fold with comparable PHB production yields to a constitutively expressing system. Those provided a new vision for enhanced PHA production.
5. Bacterial morphology engineering for easy downstream separation
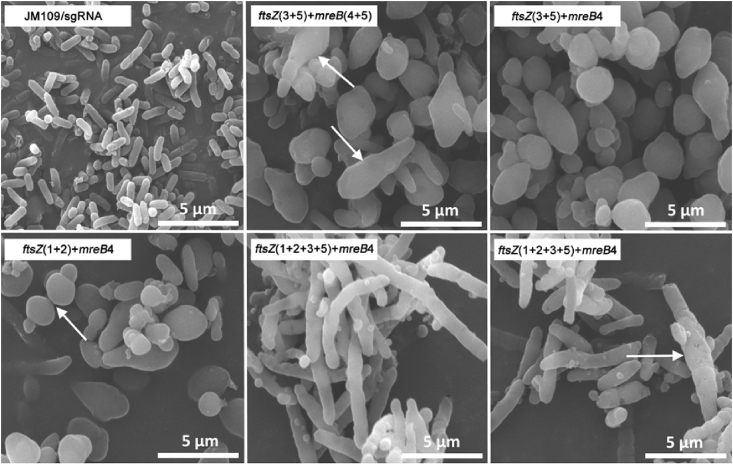
Tiny bacterial cell sizes create complexity for downstream separation. Morphology engineering changes cell sizes and shapes, allow easy downstream separation [24]. Genes ftsZ and mreB encoding proteins of bacterial fission ring and skeletons, respectively, are essential for cell growth and for maintaining the bacterial shapes [46], [47], [48], [49], [50]. Clustered regularly interspaced short palindromic repeats interference (CRISPRi), was used to regulate expression intensities of ftsZ or/and mreB in E. coli resulting in various reduced expression levels of ftsZ or/and mreB, respectively [51]. It was found that the stronger the repression on genes ftsZ or/and mreB, the longer the E. coli fibers, and the larger the E. coli cells [51]. Combined repressions on expressions of ftsZ and mreB generated long and larger E. coli with diverse morphologies including various sizes of gourds, bars, coccus, spindles, multi-angles and ellipsoids (Fig. 4). In all cases, PHB accumulations were improved. Enlarged morphology increased PHB synthesis from 40% to 80% PHB, it also promotes gravity separation of cells from fermentation broth [23], [24], [52].
Fig. 4.
CRISPRi was used to regulate expression intensities of ftsZ or/and mreB in E. coli resulting in various reduced expression levels of ftsZ or/and mreB, respectively [51]. Combined repressions on expressions of ftsZ and mreB generated long and larger E. coli with diverse morphologies and enhanced PHB accumulations. Large cells are prone to separate from broth via gravity or filtration.
6. Future prospects: next generation industry biotechnology (NGIB) based on extremophiles (halophiles)
Extremophiles grown under extreme conditions are more resistant to microbial contamination. Among them, halophilic bacteria are able to grow rapidly in medium with high salt concentrations under high pH [16], they are thus contamination resistant as few other microorganisms can do so.
Halophilic bacteria were found able to grown contamination free in open unsterile and continuous processes in seawater medium for at least two months [53]. Their values could be further improved by introducing new pathways or adding new genetic parts.
However, genetic parts are often influenced by host strains, and altered activity of biological parts frequently causes failures in process control [54], [55]. Therefore, in order to fully realizing the potential of Halomonas, genetic parts with a tight regulation and high efficiency need to be developed. Recently, Technology has also been developed for genetic manipulation of halophilic bacteria [22], [56]. Molecular engineering tools have been developed to construct recombinant Halomonas spp. for production of foreign protein [57], small molecular compound 5-aminolevulinic acid [58], and PHBV copolymers consisting of 3-hydroxybutyrate (3HB) and 3-hydroxyvalerate (3HV) with high substrate to PHA conversion efficiency [22], [59], [60]. More engineering studies will lead to the generation of new products produced by engineered halophiles.
The biotechnology based on extremophiles grown under open unsterile conditions will surely promote the emerging “Next Generation Industrial Biotechnology” or NGIB for bio-production with reduced cost and thus improved competitiveness.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This research was financially supported by a grant from Ministry of Sciences and Technology (Grant No. 2016YFB0302504), and grants from National Natural Science Foundation of China (Grant No. 31430003). Tsinghua President Fund also supported this project (Grant No. 2015THZ10).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Chen G.Q., Patel M.K. Plastics derived from biological sources: present and future: a technical and environmental review. Chem Rev. 2012;112:2082–2099. doi: 10.1021/cr200162d. [DOI] [PubMed] [Google Scholar]
- 2.Gao X., Chen J.C., Wu Q., Chen G.Q. Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr Opin Biotechnol. 2011;22:768–774. doi: 10.1016/j.copbio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Laycock B., Halley P., Pratt S., Werker A., Lant P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog Polym Sci. 2013;38:536–583. [Google Scholar]
- 4.Park S.J., Kim T.W., Kim M.K., Lee S.Y., Lim S.C. Advanced bacterial polyhydroxyalkanoates: towards a versatile and sustainable platform for unnatural tailor-made polyesters. Biotechnol Adv. 2012;30:1196–1206. doi: 10.1016/j.biotechadv.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Yin J., Chen G.Q. Microbial polyhydroxyalkanoates, Challenges and opportunities. Curr Opin Biotechnol. 2014;30C:59–65. doi: 10.1016/j.copbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Bernd H. Polyester synthases: natural catalysts for plastics. Biochem J. 2003;376:15–33. doi: 10.1042/BJ20031254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyo K.E., Fischer C.R., Simeon F., Stephanopoulos G. Analysis of polyhydroxybutyrate flux limitations by systematic genetic and metabolic perturbations. Metab Eng. 2010;12:187–195. doi: 10.1016/j.ymben.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Choi J.-i., Lee S.Y. High level production of supra molecular weight poly (3-hydroxybutyrate) by metabolically engineered Escherichia coli. Biotechnol Bioprocess Eng. 2004;9:196–200. [Google Scholar]
- 9.Lee S., Middelberg A., Lee Y. Poly (3-hydroxybutyrate) production from whey using recombinant Escherichia coli. Biotechnol Lett. 1997;19:1033–1035. [Google Scholar]
- 10.Li D., Lv L., Chen J.-C., Chen G.-Q. Controlling microbial PHB synthesis via CRISPRi. Appl Microbiol Biotechnol. 2017;101:5861–5867. doi: 10.1007/s00253-017-8374-6. [DOI] [PubMed] [Google Scholar]
- 11.Choi J., Lee S.Y. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl Microbiol Biotechnol. 1999;51:13–21. [Google Scholar]
- 12.Koutinas A.A., Vlysidis A., Pleissner D., Kopsahelis N., Lopez Garcia I., Kookos I.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem Soc Rev. 2014;43:2587–2627. doi: 10.1039/c3cs60293a. [DOI] [PubMed] [Google Scholar]
- 13.Steinbüchel A., Lütke-Eversloh T. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem Eng J. 2003;16:81–96. [Google Scholar]
- 14.Sudesh K., Abe H., Doi Y. Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci. 2000;25:1503–1555. [Google Scholar]
- 15.Serafim L.S., Lemos P.C., Albuquerque M.G., Reis M.A. Strategies for PHA production by mixed cultures and renewable waste materials. Appl Microbiol Biotechnol. 2008;81:615–628. doi: 10.1007/s00253-008-1757-y. [DOI] [PubMed] [Google Scholar]
- 16.Yin J., Chen J.-C., Wu Q., Chen G.-Q. Halophiles, coming stars for industrial biotechnology. Biotechnol Adv. 2014;33:1433–1442. doi: 10.1016/j.biotechadv.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Li T., Chen X.b., Chen J.c., Wu Q., Chen G.Q. Open and continuous fermentation: products, conditions and bioprocess economy. Biotechnol J. 2014;9:1503–1511. doi: 10.1002/biot.201400084. [DOI] [PubMed] [Google Scholar]
- 18.Li Z.J., Shi Z.Y., Jian J., Guo Y.Y., Wu Q., Chen G.Q. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metab Eng. 2010;12:352–359. doi: 10.1016/j.ymben.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Tripathi L., Wu L.P., Dechuan M., Chen J., Wu Q., Chen G.Q. Pseudomonas putida KT2442 as a platform for the biosynthesis of polyhydroxyalkanoates with adjustable monomer contents and compositions. Bioresour Technol. 2013;142:225–231. doi: 10.1016/j.biortech.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Chung A.-L., Jin H.-L., Huang L.-J., Ye H.-M., Chen J.-C., Wu Q. Biosynthesis and characterization of poly (3-hydroxydodecanoate) by β-oxidation inhibited mutant of Pseudomonas entomophila L48. Biomacromolecules. 2011;12:3559–3566. doi: 10.1021/bm200770m. [DOI] [PubMed] [Google Scholar]
- 21.Wu H., Fan Z., Jiang X., Chen J., Chen G.Q. Enhanced production of polyhydroxybutyrate by multiple dividing E. coli. Microb Cell Fact. 2016;15:128. doi: 10.1186/s12934-016-0531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu X.-Z., Tan D., Aibaidula G., Wu Q., Chen J.-C., Chen G.Q. Development of Halomonas TD01 as a host for open production of chemicals. Metab Eng. 2014;23:78–91. doi: 10.1016/j.ymben.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X.R., Chen G.Q. Morphology engineering of bacteria for bio-production. Biotechnol Adv. 2016;34:435–440. doi: 10.1016/j.biotechadv.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Jiang X.R., Wang H., Shen R., Chen G.Q. Engineering the bacterial shapes for enhanced inclusion bodies accumulation. Metab Eng. 2015;29:227–237. doi: 10.1016/j.ymben.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Andreeßen B., Lange A.B., Robenek H., Steinbüchel A. Conversion of glycerol to poly (3-hydroxypropionate) in recombinant Escherichia coli. Appl Environ Microbiol. 2010;76:622–626. doi: 10.1128/AEM.02097-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez R.J., Schripsema J., da Silva L.F., Taciro M.K., Pradella J.G., Gomez J.G.C. Medium-chain-length polyhydroxyalkanoic acids (PHA mcl) produced by Pseudomonas putida IPT 046 from renewable sources. Euro Polym J. 2003;39:1385–1394. [Google Scholar]
- 27.Kroumova A.B., Wagner G.J., Davies H.M. Biochemical observations on medium-chain-length polyhydroxyalkanoate biosynthesis and accumulation in Pseudomonas mendocina. Arch Biochem Biophys. 2002;405:95–103. doi: 10.1016/s0003-9861(02)00350-8. [DOI] [PubMed] [Google Scholar]
- 28.Cavalheiro J.M., Raposo R.S., de Almeida M.C.M., Cesário M.T., Sevrin C., Grandfils C. Effect of cultivation parameters on the production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) and poly (3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour Technol. 2012;111:391–397. doi: 10.1016/j.biortech.2012.01.176. [DOI] [PubMed] [Google Scholar]
- 29.Doi Y., Segawa A., Kunioka M. Biodegradable poly (3-hydroxybutyrate-co-4-hydroxybutyrate) produced from γ-butyrolactone and butyric acid by Alcaligenes eutrophus. Polym Commun Guildf. 1989;30:169–171. [Google Scholar]
- 30.Valentin H.E., Dennis D. Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) in recombinant Escherichia coli grown on glucose. J Biotechnol. 1997;58:33–38. doi: 10.1016/s0168-1656(97)00127-2. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q., Luo G., Zhou X.R., Chen G.Q. Biosynthesis of poly (3-hydroxydecanoate) and 3-hydroxydodecanoate dominating polyhydroxyalkanoates by β-oxidation pathway inhibited Pseudomonas putida. Metab Eng. 2011;13:11–17. doi: 10.1016/j.ymben.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Wang H.-h., Li X.-t., Chen G.Q. Production and characterization of homopolymer polyhydroxyheptanoate (P3HHp) by a fadBA knockout mutant Pseudomonas putida KTOY06 derived from P. putida KT2442. Process Biochem. 2009;44:106–111. [Google Scholar]
- 33.Chen G.Q., Hajnal I., Wu H., Lv L., Ye J. Engineering biosynthesis mechanisms for diversifying polyhydroxyalkanoates. Trends Biotechnol. 2015;33:565–574. doi: 10.1016/j.tibtech.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Povolo S., Romanelli M.G., Basaglia M., Ilieva V.I., Corti A., Morelli A. Polyhydroxyalkanoate biosynthesis by Hydrogenophaga pseudoflava DSM1034 from structurally unrelated carbon sources. New Biotechnol. 2013;30:629–634. doi: 10.1016/j.nbt.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Cerrone F., Davis R., Kenny S.T., Woods T., O'Donovan A., Gupta V.K. Use of a mannitol rich ensiled grass press juice (EGPJ) as a sole carbon source for polyhydroxyalkanoates (PHAs) production through high cell density cultivation. Bioresour Technol. 2015;191:45–52. doi: 10.1016/j.biortech.2015.04.128. [DOI] [PubMed] [Google Scholar]
- 36.Park S.J., Jang Y.A., Noh W., Oh Y.H., Lee H., David Y. Metabolic engineering of Ralstonia eutropha for the production of polyhydroxyalkanoates from sucrose. Biotechnol Bioeng. 2015;112:638–643. doi: 10.1002/bit.25469. [DOI] [PubMed] [Google Scholar]
- 37.Zhuang Q., Wang Q., Liang Q., Qi Q. Synthesis of polyhydroxyalkanoates from glucose that contain medium-chain-length monomers via the reversed fatty acid β-oxidation cycle in Escherichia coli. Metab Eng. 2014;24:78–86. doi: 10.1016/j.ymben.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Tripathi L., Wu L.-P.P., Chen J., Chen G.Q. Synthesis of Diblock copolymer poly-3-hydroxybutyrate -block-poly-3-hydroxyhexanoate [PHB-b-PHHx] by a β-oxidation weakened Pseudomonas putida KT2442. Microb Cell Fact. 2012;11:44. doi: 10.1186/1475-2859-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Chung A., Chen G.Q. Synthesis of medium-chain-length polyhydroxyalkanoate homopolymers, random copolymers, and block copolymers by an engineered strain of Pseudomonas entomophila. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201601017. [DOI] [PubMed] [Google Scholar]
- 40.Li S., Cai L., Wu L., Zeng G., Chen J., Wu Q. Microbial synthesis of functional homo-, random, and block polyhydroxyalkanoates by β-oxidation deleted Pseudomonas entomophila. Biomacromolecules. 2014;15:2310–2319. doi: 10.1021/bm500669s. [DOI] [PubMed] [Google Scholar]
- 41.Tsuge T. Fundamental factors determining the molecular weight of polyhydroxyalkanoate during biosynthesis. Polym J. 2016;48:1051–1057. [Google Scholar]
- 42.Shi F., Ashby R., Gross R.A. Use of poly (ethylene glycol) s to regulate poly (3-hydroxybutyrate) molecular weight during Alcaligenes eutrophus cultivations. Macromolecules. 1996;29:7753–7758. [Google Scholar]
- 43.Thomson N.M., Hiroe A., Tsuge T., Summers D.K., Sivaniah E. Efficient molecular weight control of bacterially synthesized polyesters by alcohol supplementation. J Chem Technol Biotechnol. 2014;89:1110–1114. [Google Scholar]
- 44.Wu H., Chen J., Chen G.Q. Engineering the growth pattern and cell morphology for enhanced PHB production by Escherichia coli. Appl Microbiol Biotechnol. 2016;100:9907–9916. doi: 10.1007/s00253-016-7715-1. [DOI] [PubMed] [Google Scholar]
- 45.Bothfeld W., Kapov G., Tyo K.E. A glucose-sensing toggle switch for autonomous, high productivity genetic control. ACS Synth Biol. 2017 doi: 10.1021/acssynbio.6b00257. [DOI] [PubMed] [Google Scholar]
- 46.Henderson T.A., Young K.D., Denome S.A., Elf P.K. AmpC and AmpH, Proteins related to the class C beta-lactamases, bind penicillin and contribute to normal morphology of E. coli. J Bacteriol. 1997;179:6112–6121. doi: 10.1128/jb.179.19.6112-6121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominguez-Escobar J., Chastanet A., Crevenna A.H., Fromion V., Wedlich-Soldner R., Carballido-Lopez R. Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria. Science. 2011;333:225–228. doi: 10.1126/science.1203466. [DOI] [PubMed] [Google Scholar]
- 48.Garner E.C., Bernard R., Wang W., Zhuang X., Rudner D.Z., Mitchison T. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science. 2011;333:222–225. doi: 10.1126/science.1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kocaoglu O., Carlson E.E. Penicillin-binding protein imaging probes. Curr Protoc Chem Biol. 2013;5:239–250. doi: 10.1002/9780470559277.ch130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bi E., Lutkenhaus J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol. 1993;175:1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elhadi D., Lv L., Jiang X.-R., Wu H., Chen G.-Q. CRISPRi engineering E. coli for morphology diversification. Metab Eng. 2016;38:358–369. doi: 10.1016/j.ymben.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Wu H., Jiang X., Chen G.Q. Engineering Escherichia coli for enhanced production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in larger cellular space. Metab Eng. 2014;25:183–193. doi: 10.1016/j.ymben.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Yue H.T., Ling C., Yang T., Chen X.B., Chen Y.L., Deng H.T. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates. Biotechnol Biofuels. 2014;7:108. [Google Scholar]
- 54.Brophy J.A., Voigt C.A. Principles of genetic circuit design. Nat Meth. 2014;11:508–520. doi: 10.1038/nmeth.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kittleson J.T., Wu G.C., Anderson J.C. Successes and failures in modular genetic engineering. Curr Opin Chem Biol. 2012;16:329–336. doi: 10.1016/j.cbpa.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Zhao H., Zhang H.M., Chen X., Li T., Wu Q., Ouyang Q. Novel T7-like expression systems used for Halomonas. Metab Eng. 2017;39:128–140. doi: 10.1016/j.ymben.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Lan L.H., Zhao H., Chen J.C., Chen G.Q. Engineering Halomonas spp. as A Low-Cost production host for production of bio-surfactant protein PhaP. Biotechnol J. 2016;11:1595–1604. doi: 10.1002/biot.201600459. [DOI] [PubMed] [Google Scholar]
- 58.Li T., Guo Y.-Y., Qiao G.-Q., Chen G.Q. Microbial synthesis of 5-aminolevulinic acid and its coproduction with polyhydroxybutyrate. ACS Synth Biol. 2016;5:1264–1274. doi: 10.1021/acssynbio.6b00105. [DOI] [PubMed] [Google Scholar]
- 59.Tan D., Wu Q., Chen J.C., Chen G.Q. Engineering Halomonas TD01 for the low-cost production of polyhydroxyalkanoates. Metab Eng. 2014;26C:34–47. doi: 10.1016/j.ymben.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Zhao Y.-X., Rao Z.-M., Xue Y.-F., Gong P., Ji Y.-Z., Ma Y.-H. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) production by Haloarchaeon Halogranum amylolyticum. Appl Microbiol Biotechnol. 2015;99:7639–7649. doi: 10.1007/s00253-015-6609-y. [DOI] [PubMed] [Google Scholar]