Abstract
The main objective of this research is to investigate the anti-biofilm and anti-bacterial activity of Sesbania grandiflora (S. grandiflora) against Staphylococcus aureus. S. grandiflora extract were prepared and analyzed with UV –Vis spectroscopy, Fourier transform infrared spectroscopy, Dynamic light scattering. Biofilm forming pathogens were identified by congo-red assay. Quantification of Extracellular polymeric substance (EPS) particularly protein and carbohydrate were calculated. The efficacy of the herbal extract S. grandiflora and its inhibition against the pathogenic strain of S. aureus was also evaluated. The gradual decrease or disappearance of peaks reveals the reduction of protein and carbohydrate content in the EPS of S. aureus when treated with S. grandiflora. The antibacterial activity of S. grandiflora extract against the bacterial strain S. aureus showed that the extract were more active against the strain. To conclude, anti-biofilm and antibacterial efficacy of S. grandiflora plays a vital role over biofilm producing pathogens and act as a good source for controlling the microbial population.
Keywords: Sesbania grandiflora, Extra polymorphic substances (EPS), FTIR, Staphylococcus aureus, Congo-red assay
Highlights
-
•
Quantification of EPS particularly protein and carbohydrate were calculated.
-
•
Efficacy of the S.grandiflora and its inhibition against the Staphylococcus aureus was evaluated.
-
•
S. grandiflora acts as a good source for controlling the microbial population.
-
•
It can be used as natural preservative ingredients in food and/or pharmaceuticals.
1. Introduction
Bio films are defined as microbial communities of cells attached either to a biotic or abiotic surface enclosed in a complex EPS (Extra cellular Polymeric Substance). Biofilms are formed with interaction among microbial aggregates, filamentous bacterial strains, organic and inorganic particles, which are held together by EPS [1], [2]. EPS majorly comprised of four substance called carbohydrate, protein, lipids and extracellular DNA (e DNA). The biofilm acts as a protective barrier and provides resistance against antibiotics, degrading enzymes, protozoan grazers and host immune response [3], [4]. Some bacterial biofilms have been reported to have useful effects on sewage treatment plants, food chains etc [5]. Moreover, biofilms also posses diverse problems in food industry, medicine, biofouling of boats, cooling towers, water pipes etc. The regular use of antibiotics makes the biofilm producing bacteria resistant to antimicrobial agents [6]. Hence, the development of anti-biofilm strategies is therefore a major interest and currently constitutes an important field of investigation in which environmentally friendly anti-biofilm molecules or organisms are highly valuable.
Traditionally, humans have utilized crude extracts of medicinal plants as curative agents for various ailments. Plant extracts and other biologically active compounds isolated from leaves, stems, and roots have gained interest in anti-biofilm activity [7], [8], [9]. Taking this as an initiative, S. grandiflora were selected to investigate the anti-biofilm and anti-bacterial activity.
S. grandiflora (Family: Fabaceae) is a small, loosely branching tree. It is used as an important dietary nutritive source. The leaves are traditionally used to treat nasal catarrah, nyctalopia and cephalagia. The literature review reveals that, S. grandiflora possess antioxidant, antiuroithiatic, anticonvulsive, anti-arthritic, anti-infammatory, anti-helminthic, anti-bacterial and anxiolytic activity [10], [11], [12].
The aim of this study was to investigate the anti-biofilm and antibacterial activity of S. grandiflora against S. aureus.
2. Materials and methods
2.1. Plant material
The leaves of S. grandiflora were collected from Vellore district. The collected leaves were washed with double distilled water and were air-dried at room temperature for two weeks and coarsely powdered [13] (Fig. 1).
Fig. 1.
Sesbania grandiflora.
2.2. Preparation of S. grandiflora seed extract
The powdered sample was boiled with 100 ml of sterile distilled water at 60 °C for 1 h. Then, the extract was filtered through Whatman No. 1 filter paper and used for further experiments (Fig. 2).
Fig. 2.
Aqueous extract of Sesbania grandiflora.
2.3. Antimicrobial activity
The antimicrobial activities of the leaves extract was determined by disc diffusion method. Nutrient agar (NA) plates were prepared, sterilized and solidified. The culture Staphylococcus aureus was swabbed uniformly onto the NA plates using sterile cotton swabs. Plates were incubated at 37 °C for 24 h [11], [14], [15].
2.4. Biofilm formation in Congo red agar
NA plates were prepared, inoculated and incubated at 37 °C for 24 h. The cultured plate was flooded with 0.2% congo red dye. After 15 min, the plates were destained with 1 M NaCl [16].
2.5. EPS production
Staphylococcus aureus culture of 20 µl (222 CFU) was added in freshly prepared 50 ml of sterile nutrient broth and were treated with Sesbania grandiflora plant extract powder of 3 different concentration (250, 500,750 mg) and one is maintained as control without the addition of plant extract then the samples were incubated at 37 °C for 24 h at 140 rpm. Control and the treated samples were centrifuged at 12,000 rpm for 15 min at 4 °C and to the supernatants were collected, to that three volumes of ice cold isopropanol was added and incubated overnight at −25 °C. The incubated samples were centrifuged at 5000 rpm for 30 min and pellets were collected (EPS) and air dried at room temperature. The collected EPS of control and treated were purified, and stored for further analysis [17].
2.6. EPS characterization
The control and treated EPS samples were characterized by UV – Visible Spectroscopy analysis, Fourier Transform Infrared Spectroscopy (FTIR), Light Scattering analysis [18], [19].
2.7. Emulsifying activity
The control and treated EPS solution (2 mg/ml) was heated at 100 °C for 15 min followed by cooling (25 °C) and the volume was made upto 2 ml using phosphate-buffered saline. 1 ml of olive oil was added, vortexed for 1 min and the absorbance was measured after 30 min at 540 nm. The emulsifying activity was calculated in terms of percentage decline in the absorbance at different time intervals [19].
2.8. Quantification and inhibition of EPS components
EPS was quantified by measuring proteins (Lowry's method) and carbohydrates (Anthrone method) [17].
The percentage of EPS inhibition was calculated using the formula
3. Results
3.1. Antimicrobial activity
The anti microbial potential of S. grandiflora was evaluated according to their zone of inhibition against S. aureus pathogens and the results were compared with the activity of the standards viz, Ampicillin. 40 µl of S. grandiflora were found to be more effective against S. aureus when compared with the standards
3.2. Biofilm formation in Congo red agar
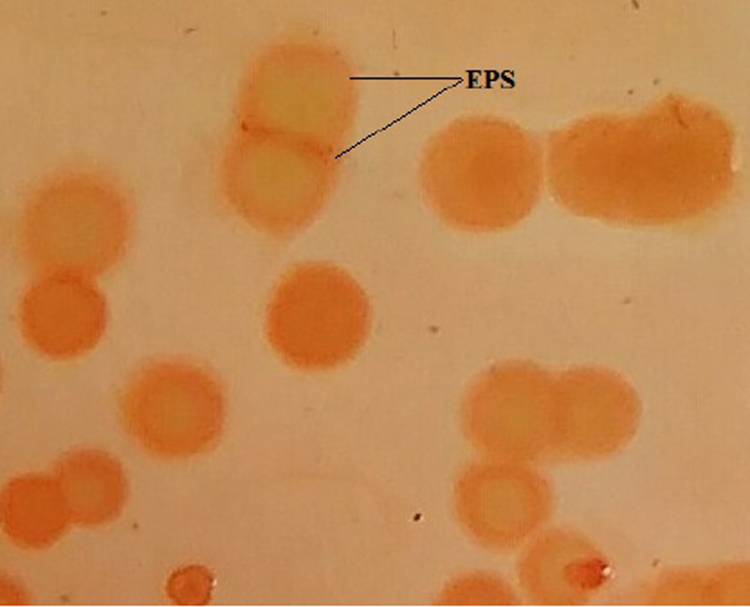
Biofilm formation of S. aureus on NA plates supplemented with congo red appeared as dark red around the bacteria colonies. The biofilm produced by bacteria is made up of exopolysaccharide matrix that protects microbes from host immune system and anti-microbial therapy. Based on our results, S. aureus displayed highest biofilm forming ability (Fig. 3).
Fig. 3.
Congo red assay.
3.3. EPS production
Maximum EPS was produced after 48 h in Nutrient broth by S. aureus.
3.4. EPS characterization
3.4.1. UV visible spectroscopy
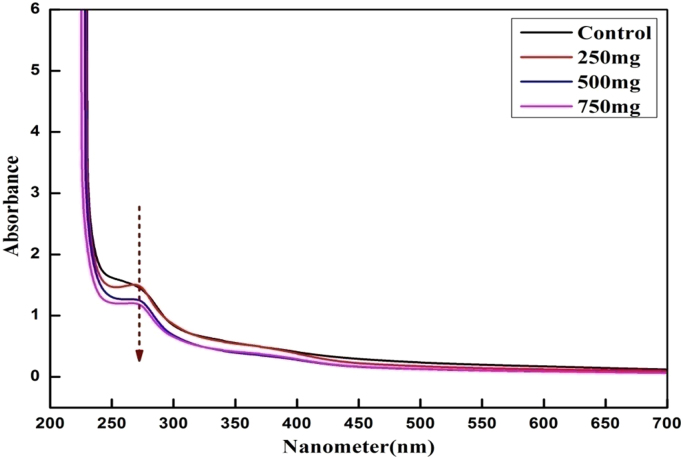
The UV-Vis analysis was used for the identification of optical property of S. grandiflora.
The high exciton binding energy was responded at 270 nm as clear absorption band (Fig. 4).
Fig. 4.
UV-Visible spectrum of EPS.
3.4.2. FTIR
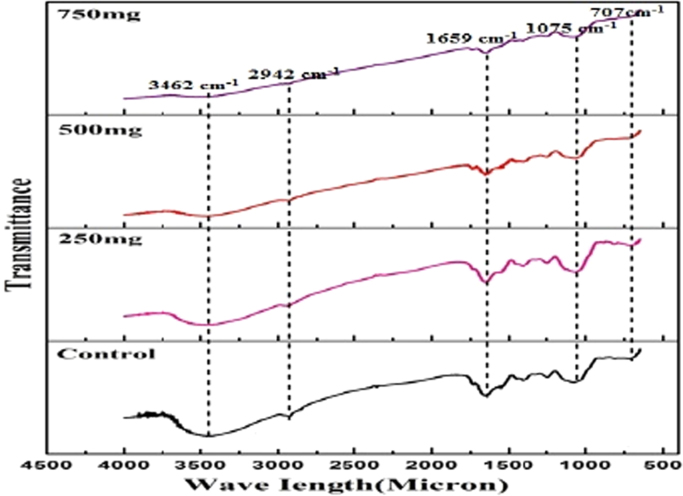
Fourier Transform Infrared spectroscopy was used to find out the biomolecules. FTIR spectrum shows peak at 707 cm−1, 1075 cm−1, 1659 cm−1, 2942 cm−1, 3462 cm−1 which confirms the presence of carboxylic group, O-acetyl ester linkage bond, C- H stretching of sugar molecules, C-H functional groups and hydroxyl group respectively. The gradual decrease or disappearance of peaks reveals the reduction of protein and carbohydrate content in the EPS of S. aureus when treated with S. grandiflora (Fig. 5).
Fig. 5.
FTIR spectrum of EPS.
3.4.3. Dynamic light scattering
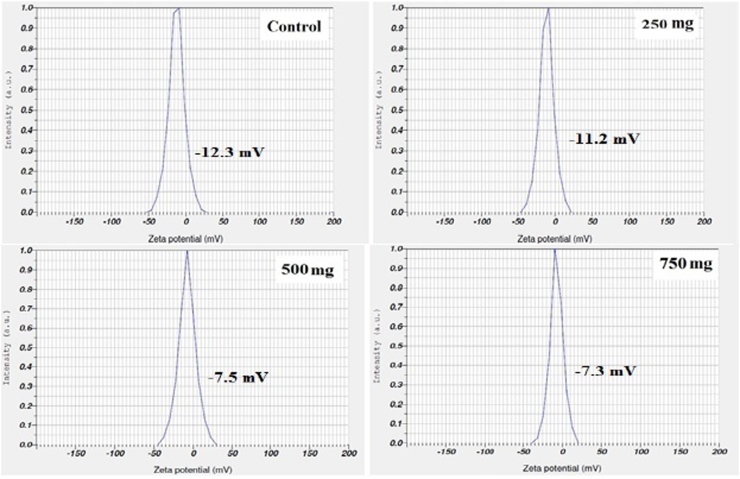
The stability of EPS was analyzed by Zeta potential which is high in the control when compare to treated EPS. The value number of the zeta potential showed the stability of the EPS. This analysis is mainly used to check the cell wall penetration of EPS and stability of the bacteria (Fig. 6; Table 1).
Fig. 6.
Dynamic light scattering of EPS.
Table 1.
Zeta potential peaks and its determination.
| EPS sample | Stability |
|---|---|
| C | −12.3 mV |
| T1 | −11.2 mV |
| T2 | −7.5 mV |
| T3 | −7.3 mV |
3.5. Emulsifying activity
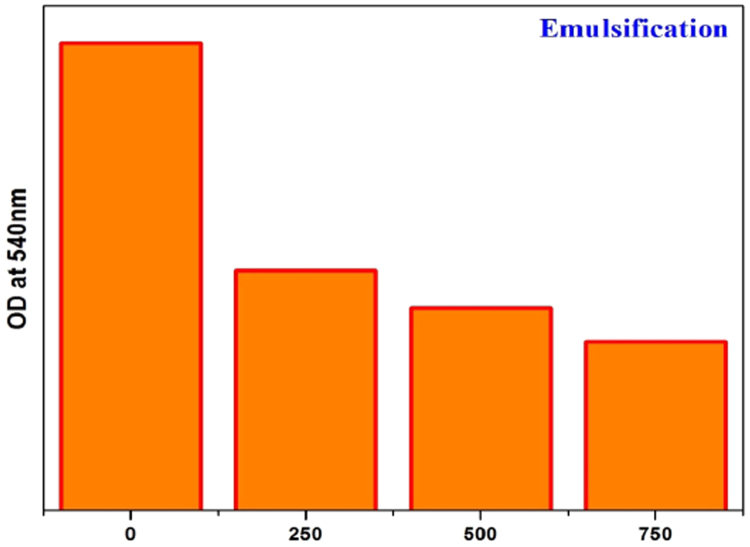
A stable emulsion of the EPS was observed with Olive oil. This yielded 48.51%, 53.62%, 72.11% (at different concentration 250, 500, 750 mg/ml) emulsifying activity with olive oil after 30 min of incubation. The emulsifying activity of EPS was measured before 30 min because emulsification will break within 30 min, due to the stability of the sample. The presence of both hydrophilic (hydroxyl) and hydrophobic (aliphatic CH2) functional groups may be responsible for its emulsifying property (Fig. 7).
Fig. 7.
Histogram of emulsification index.
3.6. Quantification and inhibition of EPS components
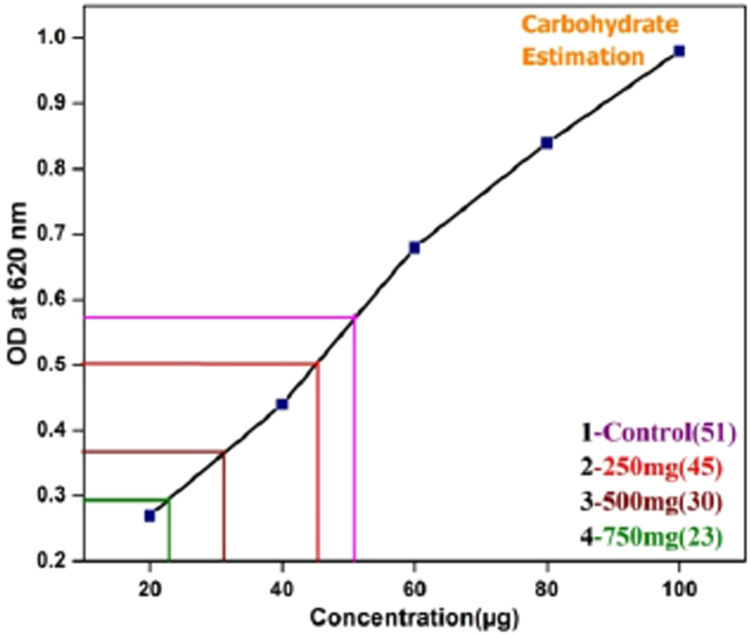
The optical density of the control and treated EPS revels that the amount and inhibition percentage of the protein in the given sample by measuring at 660 nm and inhibition percentage of carbohydrate in the given sample was measured at 620 nm (Fig. 8; Table 2).
Fig. 8.
Carbohydrate estimation in EPS.
Table 2.
Comparison of carbohydrate concentration with control.
| S.No | Concentration of S. grandiflora leaf extract (mg) | Carbohydrate concentration present in control and treated EPS (µg) | Percentage of Carbohydrate inhibition |
|---|---|---|---|
| C | – | 51 | – |
| T1 | 250 | 45 | 11.2% |
| T2 | 500 | 30 | 36.71% |
| T3 | 750 | 22 | 49.36% |
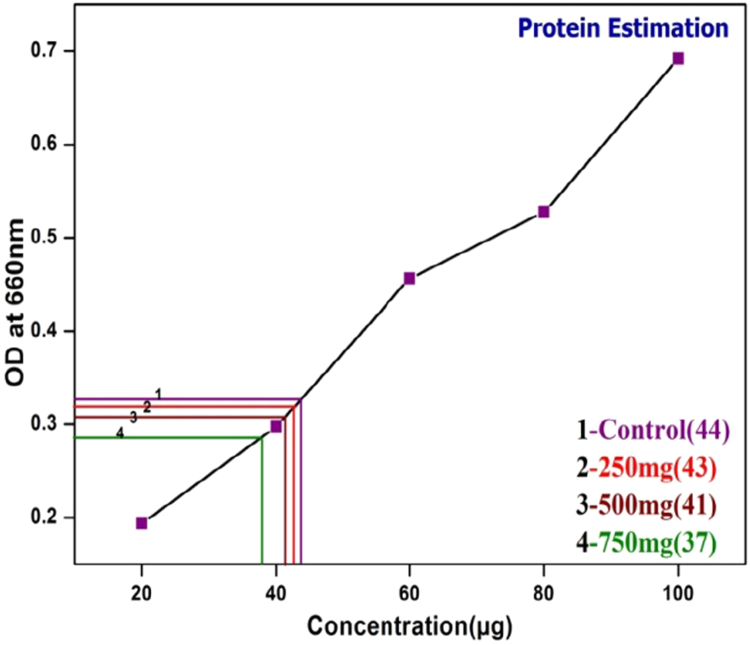
The gradual decrease in the amount of protein and carbohydrate in control and treated EPS were calculated in terms of percentage. The amount of protein in EPS decreases with the increase of plant extract. This variation in control and treated EPS is due to increase in plant extract concentration (Fig. 9; Table 3).
Fig. 9.
Estimation of Protein in EPS.
Table 3.
Comparison of protein concentration with control.
| S.No | Concentration ofS. grandifloraleaf extract (mg) | Protein concentration present in control and treated EPS(µg) | Percentage of protein inhibition |
|---|---|---|---|
| C | – | 44 | – |
| T1 | 250 | 41 | 2.1% |
| T2 | 500 | 37 | 6.5% |
| T3 | 750 | 31 | 14.44% |
4. Discussion
S. grandiflora was extensively studied by different scientists and academicians for its phytopharmacological potentials especially on leaves, flowers and seeds. Recent investigation demonstrated that use of leaf extract of S. grandiflora act as a reducing agent and synthesized spherical shaped silver nanoparticles [20]. Moreover, the S. grandiflora flower acts as a promising material to develop the active ingredient of anti-plaque toothpaste as well as mouthwash solution [21]. It has been reported that a biofilm is strongly associated with the drug resistance property [22]. Hence, eradication of biofilm is often considered to be a difficult task and therefore use of plant products to inhibit biofilm may be a viable alternative [23].
In this study, the biofilm inhibition potential of S. grandiflora against S. aureus was evaluated by using quantitative spectroscopic techniques. 40 µg of S. grandiflora leaf extract showed potential antibacterial activity against S. aureus. S. grandiflora extract have been known to contain alkaloids, flavonoids, saponins, tannins and steroids [24]. These active compounds have the potential to inhibit adhesions and prevent matrix formation. There were reports which state that biofilms acquire resistances to inhibitors under nutrient limited or depleted conditions in contrast to their susceptibility conditions. S. grandiflora leaf extract shown to have anti-biofilm efficacy under both nutrient repleted and nutrient depleted conditions, which indicate the presence of bioactive agent in the leaf extract [25].
Carbohydrate and proteins were considered as a main constituent of EPS in pure culture. In this study, the ratio of carbohydrate and protein of the pure culture was comparatively less after treatment with S. grandiflora leaf extract [26].
UV- Vis spectroscopy analysis of control and treated EPS revealed a major absorption peak at 270 nm, which revealed that the intensity of the peak is directly proportional to the concentration of the EPS. This was similar to surface Plasmon vibration with the previous work. FTIR results represented that the active biomolecules present in the leaves of S. grandiflora was responsible to inhibit the biofilm formation [27]. The broad stretching of 3462 cm−1 indicates the presence of hydroxyl group. The band at 2942 cm−1 was due to the presence of C-H functional groups, which revealed the presence of sugar. Another peak at 1659 cm−1 could attribute to the C-H stretching of mannose or galactose [28]. The absorption peak at 1075 cm−1 may be attributed to O-acetyl ester linkage bond. DLS showed that the decrease in volume number indicate the degradation of the protein molecule in EPS, and in-turn revealed the decreased stability than the Control [29].
5. Conclusion
In this study, a suitable and efficient method for the EPS extraction from S. grandiflora biofilms was achieved. This study also suggests that S. grandiflora extract possesses compounds with potential antimicrobial properties. To conclude, the aqueous extract of S. grandiflora leaf extract showed evidence of high anti-biofilm and anti-bacterial activity property against S. aureus. Further research is needed to elucidate the antimicrobial agents from S. grandiflora that are pharmacologically important using advance techniques in an eco-friendly approach
Acknowledgement
The authors gratefully acknowledge K. Sathish Kumar, N. Supraja, Department of Biotechnology, Thiruvalluvar University, Vellore and D. Sathya prabhu, Research scholar, VIT for supporting this research.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.10.004.
Contributor Information
V. Devi Rajeswari, Email: sdevirajeswari@gmail.com.
Ranganathan Babujanarthanam, Email: babukmg@gmail.com.
Appendix A. Transparency document
Supplementary material
References
- 1.Slobodníkova L., Fialova S., Rendekova K., Kovac J., Mucaji P. Antibiofilm activity of plant polyphenols. Molecules. 2016;21:1717. doi: 10.3390/molecules21121717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subramanian S.B., Yan S., Tyagi R.D., Surampalli R.Y. Extracellular polymeric substances (EPS) producing bacterial strains of municipal wastewater sludge: isolation, molecular identification, EPS characterization and performance for sludge settling and dewatering. Water Res. 2010;44:2253–2266. doi: 10.1016/j.watres.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 3.Shahwany A.W., Tawfeeq H.K., Hamed S.E. Antibacterial and anti-biofilm activity of three phenolic plant extracts and silver nanoparticles on Staphylococcus aureus and Klebsiella pneumoniae. J. Biomed. Biotechnol. 2016;4:12–18. [Google Scholar]
- 4.Novak J.T., Muller C.D., Murthy S.N. Floc structure and the role of cations. Water Sci. Technol. 2001;44:209–213. [PubMed] [Google Scholar]
- 5.Mandal S.K., Singh R.P., Patel V. Isolation and characterization of exopolysaccharide secreted by a toxic dinoflagellate, Amphidinium carterae Hulburt1957 and its probable role in harmful algal blooms (HABs) Microb. Ecol. 2011;62:518–527. doi: 10.1007/s00248-011-9852-5. [DOI] [PubMed] [Google Scholar]
- 6.Flemming H.C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 7.S. Chanda, M. Kaneria. Indian nutraceutical plant leaves as a potential source of natural antimicrobial agents. Science against microbial pathogens: communicating current research and technological advances, 2, 2011, pp. 1251–9.
- 8.Sedigheh B., Bazzaz F., Khajehkaramadin M. In vitro antibacterial activity of Rheum ribes extract obtained from various plant parts against clinical ısolates of gram-negative pathogens. Iran. J. Pharm. Res. 2005;2:87–91. [Google Scholar]
- 9.Bazzaz F., Khajehkaramadin M., Shokooheizadeh H.R. In vitro antibacterial activity of Rheum ribes extract obtained from various plant parts against clinical isolates of Gram-negative pathogens. Iran. J. Pharm. Res. 2010;20:87–91. [Google Scholar]
- 10.Gomase P.V. Sesbania sesban Linn: a review on its ethnobotany, phytochemical and pharmacological profile. Asian. J. Biomed. Pharm. Sci. 2012;2:11. [Google Scholar]
- 11.Chinaa R., Mukherjee S., Sen S., Bose S., Datta S., Koley H., Ghosh S., Dhar P. Antimicrobial activity of Sesbania grandiflora flower polyphenol extracts on some pathogenic bacteria and growth stimulatory effect on the probiotic organism Lactobacillus acidophilus. Microbiol. Res. 2012;167:500–506. doi: 10.1016/j.micres.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Rajagopal P.L., Premaletha K., Sreejith K.R. Anthelmintic activity of the flowers of Sesbania grandiflora Pers. J. Innov. Appl. Pharm. Sci. 2016;1:8–11. [Google Scholar]
- 13.Ponnanikajamideen M., Nagalingam M., Vanaja M., Malarkodi C., Rajeshkumar S. Anticancer activity of different solvent extracts of Sesbania grandiflora against neuroblastima (imr-32) and colon (ht-29) cell lines. Eur. J. Biomed. Pharm. Sci. 2015;2:509–517. [Google Scholar]
- 14.Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. J. Clin. Pathol. 1966;5:493. [PubMed] [Google Scholar]
- 15.dos Santos C.F., Alves, Bonez P.C., de Souza M.D., da Cruz R.C., Boligon A.A., Piana M., Brum T.F., Rossi G.G., da Silva Jesus R., Grando T.H., Monteiro S.G. Antimicrobial, antitrypanosomal and antibiofilm activity of Equisetum hyemale. Microb. Pathog. 2016;101:119–125. doi: 10.1016/j.micpath.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Kumar P., Selvi S.S., Govindaraju M. In vitro anti-biofilm and anti-bacterial activity of Junceella juncea for its biomedical application. Asian Pac. J. Trop. Biomed. 2012;2:930–935. doi: 10.1016/S2221-1691(13)60002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teanpaisan R., Kawsud P., Pahumunto N., Puripattanavong J. Screening for antibacterial and antibiofilm activity in Thai medicinal plant extracts against oral microorganisms. J. Tradit. Complement. Med. 2016 doi: 10.1016/j.jtcme.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart T.J., Traber J., Kroll A., Behra R., Sigg L. Characterization of extracellular polymeric substances (EPS) from periphyton using liquid chromatography-organic carbon detection–organic nitrogen detection (LC-OCD-OND) Environ. Sci. Pollut. Res. 2013;20:3214–3223. doi: 10.1007/s11356-012-1228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavita K., Singh V.K., Mishra A., Jha B. Characterisation and anti-biofilm activity of extracellular polymeric substances from Oceanobacillus iheyensis. Carbohydr. polym. 2014;101:29–35. doi: 10.1016/j.carbpol.2013.08.099. [DOI] [PubMed] [Google Scholar]
- 20.Das J., Das M.P., Velusamy P. Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim. Acta Mol. Mol. Biomol.Spectrosc. 2013;104:265–270. doi: 10.1016/j.saa.2012.11.075. [DOI] [PubMed] [Google Scholar]
- 21.Saifudin A., Forentin A.M., Fadhilah A., Tirtodiharjo K., Melani W.D., Widyasari D., Saroso T.A. Bioprospecting for anti-Streptococcus mutans: the activity of 10% Sesbania grandiflora flower extract comparable to erythromycin. Asian. Pac. J. Trop. Biomed. 2016;6:751–754. [Google Scholar]
- 22.Arun A., Karthikeyan P., Sagadevan P., Umamaheswari R., Rex Peo R. Phytochemical screening of Sesbania grandiflora (Linn) J. Biosci. Nanosci. 2014;1 [Google Scholar]
- 23.Das A., Das M.C., Sandhu P., Das N., Tribedi P., De U.C., Akhter Y., Bhattacharjee S. Antibiofilm activity of Parkia javanica against Pseudomonas aeruginosa: a study with fruit extract. RSC Adv. 2017;7:5497–5513. [Google Scholar]
- 24.Sutherland I.W. Microbial exopolysaccharides-structural subtleties and their consequences. Pure Appl Chem. 1997;69:1911–1918. [Google Scholar]
- 25.Maeda T., Garcia-Contreras R., Pu M., Sheng L., Garcia L.R., Tomás M., Wood T.K. Quorum quenching quandary: resistance to antivirulence compounds. ISME J. 2012;6:493–501. doi: 10.1038/ismej.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nwodo U.U., Okoh A.I. Characterization and flocculation properties of biopolymeric flocculant (Glycosaminoglycan) produced by Cellulomonas sp. Okoh. J. Appl. Microbiol. 2013;114:1325–1337. doi: 10.1111/jam.12095. [DOI] [PubMed] [Google Scholar]
- 27.Iyer A., Mody K., Jha B. Characterization of an exopolysaccharide produced by a marine Enterobacter cloacae. Indian J. Exp. Biol. 2005;43:467–471. [PubMed] [Google Scholar]
- 28.Freitas F., Alves V.D., Carvalheira M., Costa N., Oliveira R., Reis M.A. Emulsifying behaviour and rheological properties of the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohydr. Polym. 2009;78:549–556. [Google Scholar]
- 29.Sobeck D.C., Higgins M.J. Examination of three theories for mechanisms of cation-induced bioflocculation. Water Res. 2002;36:527–538. doi: 10.1016/s0043-1354(01)00254-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material