Abstract
Purpose: Meibomian gland disease is generally accepted as the leading cause for evaporative dry eye disease (DED). In a previous study, perfluorohexyloctane, a semifluorinated alkane, has been demonstrated to significantly increase tear film breakup time and to reduce corneal fluorescein staining in patients with evaporative DED, thereby vastly reducing dry eye-related symptoms. This study was set up to evaluate perfluorohexyloctane in a larger population of patients with Meibomian gland dysfunction.
Methods: Seventy-two patients with Meibomian gland disease and associated dry eye received 1 drop of perfluorohexyloctane 4 times daily during an observational, prospective, multicenter, 6–8-week study. Clinical assessment included best-corrected visual acuity, intraocular pressure, Schirmer test I, tear film breakup time, anterior and posterior blepharitis assessment, number of expressible Meibomian glands, meibum quality and quantity, ocular surface fluorescein staining, lid margin and symptom assessment, and Ocular Surface Disease Index (OSDI©).
Results: From the 72 patients recruited, 61 completed the trial per protocol. Nine patients did not apply the medication as recommended and 2 patients were lost to follow-up. Tear film breakup time, corneal and conjunctival fluorescein staining, number of expressible Meibomian glands, and severity of anterior and posterior blepharitis significantly improved after 6–8 weeks of perfluorohexyloctane application. In addition, symptoms improved as demonstrated by a significant decrease of OSDI-values from 37 (±13) to 26 (±16).
Conclusions: In concordance with previous findings, 6–8 weeks of topical application of perfluorohexyloctane significantly improves clinical signs of Meibomian gland disease and associated mild to moderate DED.
Keywords: : dry eye disease, Meibomian gland disease, blepharitis, semifluorinated alkanes
Introduction
Evaporative dry eye disease (DED) is a pathological condition that affects tens of millions of individuals worldwide.1 Typical symptoms include blurred vision, foreign body sensation, ocular pain, itching, burning sensation, and tired eyes with increasing intensity during activities such as reading, driving a vehicle, watching television, or using computer screens and tablets.
The main pathomechanism for evaporative DED is thought to be a dysfunction of the Meibomian glands (syn. Meibomian gland dysfunction/MGD),2–4 whereas numerous causes leading to this dysfunction are discussed. Several forms of MGD are differentiated, such as hyposecretory and hypersecretory forms, with subdifferentiation into primary and secondary diseases.3 All forms of MGD eventually lead to increased evaporation of the tear film, which is currently thought to be the leading cause of DED.5 Other associated systemic diseases include the following: Atopy, Acne rosacea, seborrheic dermatitis, and cicatricial pemphigoid, along with ageing processes and drug side-effects.4
Inflammation of the ocular surface, including the eyelids, has been shown to play a central role in the development and maintenance of DED. The role of inflammation in MGD, however, is still under discussion.3
Besides thermo-/mechanical treatment of MGD in case of obstructed gland orifices6–8 and anti-inflammatory treatment in patients with obvious blepharitis, including rosacea using antibiotics,9 the substitution of substances that may stabilize the tear film is a central treatment strategy. The latter approach includes topical application or oral supplementation of omega-3 fatty acids10–12 and other topically applied substances, which add or complement lipid components that are lacking or malsecreted by dysfunctional Meibomian glands13,14
Substances used for supplementing the lipid layer include different oils such as castor oil and mineral oil, or other stabilizing compounds such as polyethylene glycol, all of which have been shown to stabilize the tear film in a number of clinical trials.15–18
The recently introduced product NovaTears® (also marketed under the name EvoTears®) is a 1 component eye drop containing 100% perfluorohexyloctane. The product follows a new paradigm as it is a water-free lipophilic liquid that does not require preservatives or surfactants. In a prospective open-labeled observational clinical trial,19 NovaTears was shown to increase the tear film breakup time significantly, thereby significantly reducing patient symptoms, increasing the overall amount of tears, as measured by Schirmer test without anesthesia, and reducing ocular surface damage, as demonstrated by fluorescein staining of the cornea.
These clinical effects seem to be related to the physical properties of perfluorohexyloctane. The low surface and interface tension result in optimal spreading abilities and the amphiphilic nature of the molecule leads to film forming properties and the ability to dissolve lipids.20,21 Both of these properties likely result in a direct interaction of perfluorohexyloctane with tear film lipids, leading to rearrangement and consecutive stabilization of the tear film. In addition, due to its lipid dissolving properties,20,21 perfluorohexyloctane might dissolve thickened meibum that could obstruct the gland orifices, therefore resulting in nonmechanical reopening of the glands.
The objective of this observational study in 72 patients with Meibomian gland disease, blepharitis, and associated DED was to confirm perfluorohexyloctane's ability to stabilize the tear film and relieve symptoms. A further objective was to evaluate its effects on the clinical signs of blepharitis and on the number of expressible Meibomian glands. Moreover, local tolerability and safety of perfluorohexyloctane was assessed.
Methods
Study design
A prospective, multicenter, observational, 6–8-week study of patients with Meibomian gland disease and associated dry eye was designed.
The study was performed in routine clinical care at 8 eye care centers in Germany (Department of Ophthalmology, Ludwig-Maximilians-University Munich, Munich, Germany; Department of Ophthalmology, University of Erlangen-Nuremberg, Erlangen, Germany; Praxis Prof. Schrage, Cologne, Germany; Department of Ophthalmology, Heinrich-Heine University Duesseldorf, Duesseldorf, Germany; Ophthalmology Clinics, Heidelberg, Germany; Department of Ophthalmology, International Vision Correction Research Centre (IVCRC) and David J Apple International Laboratory for Ocular Pathology, University of Heidelberg, Heidelberg, Germany; Department of Ophthalmology, Staedtisches Klinikum Karlsruhe, Karlsruhe, Germany; and JENVIS Research, Jena, Germany).
The study was reviewed and approved by the ethics committees of the participating centers, adhered to the tenets of the Declaration of Helsinki, and was registered at www.clinicaltrials.gov (NCT02356341).
After informed consent was obtained, patients were advised how to use perfluorohexyloctane according to the Instructions for Use. Patients were advised to apply 1 drop 3–4 times daily in both eyes and report any adverse event (AE) immediately. Patients returned after 6–8 weeks for a follow-up visit. Data were collected between January 2015 and December 2015.
Patients and examination parameters
Seventy-two patients with mild to moderate MGD were selected from outpatient clinics from participating centers according to the inclusion criteria demonstrated in Table 1.
Table 1.
Inclusion Criteria
| 1 | Males or females ≥18 years of age |
| 2 | Patients with dry eye disease due to mild to moderate MGD as determined by: |
| • TFBUT ≤10 s | |
| • OSDI© ≥16 and ≤55 | |
| • Schirmer I Test ≥2 mm | |
| • Sum of peripheral corneal and conjunctival staining ≤grade 10 (Oxford grading scale, both eyes combined) | |
| • Altered secretion and expressibility of the Meibomian glands | |
| 3 | Ability and willingness to apply eyelid hygiene for at least 14 days before enrollment |
| 4 | Patients must be under stable therapy (both topically and systemically) for at least 4 weeks before enrollment |
| 5 | Ability and willingness to provide written informed consent before enrollment |
| 6 | Ability and willingness to participate in all examinations |
| 7 | Ability and willingness to understand and fill in the OSDI questionnaire |
| 8 | Willingness and ability to return for the follow-up assessment at week 7 (weeks 6–8) |
MGD, Meibomian gland dysfunction; OSDI, Ocular Surface Disease Index; TFBUT, tear film breakup time.
Patients with DED not caused by MGD, but due to any other underlying systemic disease, concomitant pharmacologic treatment, and malignancies, or of idiopathic nature, and patients taking lipid-containing eye drops or requiring topical treatment of the eye with antibiotics, steroids, or cyclosporin A were excluded from the trial.
Parameters assessed, both at the baseline and at the follow-up visit included best-corrected visual acuity (BCVA), intraocular pressure (IOP), Schirmer I test, tear film breakup time (TFBUT), corneal and conjunctival staining, and Meibomian gland examination. Corneal and conjunctival staining was graded according to the Oxford grading scheme22 and meibum secretion was examined by compressing the upper and lower eyelid using a Q-tip and differentiating meibum quality (clear, whitish, thick, or none). All expressible Meibomian glands were counted. A thorough slit-lamp examination was performed at both visits allowing for grading severity of anterior and posterior blepharitis and documentation of lid margin abnormalities. In addition, each patient filled in 2 questionnaires [an Ocular Surface Disease Index (OSDI©)-like questionnaire23 and a usability and satisfactory questionnaire] and physicians monitored disease symptoms and AEs. All patients reporting problems were called and interviewed by the physicians who then offered an immediate visit at the study site.
Statistical analysis and study analysis populations
Two populations were defined for the analysis:
- Safety Analysis Population: The safety analysis set includes all patients enrolled in the study. This dataset includes 72 patients.
- Per-Protocol Population: The per-protocol analysis set includes all patients in the safety analysis set. A patient was excluded from the per-protocol analysis set if
• The patient did not reliably take the study medication throughout study period as documented by the investigator (n = 9).
• The patient was lost to follow-up (n = 2).
This dataset includes 61 patients and 122 eyes.
Statistical analysis of visual acuity, IOP, Schirmer I test, TFBUT, OSDI-like questionnaire, corneal and conjunctival fluorescein staining, and number of expressible Meibomian glands was performed retrospectively to identify statistically significant differences between baseline and follow-up.
For all parameters, except for corneal and conjunctival staining, a paired 2-sided t-test testing the null hypothesis that the mean change from baseline equals zero was used. The change in corneal and conjunctival staining was analyzed using the Wilcoxon signed-rank test for location shifts between baseline and follow-up. Statistical analysis was performed using SAS® (version 9.3 for Microsoft Windows). A P value of <0.05 was used to declare significance.
A retrospective power calculation on the OSDI score to detect significant differences of at least 7.3 points24 revealed that with a standard deviation of 16.7, this study would provide an 80% power if at least 44 patients were enrolled and would provide 90% power if 57 patients were enrolled (data on file).
Results
Demographic data
Seventy-two patients (53 female and 19 male, age 59.65 ± 16.55 years) were included in the study.
Clinical findings
Visual acuity, IOP
As can be seen in Table 2, BCVA and IOP did not change over the study period.
Table 2.
Best-Corrected Visual Acuity and Intraocular Pressure at Baseline and Follow-Up in the Left Eye (OS) and the Right Eye (OD; Data Set: Safety Analysis Population)
| Parameter | Baseline | Follow-up | Data | P |
|---|---|---|---|---|
| BCVA | OD: 1.00 (0.05–1.60; n = 72) | OD: 1.00 (0.02–1.60; n = 70) | Median (min. to max.) | OD: P = 0.85 (n = 70) |
| OS: 1.00 (0.16–1.60; n = 72) | OS: 1.00 (0.05–1.60; n = 70) | OS: P = 0.19 (n = 70) | ||
| IOP (mmHg) | OD: 14.01 ± 2.55 (n = 72) | OD: 13.78 ± 2.21 (n = 68) | Mean ± SD | OD: P = 0.49 (n = 68) |
| OS: 14.14 ± 2.63 (n = 72) | OS: 13.75 ± 2.33 (n = 68) | OS: P = 0.21 (n = 68) |
BCVA, best-corrected visual acuity; SD, standard deviation.
Schirmer test without anesthesia, TFBUT, OSDI-like questionnaire, and corneal and conjunctival staining
In the study, the Schirmer test did not change over the study period. However, TFBUT, OSDI-like questionnaire, and corneal and conjunctival fluorescein staining improved significantly (Table 3).
Table 3.
Tear Film Parameters, Ocular Surface Disease Index Score, and Corneal and Conjunctival Staining Score at Baseline and Follow-Up (Data Set: Per-Protocol Population)
| Parameter | Baseline | Follow-up | Data | P |
|---|---|---|---|---|
| Schirmer without anesthesia (mm/5 min) | OD: 11.76 ± 7.57 | OD: 13.54 ± 9.28 | n = 59 | OD: P = 0.18 |
| OS: 12.24 ± 7.71 | OS: 13.85 ± 9.80 | Mean ± SD | OS: P = 0.21 | |
| TFBUT (s) | OD: 5.84 ± 2.54 | OD: 8.72 ± 4.58 | n = 60 | OD: P < 0.0001 |
| OS: 5.30 ± 2.39 | OS: 8.33 ± 5.02 | Mean ± SD | OS: P < 0.0001 | |
| OSDI | 37.30 ± 13.37 | 26.37 ± 16.53 | n = 57 | P < 0.0001 |
| Mean ± SD | ||||
| Corneal+conjunctival staining sum score | OD: 2.77 ± 1.68 | OD: 1.84 ± 1.58 | n = 61 | OD: P < 0.0001 |
| OS: 2.77 ± 1.58 | OS: 2.07 ± 1.56 | Mean ± SD | OS: P < 0.0001 |
Blepharitis assessment
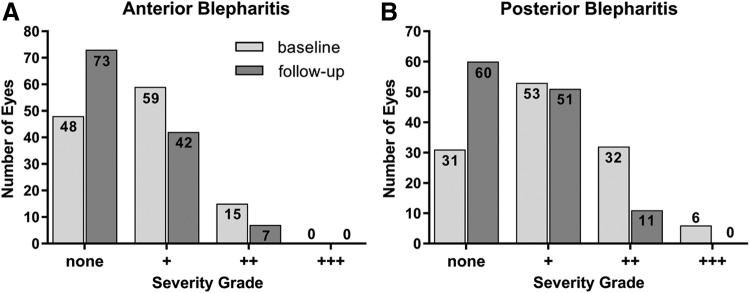
Anterior and posterior blepharitis were assessed with severity grading: +, ++, or +++, respectively. Results are demonstrated in Fig. 1. The Wilcoxon rank sum test (n = 61 each) showed significant changes for anterior blepharitis (OD: P = 0.0040; OS: P = 0.020) and posterior blepharitis (OD and OS: P < 0.0001).
FIG. 1.
Blepharitis assessment. The graphics demonstrate changes of blepharitis severity from baseline to follow-up. Improvement is visible by shift from higher to lower severity grades of anterior and posterior blepharitis (data set: PPP, n = 122 eyes). PPP, per-protocol population.
Meibum analysis
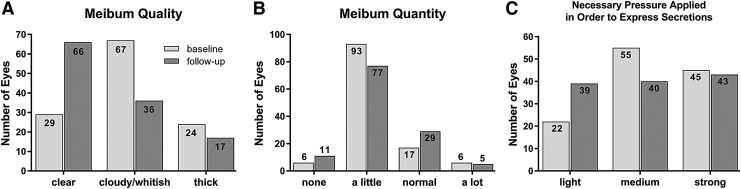
Meibum was analyzed by differentiating quality, quantity, and necessary pressure applied to express secretions. Results are demonstrated in a Fig. 2 and demonstrate improvement of meibum quality and reduction in necessary pressure for meibum expression. Meibum quantity improved slightly toward more eyes with normal meibum amount.
FIG. 2.
Meibum assessment. The graphics demonstrate improvement of meibum quality (A), only little change in meibum quantity (B), and reduction in necessary pressure for meibum expression (C). Data set: PPP, n = 122 eyes).
The number of expressible Meibomian glands increased significantly over the study period (Table 4).
Table 4.
Number of Expressible Meibomian Glands at Baseline and Follow-Up (Data Set: Per-Protocol Population)
| Expressible Meibomian glands (n) | OD: 8.34 ± 4.73 | OD: 9.20 ± 4.59 | OD: n = 56, OS: n = 55 | OD: P = 0.026 |
| OS: 8.24 ± 4.66 | OS: 9.42 ± 4.61 | Mean ± SD | OS: P = 0.012 |
Lid margin assessment
Lid margin abnormalities related to blepharitis and MGD were recorded during slit-lamp examination and displayed in a shift table (Table 5). No statistical analysis was applied. Approximately one third of the eyes showed clinical improvements.
Table 5.
Lid Margin Assessment
| Feature | Yes → no | No → yes | Yes → yes | No → no |
|---|---|---|---|---|
| Teleangiectasia | 29 | 4 | 45 | 44 |
| Plugging | 40 | 9 | 50 | 23 |
| Lid swelling | 20 | 10 | 10 | 82 |
The shift-table demonstrates improvements of lid margin abnormalities in approximately a third of the eyes examined (bold values; data set: PPP, n = 122 eyes).
PPP, per-protocol population.
Symptom assessment
Patients were asked whether they currently suffer from typical dry eye symptoms both at the baseline and follow-up visit. As can be seen in the Table 6, a larger number of patients reported symptom improvements than worsening of DED-associated symptoms after 5–7 weeks of treatment.
Table 6.
Symptom Assessment
| Symptom | Yes → no | No → yes | No change |
|---|---|---|---|
| Burning | 25 | 3 | 33 |
| Foreign body sensation | 13 | 4 | 44 |
| Red eyes | 19 | 5 | 37 |
| Tired eyes | 23 | 4 | 34 |
| Itching | 15 | 2 | 44 |
| Blurred vision | 14 | 4 | 43 |
| Clotted eyes | 10 | 5 | 46 |
| Headache | 6 | 1 | 54 |
| Other | 7 | 4 | 50 |
| Stringy mucous | 5 | 4 | 52 |
The shift-table displays the change of symptoms from baseline to follow-up, improved patients are depicted in bold (data set: PPP, n = 61 patients).
Usability, tolerability, and overall satisfaction
Patients' opinion about the usability of perfluorohexyloctane and treatment satisfaction was obtained by a questionnaire at the end of the study (follow-up visit). The majority of patients' responses were positive regarding ease of administration, sensation of the drops, vision to be affected by the drops, and relief of dry eye symptoms. Approximately half of the patients were likely or very likely to continue using the drops. One third of the patients were dissatisfied or very dissatisfied, and were unlikely or very unlikely to use NovaTears in the future (Table 7).
Table 7.
Patient Usability and Satisfaction: Responses of the Safety Analysis Population (n = 70, Excluding the 2 Patients Lost to Follow-Up)
| Q1 (Did the patient constantly apply NovaTears® during the last 6–8 weeks?) | Q6 (Was the patient's vision affected immediately after administration of NovaTears eye drops?) | ||
| Daily, recommended dose | 49 | Not at all | 29 |
| Daily, less than recommended dose | 16 | A little | 26 |
| Almost daily | 1 | Somewhat | 10 |
| Sometimes | 4 | Strongly | 5 |
| Not at all | 0 | Very strongly | 0 |
| Q2 (How easy was the administration of NovaTears eye drops into the eyes for the patient?) | Q7 (How fast did NovaTears eye drops relieve dry eye symptoms of the patient?) | ||
| Very easy | 36 | Very fast | 20 |
| Easy | 12 | Fast | 18 |
| Neutral | 5 | Slow | 12 |
| Sometimes difficult | 10 | Very slow | 5 |
| Very difficult | 7 | Not at all | 15 |
| Q3 (Did the patient touch the eye with the device during the application of NovaTears eye drops?) | Q8 (How satisfied was the patient with NovaTears treatment?) | ||
| Never | 55 | Very satisfied | 18 |
| Sometimes | 12 | Satisfied | 17 |
| Often | 2 | Neutral | 15 |
| Always | 1 | Dissatisfied | 15 |
| Very dissatisfied | 5 | ||
| Q4 [How does the patient describe the sensation of NovaTears eye drops after application (1)?] | Q9 (How likely is it that the patient will continue using NovaTears?) | ||
| Cold | 1 | Very likely | 23 |
| Warm | 9 | Likely | 11 |
| Neutral | 22 | Neutral | 8 |
| Velvety | 13 | Unlikely | 14 |
| Aqueous | 1 | Very unlikely | 14 |
| Oily | 47 | ||
| Q5 (How does the patient describe the sensation of NovaTears eye drops after application (2)?) | |||
| Very pleasant | 12 | ||
| Pleasant | 27 | ||
| Neutral | 20 | ||
| Unpleasant | 9 | ||
| Very unpleasant | 2 | ||
Adverse events
A total of six AEs were reported in 5 patients during the course of this study; none of them was classified as serious adverse device effects ( = incident according to applicable legislation) (Table 8). Foreign body sensation, application site reaction, and hypersensitivity were definitely related, and conjunctivitis (Patient 1) possibly related to the use of perfluorohexyloctane. Treatment was terminated in all cases. Sprain of ligament (Patient 4) and severe pneumonia (Patient 5) were judged as not related to perfluorohexyloctane.
Table 8.
Adverse Events
| Patient | Event | Severity | Relationship | Action taken |
|---|---|---|---|---|
| 1 | Conjunctivitis | Mild | Possible | Drug withdrawn |
| 2 | Sensation of foreign body | Moderate | Definitely | Drug withdrawn |
| 3 | Application site reaction | Mild | Definitely | Drug withdrawn |
| 4 | Hypersensitivity | Moderate | Definitely | Drug withdrawn |
| Ligament sprain | Moderate | Not related | Unknown | |
| 5 | Pneumonia | Severe | Not related | Dose not changed |
Discussion
Recently, a new class of preservative and water-free artificial tears demonstrating superior spreading properties was introduced. This new class is formed by semifluorinated alkanes (SFAs) that supplement the complex concept of treating DED as demonstrated in the first observational trial on patients with evaporative DED. Perfluorohexyloctane, the SFA contained in NovaTears, has particularly shown to improve tear film stability and tear film breakup time, decrease corneal staining, and vastly reduce dry eye-related symptoms.19 As hyperevaporation due to MGD is a leading pathomechanism in DED,25 further and more precise clinical data on SFA in this context are warranted. Due to their spreading properties, SFA are likely to be distributed into the Meibomian gland orifices. Consequently, the lipophilic SFAs might not only interact with the Meibomian lipids on the tear film but also with lipids not yet secreted and that are still within the Meibomian gland orifices.
In addition, lipids that are structurally altered leading to blockage of the orifices (clinically apparent as plugging) could potentially be dissolved with SFAs.20,21 The repeated presence of SFA eye drops at the blocked orifices could therefore incrementally dissolve altered lipids by passing over solid lipids into the liquid perfluorohexyloctane phase and eventually leading to reopening of the orifices. This second prospective observational trial therefore focused on patients with Meibomian gland disease (MGD). Based on the baseline characteristics the trial population can be classified as moderately affected according to the 2007 International Dry Eye WorkShop (DEWS) criteria.26 After 6–8 weeks of treatment for tear film stability, the number of expressible Meibomian glands increased significantly, while corneal and conjunctival fluorescein staining and OSDI score decreased significantly. On average, patients performed lid margin hygiene 64 weeks (2–393 weeks, median 20 weeks) before inclusion into the trial (data on file) and was therefore unlikely to be an influencing factor. Patients were on stable concomitant therapies, including various artificial tears, which remained stable throughout the study for the majority of patients and are thus considered not to influence the study outcome.
In line with the previous study, the Schirmer without anesthesia test showed a small increase; however, in contrast to the first study, it did not reach statistical significance. The decrease of the OSDI-like questionnaire by a mean of 11 points clearly exceeds the minimal clinical important difference of 4.5–7.3 points for mild or moderate disease.24 Furthermore, the observed improvement in OSDI-like questionnaire corresponded well to the subjective symptom assessment.
Regarding symptoms, improvements were mainly reported on burning (40% of the patients), tired eyes (38%), red eyes (30%), itching (26%), blurred vision (23%), and foreign body sensation (20%). In contrast, few patients reported worsening of their symptoms, which included burning (5%), tired eyes (7%), and red eyes (8%). This in-depth symptom analysis is in concordance to the overall improvement of the OSDI score of 11.
A central question to be answered by the trial was the impact of perfluorohexyloctane on the severity of anterior and posterior blepharitis. Following 6–8 weeks of application of perfluorohexyloctane, a significant improvement of blepharitis was determined, which was accompanied by a significant increase from 8 to 9 at average out of 15 expressible Meibomian glands. Whether this increase of 1 expressible gland on average is clinically relevant has to be questioned; however, meibum analysis also showed improvement of quality and reduction of necessary pressure to express meibum in some patients. In addition, plugging decreased in several patients, a finding that supports the beneficial effects of perfluorohexyloctane application to improve Meibomian gland function. Overall tear film stability increased, potentially due to the interaction between NovaTears and Meibomian gland lipids at different levels.
Regarding safety parameters, BCVA and IOP did not change. Also, the reported 4 AEs out of 70 patients that were related to the study medication were considered mild to moderate. In the previous trial, 3 out of 30 (3/30) patients reported AEs, which were of the same clinical spectrum with mild to moderate ocular irritation that resolved immediately after discontinuation of treatment.
Regarding symptoms, improvements were mainly reported on burning (40% of the patients), tired eyes (38%), red eyes (30%), itching (26%), blurred vision (23%), and foreign body sensation (20%). In contrast, few patients reported worsening of their symptoms, which included burning (5%), tired eyes (7%), and red eyes (8%). This in-depth symptom analysis toward the tolerability of perfluorohexyloctane is in concordance to the overall improvement of the OSDI score of 11.
In total, 35 of the 70 patients, who could be included in the usability and tolerability analysis, were satisfied or very satisfied with perfluorohexyloctane, 39/70 patients described a pleasant or very pleasant sensation after the application, and 34/70 patients will continue the treatment in the future. While about one third of the patients were dissatisfied or very dissatisfied, and were unlikely or very unlikely to use NovaTears, a subgroup analysis of those patients demonstrated that still 5/20 patients described a pleasant sensation and/or fast relief of symptoms after application of NovaTears in contrast to their quotation that they would not use the product in the future. 12/20 patients demonstrated a reduction of the staining score and 6/20 patients demonstrated an increase of TFBUT in both eyes. Considering that DED is a heterogenous disease with large variability, a rate of about 1/3 patients may not want to continue therapy is rated as rather low. Subjective sensation after the first application was velvety, neutral, and/or oily in most patients. 55/70 patients reported no problems with the use of the medication bottle, whereas 12/70 patients reported touching the eye with the bottle sometimes. Forty-eight out of 70 patients found the application to be easy or very easy.
The study has several limitations. Due to the open-label and observational character of the study, the efficacy parameters were compared with baseline measurements only and not to a control group, and data were only collected at 2 time points (baseline and follow-up visit) after a 6–8-week application. This implies that potential placebo effects in terms of symptom assessments cannot be differentiated from the treatment effect, although this is in general very difficult for this 1-component product given that there is no vehicle. Moreover, fluctuations due to seasonality could not be controlled. Follow-up prospective controlled studies will need to overcome these limitations.
Overall, perfluorohexyloctane (NovaTears) seems to be safe and effective in treating Meibomian gland disease in mild to moderate hyperevaporative DED as demonstrated by this prospective observational study. In particular, improvement of blepharitis, Meibomian gland function, TFBUT, corneal staining, and subjective symptoms show beneficial effects and implicate a broad application opportunity.
Acknowledgment
Support by Novaliq GmbH, Heidelberg, Germany.
Author Disclosure Statement
P.S.: received grants by Novaliq GmbH. T.K.: Consultant to Novaliq. N.S.: Consultant and research contracts for Novaliq GmbH. S.K.: Employee of Novaliq GmbH. M.B.: Clinical Development Consultant to Novaliq. All other authors have no competing financial interests.
References
- 1.DEWS R. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 5:75–92, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Green-Church K.B., Butovich I., Willcox M., Borchman D., Paulsen F., Barabino S., and Glasgow B.J. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest. Ophthalmol. Vis. Sci. 52:1979–1993, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knop E., Knop N., Millar T., Obata H., and Sullivan D.A. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest. Ophthalmol. Vis. Sci. 52:1938–1978, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaumberg D.A., Nichols J.J., Papas E.B., Tong L., Uchino M., and Nichols K.K. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest. Ophthalmol. Vis. Sci. 52:1994–2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heiligenhaus A., Koch J.M., Kruse F.E., Schwarz C., and Waubke T.N. Diagnosis and and differentiation of dry eye disorders [in German]. Ophthalmologe. 92:6–11, 1995 [PubMed] [Google Scholar]
- 6.Carvounis P.E., Cartwright N.E.K., Bron A., and Tiffany J. Recovery of meibomian oil after lid margin cleansing, studied by meibometry in normal individuals. Invest. Ophthalmol. Vis. Sci. 45:U122, 2004 [Google Scholar]
- 7.Korb D.R., and Blackie C.A. Debridement-scaling: a new procedure that increases Meibomian gland function and reduces dry eye symptoms. Cornea. 32:1554–1557, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Lane S.S., DuBiner H.B., Epstein R.J., Ernest P.H., Greiner J.V., Hardten D.R., Holland E.J., Lemp M.A., McDonald J.E., 2nd, Silbert D.I., Blackie C.A., Stevens C.A., and Bedi R. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 31:396–404, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Ta C.N., Shine W.E., McCulley J.P., Pandya A., Trattler W., and Norbury J.W. Effects of minocycline on the ocular flora of patients with acne rosacea or seborrheic blepharitis. Cornea. 22:545–548, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Brignole-Baudouin F., Baudouin C., Aragona P., Rolando M., Labetoulle M., Pisella P.J., Barabino S., Siou-Mermet R., and Creuzot-Garcher C. A multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta. Ophthalmol. 89:e591–e597, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Li Z., Choi J.H., Oh H.J., Park S.H., Lee J.B., and Yoon K.C. Effects of eye drops containing a mixture of omega-3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress-induced murine dry eye. Curr. Eye. Res. 39:871–878, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Liu A., and Ji J. Omega-3 essential fatty acids therapy for dry eye syndrome: a meta-analysis of randomized controlled studies. Med. Sci. Monit. 20:1583–1589, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bron A.J., and Tiffany J.M. The meibomian glands and tear film lipids. Structure, function, and control. Adv. Exp. Med. Biol. 438:281–295, 1998 [DOI] [PubMed] [Google Scholar]
- 14.McCulley J.P., and Shine W.E. Meibomian gland and tear film lipids: structure, function and control. Adv. Exp. Med. Biol. 506:373–378, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kaercher T., Thelen U., Brief G., Morgan-Warren R.J., and Leaback R. A prospective, multicenter, noninterventional study of Optive Plus(R) in the treatment of patients with dry eye: the prolipid study. Clin. Ophthalmol. 8:1147–1155, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Versura P., Profazio V., Giannaccare G., Fresina M., and Campos E.C. Discomfort symptoms reduction and ocular surface parameters recovery with Artelac Rebalance treatment in mild-moderate dry eye. Eur. J. Ophthalmol. 23:488–495, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Sindt C.W., and Foulks G.N. Efficacy of an artificial tear emulsion in patients with dry eye associated with meibomian gland dysfunction. Clin. Ophthalmol. 7:1713–1722, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dausch D., Lee S., Dausch S., Kim J.C., Schwert G., and Michelson W. Comparative study of treatment of the dry eye syndrome due to disturbances of the tear film lipid layer with lipid-containing tear substitutes [in German]. Klin. Monbl. Augenheilkd. 223:974–983, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Steven P., Scherer D., Krosser S., Beckert M., Cursiefen C., and Kaercher T. Semifluorinated alkane eye drops for treatment of dry eye disease—a prospective, multicenter noninterventional study. J. Ocul. Pharmacol. Ther. 31:498–503, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broniatowski M., and Dynarowicz-Latka P. Semifluorinated alkanes—primitive surfactants of fascinating properties. Adv. Colloid. Interface. Sci. 138:63–83, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Obraztsov V.V., Neslund G.G., Kornbrust E.S., Flaim S.F., and Woods C.M. In vitro cellular effects of perfluorochemicals correlate with their lipid solubility. Am. J. Physiol. Lung. Cell. Mol. Physiol. 278:L1018–L1024, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Bron A.J., Evans V.E., and Smith J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 22:640–650, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Schiffman R.M., Christianson M.D., Jacobsen G., Hirsch J.D., and Reis B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 118:615–621, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Miller K.L., Walt J.G., Mink D.R., Satram-Hoang S., Wilson S.E., Perry H.D., Asbell P.A., and Pflugfelder S.C. Minimal clinically important difference for the Ocular Surface Disease Index. Arch. Ophthalmol. 128:94–101, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Nichols K.K. The international workshop on meibomian gland dysfunction: introduction. Invest. Ophthalmol. Vis. Sci. 52:1917–1921, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Design and conduct of clinical trials: report of the Clinical Trials Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 5:153–162, 2007 [DOI] [PubMed] [Google Scholar]