Abstract
Collagen is the most abundant protein in the extracellular matrix in humans and is critical to the integrity and function of many musculoskeletal tissues. A molecular ensemble comprising more than 20 molecules is involved in collagen biosynthesis in the rough endoplasmic reticulum. Two proteins, heat shock protein 47 (Hsp47/SERPINH1) and 65-kDa FK506-binding protein (FKBP65/FKBP10), have been shown to play important roles in this ensemble. In humans, autosomal recessive mutations in both genes cause similar osteogenesis imperfecta phenotypes. Whereas it has been proposed that Hsp47 and FKBP65 interact in the rough endoplasmic reticulum, there is neither clear evidence for this interaction nor any data regarding their binding affinities for each other. In this study using purified endogenous proteins, we examined the interaction between Hsp47, FKBP65, and collagen and also determined their binding affinities and functions in vitro. Hsp47 and FKBP65 show a direct but weak interaction, and FKBP65 prefers to interact with Hsp47 rather than type I collagen. Our results suggest that a weak interaction between Hsp47 and FKBP65 confers mutual molecular stability and also allows for a synergistic effect during collagen folding. We also propose that Hsp47 likely acts as a hub molecule during collagen folding and secretion by directing other molecules to reach their target sites on collagens. Our findings may explain why osteogenesis imperfecta-causing mutations in both genes result in similar phenotypes.
Keywords: collagen, endoplasmic reticulum (ER), molecular chaperone, protein folding, protein-protein interaction
Introduction
The rough endoplasmic reticulum (rER)2 is a core subcellular compartment for the synthesis and secretion of proteins into the extracellular space (1, 2). Some secreted proteins form the extracellular matrix as a scaffold upon which cells may grow and build the connective tissues required for development and homeostasis in our body. Collagen is the most abundant extracellular matrix protein in humans and plays critical roles in tissues of our musculoskeletal system such as bone, cartilage, tendon, and also skin (3–5). Its biosynthesis is one of the most complex processes in the rER and >20 different molecules are involved in this process. These molecules include molecular chaperones, enzymes, and posttranslational modifiers (6, 7) and have collectively been termed a molecular ensemble (8). Two molecules, Hsp47 (coded by SERPINH1) and FKBP65 (FKBP10), have been shown to play important roles in this ensemble, and human mutations in both genes result in autosomal recessive osteogenesis imperfecta (OI), a skeletal disorder characterized by brittle bones and fractures (9–11).
Hsp47 was discovered in the late 1980s as a 47-kDa collagen-binding and heat shock protein (12). In a Hsp47 knock-out mouse model, the lack of Hsp47 caused a malfunction of type IV collagen in the basement membrane zone resulting in embryonic lethality at E11.5 (13–15). Various cell biology, molecular biology, biochemical, and biophysical approaches have since shown that Hsp47 is a collagen-specific molecular chaperone (16, 17). FKBP65 is an rER resident peptidyl-prolyl cis/trans isomerase (PPIase) and was shown to have a direct involvement in collagen folding and quality control in vitro (18, 19). Recently, enzyme assays using short peptides showed that the PPIase activity of FKBP65 was increased toward hydroxyproline-containing substrates compared with proline-containing substrates (18). FKBP65 null mice also displayed connective tissue defects at various embryonic stages and died at birth (20). Moreover, human mutations in FKBP65 not only caused OI but also other connective tissue disorders, Bruck syndrome and Kuskokwim syndrome (21, 22). In addition, tropoelastin was proposed as a substrate for FKBP65 (23, 24), whereas the function is still not clear in vivo.
Protein-protein interactions in the rER have been broadly mapped, and this is termed an interactome (25, 26). Within this interactome there is a range of affinities between the components that determine the stability and function. Two examples of tight complexes in the molecular ensemble for collagen biosynthesis are the tetramer formed by two protein disulfide isomerase (PDI) and two prolyl 4-hydroxylase (P4H) molecules and the 1:1:1 trimeric complex of prolyl 3-hydroxylase 1 (P3H1), cartilage associated protein (CRTAP), and cyclophilin B (CypB) (8, 27, 28). These two complexes require tight interactions for P4H and CRTAP, because these molecules tend to be insoluble and lack an ER retention signal (29–32). Without this interaction these molecules may be susceptible to precipitation or aberrant secretion. On the other hand, transient or weak interactions are sufficient for the interplay between enzymes and chaperones to function in protein folding and secretion (33, 34). Recently, multiple proteins that are involved in collagen biosynthesis in the rER were reported to interact with Hsp47 (35–37). Although FKBP65 is one of these proteins, it is unknown whether this binding is direct, how strong its binding affinity is, and what kind of functions this binding imparts on collagen biosynthesis. Hence, to characterize the interaction between Hsp47 and FKBP65, biochemical and biophysical experiments were performed using purified endogenous proteins. We analyzed the interaction between the two proteins and collagen and also determined affinities and specific functions in vitro.
Results and discussion
Characterization of direct binding between Hsp47 and FKBP65
Several published studies have suggested that Hsp47 and FKBP65 interact in the rER; however, none of these studies provide clear and direct evidence for this binding (35, 36, 38). To clarify this matter, we first performed immunoprecipitation experiments using rER fractions extracted from chick embryos prepared following a previously established protocol to isolate rER vesicles (39) with slight modifications. These rER fractions contain enriched amounts of endogenous Hsp47 and FKBP65 as described previously (19, 40, 41). Fig. 1 showed that a monoclonal antibody against Hsp47 co-precipitated FKBP65 from embryonic chick rER extract (Fig. 1, lane 4). Purified chick FKBP65 was used as a control for Western blotting analysis (Fig. 1, lane 2). Although the result from Fig. 1 confirmed that Hsp47 could associate with FKBP65 in the rER, it was still not clear if this association was due to direct binding or was an indirect association as both proteins recognized collagen as a substrate in the rER. Next, direct binding studies were carried out using purified endogenous chick Hsp47 and FKBP65 (Fig. 2A). Hsp47 and FKBP65 were prepared and mixed with the same molar concentrations in the following experiments because it was shown that equal amounts of endogenous Hsp47 and FKBP65 were co-eluted from gelatin Sepharose columns (19, 41, 42). Circular dichroism (CD) spectra were obtained for each protein alone and when mixed together in solution. As shown in Fig. 2B, differences in the CD spectra were seen when Hsp47 and FKBP65 proteins were mixed, indicating that small structural changes occur due to the interaction between Hsp47 and FKBP65. Specifically, the curve of the experimental mixture of Hsp47 and FKBP65 shows a decreased CD signal below 220 nm when compared with the theoretical curve created by the addition of the individual spectra of Hsp47 and FKBP65. Now, having clear evidence of a direct interaction, we next wanted to determine the binding affinity between Hsp47 and FKBP65. To achieve this, surface plasmon resonance (SPR) was performed using FKBP65 immobilized to a chip. Injected Hsp47 clearly interacted with FKBP65 and also showed a concentration-dependent binding (Fig. 2C). However the equilibrium dissociation constant (Kd) showed that the binding of Hsp47 to FKBP65 was weaker than that to the SH3 domain of TANGO1 or to collagens, which were previously identified as binding partners of Hsp47 in the rER (Table 1).
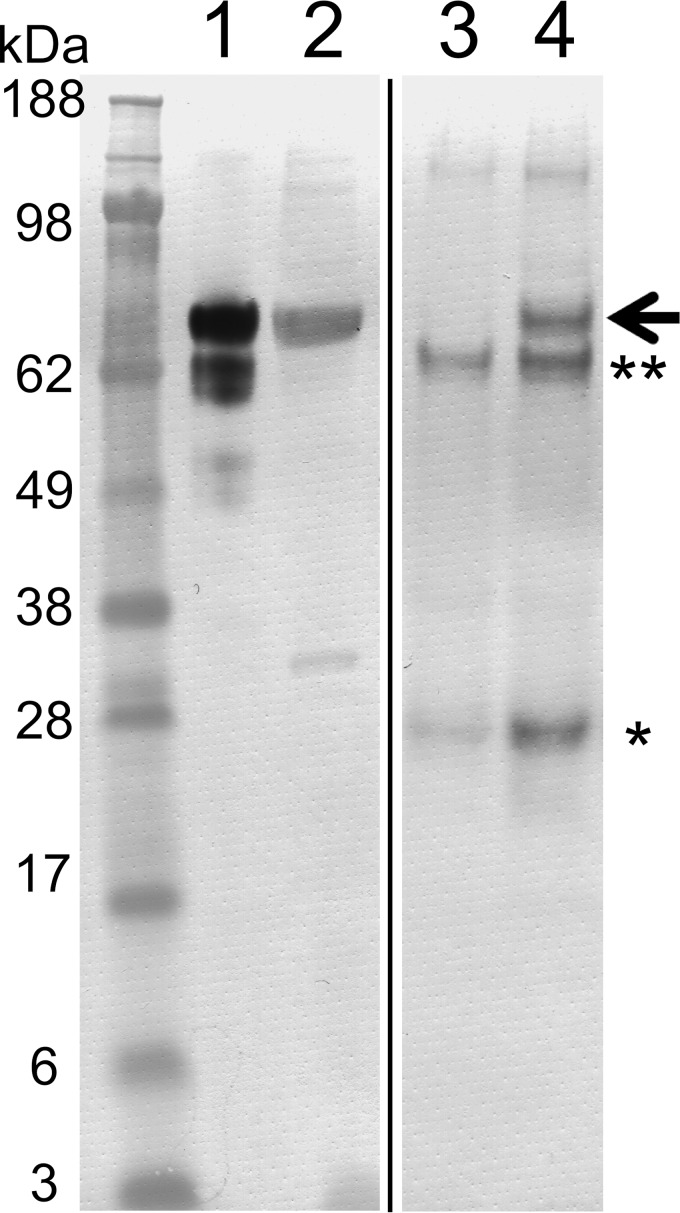
Figure 1.
Western blot analysis using an anti-FKBP65 antibody on proteins immunoprecipitated from chicken rER extracts by an Hsp47 antibody. Proteins immunoprecipitated from chicken rER extracts using a monoclonal Hsp47 antibody were electrophoresed on a Novex NuPAGE Bis-Tris 4–12% gel under reducing conditions. Proteins were transferred to a PVDF membrane and subsequently analyzed by Western blotting using a polyclonal FKBP65 antibody. Lane 1, chicken rER extract (immunoprecipitate input); lane 2, purified chicken FKBP65; lane 3, immunoprecipitate using protein G-Sepharose alone; lane 4, immunoprecipitate using protein G-Sepharose plus Hsp47 mAb. The arrow highlights the position of FKBP65. * and ** are protein G and preincubated BSA bands from the protein G-Sepharose. The black line spacing denotes irrelevant lanes that were eliminated from the image.
Figure 2.
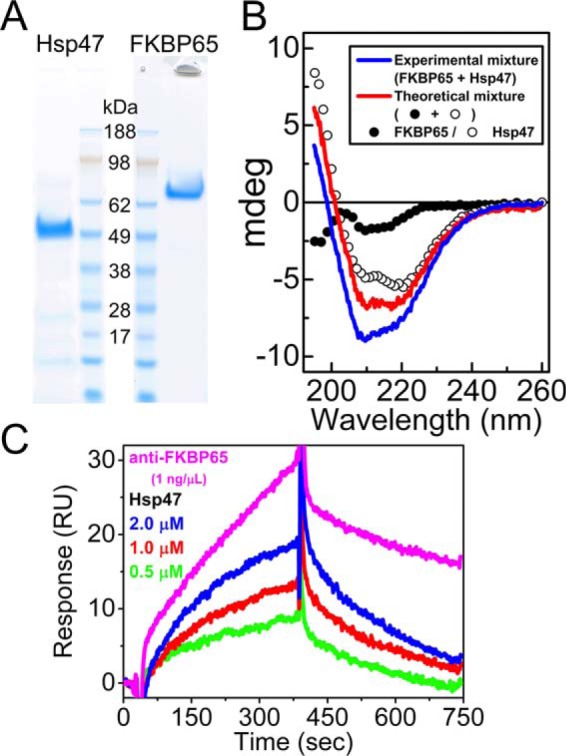
Determination of a direct interaction between Hsp47 and FKBP65. A, purified endogenous chick Hsp47 and FKBP65 were electrophoresed on a Bolt 4–12% Bis-Tris Plus Gel and stained with GelCode Blue Stain Reagent. B, CD spectra of 0.6 μm chick Hsp47 (open circles) and 0.6 μm chick FKBP65 (closed circles). Red and blue curves indicate the theoretical signal derived from addition of individual Hsp47 and FKBP65 signals, and the experimental signal from a mixture of Hsp47 and FKBP65, respectively. C, direct binding kinetics were measured by SPR analysis using a BIAcore X instrument. Various concentrations of Hsp47 were run over the FKBP65 chip. The following binding curves are shown: 0.5 μm (green), 1.0 μm (red), and 2.0 μm (blue) Hsp47. An antibody against FKBP65 (magenta line) was used as a positive control.
Table 1.
The binding affinities between Hsp47, FKBP65, and collagens
The results are shown as the mean ± S.D.
| Interaction | Kd | References |
|---|---|---|
| μm | ||
| Hsp47-FKBP65 | 3.7 ± 2.2a | Fig. 2C |
| FKBP65-type I collagenb | 24.7 ± 11.3c | Fig. 5D |
| Hsp47-type I collagenb | 4.08 ± 2.8 | Ref. 53 |
| Hsp47-collagens | 0.94d | Refs. 53 and 64 |
| Hsp47-SH3 of TANGO1 | 0.26 ± 0.09 | Ref. 37 |
a Data were calculated by a global fit of the concentration-dependent measurements using the Langmuir model.
b Type I collagen was treated by pepsin.
c Data were calculated by a steady state fit from the concentration-dependent measurements.
Binding between Hsp47 and FKBP65 conferred stability on both proteins
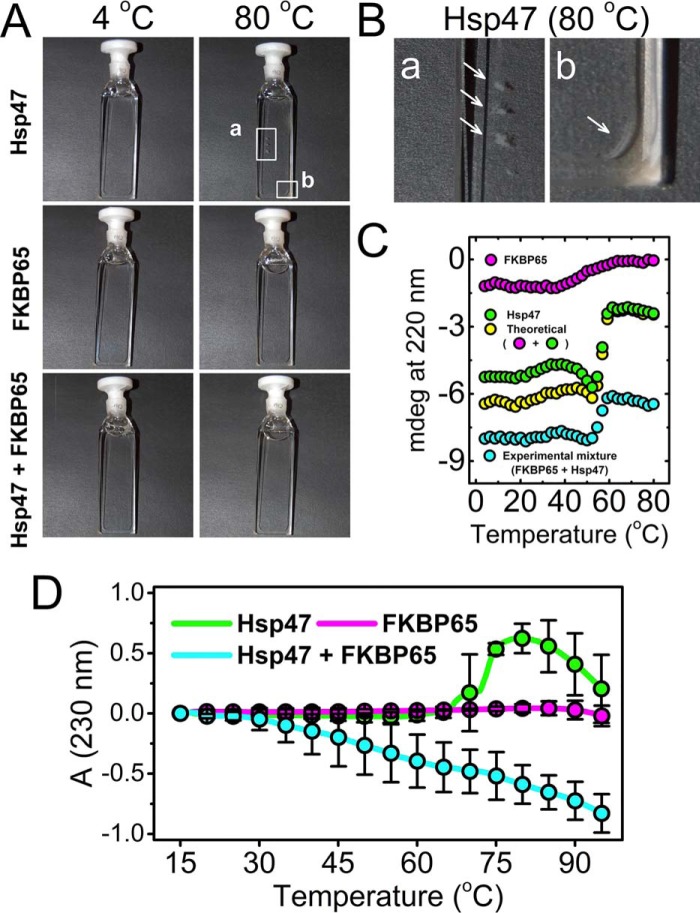
Molecular chaperones generally prevent aggregation of folding intermediates and can stabilize protein structures. To determine if the interaction between Hsp47 and FKBP65 confers mutual stability, we measured thermal transition curves by CD as a function of temperature. The thermal transitions in Fig. 3C showed a very sharp transition at ∼60 °C for Hsp47, indicating a highly cooperative unfolding of its structure. On the other hand, a broader transition was observed at ∼50 °C for FKBP65. This is likely due to the fact that FKBP65 is composed of multiple domains, four FKBP domains and two EF-hands, which do not denature in a cooperative way. Interestingly, the transition curve of the experimental mixture of Hsp47 and FKBP65 shows a smaller transition than the theoretical curve created by the addition of the individual curves of Hsp47 and FKBP65 (Fig. 3C). This is likely due to aggregation and precipitation of Hsp47, which did not occur in the presence of FKBP65 (Fig. 3, A and B). To confirm that the observation from CD is correct, turbidity measurements were performed using a UV-visible spectrophotometer. The protein solution was monitored at 230 nm because at this wavelength not only turbidity, but also the unfolding transition of the proteins, can be observed (43). FKBP65 alone showed only a small change in absorption above 85 °C (Fig. 3D). In contrast, Hsp47 behaved differently in the presence and absence of FKBP65 (Fig. 3D). The absorbance of Hsp47 alone rapidly increased at 70 °C, indicating aggregation and then dropped between 80 °C and 95 °C due to either unfolding or sedimentation of the aggregates inside the cell. However, the mixture of Hsp47 and FKBP65 showed a gradually decreasing signal with relatively large error bars. This drastic change confirmed that Hsp47 did not form aggregates in the presence of FKBP65, and that is consistent with the CD result in Fig. 3B. Hsp47 seemed to unfold without forming aggregates in the presence of FKBP65. Therefore, in vivo, FKBP65 could protect the folding-unfolding intermediates of Hsp47 and prevent Hsp47 from forming aggregates (Fig. 3). Hsp47 belongs to the serine protease inhibitor (SERPIN) superfamily and has distinct functions from other SERPINs: Hsp47 does not have protease inhibitory activity and is also not secreted from cells (17, 44). Hsp47 is sensitively up-regulated by heat shock stress (12, 17), and SERPINs have been reported to polymerize under mildly denaturing conditions (heat, low pH, or chaotropic agents or in combination) (45–47).
Figure 3.
Thermal stability of Hsp47 and FKBP65. The thermal stabilities of Hsp47 and FKBP65 and a mixture of Hsp47 and FKBP65 were measured using circular dichroism and absorbance measurements. The final concentrations of Hsp47 and FKBP65 were 0.6 μm and 0.5 μm for CD and absorbance measurements, respectively. A, pictures of the protein solutions were taken at the beginning (4 °C) and the end (80 °C) of the thermal transition experiment. B, images showing aggregates of Hsp47 that are magnified views of areas a and b from A. The arrows highlight aggregates of Hsp47. C, the thermal transition of Hsp47 (green), FKBP65 (magenta), and an experimental mixture of Hsp47 and FKBP65 (cyan) was monitored as a function of temperature by circular dichroism at 220 nm. The yellow curve indicates the theoretical signal derived from addition of individual Hsp47 and FKBP65 signals. D, the protein aggregation and/or unfolding transition of Hsp47 (green), FKBP65 (magenta), and an experimental mixture of Hsp47 and FKBP65 (cyan) was monitored as a function of temperature by absorbance (turbidity) at 230 nm. All curves were averaged from a minimum of three measurements, and error bars indicating S.D. are shown at each 5 °C interval.
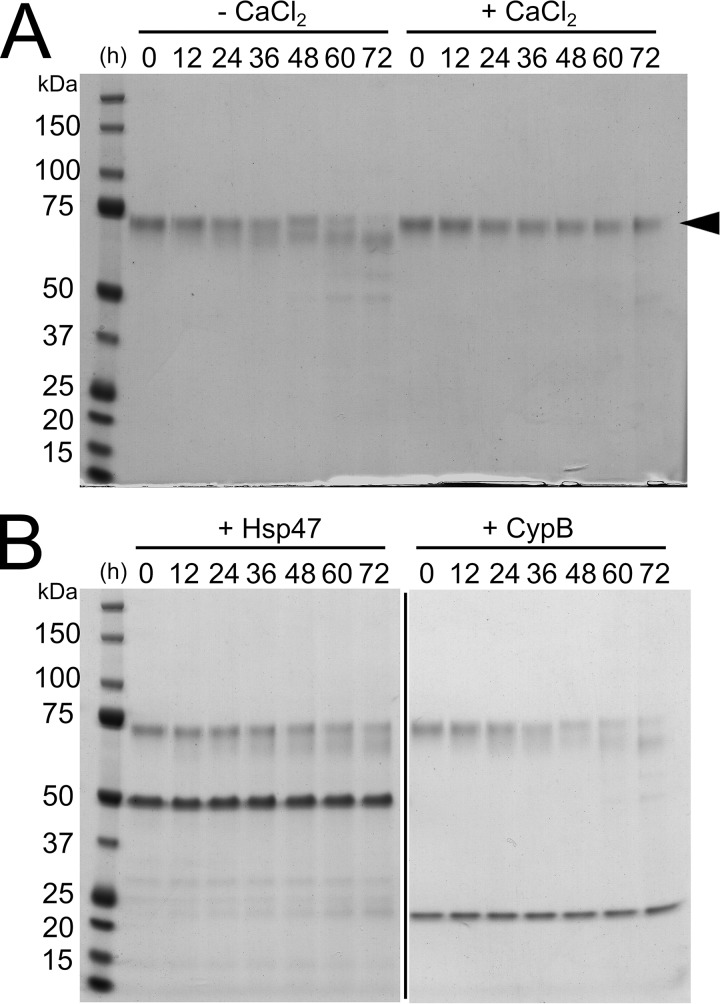
In the case of FKBP65, the stability has been shown to be affected by the presence or absence of calcium (48). The ER is the main subcellular compartment for calcium storage, and a depletion of calcium in the ER is linked to ER stress, initiation of the unfolded protein response, and various diseases (49, 50). The two EF-hand motifs, which bind to a calcium ion and constitute the carboxyl-terminal end of FKBP65, stabilize FKBP65 because structural defects of the EF-hands, caused by mutations or loss of calcium, result in intracellular degradation of FKBP65 (48). To determine the direct effect of calcium on the stability of FKBP65 in vitro, a time course experiment was performed using purified FKBP65 in the presence and absence of calcium. Full length FKBP65 (arrowhead in Fig. 4A) almost completely disappeared by 72 h in the absence of calcium due to degradation by residual proteases. Conversely in the presence of calcium, FKBP65 appeared to be more resistant to proteolysis, as minimal degradation was apparent even by 72 h (Fig. 4A). Thus the calcium-binding EF-hand motifs of FKBP65 clearly play an important role for molecular stability in vitro as well as at the cellular level. To assess if Hsp47 could compensate for the function of calcium and provide stability, another time course experiment with Hsp47 in the absence of calcium was performed. Hsp47 partially prevented FKBP65 from proteolysis (left gel in Fig. 4B). Interestingly, the M237T human mutation in Hsp47 leads to a reduction in not only the Hsp47 protein level but also the level of FKBP65 in the cell (36). Evidently, the interaction between Hsp47 and FKBP65 leads to an improvement of their individual molecular stabilities resulting in mutual preservation under physiological stress conditions in the rER. To demonstrate the specific nature of this functional interaction, we also tested CypB in a similar experiment. CypB, which is a rER resident PPIase and has been shown to interact with FKBP65 by co-immunoprecipitation from whole cell extracts (51), did not protect FKBP65 from proteolysis (right gel in Fig. 4B).
Figure 4.
Molecular stability of FKBP65. Purified FKBP65, indicated by the arrowhead, was incubated at room temperature A, in the absence (−CaCl2) or presence (+CaCl2) of calcium, or B, in the presence of Hsp47 (+Hsp47) or CypB (+CypB). Aliquots were removed at 12-h intervals and electrophoresed on NuPAGE Novex Bis-Tris 4–12% gels under reducing conditions followed by staining with GelCode Blue Stain Reagent. The black line spacing denotes irrelevant lanes that were eliminated from the image. These results indicate that the interaction between Hsp47 and FKBP65 leads to an improvement of their individual molecular stabilities.
Does the binding between Hsp47 and FKBP65 influence collagen folding?
Both Hsp47 and FKBP65 play important roles in collagen biosynthesis as a part of molecular ensemble (8, 15, 20). Formation of the collagen triple helix is accelerated by rER resident PPIases in a process that has been termed Ziploc-ing the structure (18, 52). FKBP65 was shown to be involved in this process, but its PPIase activity is marginal, and higher protein concentrations are required to observe this effect (18, 41). To determine if the interaction between Hsp47 and FKBP65 enhances their functions, we monitored collagen folding in the presence and absence of Hsp47, FKBP65, and mixtures of the two. Type III collagen was prepared as a substrate to perform in vitro collagen refolding experiments using CD as described previously (18, 53). Interestingly, in the presence of mixtures of Hsp47 and FKBP65, type III collagen showed a faster rate of refolding and a higher amount of final folded product, whereas each individual protein alone did not affect collagen refolding at the chosen concentrations (Fig. 5A). This result suggests that when the two proteins associate, Hsp47 may enhance the PPIase activity of FKBP65 and/or provide increased accessibility to collagen. To determine the binding orientation toward collagen, SPR was performed using type I collagen immobilized on a chip. FKBP65 showed binding to Hsp47 without competing with the binding of Hsp47 to collagen as shown by an increase in binding of the experimental mixture compared with the theoretical curve (Fig. 5B). This suggests that the binding between Hsp47 and FKBP65 occurs at a different site than the Hsp47-collagen–binding site, which was determined by X-ray crystallography (54). This binding manner is similar to the interaction of collagen, Hsp47, and the SH3 domain of TANGO1 at ER exit sites (37). However, the binding affinity of Hsp47 to FKBP65 is weaker than that of Hsp47 to the SH3 domain of TANGO1 (Table 1); therefore, FKBP65 is replaced by the SH3 domain of TANGO1 at ER exit sites.
Figure 5.
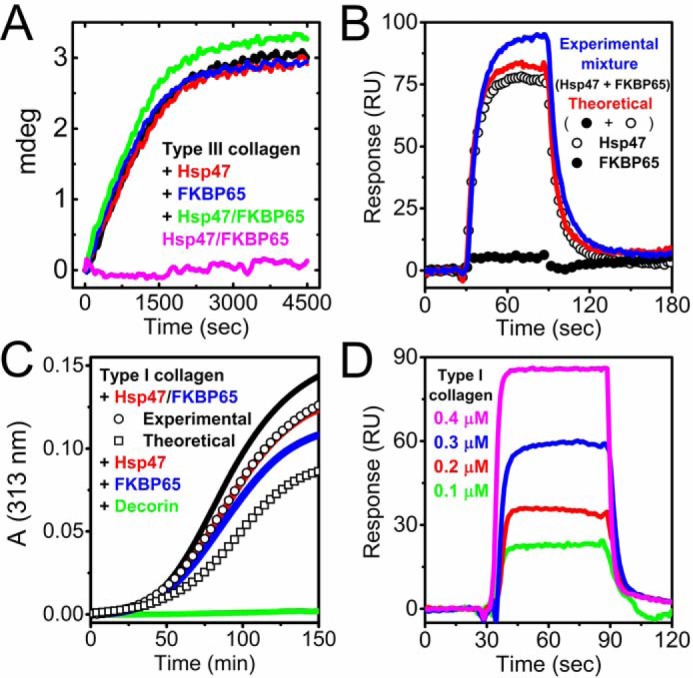
The effect of FKBP65 on collagen folding in the presence of Hsp47. A, kinetics of the refolding of full-length type III collagen in the presence of Hsp47 and/or FKBP65 monitored by CD at 220 nm is shown. The concentration of type III collagen, FKBP65, and Hsp47 were all 0.2 μm. The refolding curves shown are in the absence (black) and presence of Hsp47 (red) and FKBP65 (blue). The mixture of Hsp47 and FKBP65 with (green) and without (magenta) type III collagen are also shown. B, SPR analysis was carried out using a BIAcore X instrument to determine the binding orientation of Hsp47 and FKBP65 to the collagen chip. The open and closed circles are Hsp47 (0.05 μm) and FKBP65 (0.2 μm), respectively. Red and blue curves indicate the theoretical signal derived from the addition of individual Hsp47 and FKBP65 curves and the experimental curve from a mixture of both Hsp47 and FKBP65, respectively. C, fibril formation of type I collagen. A stock solution of type I collagen in 50 mm acetic acid was diluted to a final concentration of 0.1 μm. Fibril formation in the absence (black) and presence of 0.2 μm Hsp47 (red) and 0.2 μm FKBP65 (blue) is shown. 0.2 μm decorin was used as a positive control (green). The squares and circles indicate the theoretical signal derived from the addition of individual FKBP65 and Hsp47 signals, and the experimental signal from a mixture of FKBP65 and Hsp47, respectively. D, direct binding kinetics were measured by SPR analysis using a BIAcore X instrument. Various concentrations of pepsin-treated type I collagen were run over the FKBP65 chip. The following binding curves are shown: 0.1 μm (green), 0.2 μm (red), 0.3 (blue) and 0.4 μm (magenta) Hsp47. All curves in A–D are averaged by a minimum of three measurements. These results indicate that FKBP65 preferentially interacts with Hsp47 rather than type I collagen, and the interaction between Hsp47 and FKBP65 creates a synergistic function for collagen folding.
Does the binding between Hsp47 and FKBP65 influence collagen quality control?
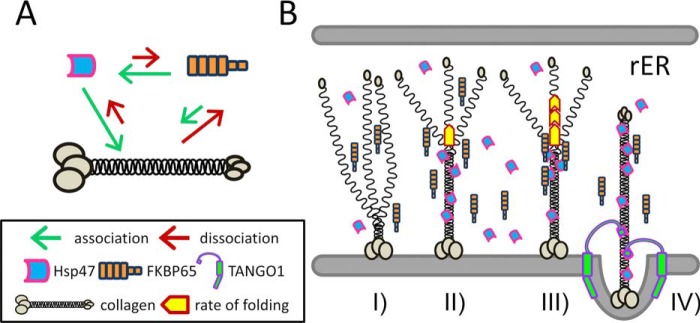
Next, we examined if this interaction affected molecular chaperone function, because both Hsp47 and FKBP65 have been characterized as molecular chaperones that recognize collagen triple helices, stabilize newly formed structures, and also prevent premature aggregation in the rER. The collagen fibril formation assay has been an established method to evaluate whether molecules or chemicals have the ability to bind and chaperone collagens (53, 55, 56). Fibril formation of type I collagen was delayed in the presence of Hsp47 or FKBP65 as described previously (Fig. 5C and Refs. 19 and 57). Surprisingly, the mixture of Hsp47 and FKBP65 did not show any additional delay as expected according to the theoretical curve but did eventually overlap with the curve of Hsp47 alone (Fig. 5C). To understand this result, the binding affinity between FKBP65 and type I collagen was calculated by SPR using FKBP65 immobilized on a chip (Fig. 5D). FKBP65 showed binding to type I collagen, but its Kd was weaker than the interaction between Hsp47 and FKBP65 (Fig. 5D and Table 1). This result is consistent with the reversed experiment that FKBP65 barely bound to type I collagen immobilized on a chip (Fig. 5B). These observations explain the results from the fibril formation assay (Fig. 5C). In conclusion, the difference in binding affinities makes FKBP65 preferentially bind to Hsp47 despite the fact that FKBP65 is capable of recognizing triple helical collagen as a chaperone. A schematic diagram of this model is shown in Fig. 6A.
Figure 6.
Schematic diagram of the interactions between collagen, Hsp47, and FKBP65 and a proposed model of collagen folding in the rER. A, illustration of the interactions between three proteins; collagen, Hsp47, and FKBP65. The length of the arrows indicates the strength of the association (green) and dissociation (red) between molecules. B, graphical representation of the combined effects of Hsp47 and FKBP65 on collagen folding. I, triple helix formation is initiated from the carboxyl-terminal end; II, Hsp47 binds to the newly formed triple helix; III, FKBP65 is recruited to Hsp47 at the triple helix propagation site and accelerates the rate of folding; IV, FKBP65 is replaced by the SH3 domain of TANGO1 at the ER exit site for the loading of collagen into a special COPII vesicle.
The role of the binding of Hsp47 to FKBP65 in the rER
As mentioned earlier, tight interactions are required for two molecular complexes, the PDI2/P4H2 tetramer and the P3H1-CRTAP-CypB complex. Conversely, we have determined that Hsp47 and FKBP65 do interact but do not form a tight complex (Fig. 2C and Table 1). As also mentioned earlier, both Hsp47 and FKBP65 play crucial roles during collagen biosynthesis. In addition, Hsp47 was recently identified as an anchor molecule between collagens and TANGO1 at ER exit sites (37), and this interaction plays a crucial role for loading collagens into special COPII vesicles for secretion. FKBP65 was also proposed to associate with lysyl hydroxylases and CypB inside the cell (51, 58, 59); however, no binding affinities were determined in these studies. These observations suggest that the weak interaction between Hsp47 and FKBP65 reflects their multifunctional roles through binding to other protein components of the molecular ensemble for collagen biosynthesis in the rER. Therefore, Hsp47 likely acts as a hub molecule during collagen folding and secretion by directing other molecules to reach their target sites on collagens. When FKBP65 is located on this hub, it could become a propagation center for triple helix formation and accelerate the rate of folding. In this model FKBP65 would then be replaced by TANGO1 at ER exit sites. A schematic diagram of this hypothesis is described in Fig. 6B. Although FKBP65 has been proposed to be a collagen molecular chaperone responsible for the stabilization of collagen triple helices cooperatively with Hsp47, this chaperone function in the rER may actually be predominantly fulfilled by Hsp47 (Figs. 5C and 6A). FKBP65 may provide a functional redundancy for molecular chaperone activity under certain stress conditions or loss of Hsp47. The P3H1-CRTAP-CypB complex and FKBP22 are also involved in collagen biosynthesis as PPIases and molecular chaperones; however, it is not clear how temporally aligned and/or coordinated Hsp47, FKBP65, FKBP22, and the P3H1-CRTAP-CypB complex facilitate collagen folding and quality control. Further studies are required to elucidate these intricate processes. In conclusion, Hsp47 and FKBP65 interact in the rER creating a synergistic function for molecular stability and collagen folding. This could explain why OI causing mutations in both genes demonstrate phenotypic similarities. In addition, although FKBP65 is relatively abundant at embryonic stages (19, 40, 41), there is little FKBP65 expressed in adult tissues (60). This correlates with the amount of newly synthesized collagens (61, 62). Further analyses are required to determine the individual binding affinities in the protein-protein interactome of the molecular ensemble in the rER. This could help us to understand the more detailed mechanisms of post-translational modifications and collagen biosynthesis in the rER.
Experimental procedures
Immunoprecipitation using embryonic chicken rER fraction
Chicken rER fraction was prepared following a previously published protocol (39) with slight modifications. The pellet of enriched rER vesicles (3 g) was resuspended in 10 ml of TBS containing 1 mm CaCl2, 0.1% (v/v) Tween 20, and EDTA-free protease inhibitor mixture (Roche Applied Science). The extract was centrifuged for 1 h at 125,000 × g in a 45-Ti rotor (Beckman), and the supernatant was removed and kept. A 1.5-ml aliquot of supernatant was incubated at 4 °C overnight with 20 μl of protein G-Sepharose (GE Healthcare) which was precoupled with 20 μg of monoclonal anti-Hsp47 (ADI-SPA-470, Enzo Life Science) for 2 h at 4 °C after preincubation with 0.1% (w/v) BSA for 30 min at 4 °C. The beads were washed twice with 1.5 ml of TBS and eluted with 2× SDS-PAGE sample buffer containing DTT by heating to 95 °C for 5 min. The eluted proteins were separated by SDS-PAGE followed by transfer to PVDF and Western blotting using a rabbit polyclonal anti-FKBP65 antibody, which was generated in our laboratory using purified chicken FKBP65 as an immunogen.
Protein purifications
Hsp47 and FKBP65 were purified from 17-day-old chicken embryos using methods described previously (19, 40). Type I and III collagens were purified from pepsin digests of mouse tail tendon and fetal bovine calf skin using methods described previously (63).
Circular dichroism measurements
CD spectra were recorded on an Aviv 202 spectropolarimeter (Aviv, Lakewood, NJ) using a Peltier thermostatted cell holder and a 1-mm path length cell (Starna Cells, Atascadero, CA). Protein concentrations were determined by amino acid analysis as described previously (63). The spectra represent the average of at least 10 scans recorded at a wavelength resolution of 0.1 nm. The proteins were measured in 5 mm Tris/HCl buffer, pH 7.5, containing 30 mm NaCl and 0.05 mm CaCl2 at 4 °C. The thermal transition curves were measured at 220 nm in 5 mm Tris/HCl buffer, pH 7.2, containing 30 mm NaCl and 0.05 mm CaCl2. Transition curves were monitored as a function of temperature with a heating rate of 10 °C/h. The protein concentrations of FKBP65 and Hsp47 were 0.6 μm for both wavelength and thermal transition measurements.
Turbidity measurements
The final protein concentrations of FKBP65 and Hsp47 were 0.5 μm, and the proteins were prepared in 25 mm Tris/HCl buffer, pH 7.5, containing 150 mm NaCl and 1 mm CaCl2. The states of protein aggregation and unfolding were monitored at 230 nm as a function of temperature with a heating rate of 10 °C/h. Measurements were performed from 15 °C to 95 °C in a Cary 4 Series UV-visible spectrophotometer (Agilent Technologies).
Stabilization of FKBP65 in the presence and absence of calcium and proteins
The effects on stability of FKBP65 in the presence of calcium and exogenous proteins were tested by time course experiments. FKBP65 (0.2 μm) was gently agitated at 25 °C in the presence or absence of 2.5 mm CaCl2 in TBS. To test the effect of exogenous proteins, FKBP65 (0.2 μm) was mixed with 0.3 μm Hsp47 or cyclophilin B and gently agitated in the absence of CaCl2 at 25 °C. In each experiment aliquots were removed at 12-h intervals up to 72 h and electrophoresed on NuPAGE Novex Bis-Tris 4–12% gels under reducing conditions followed by staining with GelCode Blue Stain Reagent.
Surface plasmon resonance analysis
SPR experiments were carried out using a BIAcore X instrument (GE Healthcare). Purified FKBP65 and type I collagen were immobilized on CM5 sensor chips by amide coupling. The approximate coupled protein concentrations were 2.3 ng/mm2 (2300 response units (RU)) of FKBP65 and 6.0 ng/mm2 (6000 RU) of type I collagen. RNase A (3.0 ng/mm2 (3000 RU)) was coupled to the reference channel in the FKBP65 chip to block nonspecific binding to the dextran matrix. The experiments were performed at 20 °C in HBS-P (10 mm Hepes, pH 7.4, containing 150 mm NaCl and 0.005% Surfactant P20) containing 1 mm CaCl2 and using a flow rate of 5 μl/min for the Hsp47-FKBP65 chip and 10 μl/min for the type I collagen-FKBP65 chip and the Hsp47/FKBP65-type I collagen chip. All curves are the average of at least three replicates, and three independent measurements were performed. For analysis of binding affinity, the curves were fitted with the Langmuir-binding model and the steady-state affinity model for Hsp47-FKBP65 and type I collagen-FKBP65, respectively (BIAevaluation software; GE Healthcare). A polyclonal antibody against FKBP65 (12172-1-AP, ProteinTech) was used as a positive control for binding to the FKBP65 chip.
Refolding of full-length type III collagen measured by circular dichroism
Refolding of full-length native type III collagen was monitored by circular dichroism measurements at 220 nm. The collagen was denatured for 5 min at 45 °C and then added into precooled reaction buffer (50 mm Tris/HCl, pH 7.5, containing 0.2 m NaCl and 1 mm CaCl2) in the presence and absence of Hsp47 and/or FKBP65 for 4500 s at 25 °C. The final concentration of type III collagen, FKBP65, and Hsp47 were all 0.2 μm. All curves shown are the average of at least three independent measurements.
Type I collagen fibril formation assay
Stock solutions of type I collagen in 50 mm acetic acid were diluted to a final concentration of 0.1 μm in 0.1 m sodium bicarbonate buffer, pH 7.8, containing 0.15 m NaCl and 1 mm CaCl2. Measurements were performed at 34 °C in a Cary 4 Series UV-visible spectrophotometer (Agilent Technologies), and the absorbance (light scattering) was monitored at 313 nm as a function of time. All curves are the average of at least three independent measurements. Recombinant human decorin (143-DE-100, R&D systems) was used as a positive control.
Author contributions
Y. I. and H. P. B. were responsible for the overall design of the study. Y. I. performed and analyzed all the experiments. Y. I., P. H., and H. P. B. reviewed the results and wrote the manuscript.
Acknowledgment
We thank the Analytical Core Facility of Shriners Hospitals for Children in Portland for amino acid analysis.
This work was supported by Shriners Hospital for Children Grants 85100 and 85500 (to H. P. B.). The authors declare that they have no conflicts of interest with the contents of this article.
- rER
- rough endoplasmic reticulum
- Hsp
- heat shock protein
- FKBP
- FK506-binding protein
- CD
- circular dichroism
- SPR
- surface plasmon resonance
- OI
- osteogenesis imperfecta
- PPIase
- peptidyl-prolyl cis/trans isomerase
- PDI
- protein disulfide isomerase
- P4H
- prolyl 4-hydroxylase
- P3H1
- prolyl 3-hydroxylase 1
- CRTAP
- cartilage-associated protein
- CypB
- cyclophilin B
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
References
- 1. Araki K., and Nagata K. (2011) Protein folding and quality control in the ER. Cold Spring Harb. Perspect. Biol. 3, a007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gidalevitz T., Stevens F., and Argon Y. (2013) Orchestration of secretory protein folding by ER chaperones. Biochim. Biophys. Acta 1833, 2410–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bächinger H. P., Mizuno K., Vranka J., and Boudko S. (2010) Collagen Formation and Structure. In Comprehensive Natural Products II: Chemistry and Biology (Mander L., and Liu H.-W. eds.) pp. 469–530, Elsevier Science Publishing Co., Inc., New York [Google Scholar]
- 4. Bateman J. F., Boot-Handford R. P., and Lamandé S. R. (2009) Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nat. Rev. Genet. 10, 173–183 [DOI] [PubMed] [Google Scholar]
- 5. Bella J., and Hulmes D. J. (2017) Fibrillar Collagens. Subcell. Biochem. 82, 457–490 [DOI] [PubMed] [Google Scholar]
- 6. Gjaltema R. A., and Bank R. A. (2017) Molecular insights into prolyl and lysyl hydroxylation of fibrillar collagens in health and disease. Crit. Rev. Biochem. Mol. Biol. 52, 74–95 [DOI] [PubMed] [Google Scholar]
- 7. Makareeva E., Aviles N. A., and Leikin S. (2011) Chaperoning osteogenesis: new protein-folding disease paradigms. Trends Cell Biol. 21, 168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishikawa Y., and Bächinger H. P. (2013) A molecular ensemble in the rER for procollagen maturation. Biochim. Biophys. Acta 1833, 2479–2491 [DOI] [PubMed] [Google Scholar]
- 9. Marini J. C., Reich A., and Smith S. M. (2014) Osteogenesis imperfecta due to mutations in non-collagenous genes: lessons in the biology of bone formation. Curr. Opin. Pediatr. 26, 500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christiansen H. E., Schwarze U., Pyott S. M., AlSwaid A., Al Balwi M., Alrasheed S., Pepin M. G., Weis M. A., Eyre D. R., and Byers P. H. (2010) Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am. J. Hum. Genet. 86, 389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alanay Y., Avaygan H., Camacho N., Utine G. E., Boduroglu K., Aktas D., Alikasifoglu M., Tuncbilek E., Orhan D., Bakar F. T., Zabel B., Superti-Furga A., Bruckner-Tuderman L., Curry C. J., Pyott S., Byers P. H., et al. (2010) Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am. J. Hum. Genet. 86, 551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagata K., Saga S., and Yamada K. M. (1986) A major collagen-binding protein of chick embryo fibroblasts is a novel heat shock protein. J. Cell Biol. 103, 223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuoka Y., Kubota H., Adachi E., Nagai N., Marutani T., Hosokawa N., and Nagata K. (2004) Insufficient folding of type IV collagen and formation of abnormal basement membrane-like structure in embryoid bodies derived from Hsp47-null embryonic stem cells. Mol. Biol. Cell 15, 4467–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marutani T., Yamamoto A., Nagai N., Kubota H., and Nagata K. (2004) Accumulation of type IV collagen in dilated ER leads to apoptosis in Hsp47-knockout mouse embryos via induction of CHOP. J. Cell Sci. 117, 5913–5922 [DOI] [PubMed] [Google Scholar]
- 15. Nagai N., Hosokawa M., Itohara S., Adachi E., Matsushita T., Hosokawa N., and Nagata K. (2000) Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J. Cell Biol. 150, 1499–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishida Y., and Nagata K. (2011) Hsp47 as a collagen-specific molecular chaperone. Methods Enzymol. 499, 167–182 [DOI] [PubMed] [Google Scholar]
- 17. Ito S., and Nagata K. (2017) Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin. Cell Dev. Biol. 62, 142–151 [DOI] [PubMed] [Google Scholar]
- 18. Ishikawa Y., Mizuno K., and Bächinger H. P. (2017) Ziploc-ing the Structure 2.0: endoplasmic reticulum resident peptidyl prolyl isomerases show different activities toward hydroxyproline. J. Biol. Chem. 292, 9273–9282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishikawa Y., Vranka J., Wirz J., Nagata K., and Bächinger H. P. (2008) The rough endoplasmic reticulum-resident FK506-binding protein FKBP65 is a molecular chaperone that interacts with collagens. J. Biol. Chem. 283, 31584–31590 [DOI] [PubMed] [Google Scholar]
- 20. Lietman C. D., Rajagopal A., Homan E. P., Munivez E., Jiang M. M., Bertin T. K., Chen Y., Hicks J., Weis M., Eyre D., Lee B., and Krakow D. (2014) Connective tissue alterations in Fkbp10-/- mice. Hum. Mol. Genet. 23, 4822–4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwarze U., Cundy T., Pyott S. M., Christiansen H. E., Hegde M. R., Bank R. A., Pals G., Ankala A., Conneely K., Seaver L., Yandow S. M., Raney E., Babovic-Vuksanovic D., Stoler J., Ben-Neriah Z., Segel R., et al. (2013) Mutations in FKBP10, which result in Bruck syndrome and recessive forms of osteogenesis imperfecta, inhibit the hydroxylation of telopeptide lysines in bone collagen. Hum. Mol. Genet. 22, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barnes A. M., Duncan G., Weis M., Paton W., Cabral W. A., Mertz E. L., Makareeva E., Gambello M. J., Lacbawan F. L., Leikin S., Fertala A., Eyre D. R., Bale S. J., and Marini J. C. (2013) Kuskokwim syndrome, a recessive congenital contracture disorder, extends the phenotype of FKBP10 mutations. Hum. Mutat. 34, 1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheung K. L., Bates M., and Ananthanarayanan V. S. (2010) Effect of FKBP65, a putative elastin chaperone, on the coacervation of tropoelastin in vitro. Biochem. Cell Biol. 88, 917–925 [DOI] [PubMed] [Google Scholar]
- 24. Miao M., Reichheld S. E., Muiznieks L. D., Huang Y., and Keeley F. W. (2013) Elastin binding protein and FKBP65 modulate in vitro self-assembly of human tropoelastin. Biochemistry 52, 7731–7741 [DOI] [PubMed] [Google Scholar]
- 25. Hong F., Mohammad Rachidi S., Lundgren D., Han D., Huang X., Zhao H., Kimura Y., Hirano H., Ohara O., Udono H., Meng S., Liu B., and Li Z. (2017) Mapping the Interactome of a major mammalian endoplasmic reticulum heat shock protein 90. PLoS ONE 12, e0169260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jansen G., Määttänen P., Denisov A. Y., Scarffe L., Schade B., Balghi H., Dejgaard K., Chen L. Y., Muller W. J., Gehring K., and Thomas D. Y. (2012) An interaction map of endoplasmic reticulum chaperones and foldases. Mol. Cell. Proteomics 11, 710–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koski M. K., Anantharajan J., Kursula P., Dhavala P., Murthy A. V., Bergmann U., Myllyharju J., and Wierenga R. K. (2017) Assembly of the elongated collagen prolyl 4-hydroxylase α2β2 heterotetramer around a central α2 dimer. Biochem. J. 474, 751–769 [DOI] [PubMed] [Google Scholar]
- 28. Morello R., Bertin T. K., Chen Y., Hicks J., Tonachini L., Monticone M., Castagnola P., Rauch F., Glorieux F. H., Vranka J., Bächinger H. P., Pace J. M., Schwarze U., Byers P. H., Weis M., et al. (2006) CRTAP is required for prolyl 3- hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell 127, 291–304 [DOI] [PubMed] [Google Scholar]
- 29. Kersteen E. A., Higgin J. J., and Raines R. T. (2004) Production of human prolyl 4-hydroxylase in Escherichia coli. Protein Expr. Purif. 38, 279–291 [DOI] [PubMed] [Google Scholar]
- 30. John D. C., Grant M. E., and Bulleid N. J. (1993) Cell-free synthesis and assembly of prolyl 4-hydroxylase: the role of the β-subunit (PDI) in preventing misfolding and aggregation of the α-subunit. EMBO J. 12, 1587–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang W., Barnes A. M., Cabral W. A., Bodurtha J. N., and Marini J. C. (2010) Prolyl 3-hydroxylase 1 and CRTAP are mutually stabilizing in the endoplasmic reticulum collagen prolyl 3-hydroxylation complex. Hum. Mol. Genet. 19, 223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takagi M., Ishii T., Barnes A. M., Weis M., Amano N., Tanaka M., Fukuzawa R., Nishimura G., Eyre D. R., Marini J. C., and Hasegawa T. (2012) A novel mutation in LEPRE1 that eliminates only the KDEL ER- retrieval sequence causes non-lethal osteogenesis imperfecta. PLoS ONE 7, e36809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ellgaard L., McCaul N., Chatsisvili A., and Braakman I. (2016) Co- and post-translational protein folding in the ER. Traffic 17, 615–638 [DOI] [PubMed] [Google Scholar]
- 34. Paulsson K., and Wang P. (2003) Chaperones and folding of MHC class I molecules in the endoplasmic reticulum. Biochim Biophys Acta 1641, 1–12 [DOI] [PubMed] [Google Scholar]
- 35. Duran I., Martin J. H., Weis M. A., Krejci P., Konik P., Li B., Alanay Y., Lietman C., Lee B., Eyre D., Cohn D. H., and Krakow D. (2017) A chaperone complex formed by HSP47, FKBP65, and BiP modulates telopeptide lysyl hydroxylation of type I procollagen. J. Bone Miner. Res. 32, 1309–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duran I., Nevarez L., Sarukhanov A., Wu S., Lee K., Krejci P., Weis M., Eyre D., Krakow D., and Cohn D. H. (2015) HSP47 and FKBP65 cooperate in the synthesis of type I procollagen. Hum. Mol. Genet. 24, 1918–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ishikawa Y., Ito S., Nagata K., Sakai L. Y., and Bächinger H. P. (2016) Intracellular mechanisms of molecular recognition and sorting for transport of large extracellular matrix molecules. Proc. Natl. Acad. Sci. U.S.A. 113, E6036–E6044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelley B. P., Malfait F., Bonafe L., Baldridge D., Homan E., Symoens S., Willaert A., Elcioglu N., Van Maldergem L., Verellen-Dumoulin C., Gillerot Y., Napierala D., Krakow D., Beighton P., Superti-Furga A., De Paepe A., and Lee B. (2011) Mutations in FKBP10 cause recessive osteogenesis imperfecta and Bruck syndrome. J. Bone Miner. Res. 26, 666–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beck K., Boswell B. A., Ridgway C. C., and Bächinger H. P. (1996) Triple helix formation of procollagen type I can occur at the rough endoplasmic reticulum membrane. J. Biol. Chem. 271, 21566–21573 [DOI] [PubMed] [Google Scholar]
- 40. Macdonald J. R., and Bächinger H. P. (2001) HSP47 binds cooperatively to triple helical type I collagen but has little effect on the thermal stability or rate of refolding. J. Biol. Chem. 276, 25399–25403 [DOI] [PubMed] [Google Scholar]
- 41. Zeng B., MacDonald J. R., Bann J. G., Beck K., Gambee J. E., Boswell B. A., and Bächinger H. P. (1998) Chicken FK506-binding protein, FKBP65, a member of the FKBP family of peptidylprolyl cis-trans isomerases, is only partially inhibited by FK506. Biochem. J. 330, 109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vranka J. A., Sakai L. Y., and Bächinger H. P. (2004) Prolyl 3-hydroxylase 1, enzyme characterization and identification of a novel family of enzymes. J. Biol. Chem. 279, 23615–23621 [DOI] [PubMed] [Google Scholar]
- 43. Liu P. F., Avramova L. V., and Park C. (2009) Revisiting absorbance at 230 nm as a protein unfolding probe. Anal. Biochem. 389, 165–170 [DOI] [PubMed] [Google Scholar]
- 44. Hirayoshi K., Kudo H., Takechi H., Nakai A., Iwamatsu A., Yamada K. M., and Nagata K. (1991) HSP47: a tissue-specific, transformation-sensitive, collagen-binding heat shock protein of chicken embryo fibroblasts. Mol. Cell. Biol. 11, 4036–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huntington J. A. (2011) Serpin structure, function, and dysfunction. J. Thromb. Haemost. 9, 26–34 [DOI] [PubMed] [Google Scholar]
- 46. Belorgey D., Irving J. A., Ekeowa U. I., Freeke J., Roussel B. D., Miranda E., Pérez J., Robinson C. V., Marciniak S. J., Crowther D. C., Michel C. H., and Lomas D. A. (2011) Characterisation of serpin polymers in vitro and in vivo. Methods 53, 255–266 [DOI] [PubMed] [Google Scholar]
- 47. Yamasaki M., Li W., Johnson D. J., and Huntington J. A. (2008) Crystal structure of a stable dimer reveals the molecular basis of serpin polymerization. Nature 455, 1255–1258 [DOI] [PubMed] [Google Scholar]
- 48. Murphy L. A., Ramirez E. A., Trinh V. T., Herman A. M., Anderson V. C., and Brewster J. L. (2011) Endoplasmic reticulum stress or mutation of an EF-hand Ca2+-binding domain directs the FKBP65 rotamase to an ERAD-based proteolysis. Cell Stress Chaperones 16, 607–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mekahli D., Bultynck G., Parys J. B., De Smedt H., and Missiaen L. (2011) Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb. Perspect. Biol. 3, a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chernorudskiy A. L., and Zito E. (2017) Regulation of calcium homeostasis by er redox: a close-up of the ER/mitochondria connection. J. Mol. Biol. 429, 620–632 [DOI] [PubMed] [Google Scholar]
- 51. Terajima M., Taga Y., Chen Y., Cabral W. A., Hou-Fu G., Srisawasdi S., Nagasawa M., Sumida N., Hattori S., Kurie J. M., Marini J. C., and Yamauchi M. (2016) Cyclophilin-B modulates collagen cross-linking by differentially affecting lysine hydroxylation in the helical and telopeptidyl domains of tendon type I collagen. J. Biol. Chem. 291, 9501–9512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ishikawa Y., Boudko S., and Bächinger H. P. (2015) Ziploc-ing the structure: triple helix formation is coordinated by rough endoplasmic reticulum resident PPIases. Biochim. Biophys. Acta 1850, 1983–1993 [DOI] [PubMed] [Google Scholar]
- 53. Ishikawa Y., and Bächinger H. P. (2014) A substrate preference for the rough endoplasmic reticulum resident protein FKBP22 during collagen biosynthesis. J. Biol. Chem. 289, 18189–18201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Widmer C., Gebauer J. M., Brunstein E., Rosenbaum S., Zaucke F., Drögemüller C., Leeb T., and Baumann U. (2012) Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc. Natl. Acad. Sci. U.S.A. 109, 13243–13247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Islam M., Gor J., Perkins S. J., Ishikawa Y., Bächinger H. P., and Hohenester E. (2013) The concave face of decorin mediates reversible dimerization and collagen binding. J. Biol. Chem. 288, 35526–35533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Knüppel L., Ishikawa Y., Aichler M., Heinzelmann K., Hatz R., Behr J., Walch A., Bächinger H. P., Eickelberg O., and Staab-Weijnitz C. A. (2017) A novel antifibrotic mechanism of nintedanib and pirfenidone: inhibition of collagen fibril assembly. Am. J. Respir. Cell Mol. Biol. 57, 77–90 [DOI] [PubMed] [Google Scholar]
- 57. Thomson C. A., and Ananthanarayanan V. S. (2000) Structure-function studies on hsp47: pH-dependent inhibition of collagen fibril formation in vitro. Biochem. J. 349, 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen Y., Terajima M., Banerjee P., Guo H., Liu X., Yu J., Yamauchi M., and Kurie J. M. (2017) FKBP65-dependent peptidyl-prolyl isomerase activity potentiates the lysyl hydroxylase 2-driven collagen cross-link switch. Sci. Rep. 7, 46021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gjaltema R. A., van der Stoel M. M., Boersema M., and Bank R. A. (2016) Disentangling mechanisms involved in collagen pyridinoline cross-linking: the immunophilin FKBP65 is critical for dimerization of lysyl hydroxylase 2. Proc. Natl. Acad. Sci. U.S.A. 113, 7142–7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patterson C. E., Abrams W. R., Wolter N. E., Rosenbloom J., and Davis E. C. (2005) Developmental regulation and coordinate reexpression of FKBP65 with extracellular matrix proteins after lung injury suggest a specialized function for this endoplasmic reticulum immunophilin. Cell Stress Chaperones 10, 285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Boyer B., Kern P., Fourtanier A., and Labat-Robert J. (1991) Age-dependent variations of the biosyntheses of fibronectin and fibrous collagens in mouse skin. Exp. Gerontol. 26, 375–383 [DOI] [PubMed] [Google Scholar]
- 62. Feru J., Delobbe E., Ramont L., Brassart B., Terryn C., Dupont-Deshorgue A., Garbar C., Monboisse J. C., Maquart F. X., and Brassart-Pasco S. (2016) Aging decreases collagen IV expression in vivo in the dermo-epidermal junction and in vitro in dermal fibroblasts: possible involvement of TGF-β1. Eur. J. Dermatol. 26, 350–360 [DOI] [PubMed] [Google Scholar]
- 63. Pokidysheva E., Zientek K. D., Ishikawa Y., Mizuno K., Vranka J. A., Montgomery N. T., Keene D. R., Kawaguchi T., Okuyama K., and Bächinger H. P. (2013) Posttranslational modifications in type I collagen from different tissues extracted from wild type and prolyl 3-hydroxylase 1 null mice. J. Biol. Chem. 288, 24742–24752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Natsume T., Koide T., Yokota S., Hirayoshi K., and Nagata K. (1994) Interactions between collagen-binding stress protein HSP47 and collagen: analysis of kinetic parameters by surface plasmon resonance biosensor. J. Biol. Chem. 269, 31224–31228 [PubMed] [Google Scholar]