Abstract
In ∼50% of prostate cancers, chromosomal rearrangements cause the fusion of the promoter and 5′-UTR of the androgen-regulated TMPRSS2 (transmembrane protease, serine 2) gene to the open reading frame of ERG, encoding an ETS family transcription factor. This fusion results in expression of full-length or N-terminally truncated ERG protein in prostate epithelia. ERG is not expressed in normal prostate epithelia, but when expressed, it promotes tumorigenesis via altered gene expression, stimulating epithelial-mesenchymal transition, cellular migration/invasion, and transformation. However, limited knowledge about the molecular mechanisms of ERG function in prostate cells has hampered efforts to therapeutically target ERG. ERK-mediated phosphorylation of ERG is required for ERG functions in prostate cells, but the reason for this requirement is unknown. Here, we report a mechanism whereby ERK-mediated phosphorylation of ERG at one serine residue causes a conformational change that allows ERK phosphorylation at a second serine residue, Ser-96. We found that the Ser-96 phosphorylation resulted in dissociation of EZH2 and SUZ12, components of polycomb repressive complex 2 (PRC2), transcriptional activation of ERG target genes, and increased cell migration. Conversely, loss of ERG phosphorylation at Ser-96 resulted in recruitment of EZH2 across the ERG-cistrome and a genome-wide loss of ERG-mediated transcriptional activation and cell migration. In conclusion, our findings have identified critical molecular mechanisms involving ERK-mediated ERG activation that could be exploited for therapeutic intervention in ERG-positive prostate cancers.
Keywords: ETS transcription factor family, mitogen-activated protein kinase (MAPK), polycomb, prostate cancer, transcription factor
Introduction
ERG is a member of the ETS family of transcription factors that is implicated in endothelial differentiation, angiogenesis, hematopoiesis, and bone development (1). In ∼50% of prostate cancers, chromosomal rearrangements cause the fusion of the promoter and 5′-UTR of the androgen-regulated TMPRSS2 gene to the open reading frame of ERG resulting in expression of either full-length or N-terminally truncated ERG protein in prostate epithelia (2). Mice engineered to express ERG in the prostate epithelia develop prostate adenocarcinoma, but only when this aberrant ERG expression is coupled with a second mutation activating PI3K/AKT or androgen receptor signaling (3–5). Various genome-wide studies have identified ERG-induced gene expression programs responsible for oncogenic phenotypes such as cell migration and invasion, anchorage-independent growth, and cellular transformation (6–8). However, the molecular mechanisms by which ERG activates or represses transcription of target genes are not well understood.
Post-translational modifications such as phosphorylation commonly modulate the functions of ETS transcription factors by mechanisms such as increasing nuclear concentration (9), changing DNA affinity (10), or changing the affinity for co-regulators (11). We previously reported that, in vitro, ERK binds an FIFP docking sequence (DEF motif) in ERG to phosphorylate Ser-215 (12). We also showed that ERG Ser-215 was phosphorylated by ERK in prostate cells, and this event was necessary for ERG to promote transcriptional activation and cell migration. However, the consequence of Ser-215 phosphorylation on the molecular function of ERG is unknown.
ERG is known to cooperate with co-regulators to alter transcriptional output of target genes. Co-activators such as EWS, CBP/p300, BRD4, PARP1, and DNA-PKcs have been implicated in the transactivation function of ERG (8, 13–15). In contrast, co-repressors such as polycomb repressive complex 2 (PRC2)3 and histone deacetylases are known to repress ERG-mediated transcription (16, 17). Although these co-regulators are imperative for ERG function, it is not known whether phosphorylation of ERG has any effect on interactions with these co-regulators.
Here, we demonstrate that phosphorylation of Ser-215 leads to a conformational change in ERG that primes a second serine, Ser-96, for phosphorylation by ERK. Phosphomimetic and phosphor-null mutants indicate that the ability of ERG to activate gene expression and promote prostate cell migration is dependent solely on phosphorylated Ser-96, whereas Ser-215 phosphorylation is required to allow Ser-96 phosphorylation. Phosphorylation at Ser-96 resulted in dissociation of the PRC2 subunits EZH2 and SUZ12 from ERG. Further, the phospho-null mutant S96A was associated with increased EZH2 recruitment across the ERG cistrome and a failure to activate an ERG-dependent gene expression program. These data suggest that ERK potentiates transactivation and cell migration, promoting functions of ERG by phosphorylation-induced dissociation of the PRC2 complex.
Results
Phosphorylated Ser-215 primes Ser-96 phosphorylation by ERK
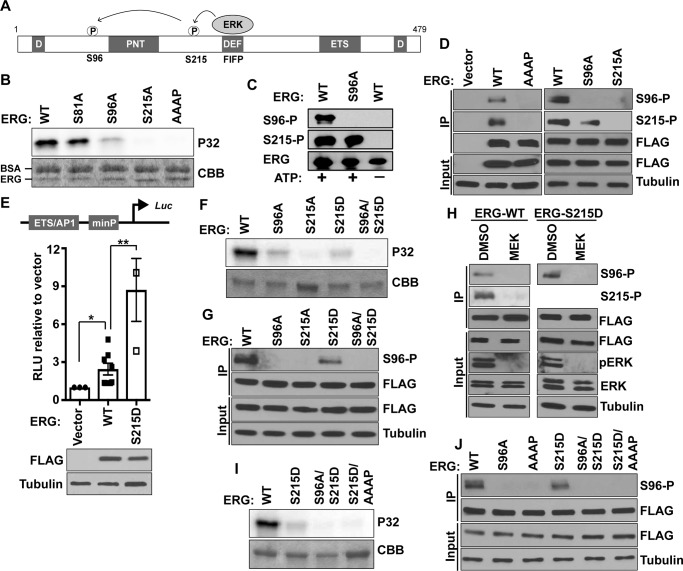
ERK binds D or DEF docking domains in target proteins and phosphorylates serine or threonine residues present within consensus PX(SP/TP) or sometimes SP/TP motifs (18). ERG has two putative D domains and one canonical DEF domain (Fig. 1A and supplemental Fig. S1) (12). We previously found that ERK binds the DEF domain in ERG (sequence FIFP) and phosphorylates nearby Ser-215 (12). In addition, our results suggested that Ser-96 could be a secondary phosphorylation site that may be dependent on prior phosphorylation of Ser-215. To further confirm this observation, ERK2 was used to in vitro phosphorylate purified ERG phospho-null mutants and a DEF docking domain mutant (FIFP to AAAP). All phosphorylation was ablated in ERG S215A and ERG AAAP, whereas the signal partially decreased in ERG S96A (Fig. 1B). In contrast, there was no change in the phosphorylation signal in negative control ERG S81A, where a non-phosphorylated serine is changed to an alanine (Fig. 1B). To test site-specific phosphorylation in cells, a phospho-specific antibody was raised to ERG phosphorylated at Ser-96 (Ser(P)-96). A phospho-specific antibody against ERG Ser-215 (Ser(P)-215) has been described previously (12). Both phospho-specific antibodies failed to detect ERG protein purified from a bacterial expression system unless the protein was phosphorylated using purified ERK2 (Fig. 1C). An immortalized normal prostate epithelial cell line (RWPE1), which does not express endogenous ERG, was modified to stably express 3×FLAG-ERG (RWPE-ERG). ERG was immunoprecipitated from RWPE-ERG cells with FLAG antibody and immunoblotted with FLAG and phospho-specific antibodies. Both Ser(P)-96 and Ser(P)-215 antibodies detected a band the same size as ERG only from cells expressing ERG and not empty vector (Fig. 1D). Bands corresponding to both Ser(P)-96 and Ser(P)-215 disappeared when ERG S215A or ERG AAAP was expressed, whereas only the Ser(P)-96 band was absent when ERG S96A was expressed (Fig. 1D). Therefore, both in vitro and in cells, Ser-96 phosphorylation is dependent on Ser-215 and the FIFP ERK-docking sequence.
Figure 1.
ERG Ser-96 phosphorylation requires prior Ser-215 phosphorylation and the ERK-binding sequence FIFP. A, depiction of ERG protein showing ERK phosphorylation sites, possible ERK-binding motifs (D and DEF domain), and known structured domains: pointed (PNT) and ETS DNA binding (ETS). Numbering is based on NCBI isoform 1, NP_891548. B, in vitro phosphorylation by ERK2 of ERG and ERG mutants. Coomassie (CBB)-stained SDS gel (bottom panel) and autoradiograph of 32P-labeled (P32) ERG (top panel) are shown. BSA was included in this, but not subsequent kinase reactions, because it had no effect on specificity. C, immunoblot of purified ERG or ERG S96A protein with Ser(P)-96 (S96-P), Ser(P)-215 (S215-P), or pan-ERG antibody after phosphorylation by ERK2 (+ ATP) or mock treatment (− ATP). D, immunoblot with either Ser(P)-96, Ser(P)-215, or FLAG antibodies of whole cell extract (Input) or protein immunoprecipitated (IP) with FLAG antibody from indicated FLAG-ERG expressing RWPE1 cells. E, firefly luciferase levels in RWPE1 cells expressing indicated constructs shown relative to control Renilla luciferase as means and S.E. (n = 3). Protein levels shown by immunoblot (lower panel). F, in vitro ERK2 phosphorylation of indicated constructs as in B. G, IP immunoblot as in D. H, immunoprecipitated immunoblot as in D, but with extracts from RWPE1-ERG cells treated with MEK inhibitor (10 μm U0126) or DMSO for 6 h prior to immunoprecipitation. I, in vitro ERK2 phosphorylation as in B with the indicated proteins. I, kinase assay as in B. J, IP immunoblot as in D, with RWPE1 cells expressing indicated ERG mutants.
To further confirm that ERG Ser-215 phosphorylation is required for subsequent Ser-96 phosphorylation, a phosphomimetic S215D mutant was tested. Using a luciferase reporter construct that we previously demonstrated is activated by ERG (8), we found that ERG S215D activates transcription more strongly than wild-type ERG, consistent with a phosphomimetic function (Fig. 1E). ERG S215D was purified and subjected to in vitro phosphorylation by ERK2. Unlike purified ERG S215A, which was not phosphorylated, ERK phosphorylated purified ERG S215D to a similar extent as ERG S96A, consistent with a single phosphorylation event (Fig. 1F). Loss of this phosphorylation in an S96A/S215D double mutant indicated that this single phosphorylation event occurs at Ser-96 (Fig. 1F). To verify this result in cells, each of these mutants was expressed in RWPE1 cells, and Ser(P)-96 was monitored by immunoblot. In RWPE1 cells, ERG S215D was phosphorylated at Ser-96, but this band was lost in the ERG S96A-S215D double mutant (Fig. 1G). These findings indicate that prior phosphorylation of ERG at Ser-215 is required for Ser-96 phosphorylation both in vitro and in cells.
Although ERK phosphorylated ERG Ser-96 in vitro, it was not clear whether ERK or some other kinase was responsible for Ser-96 phosphorylation in cells. MEK is upstream of ERK in the MAPK pathway and is responsible for phosphorylation and activation of ERK (19, 20). We found that the MEK inhibitor U0126 decreased phosphorylation of ERK and decreased phosphorylation of ERG at both Ser-96 and Ser-215 (Fig. 1H). However, because Ser-96 phosphorylation is dependent on prior Ser-215 phosphorylation, this experiment was repeated with the ERG S215D construct to decouple this dependence. Again, in the ERG S215D mutant, the MEK inhibitor reduced phosphorylation at Ser-96, consistent with ERK phosphorylating both Ser-96 and Ser-215 in RWPE1 cells (Fig. 1H).
In ERG, the FIFP ERK-binding sequence is only 14 amino acid residues from Ser-215, whereas Ser-96 is 133 residues away (Fig. 1A). We hypothesized that conformational changes associated with Ser-215 phosphorylation may either bring the FIFP-bound ERK closer to Ser-96 for phosphorylation or may expose a non-canonical ERK binding site near Ser-96. To differentiate between these models, FIFP was mutated to AAAP in the background of S215D. Interestingly, Ser-96 phosphorylation present in the S215D single mutant was lost in the S215D-AAAP double mutant, both in vitro and in RWPE1 cells (Fig. 1, I and J). These findings are consistent with a model that ERK binds to the FIFP sequence and phosphorylates Ser-215, and then ERK remains bound to FIFP and phosphorylates Ser-96.
Phosphorylation at Ser-215 is associated with secondary and tertiary structural changes in ERG
Our model is that upon phosphorylation at Ser-215, ERG undergoes a conformational change to bring Ser-96 in proximity to ERK bound to FIFP. To test changes in secondary structure of ERG, the far-UV CD spectrum of purified ERG, ERG S215A, and ERG S215D was recorded. Strikingly, ERG and ERG S215A had similar CD spectra, whereas ERG S215D was dramatically different and contained 70% more α-helix than WT (Fig. 2A). These data suggest that Ser-215 phosphorylation induces a more compact conformation by increasing α-helix content at the expense of random coil.
Figure 2.
Phosphorylation at Ser-215 induces conformational changes in ERG. A, far-UV CD spectra of purified ERG and indicated ERG point mutants (top panel). Percentage values of each element were calculated from the CD data using K2D2 algorithm (bottom panel). B, Coomassie-stained SDS-PAGE showing partial chymotrypsin (left panel) and trypsin (right panel) digestion of purified ERG and indicated point mutants. C, chymotrypsin digestion of the indicated ERG proteins as in B with or without prior in vitro phosphorylation using ERK2.
To determine tertiary structural changes, purified ERG, ERG S215A, and ERG S215D were subjected to partial proteolytic digestion with trypsin and chymotrypsin (Fig. 2B). ERG S215D displayed protected fragments in both chymotrypsin (Fig. 2B, left panel, peptides indicated by arrow, compare lanes 4 and 5) and trypsin (Fig. 2B, right panel, peptides indicated by square bracket, compare lanes 4 and 5) digestion that were quickly lost in ERG and ERG S215A. This indicates a structural difference caused by the phosphomimetic mutation. To directly monitor Ser-215 phosphorylation induced tertiary structural changes, ERG was phosphorylated by ERK2 and subjected to chymotrypsin partial digestion. A protected band similar to that of S215D-digested products (Fig. 2B) was also observed in phosphorylated ERG (peptides indicated by arrow, compare lanes 2 and 4 in Fig. 2C). Under similar conditions, digestion of ERG S215A did not show any changes in proteolytic products (peptides indicated by arrow, compare lanes 6 and 8 in Fig. 2C). Overall these findings suggest that ERG undergoes secondary and tertiary structural changes when Ser-215 is phosphorylated.
Ser-96 phosphorylation potentiates transcriptional activation and cell migration functions of ERG
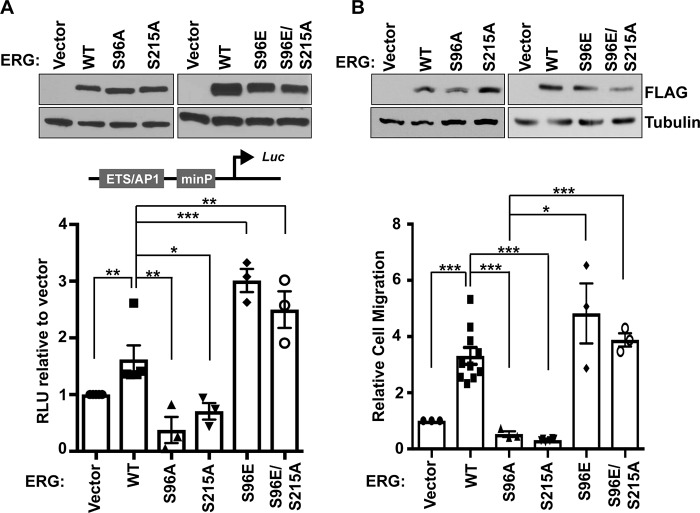
ERG can activate genes that induce cell migration by binding to cis-regulatory sequences consisting of neighboring ETS and AP-1 transcription factor–binding sites (6). To measure phosphorylation-induced transcriptional activation, a firefly luciferase reporter driven by an ETS/AP-1–containing enhancer was used (8). This ETS/AP-1 reporter was co-transfected with constructs expressing 3×FLAG-ERG and its mutants into RWPE1 cells. ERG activated the reporter compared with vector control (Fig. 3A). Reporter activation decreased with S96A and S215A mutations, indicating that these residues are important for transcriptional activity of ERG (Fig. 3A). Interestingly, an increase in reporter activity was observed with phosphomimetic ERG S96E, and this was unchanged with ERG S96E/S215A double mutant (Fig. 3A). Because genes regulated by ETS/AP-1 enhancers regulate cell migration, we measured the ability of WT and mutants to drive cell migration when stably expressed in RWPE1 cells (Fig. 3B, top). S96A and S215A mutations inhibited the ability of ERG to promote cell migration (Fig. 3B). Similar to the luciferase reporter data, an increase in cell migration with phosphomimetic ERG S96E was unchanged in the ERG S96E/S215A double mutant (Fig. 3B). ERG and mutant ERG expression did not affect cell proliferation (supplemental Fig. S2). Together, these findings suggest that phosphorylation at Ser-96 potentiates cell migration and transactivation functions of ERG, whereas Ser-215 phosphorylation is only required to allow Ser-96 phosphorylation.
Figure 3.
Ser-96 phosphorylation is necessary for transactivation and cell migration function of ERG. A, transient transfection of RWPE1 cells with the indicated FLAG-ERG proteins shows similar expression (top panel). Ratio of firefly to Renilla (control) luciferase in RWPE1 cells transfected with ETS-AP1–containing firefly FHL3 enhancer reporter and indicated ERG construct, normalized to vector only (bottom panel). The values are means and S.E. (n = 3). B, RWPE1 cells stably expressed the indicated FLAG-ERG proteins. Protein levels were determined by immunoblot (top panel). Transwell migration (bottom panel) is shown relative to RWPE1 cells with empty vector as mean and S.E. (n = 3). All p values were determined by t test: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
ERK phosphorylates TMPRSS2-ERG gene fusion protein products
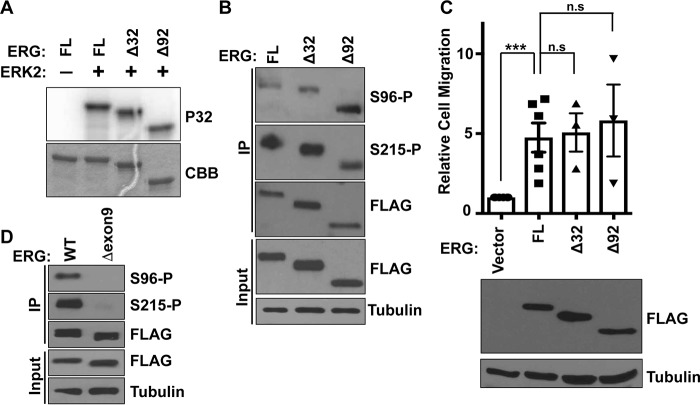
TMPRSS2-ERG fusions can express either full-length ERG or N-terminal deletions of 32 (most common) or 92 (less frequent) amino acids (2, 21). Because these deletions result in loss of a predicted D motif (Fig. 1A) and are in close proximity to Ser-96, we tested whether their absence altered phosphorylation. In vitro, ERK2 phosphorylated ERGΔ32 and ERGΔ92 similarly to full-length ERG (Fig. 4A). Similarly, Ser(P)-96 and Ser(P)-215 signal was unchanged in both deletion mutants of ERG expressed in prostate cells (Fig. 4B). Because phosphorylation at Ser-96 induces cell migration, we checked the ability of deletion mutants to drive cell migration. Both Δ32 and Δ92 ERG expressed at similar levels to full-length ERG in RWPE1 cells, and both induced cell migration similar to full-length ERG (Fig. 4C). These data suggest that N-terminal truncations found in prostate tumors have no effect on ERK-mediated phosphorylation and the cell migration function of ERG.
Figure 4.
Comparison of common TMPRSS2-ERG fusion products and alternative splicing isoforms. A, in vitro phosphorylation of full-length (FL) and the indicated N-terminal deletions of ERG by ERK2. Coomassie (CBB) staining of gel (bottom panel) and autoradiograph of 32P-labeled (P32) ERG (top panel) are shown. B, immunoblot using Ser(P)-96 or Ser(P)-215 specific antibodies or FLAG antibody of whole cell extract (input), protein immunoprecipitated (IP) with FLAG antibody from RWPE1 cells expressing 3×FLAG-ERG (FL), N-terminal deletions 3×FLAG-Δ32ERG (Δ32), or 3×FLAG Δ92ERG (Δ92). C, Transwell migration of RWPE1 cells stably expressing indicated 3×FLAG-ERG proteins, shown relative to RWPE1 cells with empty vector as mean and S.E. (n = 3). p values were determined by t test: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Expression shown by immunoblot. D, FLAG IP immunoblot with indicated antibodies from RWPE1 cells expressing ERG or ERG lacking exon 9.
Some ERG splicing isoforms lack exon 9 that codes for 24 amino acids including the ERK-binding motif “FIFP” (DEF motif). Inclusion of this exon in TMPRSS2-ERG fusion transcripts has been shown to be critical for the cell migration function of ERG (22). Furthermore, multiple studies have shown that the expression of TMPRSS2-ERG fusion transcripts with exon 9 was higher in tumors than transcripts that lacked this exon (22–24). We previously showed that an ERG isoform that lacks exon 9, and the FIFP motif is not phosphorylated at Ser-215 and fails to induce cell migration (12). As expected, Ser-96 was also not phosphorylated when ERG lacking exon 9 was expressed in RWPE1 cells (Fig. 4D). Therefore, these data indicate a critical role for exon 9 and the FIFP motif in ERG function.
ERK disrupts ERG-PRC2 interaction by phosphorylation at Ser-96
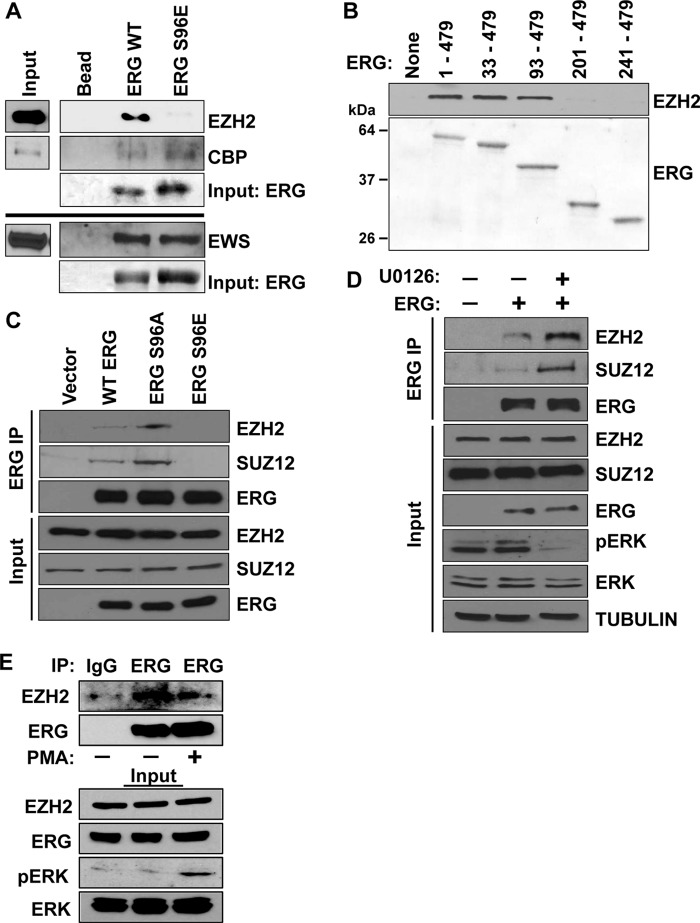
Phosphorylation can alter the affinity of ETS proteins to their co-regulators (11, 25). To test whether phosphorylation at Ser-96 alters interactions with known ERG co-regulators, we incubated purified ERG and ERG S96E with nuclear extracts from the prostate cancer cell line PC3 and monitored binding of co-regulators with specific antibodies. Interaction with two known co-activators, EWS (8) and CBP (14), was unchanged between ERG and ERG S96E (Fig. 5A). We next considered co-repressors. ERG can recruit EZH2, the enzymatic component of PRC2 to target genes to repress expression (16, 17). In our assay, ERG bound to EZH2, but ERG S96E did not, suggesting that phosphorylation could block EZH2 binding (Fig. 5A). To map the region of ERG that interacts with EZH2, ERG deletion mutants were purified and incubated with PC3 nuclear extract. EZH2 interacted with ERG Δ32 and ERG Δ92, whereas the EZH2 interaction was lost in an ERG Δ200 deletion (Fig. 5B). Thus the region between residues 93–200 in ERG that includes the Ser-96 phosphorylation site and the pointed domain is necessary for EZH2 binding.
Figure 5.
Phosphorylation of ERG at Ser-96 decreases its affinity for the PRC2 complex. A, pulldown of EZH2/CBP/EWS from PC3 nuclear extracts. The indicated N-terminal His-tagged ERG constructs were immobilized to beads, incubated with PC3 nuclear extract, and washed. EZH2/CBP/EWS binding was visualized by immunoblot. Ponceau stain of the same blots show input amount of His-ERG. B, pulldown of EZH2 as in A but with full-length and indicated deletion mutants of ERG. EZH2/CBP/EWS binding was visualized by immunoblot (top panels, IP). Ponceau stain shows amount of His-ERG present (bottom panels, Input). C, co-immunoprecipitation using ERG antibody from nuclear extracts prepared from RWPE1 cells expressing vector or indicated FLAG-tagged WT and mutants of ERG, followed by immunoblotting for EZH2, SUZ12, and FLAG. D, co-immunoprecipitation of ERG as in C but with cells treated either with 20 μm U0126 or mock treatment for 6 h. E, VCaP cells with or without PMA treatment (1 h 200 nm) were immunoprecipitated with ERG antibody or IgG control and immunoblotted for indicated proteins.
To validate the phosphorylation regulated binding of EZH2 in cells, we immunoprecipitated FLAG-tagged ERG and its mutants from RWPE1 cells and probed for EZH2 and the PRC2 complex subunit SUZ12. Interestingly the phospho-null mutant S96A bound both EZH2 and SUZ12 better than wild-type ERG, whereas the interaction was lost in the S96E mutant (Fig. 5C). To directly test that ERK regulates PRC2 binding to ERG, RWPE1-ERG cells were treated with the MEK inhibitor to decrease ERK activation. Upon MEK inhibition, phosphorylated (activated) ERK decreased, and by co-immunoprecipitation, the interaction between ERG and both EZH2 and SUZ12 increased (Fig. 5D). To test this interaction in prostate cancer cells with a TMPRSS2/ERG fusion gene, VCaP cells were treated with the phorbol ester PMA for 1 h to activate ERK. This treatment resulted in decreased interaction between ERG and EZH2 (Fig. 5E). Collectively, these data indicate that PRC2 complex binding to ERG is inhibited by ERK phosphorylation of ERG at Ser-96.
Dissociation of EZH2 from the ERG cistrome is critical for activation of ERG target genes
To test whether Ser-96 phosphorylation alters genomic binding of ERG, we compared ChIP-seq of ERG, ERG S96A, and ERG S96E expressed in RWPE1 cells using an anti-ERG antibody. A total of 4565 regions were called as ERG-bound in one or more of these three data sets. A comparison of ERG, ERG S96A, and ERG S96E across all of these possible ERG-bound regions revealed co-occurrence at most sites (Fig. 6A). We then compared these data sets at 2314 genomic regions previously identified as ERG-bound by anti-FLAG ChIP-seq in 3×FLAG-ERG–expressing RWPE1 cells (6). ERG, ERG S96A, and ERG S96E bound these 2314 ERG targets to a strikingly similar extent (Fig. 6B and supplemental Fig. S3A). Therefore, we concluded that Ser-96 phosphorylation does not significantly alter the ability of ERG to bind chromatin.
Figure 6.
Phosphorylation-induced dissociation of EZH2 increases transcriptional activation function of ERG. A, ChIP with anti-ERG antibody in RWPE1 cells expressing ERG-WT, ERG-S96A, or ERG S96E. Enrichment is shown at all 4565 regions that were called as peaks in any of the three data sets. B, ratio of ERG ChIP-seq reads to input reads from RWPE1 cells expressing indicated version of ERG is shown averaged across 2314 regions previously reported as ERG-bound in RWPE1 cells (6). C, comparison of EZH2 ChIP-seq in RWPE1 cells expressing indicated constructs, as in B. D, average ratio of EZH2 ChIP-seq reads to input reads, as in C, but at the 3116 regions bound by EZH2 in RWPE1 cells expressing empty vector. E, box plots show expression levels of 451 genes significantly activated by ERG-WT in RWPE1 compared with vector only. Boxes show median and 10th and 90th percentiles; bars encompass all values except outliers.
To test whether phosphorylation at Ser-96 affects recruitment of EZH2 to the ERG cistrome, ChIP-seq was used to map EZH2 binding in RWPE1 cells expressing empty vector, ERG, ERG S96A, or ERG S96E. Strikingly, at the 2314 previously identified 3×FLAG-ERG target sites, EZH2 enrichment was drastically increased in RWPE-ERG S96A cells compared with RWPE1 empty vector, RWPE1-ERG, or RWPE1-ERG S96E cells (Fig. 6C and supplemental Fig. S3A). In contrast, EZH2 enrichment at ERG-independent sites (sites with EZH2 genomic occupancy in RWPE1 cells expressing empty vector) was similar in RWPE-ERG, RWPE-ERG S96A, and RWPE-ERG S96E cells (Fig. 6D and supplemental Fig. S3B), indicating that the increase in EZH2 occupancy when ERG S96A is present was limited to ERG target sites. Overall, these data indicate that blocking ERK phosphorylation of ERG at Ser-96 results in EZH2 occupancy across the ERG cistrome.
The canonical role of EZH2 and the PRC2 complex is transcriptional repression (26). In this case, genes activated by ERG would be turned off when the PRC2 complex is recruited to ERG targets. To test this hypothesis, we compared gene expression by RNA-seq in RWPE1 empty vector, RWPE-ERG, RWPE-ERG S96A, and RWPE-ERG S96E cells. We analyzed a list of 451 ERG target genes (supplemental Table S1) that were both significantly up-regulated by ERG expression in RWPE1 cells (greater than 1.5-fold change and adjusted p value < 0.05) and had a neighboring ERG-bound region in either of two published RWPE1-ERG ChIP-seq data sets (6, 8). These 451 ERG target genes showed a significant (p < 0.001) loss of activation in RWPE-ERG S96A cells compared with RWPE-ERG cells but maintained activation in RWPE-ERG S96E cells (Fig. 6E and supplemental Table S1). In fact, 91% of these genes had lower expression in cells expressing ERG S96A compared with ERG (supplemental Fig. S3C). These findings indicate that the EZH2 and the PRC2 complex repress activation of an ERG-induced gene expression program until ERG is phosphorylated by ERK.
Discussion
Here, we find that phosphorylation of ERG Ser-215 induces a conformational change that facilitates Ser-96 phosphorylation. Further, ERK utilizes the same FIFP docking motif to phosphorylate both Ser-215 and Ser-96 residues. Interestingly, Ser-96 phosphorylation was required for transactivation and cell migration functions of ERG via dissociation of the PRC2 complex, whereas Ser-215 was required to allow Ser-96 phosphorylation. Together, these findings indicate a mechanism for ERK-mediated activation of ERG function in prostate cells.
In prostate cancer, somatic mutations that activate RAS-MAPK pathway are rare (27). However, we previously found that ERG is phosphorylated by ERK in prostate cells with a wild-type RAS/MAPK pathway and with low levels of ERK activity (12). This low level ERK activation could be due to growth factors in the media or a feedback loop driven by ERG itself (28). The ability of ERK to phosphorylate ERG even at low levels is due to the high-affinity ERK-binding motif (FIFP) and contrasts with other ETS proteins such as ETS1 that have lower affinity ERK-binding domains and require higher levels of ERK activity for their function (12). Therefore, it may be that low-level ERK activity promoted by the tumor microenvironment could be necessary for ERG function in tumors.
It has been previously proposed that ERG recruits the PRC2 complex to catalyze H3K27me3 modification, resulting in repression of ERG target genes (16, 17). Here we find that ERG phosphorylation at Ser-96 by ERK dissociates PRC2 from ERG targets to activate expression of genes. It is possible that both activating and repressive functions of ERG occur at the same time, but at distinct target genes. It is known that ERK can be recruited to specific cis-regulatory elements. In embryonic stem cells, ERK2 binds chromatin near ETS binding sequences and is associated with gene activation (29). Strikingly, this study found that cis-regulatory elements that have ETS binding sequences but lack ERK2 are bound by EZH2 and SUZ12. Therefore, it is interesting to speculate that the difference between an ERG-activated and an ERG-repressed gene might be the presence of ERK at the cis-regulatory element and the subsequent phosphorylation of ERG at Ser-215 and Ser-96.
In addition to dissociation of the PRC2 complex from ERG, we cannot rule out that Ser-96 phosphorylation may trigger additional post-translational modifications and/or may enhance binding of co-activators to ERG. In leukemia cells, CBP/p300 can add acetyl groups to Lys-89 and Lys-92 residues of ERG. Diacetylation of ERG results in recruitment of the co-activator BRD4, which promotes transcriptional activation of ERG target genes (14, 15). There is evidence that ERG Ser-96 is also phosphorylated in leukemia cells (30). The proximity of the p300/CBP acetylation sites and the Ser-96 phosphorylation site suggests that interdependency between Ser-96 phosphorylation and acetylation and recruitment of BRD4 should be investigated in the future.
ERG gene fusions are the most common genetic lesions in prostate cancer and are implicated in both initiation and cancer progression (31, 32). Thus elucidating mechanisms by which ERG can induce prostate cancer is critical for targeted therapeutics. Our findings demonstrate a novel mechanism by which ERK-mediated sequential phosphorylation activates transcriptional activity of ERG that contributes to prostate cell migration. Recently, several novel inhibitors targeting Ras-ERK pathway have shown significant clinical outcome against metastatic melanoma (33). Because ERK-mediated phosphorylation is critical for ERG functions in the prostate, these inhibitors could be tested for efficacy in ERG-positive prostate cancer in the future.
Experimental procedures
Protein purification and immunoblotting
Purification of full-length ETS proteins was previously described (12). Deletions and point mutations of ERG were cloned into pET28a (Novagen), expressed in Escherichia coli BL21 pRIL, and purified using nickel-nitrilotriacetic acid-agarose resin (Qiagen) using the same protocol as the full-length ETS proteins. Activated ERK2 enzyme was purified from bacteria as previously described (12). Antibodies for immunoblotting were ERG (catalog no. CM 421; Biocare), EWS (catalog no. sc-28327; Santa Cruz Biotechnology), EZH2 (catalog no. 5246; Cell Signaling), SUZ12 (catalog no. 3737; Cell signaling), ERK1/2 (catalog no. Sc-94; Santa Cruz Biotechnology), pERK1/2 (catalog no. Sc-7383; Santa Cruz Biotechnology), tubulin (catalog no. T9026; Sigma), and FLAG (catalog no. F1804; Sigma). Total protein extract from equal number of cells was separated on SDS-PAGE gels and immunoblotted by standard procedures.
Phosphorylation of ETS proteins
Kinase reactions were performed as described previously (12). Briefly, 5 nm ERK2 was incubated with 1.5 μm ERG protein in kinase buffer (25 mm Tris, pH 7.9, 1 mm DTT, 10 mm magnesium acetate, 2 mm ATP, 12 mm β-glycerophosphate, 0.5 mm Na3VO4, 5% glycerol, 87.5 mm KCl, and 8 μCi of 3 Ci/μmol [γ-32P]ATP) for 30 min at 30 °C. The reactions were stopped by the addition of 4% SDS loading dye and electrophoresed on a SDS-PAGE gel. The gels were stained with Coomassie Blue, and radioactivity was detected by PhosphorImaging (Amersham Biosciences Typhoon 9210).
Circular dichroism spectroscopy and partial protease digestion
Purified ERG and its mutants were diluted in 10 mm potassium phosphate buffer, pH 7.4, to a concentration of 100 μg/ml. CD spectra were recorded in Jasco J-715 spectropolarimeter using a 1-mm quartz cuvette with a bandwidth of 1 nm. The spectra thus obtained were smoothed and corrected for buffer contribution. The percentage values of each structural element in WT and its point mutants of ERG were calculated from the CD data using K2D2 algorithm (34). Limited digestion of 2 μg of purified ERG and its mutants was carried out with 5 ng of trypsin (catalog no. 6502; Calbiochem) or 5 ng of chymotrypsin (catalog no. C7752; Sigma) at room temperature for the indicated time points in trypsin reaction buffer (20 mm Tris, pH 7.5, and 100 mm NaCl) or chymotrypsin reaction buffer (25 mm Tris, pH 7.1, and 100 mm NaCl), respectively. Digested protein products were run on SDS-PAGE gel and stained with Coomassie Blue.
Viral vector expression in RWPE1 cells and phenotypic assays
Cell lines were cultured according to ATCC guidelines. N-terminal 3×FLAG ERG and its point mutants were cloned into HNRNPA2B1 promoter containing pLHCX vector (Clontech) and stably expressed in RWPE1 cells via retrovirus as described previously (8). Migration assays were carried out as previously described (8). Briefly, 5 × 104 cells were introduced to the Transwell (8-μm pore size; BD Bioscience) and incubated for 60–70 h. The migrated cells are reported as the mean of at least three biological replicates with two technical replicates each. Cell proliferation was measured using MTT reagent (Calbiochem) as previously described (8). Briefly, ∼1500 cells were seeded in quadruplicate onto a 96-well tissue culture plate and incubated at 37 °C for up to 5 days. MTT reagent (5 mg/ml in PBS) was added to cells each day and incubated for at least 4 h, and then the medium was removed, and DMSO was added. Absorbance (600 nm) was measured using a micro-plate reader ELx8200 (Biotek Instruments). Proliferation is reported as the mean of at least three biological replicates with four technical replicates each.
Luciferase reporter assay
Dual luciferase reporter assay (Promega) was used to measure luciferase activity as previously described (8). A pGL4.25 firefly reporter plasmid containing a 474-bp (chr1:38465034–38465507, hg19) FHL3 enhancer with ETS-AP1 sequence was used. In brief, ∼2.5 × 105 RWPE1 cells were plated in a 6-well tissue culture plate. After 24 h, the cells were co-transfected with ERG wild-type and point mutants overexpression, firefly, and Renilla (pRL-TK) plasmids using Trans-IT 2020 transfection reagent (Mirus). After 24 h of incubation, the cells were lysed using passive lysis buffer, and luciferase activity was measured using Appliskan microplate reader (Thermo Scientific). Firefly luciferase values were normalized to Renilla values.
Immunoprecipitation, co-immunoprecipitation, and pulldown assays
RWPE1 cells were treated with MEK1/2 inhibitor (10 μm, catalog no. U0126; Cell Signaling) or DMSO. Post-treatment, the cells were washed with PBS and homogenized in RIPA buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 1% sodium deoxycholic acid, 0.1% SDS, 50 mm β-glycerophosphate, 10 mm NaF, 0.5 mm DTT) through sonication. Protein lysates were added to either anti-FLAG M2 magnetic beads (Sigma) or anti-ERG–conjugated magnetic beads and rotated overnight. The beads were washed four times with RIPA buffer and immunoblotted. The nuclei were isolated from RWPE1 cells using cell lysis buffer (50 mm Hepes-KOH, pH 8.0, 140 mm NaCl, 1 mm EDTA, 0.5 mm EGTA, 10% glycerol, 0.5% Nonidet P-40, 0.25% Triton X-100). The nuclei were lysed with Nonidet P-40 lysis buffer (50 mm Tris-HCl, pH 7.4, 250 mm NaCl, 5 mm EDTA, 50 mm NaF, and 1% Nonidet P-40). Nuclear lysates were centrifuged at 15,000 rpm to obtain clear supernatants. The indicated antibodies were added to supernatants and rotated overnight in a rocker. 50 μl of Dynabeads (Invitrogen) were added and rotated for 2 h in a rocker. The beads were washed four times with Nonidet P-40 lysis buffer and immunoblotted. VCaP cells were treated with PMA (200 nm; Sigma–Aldrich) or DMSO for 1 h. Post-treatment, the cells were washed with PBS and homogenized in Nonidet P-40 lysis buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 1 mm EDTA, 0.5% Nonidet P-40, 50 mm NaF, 1/250 protease inhibitor) through sonication, and protein lysates were rotated overnight with anti-ERG–conjugated magnetic beads and washed with 1× TBS four times.
His-tagged purified ETS and its deletion fragments were diluted in 300 μl of binding/wash buffer (100 mm sodium phosphate, pH 8.0, 600 mm NaCl, and 0.02% Tween 20) and incubated with 2.5 μl of His tag Dynabeads (Life Technologies) for 2 h in a rocker. Unbound ETS proteins were removed by washing the beads two times with binding/wash buffer. To the bead-bound ETS proteins, ∼14 μg of PC3 nuclear extracts (catalog no. sc-2152; Santa Cruz Biotechnology) were added and rotated for 1–2 h. The beads were washed four times with Nonidet P-40 lysis buffer, and bead-bound proteins were eluted using SDS protein loading dye. Eluted proteins were separated in a SDS gel and transferred to a nitrocellulose membrane, which was Ponceau-stained (0.1% in 5% acetic acid) and immunoblotted.
Chromatin immunoprecipitation and ChIP-seq
ChIP of indicated proteins were performed from RWPE1 cells as described previously (6), using the same antibodies described for immunoblotting. ChIP enrichment was assessed using quantitative PCR and standard curves to measure copy number of the locus of interest and one negative control loci using DNA oligonucleotides previously described (8). For next-generation sequencing, purified ChIP DNA from at least three biological replicates were pooled and sheared in a Diagenode Bioruptor to obtain DNA fragments of ∼150 bp. Sheared DNA was end-repaired and ligated to indexing primers provided in Truseq sample preparation kit (Illumina). DNA libraries were pooled and sequenced in an Illumina Hiseq 2000 or Next Seq 500 sequencer according to the manufacturer's protocol.
Reads from ChIP and input controls were aligned to human genome (hg19; UCSC). ERG bound regions were identified using Peak calling program Useq (peak shift, 250 bp; sliding window, 500 bp) (35). The ERG ChIP-seq in RWPE1-ERG cells was previously reported (8), and all other ChIP-seq data sets can be found in the Gene Expression Omnibus under accession number GSE86232.
RNA-seq and analysis
Total RNA from three biological replicates of RWPE1 cells expressing ERG-S96A was isolated using RNeasy kit (Qiagen) according to the manufacturer's protocol. Total RNA was DNase-treated with RNase-Free DNase set (Qiagen) according to manufacturer's protocol. Next, poly(A) containing RNA was purified using oligo(dT) beads (Invitrogen). cDNAs of poly(A) selected RNA was generated with SuperScript III reverse transcriptase enzyme using random hexamers (Invitrogen). Second strand synthesis from cDNAs was carried out with E. coli DNA ligase and E. coli DNA polymerase I (New England BioLabs). The double-stranded cDNAs were sheared to ∼150 nucleotide fragments using a Diagenode BioRuptor, and the size was confirmed by DNA gel electrophoresis. Following shearing DNA, the library was prepared as described under chromatin immunoprecipitation protocol. Differential gene expression analysis was carried out with Tuxedo Suite RNA sequencing pipeline (36). Sequence reads obtained from an Illumina sequencer was mapped to the human genome (hg19; UCSC) using Bowtie2. Differential gene expression was determined using Cuffdiff. RWPE1-vector and RWPE1-ERG data sets were previously reported (8). RWPE1-S96A and RWPE1-S69E data are available at Gene Expression Omnibus accession number GSE86232.
Author contributions
V. K., B. G. S., and P. C. H. designed the research; V. K., B. G. S., and P. B. D. performed the research; V. K., B. G. S., P. B. D., and P. C. H. analyzed the data; and V. K. and P. C. H. wrote the paper.
Supplementary Material
Acknowledgments
We thank the Indiana University Center for Genomics and Informatics for help with high-throughput sequencing and Charles Dann III and Sundharraman Subramanaian for assistance with circular dichroism.
This work was supported by American Cancer Society Research Scholar Award RSG-13-215-01-DMC, by the Indiana University Vice Provost for Research through the Faculty Research Support Program, and by Indiana University Simon Cancer Center P30 Support Grant P30CA82709 (to P. C. H.). This work was also supported by the Indiana University Graduate Training Program in Quantitative and Chemical Biology under Award T32 GM109825 (to B. G. S.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Table S1 and Figs. S1–S3.
ChIP-seq and RNA-seq data from this study are deposited in the NCBI7 Gene Expression Omnibus with accession number GSE86232.
- PRC2
- polycomb repressive complex 2
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PMA
- phorbol 12-myristate 13-acetate.
References
- 1. Hollenhorst P. C., McIntosh L. P., and Graves B. J. (2011) Genomic and biochemical insights into the specificity of ETS transcription factors. Annu. Rev. Biochem. 80, 437–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tomlins S. A., Rhodes D. R., Perner S., Dhanasekaran S. M., Mehra R., Sun X. W., Varambally S., Cao X., Tchinda J., Kuefer R., Lee C., Montie J. E., Shah R. B., Pienta K. J., Rubin M. A., et al. (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 [DOI] [PubMed] [Google Scholar]
- 3. Carver B. S., Tran J., Gopalan A., Chen Z., Shaikh S., Carracedo A., Alimonti A., Nardella C., Varmeh S., Scardino P. T., Cordon-Cardo C., Gerald W., and Pandolfi P. P. (2009) Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat. Genet. 41, 619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. King J. C., Xu J., Wongvipat J., Hieronymus H., Carver B. S., Leung D. H., Taylor B. S., Sander C., Cardiff R. D., Couto S. S., Gerald W. L., and Sawyers C. L. (2009) Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat. Genet. 41, 524–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zong Y., Xin L., Goldstein A. S., Lawson D. A., Teitell M. A., and Witte O. N. (2009) ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells. Proc. Natl. Acad. Sci. U.S.A. 106, 12465–12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hollenhorst P. C., Ferris M. W., Hull M. A., Chae H., Kim S., and Graves B. J. (2011) Oncogenic ETS proteins mimic activated RAS/MAPK signaling in prostate cells. Genes Dev. 25, 2147–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y., Chi P., Rockowitz S., Iaquinta P. J., Shamu T., Shukla S., Gao D., Sirota I., Carver B. S., Wongvipat J., Scher H. I., Zheng D., and Sawyers C. L. (2013) ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nat. Med. 19, 1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kedage V., Selvaraj N., Nicholas T. R., Budka J. A., Plotnik J. P., Jerde T. J., and Hollenhorst P. C. (2016) An interaction with Ewing's sarcoma breakpoint protein EWS defines a specific oncogenic mechanism of ETS factors rearranged in prostate cancer. Cell Rep. 17, 1289–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ducret C., Maira S. M., Dierich A., and Wasylyk B. (1999) The net repressor is regulated by nuclear export in response to anisomycin, UV, and heat shock. Mol. Cell. Biol. 19, 7076–7087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carlson S. M., Chouinard C. R., Labadorf A., Lam C. J., Schmelzle K., Fraenkel E., and White F. M. (2011) Large-scale discovery of ERK2 substrates identifies ERK-mediated transcriptional regulation by ETV3. Sci. Signal. 4, rs11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foulds C. E., Nelson M. L., Blaszczak A. G., and Graves B. J. (2004) Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Mol. Cell. Biol. 24, 10954–10964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Selvaraj N., Kedage V., and Hollenhorst P. C. (2015) Comparison of MAPK specificity across the ETS transcription factor family identifies a high-affinity ERK interaction required for ERG function in prostate cells. Cell Commun. Signal. 13, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brenner J. C., Ateeq B., Li Y., Yocum A. K., Cao Q., Asangani I. A., Patel S., Wang X., Liang H., Yu J., Palanisamy N., Siddiqui J., Yan W., Cao X., Mehra R., et al. (2011) Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell 19, 664–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roe J. S., Mercan F., Rivera K., Pappin D. J., and Vakoc C. R. (2015) BET bromodomain inhibition suppresses the function of hematopoietic transcription factors in acute myeloid leukemia. Mol. Cell 58, 1028–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blee A. M., Liu S., Wang L., and Huang H. (2016) BET bromodomain-mediated interaction between ERG and BRD4 promotes prostate cancer cell invasion. Oncotarget 7, 38319–38332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu J., Mani R. S., Cao Q., Brenner C. J., Cao X., Wang X., Wu L., Li J., Hu M., Gong Y., Cheng H., Laxman B., Vellaichamy A., Shankar S., Li Y., et al. (2010) An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 17, 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chng K. R., Chang C. W., Tan S. K., Yang C., Hong S. Z., Sng N. Y., and Cheung E. (2012) A transcriptional repressor co-regulatory network governing androgen response in prostate cancers. EMBO J. 31, 2810–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang S. H., Sharrocks A. D., and Whitmarsh A. J. (2003) Transcriptional regulation by the MAP kinase signaling cascades. Gene 320, 3–21 [DOI] [PubMed] [Google Scholar]
- 19. Gómez N., and Cohen P. (1991) Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature 353, 170–173 [DOI] [PubMed] [Google Scholar]
- 20. Chang F., Steelman L. S., Lee J. T., Shelton J. G., Navolanic P. M., Blalock W. L., Franklin R. A., and McCubrey J. A. (2003) Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 17, 1263–1293 [DOI] [PubMed] [Google Scholar]
- 21. Wang J., Cai Y., Ren C., and Ittmann M. (2006) Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res. 66, 8347–8351 [DOI] [PubMed] [Google Scholar]
- 22. Wang J., Cai Y., Yu W., Ren C., Spencer D. M., and Ittmann M. (2008) Pleiotropic biological activities of alternatively spliced TMPRSS2/ERG fusion gene transcripts. Cancer Res. 68, 8516–8524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagen R. M., Adamo P., Karamat S., Oxley J., Aning J. J., Gillatt D., Persad R., Ladomery M. R., and Rhodes A. (2014) Quantitative analysis of ERG expression and its splice isoforms in formalin-fixed, paraffin-embedded prostate cancer samples: association with seminal vesicle invasion and biochemical recurrence. Am. J. Clin. Pathol. 142, 533–540 [DOI] [PubMed] [Google Scholar]
- 24. Hu Y., Dobi A., Sreenath T., Cook C., Tadase A. Y., Ravindranath L., Cullen J., Furusato B., Chen Y., Thangapazham R. L., Mohamed A., Sun C., Sesterhenn I. A., McLeod D. G., Petrovics G., et al. (2008) Delineation of TMPRSS2-ERG splice variants in prostate cancer. Clin. Cancer Res. 14, 4719–4725 [DOI] [PubMed] [Google Scholar]
- 25. Li Q. J., Yang S. H., Maeda Y., Sladek F. M., Sharrocks A. D., and Martins-Green M. (2003) MAP kinase phosphorylation-dependent activation of Elk-1 leads to activation of the co-activator p300. EMBO J. 22, 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., Jones R. S., and Zhang Y. (2002) Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science 298, 1039–1043 [DOI] [PubMed] [Google Scholar]
- 27. Grasso C. S., Wu Y. M., Robinson D. R., Cao X., Dhanasekaran S. M., Khan A. P., Quist M. J., Jing X., Lonigro R. J., Brenner J. C., Asangani I. A., Ateeq B., Chun S. Y., Siddiqui J., Sam L., et al. (2012) The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Selvaraj N., Budka J. A., Ferris M. W., Jerde T. J., and Hollenhorst P. C. (2014) Prostate cancer ETS rearrangements switch a cell migration gene expression program from RAS/ERK to PI3K/AKT regulation. Mol. Cancer 13, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Göke J., Chan Y. S., Yan J., Vingron M., and Ng H. H. (2013) Genome-wide kinase-chromatin interactions reveal the regulatory network of ERK signaling in human embryonic stem cells. Mol. Cell 50, 844–855 [DOI] [PubMed] [Google Scholar]
- 30. Huang Y., Thoms J. A., Tursky M. L., Knezevic K., Beck D., Chandrakanthan V., Suryani S., Olivier J., Boulton A., Glaros E. N., Thomas S. R., Lock R. B., MacKenzie K. L., Bushweller J. H., Wong J. W., et al. (2016) MAPK/ERK2 phosphorylates ERG at serine 283 in leukemic cells and promotes stem cell signatures and cell proliferation. Leukemia 30, 1552–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klezovitch O., Risk M., Coleman I., Lucas J. M., Null M., True L. D., Nelson P. S., and Vasioukhin V. (2008) A causal role for ERG in neoplastic transformation of prostate epithelium. Proc. Natl. Acad. Sci. U.S.A. 105, 2105–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tomlins S. A., Mehra R., Rhodes D. R., Cao X., Wang L., Dhanasekaran S. M., Kalyana-Sundaram S., Wei J. T., Rubin M. A., Pienta K. J., Shah R. B., and Chinnaiyan A. M. (2007) Integrative molecular concept modeling of prostate cancer progression. Nat. Genet. 39, 41–51 [DOI] [PubMed] [Google Scholar]
- 33. Samatar A. A., and Poulikakos P. I. (2014) Targeting RAS-ERK signalling in cancer: promises and challenges. Nat. Rev. Drug Discov. 13, 928–942 [DOI] [PubMed] [Google Scholar]
- 34. Perez-Iratxeta C., and Andrade-Navarro M. A. (2008) K2D2: estimation of protein secondary structure from circular dichroism spectra. BMC Struct. Biol. 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nix D. A., Courdy S. J., and Boucher K. M. (2008) Empirical methods for controlling false positives and estimating confidence in ChIP-Seq peaks. BMC Bioinformatics 9, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trapnell C., Hendrickson D. G., Sauvageau M., Goff L., Rinn J. L., and Pachter L. (2013) Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31, 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.