Abstract
Sirtuin 3 (SIRT3) deacetylates and regulates many mitochondrial proteins to maintain health, but its functions are depressed in aging and obesity. The best-studied sirtuin, SIRT1, counteracts aging- and obesity-related diseases by deacetylating many proteins, but whether SIRT1 has a role in deacetylating and altering the function of SIRT3 is unknown. Here we show that SIRT3 is reversibly acetylated in the mitochondria and unexpectedly is a target of SIRT1 deacetylation. SIRT3 is hyperacetylated in aged and obese mice, in which SIRT1 activity is low, and SIRT3 acetylation at Lys57 inhibits its deacetylase activity and promotes protein degradation. Adenovirus-mediated expression of SIRT3 or an acetylation-defective SIRT3-K57R mutant in diet-induced obese mice decreased acetylation of mitochondrial long-chain acyl-CoA dehydrogenase, a known SIRT3 deacetylation target; improved fatty acid β-oxidation; and ameliorated liver steatosis and glucose intolerance. These SIRT3-mediated beneficial effects were not observed with an acetylation-mimic SIRT3-K57Q mutant. Our findings reveal an unexpected mechanism for SIRT3 regulation via SIRT1-mediated deacetylation. Improving mitochondrial SIRT3 functions by inhibiting SIRT3 acetylation may offer a new therapeutic approach for obesity- and aging-related diseases associated with mitochondrial dysfunction.
Keywords: acetylation, aging, fatty acid oxidation, mitochondria, obesity, post-translational modification, sirtuin, deacetylase, liver steatosis
Introduction
The sirtuins are NAD+-dependent protein deacetylases that play critical roles in metabolism, stress responses, and aging processes (1–5). Among the seven mammalian sirtuins, SIRT13 is best-studied and mediates transcriptional responses to fasting, exercise, or caloric restriction by deacetylating histones and non-histone gene regulatory proteins, including the key metabolic regulators, PGC-1α and SREBP-1 (6–8). Expression and enzymatic activity of SIRT1 are aberrantly low in both aging and obesity, and activation of SIRT1 delays and improves many diseases related with these conditions (1–5, 8–11). SIRT1 has received great attention as a drug target because the activity of SIRT1 can be enhanced by natural or synthetic SIRT1 activators (12) or dietary supplements that increase cellular levels of a key enzymatic co-factor, NAD+ (13, 14). Although SIRT1 deacetylates many cellular proteins, whether SIRT1 can deacetylate and alter the function of other sirtuin members has not been described.
Mitochondrial SIRT3 deacetylates and regulates the activity of proteins involved in mitochondrial functions, including intermediary metabolism, fatty acid oxidation, the urea cycle, and the oxidative stress response (15–19). Mice lacking SIRT3, but not the other mitochondrial sirtuins, SIRT4 or SIRT5, exhibited global mitochondrial protein hyperacetylation (20), indicating that SIRT3 is the major mitochondrial deacetylase. Indeed, the global mitochondrial acetyl-proteome was markedly reprogrammed in SIRT3-KO mice (21). Although SIRT3-KO mice fed normal chow did not show a metabolically remarkable phenotype (20), development of metabolic syndromes, such as adiposity, insulin resistance, and liver steatosis, were accelerated by chronically feeding these mice a high-fat diet (HFD) (22). Intriguingly, a genetic polymorphism that increases SIRT3 function has been associated with extreme longevity in humans (23), and conversely, a mutation of SIRT3 that reduces its activity is associated with increased risk for metabolic syndrome (22). The expression and enzymatic activity of SIRT3 are reduced in obesity and aging (15, 22, 24), but the underlying mechanisms are not clearly understood.
In this study, we show that the acetylation state of SIRT3 is regulated post-translationally by SIRT1 deacetylation. SIRT3 is acetylated at Lys57 and is hyperacetylated in both aged and obese mice, conditions in which SIRT1 deacetylase function is low. Utilizing acetylation-mimic and -defective Lys57-SIRT3 mutants, we provide evidence that acetylation of SIRT3 leads to decreased SIRT3 protein stability and deacetylase activity and increased levels of acetylated mitochondrial long-chain Acyl-CoA dehydrogenase (LCAD), a known target of SIRT3 deacetylation (16), which contributes to impaired fatty acid oxidation, liver steatosis, and glucose intolerance in HFD-induced obese mice.
Results
SIRT3 interacts with SIRT1 in the liver mitochondrial fraction
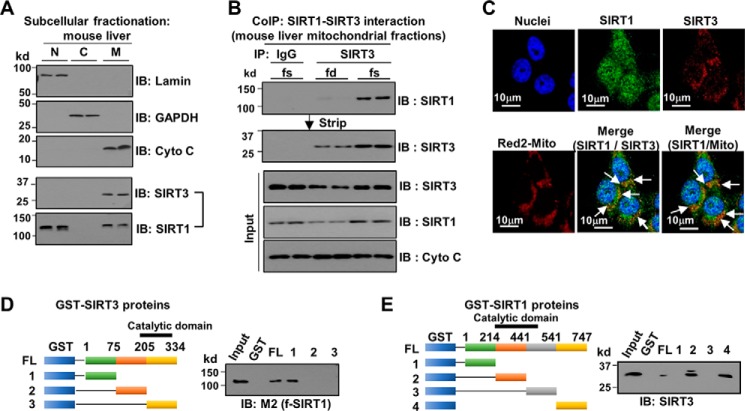
Analysis of proteins that co-immunoprecipitated with mouse SIRT3-FLAG in the mitochondrial fraction of mouse liver extracts by LC-MS identified a SIRT1 peptide (not shown). Although identification of SIRT1 by this method did not quite reach statistical significance, we examined this possible interaction further because SIRT1 had not been reported as a SIRT3-interacting protein, and it was unexpected because SIRT1 is predominantly a nuclear protein.
To examine whether a minor fraction of SIRT1 is present in the mitochondria, endogenous SIRT1 and SIRT3 protein levels in subcellular fractions of mouse liver extracts were determined. SIRT3 was predominantly mitochondrial, whereas SIRT1 was mainly nuclear, but a small fraction of SIRT1 was detected in the mitochondrial fraction (Fig. 1A). The interaction between SIRT3 and SIRT1 in the mitochondrial fraction was confirmed by CoIP (Fig. 1B and supplemental Fig. S1). Remarkably, their interaction was substantially increased in fasted mice (Fig. 1B). In immunofluorescence studies in Hepa1c1c7 cells, whereas endogenous SIRT3 is localized in the mitochondria as expected, endogenous SIRT1 is broadly distributed in the cell, and co-localization of SIRT1 with SIRT3 and of SIRT1 with the mitochondrial marker was detected (Fig. 1C). In GST-pulldown experiments, SIRT3 interacted with SIRT1 through its N-terminal region (Fig. 1D), and both the catalytic and C-terminal domains of SIRT1 interacted with SIRT3 (Fig. 1E). These results provide evidence that a small fraction of SIRT1 interacts with SIRT3 in the liver mitochondria.
Figure 1.
SIRT1 is a novel SIRT3-interacting protein in the mitochondrial fraction. A and B, CoIP using liver mitochondrial fractions. A, subcellular fractionation of mouse liver extracts was performed, and endogenous SIRT1 and SIRT3 in each fraction were determined by IB. B, interaction between SIRT1 and SIRT3 in the mitochondrial fractions from fasted (fs) or fed (fd) mice was examined by CoIP, and the membrane was stripped and reprobed with SIRT3 antibody. Protein levels in the input samples are shown below. The results from two mice are shown. C, endogenous SIRT1, SIRT3, and a mitochondrial DNA marker (Red2-Mito) were detected in Hepa1c1c7 cells by immunofluorescence. In the merged image, co-localization of SIRT1 with SIRT3 and the mitochondrial marker is indicated by arrows. Scale bar, 10 μm. D and E, GST-pulldown assays. D, schematic of the fragments of SIRT3 that were fused to GST (left panel). GST-SIRT3 fusion proteins were incubated with FLAG-SIRT1, and bound FLAG-SIRT1 was detected by IB (right panel). E, schematic of the fragments of SIRT1 that were fused to GST (left panel). GST-SIRT1 fusion proteins were incubated with SIRT3, and bound SIRT3 was detected by IB (right panel).
SIRT3 is hyperacetylated in SIRT1-LKO mice and in aged or obese mice
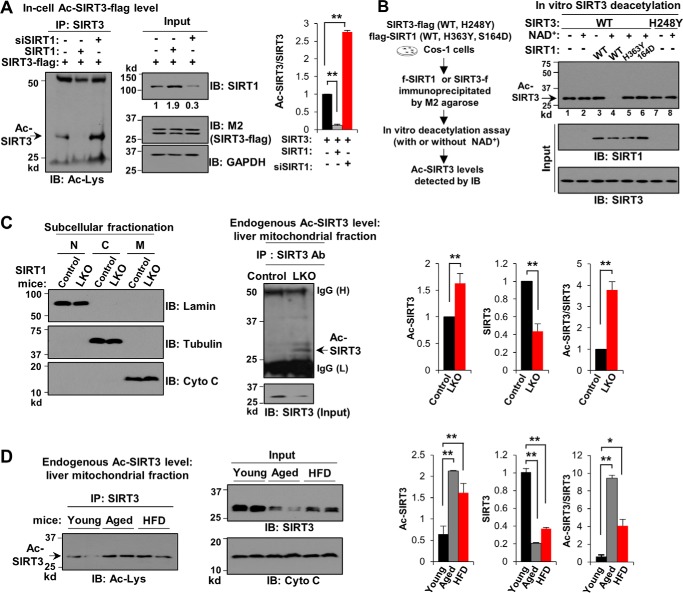
To determine the functional significance of the interaction of SIRT3 with SIRT1, we first tested whether the acetylation status of SIRT3 is regulated by SIRT1. Ectopic expression of SIRT1 in Cos-1 cells dramatically decreased the level of acetylated SIRT3-FLAG, and conversely, down-regulation of SIRT1 increased the acetylated SIRT3 level by more than 2-fold (Fig. 2A). These results suggest that SIRT3 is reversibly acetylated, and the SIRT3 acetylation level is regulated by SIRT1.
Figure 2.
Acetylation levels of SIRT3 are increased in SIRT1-LKO mice and in aged or HFD obese mice. A, in-cell deacetylation assay. Cos-1 cells were transfected with expression plasmids for SIRT3-FLAG, SIRT1, and siRNA for SIRT1 or control RNA as indicated, and acetylated (Ac)-SIRT3 levels were determined by IP/IB (left panel) as described under “Experimental procedures.” Input SIRT1 and SIRT3-FLAG protein (M2) levels are shown (middle panel). SIRT3 band intensities were measured, and Ac-SIRT3 levels relative to total SIRT3 levels were calculated (right panel). B, in vitro deacetylation assay: Cos-1 cells were transfected with expression plasmids for SIRT1-WT and its mutants having decreased activity, H363Y or S164D, and for SIRT3-WT or its catalytically inactive mutant, H248Y. FLAG-SIRT1 and SIRT3-FLAG proteins were immunoprecipitated and used for in vitro deacetylation assays in the presence or absence of NAD+ for 1 h. Ac-SIRT3 levels and protein levels in the input samples were detected by IB. C, quality of the subcellular fractionation of livers of littermates (Control) and SIRT1-LKO mice (LKO) was assessed by IB of markers (left panel). Acetylation levels of endogenous SIRT3 in the mitochondrial fraction were determined by IP/IB (middle panel), and input SIRT3 protein levels are shown below. Ac-SIRT3 levels relative to total SIRT3 levels were quantified (right panel). D, the mitochondrial fraction was isolated from livers of aged (20 months) and obese mice fed a HFD for 16 weeks, and Ac-SIRT3 levels were determined by IP/IB (left panel). Input SIRT3 and cytochrome c levels are shown (middle panel). Quantification of Ac-SIRT3 levels relative to total SIRT3 levels is shown (right panel). In A, C, and D, three independent experiments were done, and statistical significance between the indicated two groups was determined by the Student's t test (S.E., n = 3). *, p < 0.05; **, p < 0.01.
To test directly whether SIRT3 is a substrate for SIRT1 deacetylation, in vitro assays were performed using SIRT1-WT and two SIRT1 mutants having decreased activity, H363Y (25) and S164D (10) that were expressed in and immunoprecipitated from Cos-1 cells. The addition of SIRT1-WT, together with NAD+, led to a substantial decrease in acetylated SIRT3 levels (Fig. 2B, lanes 3 and 4), but these SIRT1-mediated effects were not observed with either the H363Y or S164D SIRT1 mutant (Fig. 2B, lanes 5 and 6). Similar results for SIRT1-WT were observed using commercially available purified SIRT1 (supplemental Fig. S2). Acetylation levels of SIRT3-WT or the catalytically inactive H248Y-SIRT3 mutant (16, 20) were not changed by incubation of these proteins with NAD+ (Fig. 2B, lanes 1, 2, 7, and 8), indicating that SIRT3 does not undergo autodeacetylation. These results suggest that SIRT3 is a direct target of SIRT1 deacetylation in vitro.
We next determined whether the acetylation level of endogenous SIRT3 in mouse liver is regulated by SIRT1 in vivo utilizing SIRT1-LKO mice. Subcellular fractions of livers from control littermates or SIRT1-LKO mice were prepared (Fig. 2C, left panel), and acetylation of SIRT3 in the mitochondrial fraction was determined by IP/IB. Levels of acetylated endogenous mitochondrial SIRT3 were higher in the SIRT1-LKO mice than in the control mice, even though SIRT3 protein levels were markedly decreased in the SIRT1-LKO mice (Fig. 2C, center and right panels). These results, together with the cell and in vitro studies above, provide evidence that the SIRT3 acetylation status is regulated by SIRT1.
Because SIRT1 function is depressed in both obesity and aging and levels of its enzymatic co-factor, NAD+, are decreased in these conditions (26, 27), we examined acetylated SIRT3 levels in aged mice and in HFD-induced obese mice. Intriguingly, in liver mitochondrial fractions, acetylation levels of endogenous SIRT3 are elevated in both aged and obese mice compared with young lean mice (Fig. 2D, left panel), even though SIRT3 protein levels were markedly decreased (Fig. 2D, center panel; quantitation in right panel). These results show that SIRT3 acetylation levels in the liver are increased in SIRT1-LKO mice and in aged or obese mice. Because SIRT1 function is aberrantly low in aged or obese animals, these results are consistent with the conclusions that SIRT1 regulates the acetylation level of SIRT3 and that the low SIRT1 function contributes to hyperacetylation of SIRT3 in aged and obese mice.
Lys57 is the acetylation site in SIRT3
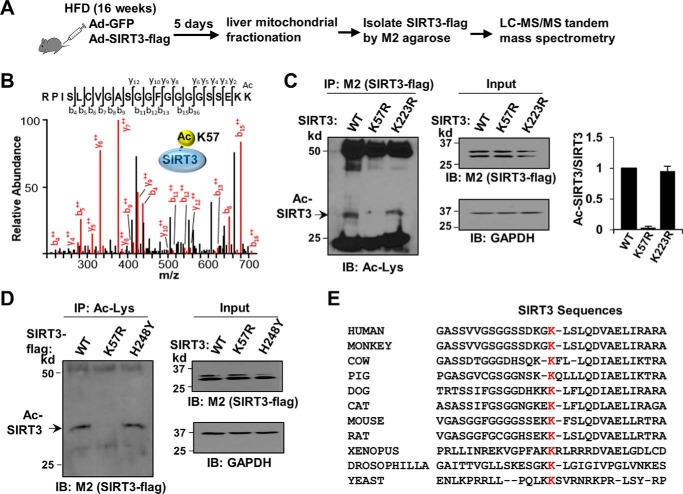
The site of acetylation in SIRT3 was identified by tandem mass spectrometry analysis. Mouse SIRT3-FLAG was adenovirally expressed in livers of mice chronically fed a HFD, and SIRT3-FLAG in the mitochondrial fraction was purified under stringent conditions with SDS in the buffers (Fig. 3A). Lys57 was the only acetylated site identified in SIRT3 in HFD obese mice by this analysis (Fig. 3B).
Figure 3.
Identification of obesity-linked acetylation of SIRT3 at Lys57. A, experimental outline. B, tandem mass spectrum identifying Lys57 as the only detectable acetylation site in SIRT3 in HFD mouse liver. C and D, in-cell acetylation assays, Cos-1 cells were transfected with expression plasmids for WT SIRT3-FLAG or the SIRT3 mutants, K57R-SIRT3, catalytically inactive H248Y-SIRT3, or K223R-SIRT3 as a negative control, and Ac-SIRT3-FLAG levels were determined by IP/IB (left panel). Input SIRT3-FLAG protein levels are shown (right panel). In C, the SIRT3 band intensities were quantified, Ac-SIRT3 levels relative to total SIRT3 levels were calculated, and mean values ± S.E. are presented (n = 3 independent assays) (right panel). In D, consistent results were observed in two independent assays. E, amino acid sequences adjacent to Lys57 in SIRT3 in the indicated species.
To confirm Lys57 as the acetylation site in SIRT3, Lys57 was mutated to Arg, and acetylation of SIRT3-FLAG was assessed by an in-cell acetylation assay. Acetylation of exogenously expressed WT SIRT3-FLAG was detected in Cos-1 cells, and mutation of Lys57 eliminated SIRT3 acetylation, whereas mutation of Lys223 to Arg as a negative control did not (Fig. 3C). We also tested whether SIRT3 can deacetylate itself utilizing a catalytically inactive SIRT3 mutant, H248Y (16, 20). Consistent with the results from in vitro studies above (Fig. 2B), the levels of acetylated H248Y-SIRT3 and SIRT3-WT were similar (Fig. 3D), suggesting that SIRT3 does not autodeacetylate. These results indicate that SIRT3 is a target of post-translational acetylation at Lys57 and are consistent with the direct interaction of SIRT3 and SIRT1 through the N-terminal domain of SIRT3 and catalytic and C-terminal domain of SIRT1 (Fig. 1, G and H). Remarkably, Lys57 in mouse SIRT3 (Lys122 in humans) is highly conserved among species from human to yeast (Fig. 3E), suggesting the functional importance of this residue.
Expression of SIRT1 or acetylation-defective K57R-SIRT3 increases the stability of SIRT3
Reversible protein acetylation profoundly modulates multiple protein functions, including subcellular localization, protein-protein and protein-DNA interaction, enzymatic activity, and protein stability (16, 28–30). Protein levels of endogenous SIRT3 in mitochondrial fractions were decreased, and conversely, its acetylation levels were increased in aged, HFD obese, and SIRT1-LKO mice (Fig. 2, C and D), which suggests that acetylation of SIRT3 may influence protein stability. To test this idea, SIRT3-FLAG was expressed in Cos-1 cells, and the effects of exogenous expression or down-regulation of SIRT1 on SIRT3-FLAG protein levels were measured with time after inhibition of protein synthesis with cycloheximide (CHX). Surprisingly, SIRT3-FLAG was rapidly degraded after CHX treatment with a half-life ∼45 min (Fig. 4, A–D). Exogenous expression of SIRT1 reduced the degradation rate of SIRT3, whereas down-regulation of SIRT1 increased it (Fig. 4, A and B).
Figure 4.
Expression of SIRT1 or the acetylation-defective K57R mutation of SIRT3 increases the stability of SIRT3. A and B, Cos-1 cells were transfected with plasmids for SIRT3-FLAG and SIRT1 or siRNA for SIRT1 or control RNA as indicated. C and D, Cos-1 cells were transfected with plasmids for SIRT3-FLAG WT, K57R-SIRT3, or K57Q-SIRT3 mutant. E, primary mouse hepatocytes were transfected with plasmids for SIRT3-FLAG WT or K57R-SIRT3 mutant in the presence of SIRT1-WT or a catalytically inactive H363Y mutant. A–E, after 36 h, the cells were treated with CHX for the indicated times, and protein levels of SIRT3-FLAG and GAPDH as control were determined by IB. The SIRT3-FLAG band intensities relative to the 0-min time point are plotted on the right. Three (E) to six (A–D) independent CHX experiments were done, and statistical significance was determined by the Student's t test (S.E., n = 3–6). *, p < 0.05; **, p < 0.01.
We further determined the effects of mutation of Lys57 on the stability of SIRT3. The degradation rate of the acetylation-defective K57R-SIRT3 mutant was markedly reduced compared with SIRT3-WT, whereas that of the acetylation-mimic K57Q-SIRT3 mutant was modestly, although significantly, increased (Fig. 4, C and D). The reason for the modest effect of the K57Q mutation, compared with the K57R mutation, on protein degradation is not clear but may be due to substantial basal levels of acetylated WT-SIRT3 in these cells, as detected by pan-acetyl-lysine antibody (Fig. 2A). These results indicate that acetylation of SIRT3 at Lys57 promotes protein degradation.
To further test whether SIRT1-mediated deacetylation of SIRT3 at Lys57 affects SIRT3 stability, we examined the effect of co-expression of SIRT1 or a catalytically inactive H363Y-SIRT1 mutant (25) with SIRT3-WT or K57R-SIRT3 on the stability of SIRT3. Because hepatic factors may affect the degradation rate of SIRT3, these experiments were done in primary mouse hepatocytes. In hepatocytes, the degradation rate of SIRT3-FLAG was very rapid, with a half-life shorter than 30 min (Fig. 4E). The degradation rate of SIRT3-WT was decreased by addition of SIRT1-WT but not by addition of catalytically inactive SIRT1 mutants as expected (Fig. 4E). Compared with SIRT3-WT, the degradation rate of the K57R-SIRT3 mutant was relatively insensitive to the addition of SIRT1-WT or H363Y mutant. These results indicate that acetylation of SIRT3 at Lys57, regulated by SIRT1, promotes SIRT3 protein degradation.
Acetylation-mimic mutation of SIRT3 at Lys57 results in decreased SIRT3 activity
In addition to effects on protein stability, acetylation of SIRT3 may also affect its activity. We thus determined the deacetylase activities of acetylation-defective K57R-SIRT3 and acetylation-mimic K57Q-SIRT3. In assays utilizing SIRT3 substrate peptides with a C-terminally attached fluorophore (31), deacetylase activity of the acetylation-mimic K57Q mutant was substantially decreased compared with SIRT3-WT or the acetylation-defective K57R SIRT3 mutant (Fig. 5A). We also examined the effects of these mutations on acetylation levels of LCAD, a known SIRT3 deacetylation target (16). SIRT3 promotes fatty acid oxidation in the liver by deacetylating and enhancing the activity of mitochondrial enzymes, including LCAD, under fasting conditions (16, 32). The acetylation level of LCAD (16, 33) has been shown to be increased by chronic HFD feeding (22). The addition of SIRT3-WT or the acetylation-defective K57R-SIRT3 mutant led to decreases in levels of acetylated LCAD similar to basal levels, whereas this decrease was not observed with the acetylation-mimic K57Q mutant (Fig. 5B). These results indicate that the intrinsic deacetylase activity of SIRT3 is reduced by the acetylation at Lys57.
Figure 5.
Acetylation-mimic mutation of SIRT3 at Lys57 results in decreased SIRT3 activity and increased acetylation of LCAD, which is associated with increased long-chain acylcarnitine levels. A, Fluor-de-Lys in vitro deacetylase assay. Fluorescently labeled Ac-substrate histone peptide was incubated with SIRT3-FLAG WT, K57R, or K57Q that was immunoprecipitated from Cos-1 cell extracts, and SIRT3 deacetylase activity was measured as described under “Experimental procedures.” Statistical significance between SIRT3-WT and K57Q was determined by the Student's t test (S.E., n = 3, ** p < 0.01). B, in vitro deacetylase assay: FLAG-LCAD proteins bound to M2 agarose were incubated with SIRT3-WT, K57R, or K57Q mutant in vitro in the presence of NAD+ for 1 h, and Ac-LCAD levels and protein levels in the input samples were detected by IB. Consistent results were observed from two independent assays. C–G, effects of SIRT3 Lys57 mutations on LCAD acetylation levels and liver acylcarnitine levels in vivo. C, experimental outline (five mice/group). D, SIRT3 and control GFP protein levels detected by IB in liver extracts from mice fed a ND or HFD and infected with control Ad-GFP or Ad-GFP-SIRT3-WT, Ad-GFP-K57R-SIRT3, or Ad-GFP-K57Q-SIRT3. E, acetylated LCAD levels were determined by IP/IB and input protein levels were detected by IB. In D and E, liver samples from three mice from the total of five mice in each group were randomly selected and used for IB. F and G, liver acylcarnitine levels (F) and serum β-hydroxyl butyrate levels (G) in HFD mice were detected by metabolomic analysis. Statistical significance between K57R and K57Q groups was determined by the Student's t test (S.E., n = 5). *, p < 0.05; **, p < 0.01.
Acetylation-mimic K57Q mutation of SIRT3 at Lys57 results in increased acetylation of LCAD in vivo and impaired β-oxidation
To further determine whether acetylation of SIRT3 at Lys57 influences its activity on LCAD deacetylation in mice in vivo, SIRT3-WT, an acetylation-defective K57R mutant or an acetylation-mimic K57Q mutant were adenovirally expressed in livers of dietary obese mice for 3 weeks (Fig. 5, C and D), and levels of acetylated LCAD were determined. Similar levels of SIRT3-WT and the mutants were detected in liver extracts, even though the stability is affected by the mutants in cells (Fig. 4). This may be due to overexpression of the proteins in vivo, which diminishes the differences in stability. Protein levels of endogenous SIRT3 in the liver extracts were markedly decreased in HFD mice compared with lean ND mice injected with control Ad-GFP (Fig. 5D) as shown in previous studies (22).
Acetylated levels of LCAD were readily detected in HFD obese mice as previously reported (22) but were substantially reduced below detection levels by exogenous expression of SIRT3-WT or the acetylation-defective K57R mutant (Fig. 5E). In contrast, levels of acetylated of LCAD were substantially higher for the acetylation-mimic K57Q-SIRT3 mutant compared with SIRT3-WT or K57R-SIRT3 (Fig. 5E). These results, together with in vitro deacetylation studies above (Fig. 5, A and B), indicate that acetylation of SIRT3 inhibits its deacetylase activity.
Because increased acetylation of LCAD in HFD mice is associated with its decreased enzymatic activity in the fatty acid oxidation pathway (16, 22), we further tested whether SIRT3 acetylation affects liver acylcarnitine levels. Impaired β-oxidation results in accumulation of acylcarnitine, particularly long-chain species (34). Long-chain acylcarnitine levels in liver homogenates were increased by expression of K57Q-SIRT3 compared with expression of SIRT3-WT or K57R-SIRT3 (Fig. 5F). Consistent with these results, the serum level of a ketone body, β-hydroxybutyrate, was decreased in mice expressing K57Q-SIRT3, compared with mice expressing SIRT3-WT or K57R-SIRT3 (Fig. 5G). These results, together with in vitro studies above (Fig. 5B), suggest that acetylation of SIRT3 at Lys57 is associated with increased LCAD acetylation and increased long-chain acylcarnitine levels in HFD obese mice, indicative of impaired fatty acid oxidation.
Expression of K57R-SIRT3 reverses fatty liver symptoms in HFD obese mice
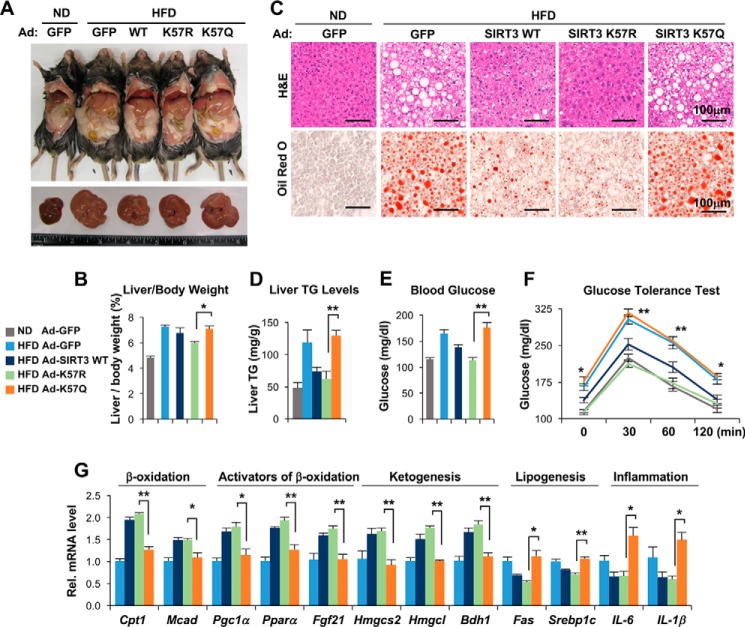
SIRT3-KO mice exhibit accelerated metabolic symptoms in response to chronic HFD feeding, which include adiposity, hyperlipidemia, insulin resistance, and liver steatosis (22). Impaired fatty acid oxidation results in abnormal accumulation of triglyceride (TG) and pathological symptoms of fatty liver (35). We therefore further investigated the effects of adenovirus-mediated exogenous expression of SIRT3-WT and its Lys57 acetylation mutants on fatty liver symptoms in HFD obese mice.
In mice fed a HFD, body weight (supplemental Fig. S3A) and liver size (Fig. 6A), liver and adipose weights (supplemental Fig. S3, B and C), liver weight/body weight (Fig. 6B), and neutral lipid content (Fig. 6C) and triglyceride levels in the liver (Fig. 6D) all increased as expected compared with lean mice fed a normal diet. Strikingly, expression of SIRT3-WT or the acetylation-defective K57R-SIRT3 largely reversed all the increases in HFD mice, whereas expression of the acetylation-mimic K57Q-SIRT3 mutant had little effect (Fig. 6, A–D). Food intake was not markedly changed among groups (supplemental Fig. S3D). Impaired glucose regulation frequently occurs in liver steatosis (36). Expression of SIRT3-WT or K57R-SIRT3 in HFD mice led to decreased fasting blood glucose levels (Fig. 6E) and improved glucose tolerance (Fig. 6F), but expression of K57Q-SIRT3 had little effect. These results, taken together, suggest that hyperacetylation of SIRT3 in mice chronically fed a HFD contributes to symptoms of fatty liver.
Figure 6.
Adenovirus-mediated expression of the acetylation-defective K57R-SIRT3 mutant ameliorates pathological symptoms of liver steatosis in HFD obese mice. A, representative pictures of HFD obese mice infected with control Ad-GFP, Ad-SIRT3-WT, K57R-SIRT3, or K57Q-SIRT3, and lean mice fed a ND infected with Ad-GFP as control (top panel) and livers of these mice (bottom panel). B, the ratios of liver/body weight. C, liver sections stained with hematoxylin and eosin and Oil Red O. Scale bar, 100 μm. D, liver TG levels. E, fasting glucose levels in the blood. F, glucose tolerance test. Blood glucose levels at indicated times after administration of glucose to fasted mice are shown. G, the mRNA levels of the indicated genes in the liver were measured by quantitative RT-PCR. Statistical significance between K57R and K57Q groups was determined by the Student's t test (S.E., n = 5 mice). *, p < 0.05; **, p < 0.01.
Changes in mitochondrial function can cause altered gene activity in the nucleus (37) so that hepatic gene expression associated with fatty liver might be affected by acetylation of mitochondrial SIRT3. Indeed, expression of SIRT3-WT or K57R-SIRT3 in HFD mice significantly increased mRNA levels of fatty acid oxidation enzymes Cpt1 and Mcad; key gene activators of mitochondrial fatty acid oxidation, Pgc1α and Pparα; a key hormone promoting fatty acid oxidation, Fgf21; and ketogenic genes, including the rate-limiting Hmgcs1/2, whereas mRNA levels of lipogenic and pro-inflammatory genes were decreased (Fig. 6G). In contrast, expression of the acetylation-mimic K57Q-SIRT3 had little effect on gene expression (Fig. 6G). These effects on gene expression are consistent with the effects on fatty liver symptoms of the Lys57 mutants of SIRT3.
Overall, adenovirus-mediated expression of SIRT3-WT or the acetylation-defective K57R mutant in livers of HFD obese mice led to remarkable metabolic effects with decreased adiposity and liver TG levels and improved glucose tolerance. Conversely, these SIRT3-mediated beneficial effects were not detected with the acetylation-mimic K57Q-SIRT3 mutant, leading to little improvement of the impaired fatty acid oxidation and fatty liver symptoms in HFD mice.
Discussion
In this study, we present evidence that SIRT3 is reversibly acetylated in the mitochondria and is a target of SIRT1 deacetylation. The acetylation level of SIRT3 is highly elevated in obesity and aging, conditions where SIRT1 function is low. SIRT3 is acetylated at Lys57, which leads to decreased SIRT3 deacetylase activity and protein stability. From metabolic studies utilizing adenoviral-mediated expression of SIRT3-WT or acetylation-defective or -mimic SIRT3-Lys57 mutants in HFD obese mice, we further show that hyperacetylation of SIRT3 contributes to impaired fatty acid β-oxidation, liver steatosis, and glucose intolerance.
An unexpected finding of this study was that levels of mitochondrial acetylated SIRT3 are regulated by SIRT1, because SIRT1 is mostly a nuclear-cytoplasmic shuttling protein (38) that mediates fasting transcriptional responses by deacetylating histones and gene regulatory proteins in the nucleus (2, 3) and is not considered to be a mitochondrial protein. Detection of SIRT1 in mitochondria, however, has been previously reported (39, 40). Consistent with these earlier studies, the present study provides proteomic, imaging, and biochemical evidence that that a minor fraction of SIRT1 is present in liver mitochondria and further directly interacts with SIRT3 under fasting conditions. SIRT3 acetylation levels were regulated by SIRT1 in vitro and in cells and increased in SIRT1-LKO mice in vivo. Because SIRT3 is a deacetylase, it is possible that the SIRT1 affects acetylation of SIRT3 indirectly by affecting SIRT3 levels or activity. However, results from in vitro and in-cell deacetylation studies indicate that this is not the case. Our experimental data support the conclusion that SIRT3 acetylation status is regulated by SIRT1 and is the first example of a sirtuin being a target of another sirtuin.
A significant functional consequence of acetylation of SIRT3 is decreased deacetylase activity. A single amino acid residue, Lys57, in the N-terminal domain of SIRT3, was identified as the acetylation site in dietary obese mice, and mutation of acetylation-mimic K57Q led to decreased SIRT3 activity. The catalytic domain of SIRT3 is located in the central and C-terminal regions, so acetylation at Lys57 may alter the conformation of SIRT3 and inhibit the deacetylase activity. Allosteric modulation of SIRT1 activity has been reported, which is mediated through its N-terminal domain that binds STACs and SIRT1-interacting proteins (12, 41). Further, it was shown that obesity-linked phosphorylation of SIRT1 by CK2 at Ser164 in the N-terminal domain inhibits its activity (10). In the present study, expression of the acetylation-mimic K57Q-SIRT3 is associated with increased LCAD acetylation and long-chain acylcarnitine levels, indicative of impaired β-oxidation, which contributes to liver steatosis and glucose intolerance. A single-nucleotide polymorphism in SIRT3, V208I, in fatty liver disease patients, reduces SIRT3 activity, thereby playing a pathological role in liver steatosis and insulin resistance (22). The basis for the decrease in activity of the Val208 mutant is not known, but it could affect the conformation of SIRT3 as we suggest for acetylation at Lys57.
SIRT3 acetylation also has significant effects on protein stability. Overexpression of SIRT1 or the acetylation-defective K57R-SIRT3 mutant increased stability, whereas down-regulation of SIRT1 or overexpression of the acetylation-mimic K57Q mutant had the opposite effects. In biochemical studies, we observed that inhibition of proteasomal degradation with MG132 substantially increased SIRT3 levels (supplemental Fig. S4). Further, expression of SIRT1 decreased, whereas down-regulation of SIRT1 increased, ubiquitinated SIRT3 levels (supplemental Fig. S5), suggesting that the ubiquitin-proteasomal degradation pathway might be involved and regulated by SIRT1-mediated deacetylation of SIRT3. It is therefore possible that acetylated SIRT3 is targeted for ubiquitination and retrotransported from the mitochondria to the cytoplasm for proteasomal degradation, as has been observed for other mitochondrial proteins (42), but further experiments will be necessary to fully elucidate the mechanisms by which SIRT3 acetylation promotes protein degradation.
SIRT1 deacetylates and increases expression of Pgc-1α and Pparα, both of which increase Sirt3 gene expression (22, 43, 44), so that SIRT1 likely increases transcription of SIRT3. Consistent with this, we observed that mRNA levels of hepatic Sirt3 and Pgc-1a were decreased in SIRT1-LKO mice (supplemental Fig. S6). Further, SIRT1 occupancy at the PPARα-bound Sirt3 promoter was detected in fasted mice (supplemental Fig. S7), suggesting a direct role of SIRT1 in transcriptional induction of Sirt3 during fasting. Thus, SIRT1 appears to increase SIRT3 levels by at least two mechanisms: increasing transcription of Sirt3 and increasing SIRT3 stability via deacetylation. The relative importance of these two mechanisms remains to be determined, but because SIRT3 has a short half-life of ∼30–45 min, changes in SIRT3 protein levels via reversible acetylation could provide a rapid mechanism to respond to environmental cues.
Although deacetylation by SIRT1 regulates the acetylation status of SIRT3, the acetylation of SIRT3 could also play a role in determining the levels of acetylated SIRT3. Little is known about acetylation of mitochondrial proteins, even though global analysis has shown that nearly every major mitochondrial metabolic enzyme is reversibly acetylated (21, 45). Because non-enzymatic acetylation of histones with acetyl-CoA occurs under physiological conditions (46), SIRT3 could be acetylated by increased acetyl CoA levels. Mitochondrial acetyltransferases in vivo have not been identified, but Scott et al. (47) reported that GCN5L1 acetylase counteracts SIRT3 activities in regulation of mitochondrial function by promoting acetylation of SIRT3 targets, suggesting GCN5L1 is a mitochondrial acetyltransferase. Thus, it remains possible that acetylation of SIRT3 may be aberrantly up-regulated in aging and obesity and contribute, with decreased SIRT1 deacetylation, to the hyperacetylation of SIRT3 and consequently to elevated acetylation levels of mitochondrial proteins observed in these conditions (22).
This study identifies SIRT3 as a target of post-translational acetylation, which is regulated by SIRT1. Hyperacetylation of SIRT3 in obesity and aging contributes to decreased SIRT3 activity, leading to increased LCAD acetylation, defective β-oxidation, and metabolic symptoms, including liver steatosis and glucose intolerance. Reversible mitochondrial protein acetylation is now recognized as a key metabolic regulatory mechanism (15, 16, 18, 32). By targeting the hyperacetylation of SIRT3 in obesity and aging, depressed SIRT3 activity might be restored, and consequently, dysregulated mitochondrial function be improved. SIRT3 acetylation may thus provide a new therapeutic target for obesity- and other aging-related diseases associated with mitochondrial dysfunction.
Experimental procedures
Reagents and materials
Antibodies for SIRT3 (5490S), cytochrome c (11940S), and acetyl-Lys (9441S) were purchased from Cell Signaling; antibodies for SIRT1 (sc-47765), lamin (sc-20680), tubulin (sc-5274), GAPDH (sc-47724), GFP (sc-8334), and LCAD (sc-82466) were from Santa Cruz Biotechnology; and antibodies for SIRT1 (07-131) were from EMD Millipore, Inc. M2 antibody, M2 agarose, CHX, trichostatin A (TSA), and nicotinamide (NAM) were from Sigma. The siRNAs for SIRT1 (s96764 and s174220) were purchased from Applied Biosystems. Expression plasmids for SIRT3-WT and its H248Y mutant and SIRT1 H363Y mutant were obtained from Addgene. Purified recombinant SIRT1 was obtained from Sigma (cs1040).
Animal experiments
To develop diet-induced obesity, 8–12-week-old C57BL/6J male mice were fed a HFD (42% fat; Harlan Teklad) for 16 weeks. For adenoviral experiments, adenovirus expressing SIRT3-WT-FLAG or Lys57 mutants (2.5–5.0 × 108 active viral particles in 100 μl of saline) was injected via the tail vein, and 3 weeks later, the mice were sacrificed at approximately 9:00 a.m., and the livers were collected. Infection of mice with these viral doses does not cause marked inflammation (28, 48). Ad-SIRT3-FLAG was constructed using mouse SIRT3-FLAG cDNA (49) inserted into the XhoI/HindIII site in the Ad-Track-CMV vector. For fasting/feeding experiments, the mice were fasted for 12 h and then fasted or refed a normal chow for 6 h. For the glucose tolerance test, the mice were fasted for 16 h and injected i.p. with 2 g/kg glucose (Sigma), and blood glucose levels were measured using an Accu-chek Aviva glucometer (Roche). Liver acylcarnitine and serum β-hydroxyl butyrate levels were measured as described (10) by metabolomics analysis in the University of Illinois at Urbana-Champaign Metabolomics Facility. All animal use and adenoviral protocols were approved by the Institutional Animal Care and Use and Institutional Biosafety Committees.
Tandem mass spectrometry
SIRT3-FLAG was expressed in mice fed a normal chow diet or a HFD by tail vein injection of Ad-SIRT3-FLAG. Five days after injection, the mice were sacrificed at 9:00 a.m., the mitochondrial fraction was prepared, and SIRT3-FLAG protein in the fraction was purified by binding to M2 agarose and then subjected to LC-MS or LC-MS/MS mass spectrometry-based proteomic analysis as described previously (7, 28, 29, 48). LC/MS or MS/MS spectra were screened against the SIRT3 sequence using SEQUEST (Thermo Finnigan, San Jose, CA), and the identified acetylated peptides were confirmed by manual inspection of the MS2 and MS3 spectra.
Mitochondrial fractionation
Mouse liver mitochondrial fractions were prepared using a mitochondrial fraction kit (Active Motif, Inc.) according to the manufacturer's instruction with modifications. Mouse livers were collected in 10 ml of ice-cold PBS and minced with a razor blade. The minced tissue was washed with ice-cold PBS and resuspended in 3 ml of 1× cytosolic buffer. The sample was incubated on ice for 15 min then transferred to a prechilled Dounce homogenizer. The tissue was homogenized by 20 strokes and centrifuged at 800 × g for 20 min at 4 °C. The resulting pellet was the nuclear fraction. The supernatant was centrifuged three times at 800 × g for 10 min at 4 °C to remove any residual nuclei. The supernatant was then centrifuged at 10,000 × g for 20 min at 4 °C to pellet the mitochondria. The resulting supernatant was centrifuged three times at 16,000 × g for 20 min at 4 °C to produce the cytosol fraction. The mitochondrial pellet was washed five times by resuspension in 300 μl of cytosolic buffer and centrifugation at 10,000 × g for 10 min at 4 °C. The final mitochondrial fraction pellet was lysed by adding 100 μl of mitochondrial buffer, incubated on ice for 15 min, and then vortexed for 10 s. All the buffers and solutions were supplemented with protease and phosphatase inhibitors, 1 mm DTT, 1 μm TSA, and 20 mm NAM. For IB analysis, 10 μl of each cellular fraction of a total of 500 μl, 1.5 ml, and 100 μl for the nuclear, cytosolic, and mitochondrial fractions, respectively, were analyzed.
In-cell and in vivo acetylation assay
For in-cell SIRT3 acetylation studies, Cos-1 cells were transfected with plasmids as indicated and/or siRNA, and 36–48 h later, the cells were treated with 1 μm TSA and 20 mm NAM, in the presence or absence of 1 μm MG132 for 3 h and harvested. Cos-1 cell extracts or mouse liver extracts were prepared by brief sonication of cell pellets or liver tissue in SDS-containing post-translational modification buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 2 mm EDTA, 0.5% Nonidet P-40, 0.1% SDS, 5% glycerol) supplemented with protease and phosphatase inhibitors, 1 mm DTT, 1 μm TSA, and 20 mm NAM. After centrifugation, supernatants were incubated with 1–2 μg of antibodies for SIRT3 or pan acetyl-Lys for 2 h and isolated by binding to protein G-agarose. Bound acetylated proteins or SIRT3 were detected by IB using antibodies for pan acetyl-Lys or SIRT3, respectively.
In vitro fluorometric SIRT3 deacetylase activity assay
SIRT3-WT-FLAG (49) and FLAG-SIRT3 mutants (K57R or K57Q) were expressed in Cos-1 cells and immunoprecipitated with M2 agarose from mitochondria fractions, and the level of SIRT3 was determined by IB. The immunoprecipitated SIRT3-FLAG proteins were incubated with fluoro-substrate peptide (Abcam, ab156067) and increasing concentrations of NAD+. SIRT3 activity was determined by measuring fluorescent emission at 460 nm, following excitation at 360 nm according to the manufacturer's instruction.
In vitro deacetylation assays
SIRT3-WT-FLAG (49), SIRT3-FLAG mutants (K57R or K57Q), FLAG-SIRT1, FLAG-SIRT1 mutants (S164D or H363Y), and FLAG-LCAD were expressed in Cos-1 cells and purified by binding to M2 agarose. Proteins were incubated with 50 μl of acetylation buffer (Tris-HCl, pH 8.8, 5% glycerol, 50 mm NaCl, 4 mm MgCl2, 1 mm DTT) in the presence of 50 μm NAD+ at 37 °C for 1 h, and levels of acetylated SIRT3 or LCAD or input levels were measured by IB. Consistent results were observed from two independent experiments.
Histological microscopy
Frozen liver sections were stained with Oil Red O, and paraffin-embedded liver sections were stained with hematoxylin and eosin as described previously (28, 48). Stained slides were imaged with a NanoZoomer Scanner.
CoIP and GST pulldown
Mitochondrial fractions were prepared from fasted or fed mice and briefly sonicated in CoIP buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 2 mm EDTA, 0.5% Nonidet P-40, 5% glycerol) supplemented with protease and phosphatase inhibitors, 1 mm DTT, 1 μm TSA, and 20 mm NAM. After centrifugation, the supernatant was incubated with 1–2 μg of antibodies for 2 h, and 30 μl of a 25% protein G-agarose slurry was added. 1 h later, samples were washed with CoIP buffer three times, and bound proteins were detected by IB. GST-pulldown assays were done as previously described (7, 28, 29, 48). 1–2 μg of GST-fusion proteins were incubated with in vitro synthesized proteins (Promega), and bound proteins were detected by IB. For construction of GST-SIRT3 plasmids, DNA fragments that contained different regions of SIRT3 sequences were amplified by PCR from the SIRT3 expression plasmid and cloned into the EcoRI and XhoI site of the pGEX-4T1 vector.
Construction of K57R and K57Q SIRT3 mutants
The acetylation-defective K57R-SIRT3 mutant and acetylation-mimic K57Q-SIRT3 mutants were constructed by site-directed mutagenesis (Stratagene) and confirmed by DNA sequencing. For construction of adenoviral SIRT3, SIRT3 DNA fragments were amplified by PCR from the SIRT3 WT or Lys57 mutant expression plasmid and cloned into the XhoI and HindIII sites of the AdTrack-CMV vector.
CHX study
Protein stability studies using CHX were done as previously described (7, 50). Briefly, Cos-1 cells or primary mouse hepatocytes were transfected with expression plasmids for SIRT3-FLAG WT (49) or Lys57 mutants, along with either SIRT1 WT or deacetylase activity-defective mutants, H363Y or S164D, or siRNA for SIRT1 (mixture of s96764 and s174220; Applied Biosystems) or control RNA, and then, 36 h later, cells were treated with CHX (10 μg/ml) for times indicated in the figure legends. The cell extracts were prepared and SIRT3-FLAG protein levels were detected by IB.
Quantitative RT-PCR assay
Total RNA was isolated from liver by TRIzol (Invitrogen) and real-time RT-PCR was performed with an iCycler iQ (Bio-Rad) using SYBR Green PCR master mix. Target gene mRNA levels were normalized to those of 36B4. Primer sequences for quantitative real-time PCR are shown in supplemental Table S1.
Statistical analysis
Statistical significance between two groups was determined by Student's t test, and the mean ± S.E. are presented. p < 0.05 was considered as statistically significant.
Author contributions
S. K., B. K., and J. K. K. designed research; S. K. and S. S. performed experiments; P. Y. performed the mass spectrometry-based proteomic analyses; X. L. provided SIRT1-LKO mice; S. K., S. S., P. Y., B. K., and J. K. K. analyzed data; and S. K., B. K., and J. K. K. wrote the paper.
Supplementary Material
Acknowledgments
We thank to Qiang Tong at Baylor College of Medicine for providing the pcDNA-mouse SIRT3-FLAG expression plasmid and Mark Leid at the Oregon State University for GST-SIRT1 constructs. We also thank to Li Zhong in the Metabolomic Center at University of Illinois at Urbana-Champaign for measuring liver acylcarnitines and serum β-hydroxyl butyrate levels.
This work was supported by National Institutes of Health Grants DK062777 and DK095842 and a Basic Science Award 1-16-IBS-156 from the American Diabetes Association (to J. K. K.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Table S1 and Figs. S1–S7.
- SIRT
- sirtuin
- HFD
- high-fat diet
- LCAD
- long-chain acyl-CoA dehydrogenase
- LKO
- liver-specific knockout
- CHX
- cycloheximide
- GCN5L
- GCN5-like
- NAM
- nicotinamide
- TSA
- trichostatin A
- IB
- immunoblotting
- CoIP
- co-immunoprecipitation
- TG
- triglyceride
- ND
- normal diet.
References
- 1. Guarente L. (2011) Sirtuins, aging, and metabolism. Cold Spring Harb. Symp. Quant. Biol. 76, 81–90 [DOI] [PubMed] [Google Scholar]
- 2. Finkel T., Deng C. X., and Mostoslavsky R. (2009) Recent progress in the biology and physiology of sirtuins. Nature 460, 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haigis M. C., and Sinclair D. A. (2010) Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 5, 253–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giblin W., Skinner M. E., and Lombard D. B. (2014) Sirtuins: guardians of mammalian healthspan. Trends Genet. 30, 271–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamamoto H., Schoonjans K., and Auwerx J. (2007) Sirtuin functions in health and disease. Mol. Endocrinol. 21, 1745–1755 [DOI] [PubMed] [Google Scholar]
- 6. Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., and Puigserver P. (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 [DOI] [PubMed] [Google Scholar]
- 7. Ponugoti B., Kim D. H., Xiao Z., Smith Z., Miao J., Zang M., Wu S. Y., Chiang C. M., Veenstra T. D., and Kemper J. K. (2010) SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J. Biol. Chem. 285, 33959–33970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Revollo J. R., and Li X. (2013) The ways and means that fine tune Sirt1 activity. Trends Biochem. Sci. 38, 160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi S. E., Fu T., Seok S., Kim D. H., Yu E., Lee K. W., Kang Y., Li X., Kemper B., and Kemper J. K. (2013) Elevated microRNA-34a in obesity reduces NAD levels and SIRT1 activity by directly targeting NAMPT. Aging Cell 12, 1062–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi S. E., Kwon S., Seok S., Xiao Z., Lee K. W., Kang Y., Li X., Shinoda K., Kajimura S., Kemper B., and Kemper J. K. (2017) Obesity-linked phosphorylation of SIRT1 by CK2 inhibits its nuclear localization and promotes fatty liver. Mol. Cell. Biol. 37, e00006–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee J., and Kemper J. K. (2010) Controlling SIRT1 expression by microRNAs in health and metabolic disease. Aging 2, 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hubbard B. P., Gomes A. P., Dai H., Li J., Case A. W., Considine T., Riera T. V., Lee J. E., E S. Y., Lamming D. W., Pentelute B. L., Schuman E. R., Stevens L. A., Ling A. J., Armour S. M., Michan S., et al. (2013) Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339, 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshino J., Mills K. F., Yoon M. J., and Imai S. (2011) Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cantó C., Houtkooper R. H., Pirinen E., Youn D. Y., Oosterveer M. H., Cen Y., Fernandez-Marcos P. J., Yamamoto H., Andreux P. A., Cettour-Rose P., Gademann K., Rinsch C., Schoonjans K., Sauve A. A., and Auwerx J. (2012) The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He W., Newman J. C., Wang M. Z., Ho L., and Verdin E. (2012) Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol. Metab. 23, 467–476 [DOI] [PubMed] [Google Scholar]
- 16. Hirschey M. D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D. B., Grueter C. A., Harris C., Biddinger S., Ilkayeva O. R., Stevens R. D., Li Y., Saha A. K., Ruderman N. B., Bain J. R., et al. (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qiu X., Brown K., Hirschey M. D., Verdin E., and Chen D. (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 12, 662–667 [DOI] [PubMed] [Google Scholar]
- 18. Hallows W. C., Lee S., and Denu J. M. (2006) Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc. Natl. Acad. Sci. U.S.A. 103, 10230–10235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hallows W. C., Yu W., Smith B. C., Devries M. K., Ellinger J. J., Someya S., Shortreed M. R., Prolla T., Markley J. L., Smith L. M., Zhao S., Guan K. L., and Denu J. M. (2011) Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol. Cell 41, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lombard D. B., Alt F. W., Cheng H. L., Bunkenborg J., Streeper R. S., Mostoslavsky R., Kim J., Yancopoulos G., Valenzuela D., Murphy A., Yang Y., Chen Y., Hirschey M. D., Bronson R. T., Haigis M., et al. (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 27, 8807–8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hebert A. S., Dittenhafer-Reed K. E., Yu W., Bailey D. J., Selen E. S., Boersma M. D., Carson J. J., Tonelli M., Balloon A. J., Higbee A. J., Westphall M. S., Pagliarini D. J., Prolla T. A., Assadi-Porter F., Roy S., et al. (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 49, 186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirschey M. D., Shimazu T., Jing E., Grueter C. A., Collins A. M., Aouizerat B., Stanĉáková A., Goetzman E., Lam M. M., Schwer B., Stevens R. D., Muehlbauer M. J., Kakar S., Bass N. M., Kuusisto J., et al. (2011) SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell 44, 177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Albani D., Ateri E., Mazzuco S., Ghilardi A., Rodilossi S., Biella G., Ongaro F., Antuono P., Boldrini P., Di Giorgi E., Frigato A., Durante E., Caberlotto L., Zanardo A., Siculi M., et al. (2014) Modulation of human longevity by SIRT3 single nucleotide polymorphisms in the prospective study “Treviso Longeva (TRELONG).” Age 36, 469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kendrick A. A., Choudhury M., Rahman S. M., McCurdy C. E., Friederich M., Van Hove J. L., Watson P. A., Birdsey N., Bao J., Gius D., Sack M. N., Jing E., Kahn C. R., Friedman J. E., and Jonscher K. R. (2011) Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem. J. 433, 505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., Hu L. S., Cheng H. L., Jedrychowski M. P., Gygi S. P., Sinclair D. A., et al. (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 26. Imai S., and Kiess W. (2009) Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Front. Biosci. (Landmark Ed.) 14, 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Houtkooper R. H., Pirinen E., and Auwerx J. (2012) Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim D. H., Xiao Z., Kwon S., Sun X., Ryerson D., Tkac D., Ma P., Wu S. Y., Chiang C. M., Zhou E., Xu H. E., Palvimo J. J., Chen L. F., Kemper B., and Kemper J. K. (2015) A dysregulated acetyl/SUMO switch of FXR promotes hepatic inflammation in obesity. EMBO J. 34, 184–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kemper J. K., Xiao Z., Ponugoti B., Miao J., Fang S., Kanamaluru D., Tsang S., Wu S. Y., Chiang C. M., and Veenstra T. D. (2009) FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 10, 392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flick F., and Lüscher B. (2012) Regulation of sirtuin function by posttranslational modifications. Front. Pharmacol. 3, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nguyen G. T., Gertz M., and Steegborn C. (2013) Crystal structures of Sirt3 complexes with 4′-bromo-resveratrol reveal binding sites and inhibition mechanism. Chem. Biol. 20, 1375–1385 [DOI] [PubMed] [Google Scholar]
- 32. Shimazu T., Hirschey M. D., Hua L., Dittenhafer-Reed K. E., Schwer B., Lombard D. B., Li Y., Bunkenborg J., Alt F. W., Denu J. M., Jacobson M. P., and Verdin E. (2010) SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 12, 654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bharathi S. S., Zhang Y., Mohsen A. W., Uppala R., Balasubramani M., Schreiber E., Uechi G., Beck M. E., Rardin M. J., Vockley J., Verdin E., Gibson B. W., Hirschey M. D., and Goetzman E. S. (2013) Sirtuin 3 (SIRT3) protein regulates long-chain acyl-CoA dehydrogenase by deacetylating conserved lysines near the active site. J. Biol. Chem. 288, 33837–33847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., Lopaschuk G. D., and Muoio D. M. (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7, 45–56 [DOI] [PubMed] [Google Scholar]
- 35. Ji H., and Friedman M. I. (2008) Reduced hepatocyte fatty acid oxidation in outbred rats prescreened for susceptibility to diet-induced obesity. Int. J. Obes. (Lond.) 32, 1331–1334 [DOI] [PubMed] [Google Scholar]
- 36. Kahn S. E., Hull R. L., and Utzschneider K. M. (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 [DOI] [PubMed] [Google Scholar]
- 37. Tian Y., Garcia G., Bian Q., Steffen K. K., Joe L., Wolff S., Meyer B. J., and Dillin A. (2016) Mitochondrial stress induces chromatin reorganization to promote longevity and UPR(mt). Cell 165, 1197–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tanno M., Sakamoto J., Miura T., Shimamoto K., and Horio Y. (2007) Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 282, 6823–6832 [DOI] [PubMed] [Google Scholar]
- 39. Aquilano K., Baldelli S., Pagliei B., and Ciriolo M. R. (2013) Extranuclear localization of SIRT1 and PGC-1α: an insight into possible roles in diseases associated with mitochondrial dysfunction. Curr. Mol. Med. 13, 140–154 [PubMed] [Google Scholar]
- 40. Aquilano K., Vigilanza P., Baldelli S., Pagliei B., Rotilio G., and Ciriolo M. R. (2010) Peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J. Biol. Chem. 285, 21590–21599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghisays F., Brace C. S., Yackly S. M., Kwon H. J., Mills K. F., Kashentseva E., Dmitriev I. P., Curiel D. T., Imai S. I., and Ellenberger T. (2015) The N-terminal domain of SIRT1 is a positive regulator of endogenous SIRT1-dependent deacetylation and transcriptional outputs. Cell Rep. 10, 1665–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taylor E. B., and Rutter J. (2011) Mitochondrial quality control by the ubiquitin-proteasome system. Biochem. Soc. Trans. 39, 1509–1513 [DOI] [PubMed] [Google Scholar]
- 43. Kong X., Wang R., Xue Y., Liu X., Zhang H., Chen Y., Fang F., and Chang Y. (2010) Sirtuin 3, a new target of PGC-1α, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5, e11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giralt A., Hondares E., Villena J. A., Ribas F., Díaz-Delfín J., Giralt M., Iglesias R., and Villarroya F. (2011) Peroxisome proliferator-activated receptor-γ coactivator-1α controls transcription of the Sirt3 gene, an essential component of the thermogenic brown adipocyte phenotype. J. Biol. Chem. 286, 16958–16966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., and Mann M. (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 [DOI] [PubMed] [Google Scholar]
- 46. Paik W. K., Pearson D., Lee H. W., and Kim S. (1970) Nonenzymatic acetylation of histones with acetyl-CoA. Biochim. Biophys. Acta 213, 513–522 [DOI] [PubMed] [Google Scholar]
- 47. Scott I., Webster B. R., Li J. H., and Sack M. N. (2012) Identification of a molecular component of the mitochondrial acetyltransferase programme: a novel role for GCN5L1. Biochem. J. 443, 655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim D. H., Kwon S., Byun S., Xiao Z., Park S., Wu S. Y., Chiang C. M., Kemper B., and Kemper J. K. (2016) Critical role of RanBP2-mediated SUMOylation of small heterodimer partner in maintaining bile acid homeostasis. Nat. Commun. 7, 12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shi T., Wang F., Stieren E., and Tong Q. (2005) SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J. Biol. Chem. 280, 13560–13567 [DOI] [PubMed] [Google Scholar]
- 50. Miao J., Xiao Z., Kanamaluru D., Min G., Yau P. M., Veenstra T. D., Ellis E., Strom S., Suino-Powell K., Xu H. E., and Kemper J. K. (2009) Bile acid signaling pathways increase stability of small heterodimer partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev. 23, 986–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.