Abstract
Objectives/Hypothesis
The time course of the reinnervation of the paralyzed face after hypoglossal‐facial jump nerve suture using electromyography (EMG) was assessed. The relation to the clinical outcome was analyzed.
Study Design
Retrospective single‐center cohort study
Methods
Reestablishment of motor units was studied by quantitative EMG and motor unit potential (MUP) analysis in 11 patients after hypoglossal‐facial jump nerve suture. Functional recovery was evaluated using the Stennert index (0 = normal; 10 = maximal palsy).
Results
Clinically, first movements were seen between 6 and >10 months after surgery in individual patients. Maximal improvement was achieved at 18 months. The Stennert index decreased from 7.9 ± 2.0 preoperatively to a final postoperative score of 5.8 ± 2.4. EMG monitoring performed for 2.8 to 60 months after surgery revealed that pathological spontaneous activity disappeared within 2 weeks. MUPs were first recorded after the 2nd month and present in all 11 patients 8–10 months post‐surgery. Polyphasic regeneration potentials first appeared at 4–10 months post‐surgery. The MUP amplitudes increased between the 3rd and 15th months after surgery to values of control muscles. The MUP duration was significantly increased above normal values between the 3rd and 24th months after surgery.
Conclusion
Reinnervation can be detected at least 2 months earlier by EMG than by clinical evaluation. Changes should be followed for at least 18 months to assess outcome. EMG changes reflected the remodeling of motor units due to axonal regeneration and collateral sprouting by hypoglossal nerve fibers into the reinnervated facial muscle fibers.
Level of Evidence
3b.
Keywords: Facial nerve, hypoglossal nerve, cross‐nerve suture, nerve repair, muscle reinnervation, motor unit potential, EMG, regeneration
INTRODUCTION
Facial nerve fibers have the capacity to regenerate after severe lesion. Reconstruction of the facial nerve leads to reinnervation of mimic muscle fibers including the establishment of new neuromuscular connections and building new motor units. Electromyography (EMG) is an important tool in the diagnosis and follow‐up of facial nerve lesions.1, 2 Motor unit potentials (MUPs) change during denervation and regeneration reflecting maturation of nerve fibers and reorganization of muscle fibers within the motor unit3, 4 In general, standardized and quantified studies of changes in MUPs in patients with defined nerve lesions are rare.5 For direct facial nerve reanimation surgery, we only know that voluntary EMG activity seems to occur at the latest about 4.5 months after nerve reconstruction and that first synkinetic activity is observed by EMG not earlier than about 5 months after surgery.6
The hypoglossal‐facial jump nerve suture (ie, a side‐to‐end nerve suture of the incised hypoglossal nerve to a nerve graft that is then sutured end‐to‐end to the distal facial nerve) is a standard procedure for facial nerve reanimation for patients with permanent facial nerve paralysis.7 Detailed data on changes in motor unit remodeling after surgical repair with hypoglossal‐facial jump nerve suture have not been reported yet.
Hence, the present clinical study was performed to systematically analyze the time course of the reinnervation of the paralyzed face after hypoglossal‐facial jump nerve suture using EMG as a monitoring tool during the follow‐up.
MATERIAL AND METHODS
Study Design and Setting
This retrospective data evaluation was approved by the ethics committee at the Medical Faculty, Friedrich‐Schiller‐University Jena. All patients who underwent a hypoglossal‐facial jump nerve suture between 2006 and 2014 because of irreversible facial palsy were screened. Patients were included if they received at least two EMG investigations during the postoperative follow‐up and if the follow‐up took at least 24 months until the final status of the facial regeneration. Finally, the EMG examinations had to include an examination of the frontal, orbicularis oculi, orbicularis oris, and zygomatic muscle. Eleven patients could be included (6 male, 5 female, median age: 64 years). More details are given in Table 1. The clinical and electrophysiological assessment started with the onset of the palsy and continued after hypoglossal‐facial nerve suture with regular follow‐up visits. A standard hypoglossal‐facial nerve suture was performed.8, 9 Briefly, the hypoglossal nerve was incised 1 cm distal to the descendens hypoglossi branch not more than one‐third. A graft of the greater auricular nerve was sutured end‐to‐side to the proximal opening of the hypoglossal nerve. Finally, the end of the graft was sutured end‐to‐end to the facial nerve main trunk.
Table 1.
Patients’ and Electromyography (EMG) Characteristics of all Patients.
| Patient | Gender |
Age at surgery (years) |
Side | Etiology of the facial palsy | Denervation time (days) |
Stennert index Δa improvement |
EMG examinations, interval after surgery (months) | No. of EMGs | Sum of analyzed MUPs |
|---|---|---|---|---|---|---|---|---|---|
| 1 | male | 68 | left | idiopathic | 367 | 2 | 12.0–60.0 | 9 | 103 |
| 2 | male | 68 | left | neoplastic | 21 | 3 | 7.7–38.7 | 9 | 112 |
| 3 | male | 63 | left | traumatic | 276 | 2 | 2.7–56.0 | 6 | 101 |
| 4 | male | 65 | right | neoplastic | 730 | 1 | 0.3–42.4 | 8 | 67 |
| 5 | female | 33 | right | traumatic | 428 | 5 | 3.4–15.0 | 3 | 49 |
| 6 | male | 55 | right | traumatic | 162 | 3 | 6.4–51.3 | 10 | 67 |
| 7 | female | 9 | left | traumatic | 805 | 0 | 3.0–30.8 | 8 | 58 |
| 8 | female | 62 | right | traumatic | 204 | 2 | 3.0–20.7 | 6 | 56 |
| 9 | female | 49 | left | traumatic | 370 | 1 | 3.2–33.3 | 7 | 47 |
| 10 | male | 65 | left | neoplastic | 219 | 0 | 0.5–2.8 | 2 | 14 |
| 11 | female | 59 | left | traumatic | 427 | 5 | 3.0–17.0 | 6 | 74 |
= initial Stennert index prior to surgery minus final Stennert index after surgery. MUP = motor unit potential
To build a control group, EMG examinations of the same four but healthy facial muscles were performed in six subjects (3 male, 3 female, median age: 44 years). The EMG results for the healthy normal facial muscles are summarized in Supplemental Table 1.
Facial Nerve Grading and Electromyography
The severity of the facial palsy was assessed using the Stennert index.10 Stennert index classifies the face at rest (0–4 points; 0 = normal to 4 = complete loss of resting tone) and during motion (0–6 points; 0 = normal to 6 = no motion) separately. The values at rest and during motion are added to the total Stennert index (0 = normal; 10 = maximal palsy). A total Stennert index of 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 correlates to a Sunnybrook composite score of 100, 84–99, 71–83, 64–70, 59–63, 46–58, 38–46, 35–37, 20–34, 10–21, and 0–9, respectively.2
Standard needle electromyography (EMG) was performed (Synergy T5, Viasys, CareFusion, San Diego, California, U.S.A) on the paretic side as described elsewhere.1 Four mimic muscles were examined: frontal muscle, orbicularis oculi muscle, orbicularis oris muscle, and zygomatic major muscle.
Quantitative Motor Unit Potentials (MUPs) Analysis
The recording of the MUPs was performed during a stepwise increase of the effort of the patients up to maximal voluntary contraction of facial muscles with the support of tongue movements. The support of the tongue was used for all recordings in patients. Several recordings with duration of at least 1 sec were performed. The analyses were performed offline (MUP examples in Supplemental Fig. 1). The MUPs were inspected for quality and the cursors were adjusted for duration of MUPs at a gain of 0.5 mV/Div. Peak‐to‐peak amplitude, duration, turns, and phases were measured. The number of polyphasic MUPs (>4 phases) was recorded. The means of amplitude, duration, turns, and phases in the individual muscle were compared to our control values. For standardization, the data for all analyzed facial muscles and Z‐scores of all four parameters were calculated in individual muscles based on the reference values from the analyses of healthy facial muscles. The movements in the healthy subjects were performed without tongue support. In addition to the MUP analyses, the following characteristics were evaluated: (A) insertion activity (no, reduced, normal, increased); (B) pathological spontaneous activity (yes, no); (C) voluntary activity (no, single fiber activity, reduced, normal); and (D) motor unit morphology (no, reduced amplitude, polyphasic, normal); (E) synkinetic activity during voluntary EMG was registered if present (yes, no).
Figure 1.
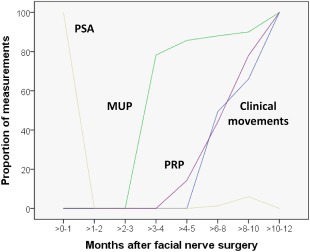
Time course of EMG and clinical parameters in months after surgery: occurrence of clinically visible movements, pathological spontaneous activity (PSA), motor unit potentials (MUP), and polyphasic regeneration potentials (PRP).
Statistical Analysis
Values are presented as mean ± standard deviation. A Spearman rank‐order correlation was performed to analyze the association between time after surgery and facial function as assessed using the Stennert index. The association of changes of facial functions over time after surgery as measured with the Stennert index and with the changes of the MUP parameters were modeled with linear mixed models. This method allows for inclusion of multiple time points per subject, while accounting for unbalanced data structure of irregular time intervals between EMG examinations and unequal numbers of EMG analyses per subject. The respective MUP parameter was included as dependent variable and time after surgery and the Stennert index as covariates. The significance level was set to p < 0.05.
RESULTS
Clinical Course of Reinnervation
Surgery was performed in all 11 patients without any complications. More details on the patients are given in Table 1. First clinical movements were not seen before 5 months after surgery in some patients and took at least 10 months until seen in all 11 patients (Fig. 1). Mean denervation time prior to facial nerve reconstruction was 364 ± 234 days. Initial preoperative and final postoperative Stennert index at rest were 2.6 ± 1.6, and 1.5 ± 1.6, respectively. There was a negative correlation between time after surgery and the Stennert index at rest (r = −0.424; p < 0.001). Initial preoperative and final postoperative Stennert index during motion were 5.3 ± 0.8, and 4.4 ± 1.4, respectively. There was also a negative correlation between time after surgery and the Stennert index during motion (r = −0.417; p < 0.001). Hypoglossal‐facial jump nerve suture had a stronger effect on the face at rest than during movements. Maximal improvement of facial reanimation seemed to be reached about 18 months after surgery (Fig. 2). Initial preoperative overall Stennert index decreased from 7.9 ± 2.0 to a final postoperative score of 5.8 ± 2.4. Mean improvement of the overall Stennert index by reconstruction surgery was Δ 2.2 ± 1.7. There was also a correlation between time after surgery and a lower overall Stennert index (r = −0.483; p < 0.001).
Figure 2.
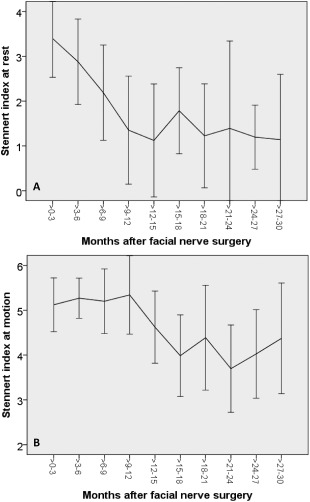
Mean Stennert index at rest (A) and at motion over time in months after surgery. Vertical lines indicate the standard deviation. A lower index reflects a better facial nerve function (0 = normal).
EMG Monitoring of Reinnervation
The EMG monitoring was performed for 2.8 to 60 months after surgery. In total, 74 EMG measurements including 748 MUPs were analyzed. The mean number of EMG examination per patient was 6.7 ± 2.5. 68 ± 29 MUPs were analyzed per patient. All patients showed pathological spontaneous activity directly after surgery (Fig. 1). This spontaneous activity disappeared within 2 weeks. First MUPs were recorded after the second postoperative month. It took 8–10 months before MUPs were seen in all 11 patients. First polyphasic regeneration potentials occurred not before 5 months after surgery and were detected in all patients after at least 10 months.
The raw data of all MUP recordings of facial muscles on the paralyzed side are summarized in Supplemental Table 2. The MUP amplitude z‐score analysis showed that the amplitudes of the reanimated facial muscles initially were lower than in normal healthy facial muscles (negative z‐scores), but started to increase 3–6 months after surgery, further increased until about 15 months after hypoglossal‐facial jump nerve suture and reached then a plateau at the level of normal amplitudes (z‐score = 0; Fig. 3A). The MUP duration z‐scores showed normal MUP duration values 0–3 months after surgery, and then the MUP duration increased to higher values than in normal muscles (z‐scores >0) until about 21–24 months after surgery (Fig. 3B). The number of MUP turns was lower up to 6 months after surgery, but then increased to constant higher level than normal over the complete follow‐up (Fig. 3C). The MUP phase analysis showed nearly the same course as the MUP turns, although the pathological increase of the number of phases was not as distinct (z‐scores >0) as for the MUP turns (Fig. 3D). Both, the MUP turns and phase analysis are reflecting the development of polyphasic regeneration potentials (cf. Fig. 1).
Table 2.
Association between MUP Characteristics and Time Course of Facial Function Improvement after Nerve Reconstruction.
| Estimated regression coefficient |
Lower 95% CI |
Upper 95% CI |
p‐valuea | |
|---|---|---|---|---|
| Amplitude (z‐score) | ||||
| Stennert index at rest | −0.393224 | −0.774281 | −0.012168 | 0.043 |
| Stennert index during motion | −0.167128 | −0.615414 | 0.281158 | 0.463 |
| Time since reconstruction, days | 0.001438 | 0.000449 | 0.002426 | 0.005 |
| Duration (z‐score) | ||||
| Stennert index at rest | −0.012622 | −0.259963 | 0.234718 | 0.920 |
| Stennert index during motion | −0.052768 | −0.343746 | 0.238210 | 0.721 |
| Time since reconstruction, days | 0.003493 | 0.001341 | 0.00564 | 0.002 |
| Phases (z‐score) | ||||
| Stennert index at rest | −0.246873 | −0.572715 | 0.078968 | 0.137 |
| Stennert index during motion | −0.026071 | −0.409400 | 0.357259 | 0.893 |
| Time since reconstruction, days | 5.9642E‐05 | −0.000786 | 0.000905 | 0.889 |
| Turns (z‐score) | ||||
| Stennert index at rest | −0.247140 | −0.595207 | 0.100928 | 0.163 |
| Stennert index during motion | 0.012798 | −0.396678 | 0.422274 | 0.951 |
| Time since reconstruction, days | −9.7423E‐05 | −0.001000 | 0.000805 | 0.832 |
significant p‐values (p < 0.05) in bold; CI = confidence interval
Figure 3.
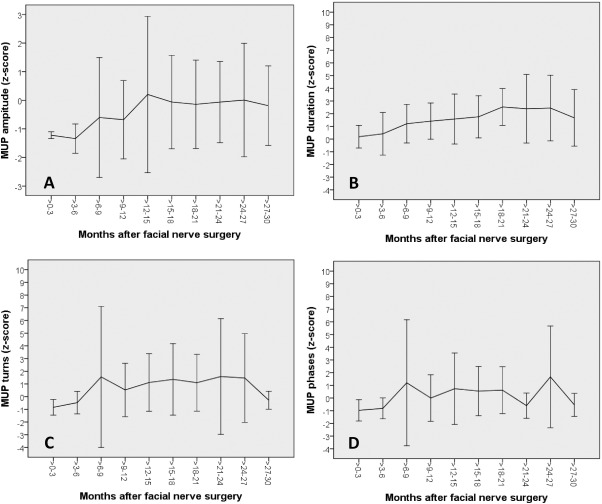
Mean motor unit potentials (MUPs) amplitudes (A), duration (B), phases (C), and turns (D) indicated as z‐scores over time in months after surgery. Vertical lines indicate the standard deviation.
The linear mixed model analyses showed an association between the MUP amplitudes z‐scores and the Stennert index at rest (Table 2). For a 1‐point reduction of the Stennert index at rest the MUP amplitude z‐score increased by 0.393224 (p=0.043). The amplitude z‐score increased by 0.001438 each day after surgery (p = 0.005). The MUP duration z‐score increased by 0.003493 each day after surgery (p = 0.002). Other dependencies over time after surgery were not revealed.
DISCUSSION
The presented analysis showed that facial EMG performed during clinical routine follow‐up visits of patients who underwent hypoglossal‐facial jump nerve suture is foreseeing the reinnervation prior to first clinical movements. Hence, the success of the surgery can be predicted much earlier by facial EMG than by clinical evaluation. The elaborate offline MUP analysis revealed that the course of facial muscle reinnervation can be monitored much more sensitively using EMG than by clinical inspection including ongoing remodeling more than one year after surgery.
The used methodology and retrospective study design had limitations. Because we used EMG data from routine clinical examinations, the number of investigations, the examined muscles, and the time intervals between the investigations were varying between the patients. Using linear mixed models for statistical analysis we could overcome this limitation and found a significant correlation between quantitative EMG data and time course of reinnervation. Recordings from different healthy normal facial muscles reveal different MUP patterns as it has been shown in the present study and by Papagianni et al.11 To overcome the variability related to the investigated muscles, a z‐score transformation was performed which is an established method for quantitative MUP analyses.12 Here we did not record evoked compound muscle action potentials (CMAPs) which might give more detailed insights into the remodeling of the motor units during nerve regeneration compared with MUP analyses.5, 13 It would be of interest to compare results of voluntary and evoked potentials in a future larger and prospective controlled clinical study.
Although voluntary EMG is an established tool to monitor and prognosticate facial nerve function in patients with acute facial palsy,1, 2 the use of EMG for monitoring facial nerve regeneration after nerve reconstruction surgery is rare in the literature. In an earlier series of 53 patients with different types facial nerve reconstruction surgeries, the onset of voluntary MUP activity was found to be at about 4.5 months after surgery (including 7 patients with hypoglossal‐facial jump anastomosis with an onset after 4.8 months).6 This earlier results are only based on EMG reports in the patient's charts. The detailed manual MUP analysis performed here revealed that MUPs due to voluntary activity can be detected much earlier than by clinical examination. Pavese et al. used voluntary EMG to confirm the functional reinnervation 5–6 months after masseteric‐facial nerve suture, but did not present a EMG time course of the reinnervation.14 Evoked CMAP analysis by Zhang et al. in 12 patients with incomplete facial palsy who received a hypoglossal‐facial side‐to‐side nerve suture revealed a variable onset between 3–9 months after surgery.15 Here, it has to be taken into account that the peripheral nerve suture was performed much more distally compared to a standard end‐to‐side hypoglossal‐facial nerve jump suture, ie, the time course of reinnervation should be much shorter than for the standard hypoglossal‐facial nerve jump nerve suture. Zhang et al. did not perform a MUP analysis using voluntary EMG data. If Zhang et al. would have performed an MUP analysis using voluntary EMG, they probably would have found MUP activity even much earlier. The recovery of first MUPs can be detected much earlier using voluntary EMG than by evoked EMG and CMAP analysis.5 CMAP recovery is proceeding more than 10–12 months after surgery.16 This confirms that voluntary EMG analysis, as performed in the present study, can most sensitively detect the onset of facial muscles reinnervation during the early follow‐up after facial reconstruction surgery. EMG allows confirming the patients the successful nerve repair about 2–3 months earlier than by clinical examination. The other way around, it allows reacting 2–3 months earlier if EMG does not show signs of a success reinnervation. As the reinnervation can be detected earlier by electrophysiology than by qualitative or quantified clinical assessment, voluntary needle EMG is not only helpful in monitoring recovery after facial nerve reconstruction but also for assessment of reinnervation of freely transferred facial muscles. In a study by Michaelidou et al., the results of the MUP analysis 6 months after gracilis muscle transplantation were the best prognostic marker for the final muscle function.17
A maximal improvement of facial reanimation up to 18 months after surgery was observed. Therefore, we recommend a monitoring of patients after hypoglossal‐facial nerve jump suture for at least 18 months. The final functional outcome should be reached before decisions are made for additional surgical or non‐surgical measures.18 To monitor the patients also with EMG has the additional advantage to allow a clear distinction between incomplete reinnervation (incomplete recovery of the MUP recruitment pattern) and synkinetic reinnervation (involuntary MUP activity in a facial muscle while voluntarily tightening another facial muscle). This information is valuable for a personalized physical training. In contrast to a facial muscle with incomplete reinnervation, a synkinetically reinnervated muscle does not need training with the aim of muscle growth. Instead, botulinum toxin therapy and/or neuromuscular biofeedback training is needed with the aim of decoupling of the synkinetic movements.19
CONCLUSIONS
This cohort study demonstrates that facial EMG is a helpful tool for monitoring of patients after facial nerve reconstruction surgery. Now a larger, prospective controlled clinical trial is needed to confirm the clinical value of facial EMG for the monitoring of a facial nerve reconstruction. Facial EMG seems to foresee the time course of functional regeneration at least 2 months earlier than the clinical evaluation in patients with hypoglossal‐facial jump nerve suture. The quantitative MUP analysis shows that the reinnervation of the facial muscles seems to proceed for at least 15 months after nerve surgery. Facial EMG seems to have the potential as a valuable monitoring tool in patients who underwent facial nerve reconstruction surgery.
Supporting information
Supplemental Fig 1. Example of two MUPs in the orbicularis oculi muscle of a patient 14 months after hypoglossal‐facial nerve suture (Gain: 0.5 mV/Div; tilting speed: 8 ms/Div).
Supplemental Table I. Control measurements in healthy facial muscles.
Supplemental Table II. Raw data of the needle EMG measurements in facial muscles on the paralyzed side/side of the facial nerve reconstruction.
Conflict of Interest: None.
Statement of financial interest: The authors have no financial interest to declare in relation to the content of this article.
BIBLIOGRAPHY
- 1. Grosheva M, Wittekindt C, Guntinas‐Lichius O. Prognostic value of electroneurography and electromyography in facial palsy. Laryngoscope 2008;118:394–397. [DOI] [PubMed] [Google Scholar]
- 2. Volk GF, Klingner C, Finkensieper M, Witte OW, Guntinas‐Lichius O. Prognostication of recovery time after acute peripheral facial palsy: a prospective cohort study. BMJ Open 2013;3:e003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erminio F, Buchthal F, Rosenfalck P. Motor unit territory and muscle fiber concentration in paresis due to peripheral nerve injury and anterior horn cell involvement. Neurology 1959;9:657–671. [DOI] [PubMed] [Google Scholar]
- 4. Buchthal F, Kuhl V. Nerve conduction, tactile sensibility, and the electromyogram after suture or compression of peripheral nerve: a longitudinal study in man. J Neurol Neurosurg Psychiatry 1979;42:436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krarup C, Boeckstyns M, Ibsen A, Moldovan M, Archibald S. Remodeling of motor units after nerve regeneration studied by quantitative electromyography. Clin Neurophysiol 2016;127:1675–1682. [DOI] [PubMed] [Google Scholar]
- 6. Guntinas‐Lichius O, Streppel M, Stennert E. Postoperative functional evaluation of different reanimation techniques for facial nerve repair. Am J Surg 2006;191:61–67. [DOI] [PubMed] [Google Scholar]
- 7. Volk GF, Pantel M, Guntinas‐Lichius O. Modern concepts in facial nerve reconstruction. Head Face Med 2010;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. May M, Sobol SM, Mester SJ. Hypoglossal‐facial nerve interpositional‐jump graft for facial reanimation without tongue atrophy. Otolaryngol Head Neck Surg 1991;104:818–825. [DOI] [PubMed] [Google Scholar]
- 9. Manni JJ, Beurskens CH, van dV, Stokroos RJ. Reanimation of the paralyzed face by indirect hypoglossal‐facial nerve anastomosis. Am J Surg 2001;182:268–273. [DOI] [PubMed] [Google Scholar]
- 10. Stennert E, Limberg CH, Frentrup KP. [An index for paresis and defective healing–an easily applied method for objectively determining therapeutic results in facial paresis (author's transl)]. HNO 1977;25:238–245. [PubMed] [Google Scholar]
- 11. Papagianni AE, Kokotis P, Zambelis T, Karandreas N. MUAP values of two facial muscles in normal subjects and comparison of two methods for data analysis. Muscle Nerve 2012;46:346–350. [DOI] [PubMed] [Google Scholar]
- 12. Uesugi H, Sonoo M, Stalberg E, et al. ‘Clustering Index method': a new technique for differentiation between neurogenic and myopathic changes using surface EMG. Clin Neurophysiol 2011;122:1032–1041. [DOI] [PubMed] [Google Scholar]
- 13. Yayla V, Oge AE. Motor unit number estimation in facial paralysis. Muscle Nerve 2008;38:1420–1428. [DOI] [PubMed] [Google Scholar]
- 14. Pavese C, Cecini M, Lozza A, et al. Rehabilitation and functional recovery after masseteric‐facial nerve anastomosis. Eur J Phys Rehabil Med 2016;52:379–388. [PubMed] [Google Scholar]
- 15. Zhang L, Li D, Wan H, et al. Hypoglossal‐facial nerve 'side'‐to‐side neurorrhaphy using a predegenerated nerve autograft for facial palsy after removal of acoustic tumours at the cerebellopontine angle. J Neurol Neurosurg Psychiatry 2015;86:865–872. [DOI] [PubMed] [Google Scholar]
- 16. Kondo K, Takeuchi N, Tojima H, Ito K, Yamasoba T. + ‐ Reconstruction of the intratemporal facial nerve using interposition nerve graft: time course of recovery in facial movement and electrophysiological findings. Acta Otolaryngol Suppl 2007;559:85–90. [DOI] [PubMed] [Google Scholar]
- 17. Michaelidou M, Herceg M, Schuhfried O, et al. Correlation of functional recovery with the course of electrophysiological parameters after free muscle transfer for reconstruction of the smile in irreversible facial palsy. Muscle Nerve 2011;44:741–748. [DOI] [PubMed] [Google Scholar]
- 18. Jowett N, Hadlock TA. An evidence‐based approach to facial reanimation. Facial Plast Surg Clin North Am 2015;23:313–334. [DOI] [PubMed] [Google Scholar]
- 19. Miltner WHR, Weiss T, Miltner EM. Feedback training and other training programs In: Facial Nerve Disorders and Diseases: Diangosis and Management. Guntinas‐Lichius O, Schaitkin BM, eds. Stuttgart, Germany: Thieme. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig 1. Example of two MUPs in the orbicularis oculi muscle of a patient 14 months after hypoglossal‐facial nerve suture (Gain: 0.5 mV/Div; tilting speed: 8 ms/Div).
Supplemental Table I. Control measurements in healthy facial muscles.
Supplemental Table II. Raw data of the needle EMG measurements in facial muscles on the paralyzed side/side of the facial nerve reconstruction.


