Abstract
Objectives
To review the safety and efficacy of surgical management for spontaneous cerebrospinal fluid (CSF) leaks of the anterior and lateral skull base.
Data Sources
A systematic review of English articles using MEDLINE.
Review Methods
Search terms included spontaneous, CSF, cerebrospinal fluid, endoscopic, middle fossa, transmastoid, leak, rhinorrhea. Independent extraction of articles by 3 authors.
Results
Patients with spontaneous CSF leaks are often obese (average BMI of 38 kg/m2) and female (72%). Many patients also have obstructive sleep apnea (∼45%) and many have elevated intracranial pressure when measured by lumbar puncture. In addition to thinning of the skull base, radiographic studies also demonstrate cortical bone thinning. Endoscopic surgical repair of anterior skull base leaks and middle cranial fossa (MCF) approach for repair of lateral skull base leaks are safe and effective with an average short‐term failure rate of 9% and 6.5%, respectively. Long‐term failure rates are low. One randomized trial failed to show improved success of anterior leak repairs with the use of a lumbar drain (LD) (95% with vs. 92% without; P = 0.2). In a large retrospective cohort of MCF lateral skull base repairs, perioperative LD use was not necessary in >94% of patients.
Conclusions
Spontaneous CSF leaks are associated with female gender, obesity, increased intracranial hypertension, and obstructive sleep apnea. Endoscopic repair of anterior skull base leaks and MCF or transmastoid approaches for lateral skull base leaks have a high success rate of repair. In most cases, intraoperative placement of lumbar drain did not appear to result in improved success rates for either anterior or lateral skull base leaks.
Level of Evidence
2a, Systematic Review.
Keywords: Cerebrospinal fluid leak, CSF leak, spontaneous, endoscopic repair, MCF repair, anterior skull base, lateral skull base, obstructive sleep apnea, review
INTRODUCTION
Spontaneous cerebrospinal fluid (sCSF) leaks occur in the absence of trauma, surgery, or another inciting event. Typically, in both the anterior and lateral skull base, these leaks occur in areas where the skull base and dura are breached in an area over a pneumatized space; anteriorly along the cribriform plate or over the paranasal sinuses (Fig. 1) and laterally in the area of the temporal bone (Fig. 2). Herniation of brain through the skull base defects, termed an encephalocele, can also occur (Fig. 2C).
Figure 1.
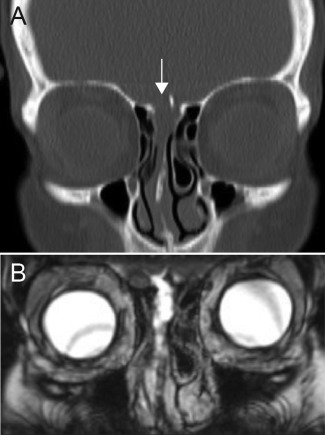
Representative images from a patient with an anterior sCSF leak. (A) Coronal CT showing a defect in the right cribriform plate (arrow). (B) Coronal T2 MRI showing the resulting meningocele through the right cribriform plate into the nasal cavity.
Figure 2.
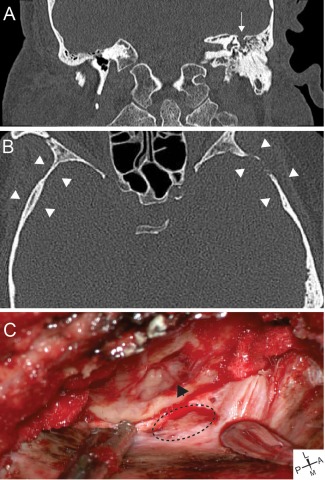
Representative images from a patient with a lateral sCSF leak. (A) Representative coronal CT showing left tegmen mastoideum defect (arrow) with fluid in the middle ear and mastoid. (B) Axial CT demonstrating cortical skull thinning (arrowheads). (C) Intraoperative images showing tegmen defect with encephalocele (arrowhead) and dural defect (dotted line). L = Lateral, M = Medial, P = posterior, A = Anterior.
The clinical presentation of sCSF leak should be recognized. Lateral sCSF leaks manifest with a clear middle ear effusion and aural fullness. Often patients will undergo myringotomy and tube placement resulting in continuous clear tube otorrhea that is pulsatile on otologic examination. Anterior sCSF leaks often present with clear rhinorrhea and headaches. Communication with the intracranial space increases the risk of meningitis and other cerebral complications. Intracranial complications may be the first presenting symptom of a sCSF leak. The average rate of preoperative meningitis in patients with a lateral skull base sCSF leaks is around 20%, with a reported range of 6%1 to 58%.2 Others patients can present with pneumocephalus.3
The diagnosis of sCSF leak involves the combination of clinical history, a skull base defect on high resolution computed tomography (CT) and associated clear pulsatile otorrhea or clear rhinorrhea. Confirmatory testing of the fluid with β‐2 transferrin can be performed although it is often not necessary if imaging findings and clinical history are consistent.
Unlike traumatic or iatrogenic leaks, sCSF leaks are highly associated with obesity and idiopathic intracranial hypertension (IIH). The obesity epidemic in the US started in the 1980s and after a 1–2 decade delay, there has been a corresponding rise in the number of patients with sCSF leaks.4 Spontaneous CSF leaks do not typically self‐resolve, and require surgical repair. For anterior skull base sCSF leaks, open repairs have largely been replaced by minimally invasive endoscopic repairs over the past 30 years, after Wigand reported the first endoscopic repair in the 1980s.5 Controversy still exists between open craniotomy (MCF), transmastoid or combined approach for repair of lateral skull base sCSF leaks.
The safety and efficacy of surgical treatment of sCSF leaks has not been systematically evaluated. The use of intra‐operative fluorescein and peri‐operative CSF diversion are controversial, but both will be discussed. This new systematic review hopes to elucidate the optimal approach and perioperative care for repair of skull base spontaneous CSF leaks.
MATERIALS AND METHODS
Study Method
Using a search methodology in accordance with PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses),6 a systematic review of the literature was performed for sCSF leaks of the anterior and lateral skull base.
The following PICOS (Participants, Interventions, Comparisons, Outcomes, and Study design) criteria were utilized. The patient population included patients with sCSF leaks of both the anterior and lateral skull base. The intervention included those who underwent surgical repair of spontaneous CSF leaks. The comparison was between various approaches with and without lumbar drains in addition to performing a comparison of the anterior and lateral skull base cohorts. The outcomes included post‐op CSF leak (i.e., success of repair) with various approaches for anterior skull base repair (i.e., endoscopic with and without septal flap) and lateral skull base repair (i.e., MCF vs. transmastoid vs. combined). In addition, we compared the postoperative CSF leak rates between anterior and lateral skull base cohorts. A secondary outcome evaluated was operative complications. The setting included one randomized controlled trial and all other studies were retrospective cohort studies.
Search Method
A search of all articles from January 1, 2000 until June 30, 2016 reporting outcomes after repair of sCSF leaks was performed using the Pubmed, Medline, and Cochrane Review Databases. This start date was chosen due to the previous meta‐analysis of anterior sCSF leaks in 2000.7 To our knowledge, there has not been a systematic review of lateral sCSF leaks. Using a keyword search strategy, studies were identified and verified independently by two authors (BCL, MMB) for the anterior skull base CSF leaks and by one author (RFN) for the lateral skull base CSF leaks.
The detailed keywords used for the anterior skull base search were as follows: Spontaneous, Cerebrospinal Fluid Leak, CSF Leak, Rhinorrhea, Endoscopic, Endonasal. The detailed keywords used for the lateral skull base search were as follows: Spontaneous, Cerebrospinal Fluid Leak, CSF Leak, Temporal, Otorrhea
Sufficient number of patients (≥5), English language and ability to extract data were used as inclusion criteria. Data points extracted from each study included number of patients, patient demographics, leak location, types of repair (including layers, flap, and rigid repairs), surgical approach, rates of postoperative leak, average follow up duration, and adjunct use of CSF diversion, packing, fluorescein, or acetazolamide.
In studies where the relevant data points were not explicitly calculated and reported, but individual data points were presented, calculations were made in order to extrapolate the relevant data points. In cases where data could not be extracted, specifically gender breakdown or number of lumbar drains used, this was documented.
RESULTS
The literature search (Fig. 3) began by broadly searching for any references of CSF or cerebrospinal fluid, ultimately coning down to identify citations relevant to endoscopic or endonasal management of spontaneous skull base CSF leaks. Articles not in English, or where fewer than 5 patients with spontaneous leaks were incorporated into analysis were immediately excluded. Once relevant citations had been identified and reviewed, articles in which relevant information could not be extracted or was not reported within the article were also excluded.
Figure 3.

Search methodology for anterior and lateral skull base sCSF leak repairs.
Literature searches identified 45 anterior and 21 lateral articles suitable for full text review. After review, 14 anterior and 6 lateral articles were excluded due to insufficient detail regarding spontaneous leaks or insufficient data for meaningful analysis. Thus, our search yielding 31 anterior and 15 lateral sCSF leak articles for analysis, which included a cumulative total of 646 anterior and 394 lateral sCSF leak patients across all studies (Tables 1 and 2).
Table 1.
Spontaneous CSF Leaks (Anterior Skull Base Repairs).
| Study Name, Year | Study Type |
Patients (#) (Gender) |
Approach | Recon Layers |
LD (#) Duration |
Follow up (avg) | Post‐op CSF leak (%) | Fluorescein |
|---|---|---|---|---|---|---|---|---|
| Lopatin et al,36 2003 | Retro |
21 (15F/6M) |
Endonasal | 3 layers |
Yes (21/21) 5‐8 d |
9–42 mo | 4.8% | N.R |
| Tosun et al,37 2003 | Retro |
7 (CND) |
Endonasal | 3 layers |
Yes (CND) 1‐5 d |
36 mo | 14% | N.R. |
| Schlosser et al,12 2003 | Retro |
16 (13F/3M) |
Endonasal | 1 or 2 layers |
Yes (16/16) 2‐3 d |
14.1 mo | 0% | Yes |
| Zuckerman et al,38 2005 | Retro |
11 (8F/3M) |
Endonasal | 2 layers or 3 layers |
Yes (11/11) CND |
15 mo | 18.2% | Yes |
| Silva et al,39 2006 | Retro |
6 (5F/1M) |
Endonasal | 3 layers or 4 layers | No | 27.4 mo | 0% | Yes |
| Basu et al,40 2006 | Retro |
8 (CND) |
Endonasal | 2 layers | N.R. | 25 mo | 12.5% | No |
| Woodworth et al,13 2008 | Retro |
56 (43F/13M) |
Endonasal + Caldwell Luc | 2 layers or 3 layers |
Yes (56/56) 2‐3 d |
34 mo | 5% | Yes |
| Purkey et al,14 2009 | Retro |
7 (5F/2M) |
Endonasal +Trephine | 2 layers |
Yes (7/7) 2‐3 d |
27.8 mo | 0% | Yes |
| Singh et al,41 2009 | Retro |
7 (5F/2M) |
Endonasal | 3 layers | No | N.R. | 0% | No |
| Banks et al,42 2009 | Retro |
77 (57F/20M) |
Endonasal | multiple |
Yes (CND) CND |
21 mo | 9% | Yes |
| Alameda et al,43 2009 | Retro |
10 (CND) |
Endonasal | 2 layers or 3 layers |
Yes (10/10) 4‐5 d |
23 mo | 6% | Yes |
| Forer et al,16 2010 | Retro |
7 (5F/2M) |
Endonasal | 5 layers | No | 33.7 mo | 14% | No |
| Seth et al,44 2010 | Retro |
39 (33F/6M) |
Endonasal | 3 layers |
Yes (38/39) CND |
23 mo | 12.8% | Yes |
| Giannetti et al,45 2011 | Retro |
26 (24F/2M) |
Endonasal | unk |
Yes (CND) CND |
70 mo | 38% | Yes |
| Caballero et al,46 2012 | Retro |
40 (CND) |
Endonasal | unk |
Yes (30/40) CND |
13 mo | 20% | Yes |
| Kirtane et al,47 2012 | Retro |
13 (7F/6M) |
Endonasal | 3 layers | No | 6–40 mo | 0% | No |
| Albu et al,21 2013 | Retro |
36 (CND) |
Endonasal | 2 layers |
Yes (17/36) 3 d |
48 mo | 16% | Yes |
| Deenadayal et al,48 2013 | Retro |
7 (5F/2M) |
Endonasal | 2 layers |
Yes (7/7) 2 d |
5–40 mo (15) | 0% | No |
| Virk et al,49 2013 | Retro |
36 (27F/9M) |
Endonasal | 3 layers |
Yes (22/36) CND |
21 mo | 11% | Yes |
| Chaaban et al,8 2014 | Prosp |
46 (34F/12M) |
Endonasal | 3 layers |
Yes (38/46) 3 d |
22 mo | 7.1% | Yes |
| Elmorsy et al,50 2014 | Retro |
31 (22F/9M) |
Endonasal | 4 layers | No | 32.4 mo | 12.9% | Yes |
| Fyrmpas et al,51 2014 | Retro |
11 (8F/3M) |
Endonasal | 3 layers |
Yes (11/11) 2‐4 d |
37.1 mo | 9.1% | No |
| Sannareddy et al,52 2014 | Retro |
11 (9F/2M) |
Endonasal | 2–3 layers |
Yes (7/11) 3 d |
15 mo. | 18.2% | No |
| Lieberman et al,53 2015 | Retro |
44 (35F/9M) |
Endonasal | 3 layers |
Yes (1/44) CND |
9.2 mo | 0% | Yes |
| Emanuelli et al,54 2015 | Retro |
10 (9F/1M) |
Endonasal | 3 layers |
No CND |
6–24 mo | 0% | Yes |
| Martínez‐Capoccioni et al,55 2015 | Retro |
25 (20F/5M) |
Endonasal | 3 layers or 2 layers |
Yes (25/25) 3 d |
1–72 mo | 4% | No |
| Pagella et al,56 2016 | Retro |
6 (6F/0M) |
Endonasal | 2 layers or NSF | No | 34–124 mo (80.8) | 16.7% | Yes |
| Ziade et al,23 2016 | Retro |
10 (8F/2M) |
Endonasal | 2 layers | No | 6–38 mo | 0% | Yes |
| Nix et al,57 2016 | Retro |
7 (CND) |
Endonasal | 2–4 Layers | No | N.R. | 0% | N.R |
| Sarkar et al,17 2016 | Retro |
5 (3F/2M) |
Endonasal | 1 layer |
Yes (5/5) 2 d |
11.4 mo | 0% | N.R. |
| Kljajic et al,58 2016 | Retro |
10 (7F/3M) |
Endonasal | 3 layers |
Yes (10/10) 5 d |
N.R. | 0% | Yes |
| Total |
646
(414F/124M) (77%F/23%M) |
0‐38%
Avg = 9% |
19 Studies |
Avg = average; CND; could not determine; F = Female; LD = lumbar drain; M = Male; N.R. = not reported; Prosp = prospective; Retro = retrospective study.
Table 2.
Spontaneous CSF Leaks (Lateral Skull Base Repairs).
| Study Name, Year | Study Type | Patients (#) (Gender) |
Approach (% of total) |
LD (#) | Follow up (avg) | Post‐op CSF leak (%) |
|---|---|---|---|---|---|---|
| Gacek et al,59 1999 | Retro |
21 (14F/7M) |
MCF (89%) TM (11%) |
N.R. | N.R. | 0 |
| Brown et al,60 2004 | Retro |
9 (3F/6M) |
MCF (88%) TM (22%) |
N.R. | 14.8 mo | 2 (22%) |
| Leonetti et al,15 2005 | Retro |
48 (28F/20M) |
MCF (100%) | No | 57 mo | 2 (3.9%) |
| Gubbels et al,61 2007 | Retro |
15 (10F/5M) |
MCF (100%) | Yes (14/15) | 13 mo | 1 (7%) |
| Kutz et al,11 2008 | Retro |
17 (12F/5M) |
MCF (76%) Combined (12%) TM (12%) |
No | 11 mo | 1 (5.9%) |
| LeVay et al,9 2008 | Retro |
14 (3F/11M) |
TM (100%) | No | 24‐140 mo | 0 |
| Kari et al,62 2011 | Retro |
33 (CND) |
TM (75%) MCF (9%) Combined (9%) Subtotal (5%) |
Yes (33/33) | 54 mo | 1 (3%) |
| Oliaei et al,63 2012 | Retro |
15 (12F/3M) |
TM (61%) MCF (23%) Combined (11%) |
No | 12.7 mo | 1 (5.5%) |
| Kenning et al,27 2012 | Retro |
23 12F/11M) |
Combined (100%) | Yes (23/23) | 10.4 mo | 1 (4%) |
| Stucken et al,64 2012 | Retro |
11 (8F/3M) |
Combined (64%) MCF (36%) |
Yes (4/20) | 27.2 mo | 1 (5%) |
| Son et al,1 2014 | Retro |
33 (CND) |
Combined (53%) TM (41%) MCF (6%) |
Yes (33/33) | 17.5 mo | 2 (6%) |
| Kim et al,65 2014 | Retro |
15 (9F/6M) |
TM (100%) | No | 9 mo | 1 (7%) |
| Vivas et al,28 2014 | Retro |
32 (22F/10M) |
MCF (84%) TM (16%) |
Yes (32/32) | 23 mo | 3 (9.4%) |
| Stevens et al,66 2016 | Retro |
48 (38F/10M) |
TM (73%) MCF (15%) Combined (12%) |
N.R. | 23.1 mo | 7 (14.5%) |
| Nelson et al,10 2016 | Retro |
60 (41/19) |
MCF (100%) | No | 19.5 mo | 3 (6.5%) |
| TOTAL |
394
(212/116) (65% F/35% M) |
0–22%
Avg = 6.6% |
Avg = Average; Combined = MCF + TM; F = Female; M = Male; MCF = Middle Cranial Fossa; LD = lumbar drain; mo = months; Retro = retrospective study; TM = Transmastoid.
All studies utilized retrospective review, however, one article8 was described as a prospective review due to prospective data collection. All studies had at least five patients with spontaneous CSF leaks. The average study cohort was 20.8 patients (range 5–77) for anterior sCSF leaks and 26.2 patients (range 9–60) for lateral sCSF leaks. Follow‐up was variable and ranged from 1 to 124 months. A breakdown of analyzed studies can be seen in Tables 1 and 2.
Demographics
The patient population with sCSF leaks are often obese females of middle age. In our review 77% of anterior sCSF leaks and 65% of lateral sCSF leaks occurred females (Tables 1 and 2). Studies have demonstrated that patients with sCSF leaks have an elevated body mass index (average approximately 35‐38 kg/m2).9, 10 The typical age at presentation is 45–65 years (average approximately 57–60 years).10, 11
Diagnosis and Location
Clear rhinorrhea or unilateral clear middle ear effusion/otorrhea were reported throughout most studies as the presenting symptoms, with the minority of studies mentioning meningitis or other intracranial complications as a precipitating symptom.
The most common diagnostic test is a high resolution CT scan of the skull base (anterior or lateral) to evaluate for a skull base defect. It is essential to obtain fine cut images with <1mm slice thickness. Multi‐planar reformats can also be helpful. MRI studies are also used when differentiation of a skull base mass is required preoperatively. When there is collectable CSF rhinorrhea, a β‐2 transferrin assay is performed to confirm that the drainage is CSF and not due to allergic or vasomotor rhinitis.
The most common locations for anterior sCSF leak were the cribriform plate and lateral sphenoid sinus. However, one study noted the posterior table of the frontal sinus as the most common site of sCSF leak.8 Importantly, up to 31% of sCSF leaks have multiple defects in the anterior skull base.12 Lateral sCSF leaks occur over the tegmen mastoideum or the tegmen tympani most commonly.11 Posterior fossa defects are less common but have been reported.11 Up to 45% of patients have multiple tegmen defects.11
Surgical Approach and Repair Technique
Surgical repair of anterior sCSF leaks is almost exclusively through endoscopic endonasal approach (Table 1). In all but 2 studies, treatment was approached in a purely endonasal fashion. One study13 noted use of a Caldwell Luc approach to access the far lateral aspects of the sphenoid sinus. Purkey et al,14 utilized trephination to access a far lateral supraorbital ethmoid leak. These approaches were ultimately used to facilitate endoscopic treatment. Additional use of the transethmoid, transphenoid, or transptergoid approach may be needed to access the leak site for repair.
Approaches to the lateral temporal bone sCSF leaks include middle cranial fossa (MCF), transmastoid (TM), or combined approach. The MCF or combined approach is the most common approach used (Table 2). The advantages of MCF approach include the ability to see the entire skull base floor in the event of multiple defects, placement of large multilayer grafts and avoidance of removal of ossicles for repair of tegmen tympani defects. Subtotal petrousectomy with ear canal closure has also been performed in poor hearing ears with large meningoencephloceles.15 It is recommended that MRI with HASTE imaging be performed at 18 months after repair to evaluate for residual epidermoid/cholesteatoma.
In the anterior skull base, multi‐layer repairs are most commonly reported, with one study16 reporting 5 layers used for repair of lateral sphenoid leaks, and one study17 mentioned the use of single layer repairs. It is essential to note that in practice the number of layers utilized for anterior repairs is heavily dependent on the size of the skull base defect. Small defects (<5 mm) are typically closed using free grafting. Typically, leaks in the cribiform region were repaired using 2–3 layers. Fibrin glue or tissue sealant use was variable and most studies reported the use of dissolvable packing. Less than half of studies reported utilizing nonabsorbable packing. Removal of the nonabsorbable packing ranged from 2–13 days post operatively. Pedicled posterior nasoseptal flaps can be utilized for cribiform and ethmoid defects, but typically smaller rotational or “trap door” flaps are used for spontaneous leaks. Bone grafting is useful in many locations along the anterior skull base, however along the cribiform this is technically very difficult and rarely used due to lack of circumferential bony edges for graft placement.
Multilayer closure also applies to lateral temporal bone sCSF leaks. Typical repair materials include autologous temporalis fascia, split calverial bone grafts, cellulose graft (Synthecel Dura Repair, DepuySynthes) and collagen graft (DuraGen, Integra). Additional reported materials used include vascularized flaps (temporal parietal flap) and bone cement. Hydroxyapatite cement (HAC) has been used to repair the skull base.18 HAC is a calcium‐phosphate cement that sets to hydroxyapatite (HA), the major component of human skeletal bone. This is advantageous because it can harden in a wet environment and it will osseointegrate into bone. The potential disadvantage is the risk of infection which has been report up to 5% of cases.
It should be noted that there is a strong association between tegmen dehiscence and superior semicircular canal dehiscence (SSCD)19 and up to 15.2% of patients with sCSF leaks will also have SSCD.20 While SSCD repair may not be required during sCSF leak repair, preoperative patient counseling about the risk of hearing loss and need for repair should be discussed.
Lumbar Drain and Fluorescein
The use of a lumbar drain in the treatment of sCSF leaks is controversial. The proposed advantages of placement of a lumbar drain intraoperatively are measurement of the CSF opening pressure (although the pressure measurement during an active leak may underrepresent the true intracranial pressure), administration of localizing agents such as fluorescein, and decompression of the temporal lobe during middle fossa craniotomy. Postoperative use of a lumber drain may be used to decrease CSF pressure to facilitate arachnoid formation at the surgical site.
Lumbar drain use
Many anterior skull base sCSF leak repair studies (21 of 31 studies) described the use of a lumbar drain (Table 1). A distinct advantage of lumbar drain placement is the use of interoperative fluorescein to localize the site of the leak endoscopically. Drains were typically used post‐operatively for 2–5 days. In contrast, most lateral skull base repairs are done without the use of a lumbar drain (Table 2). In addition, with direct visualization of the entire skull base floor through a middle fossa approach negates the need for fluorescein.
Ascribing drain use to success or failure is largely impossible due to limited data regarding individual patient lumbar drain use in each study. One randomized study of anterior skull base repairs has demonstrated that the use of LD for anterior skull base repairs does not significantly decrease the recurrence rate of CSF leak post‐operatively.21 A large retrospective review of MCF repair of lateral skull base repairs demonstrated that LD are not required for successful repair.10
The risk of a lumbar drain has been reported to be as high as 12.3% and the complications include persistent lumbar leakage after removal, over drainage, and retained catheters.22 In addition, there is increased financial cost with placement and increased length of hospital stay.10 Thus, judicious use of lumber drains is recommended.
Fluorescein use
It should be noted that intrathecal fluorescein is an off‐label use of the medication. Fluorescein was used in patients in 19 studies of anterior sCSF leak repairs (Table 1). In a single study,23 fluorescein was used topically to help identify a leak. Typically, 10 mg of the substance is utilized, diluting 0.1 mL of 10% fluorescein in 10 mL of CSF or sterile preservative free saline and injected into the intrathecal space via a catheter or puncture over 10 minutes. Reported side effects include seizures, and with doses of 700 mg (70 times the recommended dose24) death. No seizures or deaths due to fluorescein were noted in any of the analyzed studies. In our review, fluorescein was found to be used more frequently in the United States.
Success Rates
The overall success rate for surgical repair of anterior and lateral sCSF leaks is high (Tables 1 and 2). For anterior sCSF leaks, the average overall failure rate was 9%, with 12 studies noting 0% failure rates (Table 1). It should be noted that these were primary failure rates. Several studies reported taking patients back to the operating room for second and third repairs. These secondary and tertiary repairs were not analyzed. Most surgeons used the endoscopic approach with multi‐layer closure and a septal flap.
For lateral sCSF leaks, the average overall failure rate was 6.6% (Table 2). The average follow‐up was typically more than 12 months. Both transmastoid and middle fossa approaches appeared to have low failure rates. There is not sufficient data to determine which approach has a higher success rate.
Treatment Adjuncts
Spontaneous CSF leaks are consistently associated with obesity. Studies have demonstrated elevated ICP in patients with sCSF leaks of the anterior25 and lateral26, 27, 28 skull base. However, diurnal variation of ICP occurs normally29 and not all patients with sCSF leaks have elevated ICP when measured.27, 28 Options for decreasing elevated ICP after surgical repair can include acetazolamide or ventriculoperitoneal (VP) shunt. Long‐term recurrence of CSF leaks is believed to result from lack of management of ICP.8 This management was advocated more in the anterior skull base sCSF leak literature compared to the lateral skull base literature.
DISCUSSION
Evaluating and treating patients with sCSF leaks has typically been challenging due to the underappreciated association with IIH and limited data catered specifically to this type of leak. In reviewing the literature several trends became apparent.
The etiology of sCSF leaks is not completely understood, but there is a clear association of sCSF leaks with obesity (∼80% of patients), elevated ICP (∼40% of patients) and OSA (∼43% of patients). Data from the National Health and Nutrition Examination Survey (NHANES) have demonstrated high rates of obesity in the United States beginning in the early 1980s.30 It is currently estimated that 35.2% of males and 40.5% of females are obese.31 Recent NHANES data demonstrate a stabilization of the obesity rate in the United States for men since 2005,31, 32 but a continued rise in the obesity rate for women.31 Correlating with the rise in obesity rates, there has been a more than doubling of the number of lateral skull base sCSF leak repairs from 2002 to 2012.4 Thus, sCSF leaks will likely continue to be prevalent for skull base surgeons.
Weight loss should be encouraged but currently there is no data showing that weight loss or bariatric surgery can alter ICP or the incidence of sCSF leaks.
IIH as determined by lumbar puncture is seen in ∼40% of patients with sCSF leaks. It is also important to note that when opening pressure is obtained in the operating room using general anesthetic, the measured pressure is often lower than that experienced by the patient when awake. Measurement of ICP with lumbar puncture at 4–6 weeks after repair should be considered. Elevated ICP can be management medically with acetazolamide, a carbonic anhydrase inhibitor that decreases CSF production. Some patients with significantly elevated ICP should be considered for VP shunting by a neurosurgeon. However, there are risks of these management strategies. While acetazolamide is a relatively low‐risk medication, it does come with the risk of electrolyte and metabolic derangements. Delayed measurement of CSF opening pressure is invasive. VP shunt risks include surgical site infections, meningitis, low‐pressure headaches, shunt revisions, shunt failure leading to repeat CSF leaks and death.
Because of elevated ICP, some authors advocate patients to be evaluated by an ophthalmologist to evaluate for papilledema. Interestingly, one prospective study demonstrated that papilledema was absent in patients with sCSF leaks.33
There is also a strong association with obstructive sleep apnea (OSA) in patients with sCSF leaks. As many as 43% of patients with sCSF presented with the diagnosis of OSA10 and the incidence of OSA may be higher if all patients with sCSF leaks were prospectively tested for OSA. This association is important because it is know that ICP spike during apneic events34 suggesting that episodic rises in ICP may also contribute to skull base erosion over time (Fig. 4). Thus, it is recommended that all patients with sCSF leaks undergo a polysomnogram to assess for OSA.10 After surgical repair of lateral skull base sCSF leaks, it appears safe to resume CPAP treatment of OSA.3 It is unknown if CPAP treatment of OSA can delay or prevent the occurrence of sCSF leaks.
Figure 4.
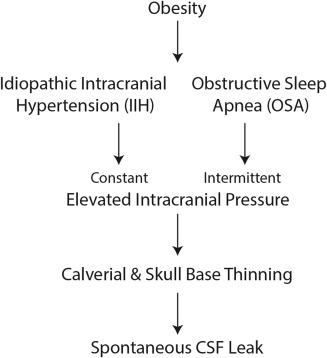
Proposed etiology of sCSF Leaks. Obesity is associated with IIH and OSA. Both of these lead to constant or intermittent elevations of intracranial pressure which is believed to lead to calvarial and skull base thinning over time leading to spontaneous CSF leaks.
Diagnosis of sCSF leaks follows a relatively straightforward paradigm, with initial testing of nasal secretions for β‐2 transferrin and high resolution imaging able to detect the vast majority of skull base defects. Cisternograms, myelograms, and radionucleide testing are also available adjuncts but should not be considered first line diagnostic modalities. Additional radiologic clues, such as empty sella, and calvarial thinning both anteriorly and laterally along the skull base should increase suspicion for IIH. If there is a concern for a significant meningocele or encephalocele neurosurgery consultation can also be considered. A note regarding lateral sphenoid sCSF leaks, Sternberg's canal has been proposed as a site of potential leak and encephalocele origin. More recent radiological analysis has demonstrated that leaks in the widely aerated sphenoid sinus tends to be lateral to this canal and not in fact associated.
Operative management of sCSF leaks for both the anterior and lateral skull base involves five key principles: 1) Identify the site of leak or leaks; 2) Determine the optimal surgical corridor; 3) Wide exposure including addressing the adjacent sinus (for anterior CSF leaks); 4) Meticulous preparation of the graft bed; and 5) Multilayer closure when possible. Regarding anterior skull base sCSF leaks, the endoscopic endonasal approach is considered the standard of care, but understanding both the necessity and anatomy of a pterygomaxillary fossa dissection to access far lateral recess sphenoid sinus sCSF leaks is essential. Meticulous wound bed preparation cannot be stressed enough as trapped sinus mucosa will impede graft healing and increase the long‐term risk for a mucocele. While the number of layers required for successful repair appears variable, multi‐layer repairs were consistently noted throughout the literature. It should also be noted that while pedicled tissue repair, namely the Hadad‐Bassagasteguy flap,35 is the workhorse of endoscopic skull base reconstruction, free mucosal grafting is typically suitable in sCSF leaks.
CSF diversion is still controversial in anterior CSF leak repairs. While there is data to suggest that in lateral leaks this is not necessary, such data is lacking for the anterior skull base. Anecdotally, it is argued that while positive pressure is helpful for graft healing and positioning in the lateral skull base, the same is not true of the anterior skull base where positive pressure will cause graft separation. Unfortunately, there is neither data to suggest or refute this.
In the very short‐term after repair, persistent CSF rhinorrhea is not necessarily indicative of graft failure. In the longer term, this may in fact represent a secondary leak rather than failure of the primary repair.
CONCLUSION
Spontaneous CSF leaks are associated with female gender, obesity, increased intracranial pressure, and obstructive sleep apnea. Diagnosis can often be achieved with testing by β‐2 transferrin and high‐resolution imaging. Endoscopic repair of anterior skull base leaks and MCF or transmastoid approaches for lateral skull base leaks have a high success rate of repair. In most cases, intraoperative placement of lumbar drain did not appear to result in improved success rates for either anterior or lateral skull base leaks.
Financial disclosures: none
Authors disclose no conflict of interest
This manuscript is an Independent Paper and has not been presented at a Triological Society meeting and is not a Triological Society Candidate Thesis.
BIBLIOGRAPHY
- 1. Son HJ, Karkas A, Buchanan P, et al. Spontaneous cerebrospinal fluid effusion of the temporal bone: repair, audiological outcomes, and obesity. Laryngoscope 2014;124:1204–1208. [DOI] [PubMed] [Google Scholar]
- 2. Markou K, Goudakos J, Franco‐Vidal V, et al. Spontaneous osteodural defects of the temporal bone: diagnosis and management of 12 cases. Am J Otolaryngol 2011;32:135–140. [DOI] [PubMed] [Google Scholar]
- 3. Wannemuehler TJ, Hubbell RD, Nelson RF. Tension pneumocephalus related to spontaneous skull base dehiscence in a patient on BiPAP. Otol Neurotol 2016;37:e322–e324. [DOI] [PubMed] [Google Scholar]
- 4. Nelson RF, Gantz BJ, Hansen MR. The rising incidence of spontaneous cerebrospinal fluid leaks in the United States and the association with obesity and obstructive sleep apnea. Otol Neurotol 2015;36:476–480. [DOI] [PubMed] [Google Scholar]
- 5. Wigand ME. Transnasal ethmoidectomy under endoscopical control. Rhinology 1981;19:7–15. [PubMed] [Google Scholar]
- 6. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hegazy HM, Carrau RL, Snyderman CH, et al. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta‐analysis. Laryngoscope 2000;110:1166–1172. [DOI] [PubMed] [Google Scholar]
- 8. Chaaban MR, Illing E, Riley KO, et al. Spontaneous cerebrospinal fluid leak repair: a five‐year prospective evaluation. Laryngoscope 2014;124:70–75. [DOI] [PubMed] [Google Scholar]
- 9. LeVay AJ, Kveton JF. Relationship between obesity, obstructive sleep apnea, and spontaneous cerebrospinal fluid otorrhea. Laryngoscope 2008;118:275–278. [DOI] [PubMed] [Google Scholar]
- 10. Nelson RF, Roche JP, Gantz BJ, et al. Middle Cranial Fossa (MCF) approach without the use of lumbar drain for the management of spontaneous cerebral spinal Fluid (CSF) Leaks. Otol Neurotol 2016;37:1625–1629. [DOI] [PubMed] [Google Scholar]
- 11. Kutz JW, Jr , Husain IA, Isaacson B, et al. Management of spontaneous cerebrospinal fluid otorrhea. Laryngoscope 2008;118:2195–2199. [DOI] [PubMed] [Google Scholar]
- 12. Schlosser RJ, Bolger WE. Spontaneous nasal cerebrospinal fluid leaks and empty sella syndrome: a clinical association. Am J Rhinol 2003;17:91–96. [PubMed] [Google Scholar]
- 13. Woodworth BA, Prince A, Chiu AG, et al. Spontaneous CSF leaks: a paradigm for definitive repair and management of intracranial hypertension. Otolaryngol Head Neck Surg 2008;138:715–720. [DOI] [PubMed] [Google Scholar]
- 14. Purkey MT, Woodworth BA, Hahn S, et al. Endoscopic repair of supraorbital ethmoid cerebrospinal fluid leaks. ORL 2009;71:93–98. [DOI] [PubMed] [Google Scholar]
- 15. Leonetti JP, Marzo S, Anderson D, et al. Spontaneous transtemporal CSF leakage: a study of 51 cases. Ear Nose Throat J 2005;84:700,702–704,706. [PubMed] [Google Scholar]
- 16. Forer B, Sethi DS. Endoscopic repair of cerebrospinal fluid leaks in the lateral sphenoid sinus recess. J Neurosurg 2010;112:444–448. [DOI] [PubMed] [Google Scholar]
- 17. Sarkar A, Sharma N. Spontaneous CSF rhinorrhea our experience. Indian J Otolaryngol Head Neck Surg 2016;68:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kveton JF, Coelho DH. Hydroxyapatite cement in temporal bone surgery: a 10 year experience. Laryngoscope 2004;114:33–37. [DOI] [PubMed] [Google Scholar]
- 19. Nadaraja GS, Gurgel RK, Fischbein NJ, et al. Radiographic evaluation of the tegmen in patients with superior semicircular canal dehiscence. Otol Neurotol 2012;33:1245–1250. [DOI] [PubMed] [Google Scholar]
- 20. Allen KP, Perez CL, Isaacson B, et al. Superior semicircular canal dehiscence in patients with spontaneous cerebrospinal fluid otorrhea. Otolaryngol Head Neck Surg 2012;147:1120–1124. [DOI] [PubMed] [Google Scholar]
- 21. Albu S, Emanuelli E, Trombitas V, et al. Effectiveness of lumbar drains on recurrence rates in endoscopic surgery of cerebrospinal fluid leaks. Am J Rhinol Allergy 2013;27:e190–e194. [DOI] [PubMed] [Google Scholar]
- 22. Ransom ER, Palmer JN, Kennedy DW, et al. Assessing risk/benefit of lumbar drain use for endoscopic skull‐base surgery. Int Forum Allergy Rhinol 2011;1:173–177. [DOI] [PubMed] [Google Scholar]
- 23. Ziade G, Hamdan AL, Homsi MT, et al. Spontaneous transethmoidal meningoceles in adults: case series with emphasis on surgical management. Scientific World J 2016;2016:3238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keerl R, Weber RK, Draf W, et al. Use of sodium fluorescein solution for detection of cerebrospinal fluid fistulas: an analysis of 420 administrations and reported complications in Europe and the United States. Laryngoscope 2004;114:266–272. [DOI] [PubMed] [Google Scholar]
- 25. Schlosser RJ, Wilensky EM, Grady MS, et al. Cerebrospinal fluid pressure monitoring after repair of cerebrospinal fluid leaks. Otolaryngol Head Neck Surg 2004;130:443–448. [DOI] [PubMed] [Google Scholar]
- 26. Brainard L, Chen DA, Aziz KM, et al. Association of benign intracranial hypertension and spontaneous encephalocele with cerebrospinal fluid leak. Otol Neurotol 2012;33:1621–1624. [DOI] [PubMed] [Google Scholar]
- 27. Kenning TJ, Willcox TO, Artz GJ, et al. Surgical management of temporal meningoencephaloceles, cerebrospinal fluid leaks, and intracranial hypertension: treatment paradigm and outcomes. Neurosurg Focus 2012;32:E6. [DOI] [PubMed] [Google Scholar]
- 28. Vivas EX, McCall A, Raz Y, et al. ICP, BMI, surgical repair, and CSF diversion in patients presenting with spontaneous CSF otorrhea. Otol Neurotol 2014;35:344–347. [DOI] [PubMed] [Google Scholar]
- 29. Maira G, Anile C, Cioni B, et al. Relationships between intracranial pressure and diurnal prolactin secretion in primary empty sella. Neuroendocrinology 1984;38:102–107. [DOI] [PubMed] [Google Scholar]
- 30. Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723–1727. [DOI] [PubMed] [Google Scholar]
- 31. Flegal KM, Kruszon‐Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011‐2012. JAMA 2014;311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aaron G, Doyle J, Vaphiades MS, et al. Increased intracranial pressure in spontaneous CSF leak patients is not associated with papilledema. Otolaryngol Head Neck Surg 2014;151:1061–1066. [DOI] [PubMed] [Google Scholar]
- 34. Jennum P, Borgesen SE. Intracranial pressure and obstructive sleep apnea. Chest 1989;95:279–283. [DOI] [PubMed] [Google Scholar]
- 35. Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 2006;116:1882–1886. [DOI] [PubMed] [Google Scholar]
- 36. Lopatin AS, Kapitanov DN, Potapov AA. Endonasal endoscopic repair of spontaneous cerebrospinal fluid leaks. Arch Otolaryngol Head Neck Surg 2003;129:859–863. [DOI] [PubMed] [Google Scholar]
- 37. Tosun F, Carrau RL, Snyderman CH, et al. Endonasal endoscopic repair of cerebrospinal fluid leaks of the sphenoid sinus. Arch Otolaryngol Head Neck Surg 2003;129:576–580. [DOI] [PubMed] [Google Scholar]
- 38. Zuckerman J, Stankiewicz JA, Chow JM. Long‐term outcomes of endoscopic repair of cerebrospinal fluid leaks and meningoencephaloceles. Am J Rhinol 2005;19:582–587. [PubMed] [Google Scholar]
- 39. Silva LR, Santos RP, Zymberg ST. Endoscopic endonasal approach for cerebrospinal fluid fistulae. MIN 2006;49:88–92. [DOI] [PubMed] [Google Scholar]
- 40. Basu D, Haughey BH, Hartman JM. Determinants of success in endoscopic cerebrospinal fluid leak repair. Otolaryngol Head Neck Surg 2006;135:769–773. [DOI] [PubMed] [Google Scholar]
- 41. Singh R, Hazarika P, Nayak DR, et al. Endoscopic repair of cerebrospinal fluid rhinorrhea ‐ Manipal experience. Indian J Otolaryngol Head Neck Surg 2009;61:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Banks CA, Palmer JN, Chiu AG, et al. Endoscopic closure of CSF rhinorrhea: 193 cases over 21 years. Otolaryngol Head Neck Surg 2009;140:826–833. [DOI] [PubMed] [Google Scholar]
- 43. Alameda YA, Busquets JM, Portela JC. Anterior skull base cerebrospinal fluid fistulas in Puerto Rico: treatment and outcome. Boletin de la Asociacion Medica de Puerto Rico 2009;101:29–33. [PubMed] [Google Scholar]
- 44. Seth R, Rajasekaran K, 3rd, Luong A, et al Spontaneous CSF leaks: factors predictive of additional interventions. Laryngoscope 2010;120:2141–2146. [DOI] [PubMed] [Google Scholar]
- 45. Giannetti AV, de Morais Silva Santiago AP, Becker HM, et al. Comparative study between primary spontaneous cerebrospinal fluid fistula and late traumatic fistula. Otolaryngol Head Neck Surg 2011;144:463–468. [DOI] [PubMed] [Google Scholar]
- 46. Caballero N, Bhalla V, Stankiewicz JA, et al. Effect of lumbar drain placement on recurrence of cerebrospinal rhinorrhea after endoscopic repair. Int Forum Allergy Rhinol 2012;2:222–226. [DOI] [PubMed] [Google Scholar]
- 47. Kirtane MV, Lall A, Chavan K, et al. Endoscopic repair of lateral sphenoid recess cerebrospinal fluid leaks. Indian J Otolaryngol Head Neck Surg 2012;64:188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deenadayal DS, Vidyasagar D, Naveen Kumar M, et al Spontaneous CSF rhinorrhea our experience. Indian J Otolaryngol Head Neck Surg 2013;65:271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Virk JS, Elmiyeh B, Saleh HA. Endoscopic management of cerebrospinal fluid rhinorrhea: the charing cross experience. J Neurol Surg Part B Skull Base 2013;74:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Elmorsy SM, Khafagy YW. Endoscopic management of spontaneous CSF rhinorrhea with septal graft and middle turbinate rotational flap technique: a review of 31 cases. Ear Nose Throat J 2014;93:E14–E19. [PubMed] [Google Scholar]
- 51. Fyrmpas G, Konstantinidis I, Selviaridis P, et al. Management of spontaneous cerebrospinal fluid leaks of the sphenoid sinus: our experience. J Laryngol Otol 2014;128:797–802. [DOI] [PubMed] [Google Scholar]
- 52. Sannareddy RR, Rambabu K, Kumar VE, et al. Endoscopic management of CSF rhinorrhea. Neurology India 2014;62:532–539. [DOI] [PubMed] [Google Scholar]
- 53. Lieberman SM, Chen S, Jethanamest D, et al. Spontaneous CSF rhinorrhea: prevalence of multiple simultaneous skull base defects. Am J Rhinol Allergy 2015;29:77–81. [DOI] [PubMed] [Google Scholar]
- 54. Emanuelli E, Milanese L, Rossetto M, et al. The endoscopic endonasal approach for cerebrospinal fluid leak repair in the elderly. Clin Neurol Neurosurg 2015;132:21–25. [DOI] [PubMed] [Google Scholar]
- 55. Martinez‐Capoccioni G, Serramito‐Garcia R, Huertas‐Pardo B, et al. Spontaneous cerebrospinal fluid leaks in the anterior skull base: a surgical challenge. J Laryngol Otol 2015;129:358–364. [DOI] [PubMed] [Google Scholar]
- 56. Pagella F, Pusateri A, Matti E, et al. Endoscopic management of spontaneous clival cerebrospinal fluid leaks: case series and literature review. World Neurosurg 2016;86:470–477. [DOI] [PubMed] [Google Scholar]
- 57. Nix P, Tyagi A, Phillips N. Retrospective analysis of anterior skull base CSF leaks and endoscopic repairs at Leeds. Br J Neurosurg 2016;30:422–426. [DOI] [PubMed] [Google Scholar]
- 58. Kljajic V, Vulekovic P, Vlaski L, et al. Endoscopic repair of cerebrospinal fluid rhinorrhea. Braz J Otorhinolaryngol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gacek RR, Gacek MR, Tart R. Adult spontaneous cerebrospinal fluid otorrhea: diagnosis and management. Am J Otology 1999;20:770–776. [PubMed] [Google Scholar]
- 60. Brown NE, Grundfast KM, Jabre A, et al. Diagnosis and management of spontaneous cerebrospinal fluid‐middle ear effusion and otorrhea. Laryngoscope 2004;114:800–805. [DOI] [PubMed] [Google Scholar]
- 61. Gubbels SP, Selden NR, Delashaw JB, Jr , et al. Spontaneous middle fossa encephalocele and cerebrospinal fluid leakage: diagnosis and management. Otol Neurotol 2007;28:1131–1139. [DOI] [PubMed] [Google Scholar]
- 62. Kari E, Mattox DE. Transtemporal management of temporal bone encephaloceles and CSF leaks: review of 56 consecutive patients. Acta Otolaryngol 2011;131:391–394. [DOI] [PubMed] [Google Scholar]
- 63. Oliaei S, Mahboubi H, Djalilian HR. Transmastoid approach to temporal bone cerebrospinal fluid leaks. Am J Otolaryngol 2012;33:556–561. [DOI] [PubMed] [Google Scholar]
- 64. Stucken EZ, Selesnick SH, Brown KD. The role of obesity in spontaneous temporal bone encephaloceles and CSF leak. Otol Neurotol 2012;33:1412–1417. [DOI] [PubMed] [Google Scholar]
- 65. Kim L, Wisely CE, Dodson EE. Transmastoid approach to spontaneous temporal bone cerebrospinal fluid leaks: hearing improvement and success of repair. Otolaryngol Head Neck Surg 2014;150:472–478. [DOI] [PubMed] [Google Scholar]
- 66. Stevens SM, Rizk HG, McIlwain WR, et al. Association between lateral skull base thickness and surgical outcomes in spontaneous CSF otorrhea. Otolaryngol Head Neck Surg 2016;154:707–714. [DOI] [PubMed] [Google Scholar]


