Abstract
Objective
To assess long‐term hearing outcomes in patients treated with third‐generation bisphosphonates for otosclerosis‐related progressive sensorineural hearing loss (SNHL).
Study Design
Retrospective case series review
Methods
We performed a retrospective case series review of patients with otosclerosis and progressive SNHL. Patients were treated with either risedronate or zoledronate after a diagnosis of otosclerosis with a significant SNHL component. Bone conduction pure tone threshold averages (BC‐PTAs) and word recognition scores (WRS) before and after bisphosphonate administration in long‐term follow‐up was analyzed. Significant change in BC‐PTA was defined as greater than 10dB or between 4% and 18% in WRS based on binomial variance.
Results
Seven patients were identified and 14 ears met inclusion criteria. Three patients were female and the mean age was 48.3 ± 10.3 years. The mean duration between treatment with bisphosphonate administration and long‐term post‐treatment follow‐up audiometry was 87.6 ± 18.3 months, with a range of 61.6 to 109.1 months and median of 89.2 months. Analysis using BC‐PTA and WRS demonstrated that 11 ears remained stable while 2 improved and 1 worsened. No patient experienced any major complication as the result of bisphosphonate therapy.
Conclusion
Treatment with third‐generation bisphosphonates is associated with stability in otosclerosis‐related sensorineural hearing over 5‐ to 9‐year period. These results suggest that such medications may prevent the progression of SNHL in patients with otosclerosis.
Level of Evidence
4 (Case series).
Keywords: Otosclerosis, sensorineural hearing loss, bisphosphonates, cochlear otosclerosis
INTRODUCTION
Otosclerosis is a common disorder with a prevalence of approximately 3 in 1000 adult Caucasians.1 It is an osteoclast‐driven disorder of the bony otic capsule with multiple reported stages.2 Clinical otosclerosis typically presents with conductive hearing loss due to an otosclerotic focus that interferes with stapes mobility.3, 4 These otosclerotic foci are visible radiographically by high resolution CT greater than 85% of the time.5, 6, 7, 8 In about 80% of cases, the affected site is the fissula ante fenestram9, where fixation of the stapes footplate occurs. Other areas that may also be affected include the round window (30%), pericochlear region (21%), and internal auditory canal (19%).9 It is estimated that approximately one‐third of patients with clinical otosclerosis eventually develop sensorineural hearing loss (SNHL).10, 11 While surgical procedures, such as stapedectomy can ameliorate the conductive component of hearing loss, treatment of sensorineural loss is only possible via amplification or, in extreme conditions, cochlear implantation.12 As such, there is a clear need for therapies that may slow or halt progressive SNHL for patients with cochlear otosclerosis.
Medical therapies for SNHL in otosclerosis have been proposed but have not gained widespread acceptance. Prior studies have examined the use of sodium fluoride and first‐generation bisphosphonates for halting SNHL, however, their effects have been modest.13, 14 A newer class of third‐generation bisphosphonates acts by inhibiting farnesyl pyrophosphate synthetase, thereby rendering osteoclasts inactive.15 It has also been hypothesized that reducing osteoclast activity will reduce the amount of TNF‐alpha that diffuses into the inner ear, thereby stabilizing or reversing hearing loss.16, 17 Recently, our group first reported on the use of third‐generation bisphosphonates for the treatment of progressive SNHL in patients with otosclerosis. In a preliminary report, we demonstrated that treatment stops progressive hearing loss in the short term (mean 13 months) for a majority of cases.7
Given the long‐term and slowly progressive nature of SNHL in otosclerosis, there is a need to evaluate medical therapies over extended periods of time to determine their efficacy and durability. In this study, we report long‐term hearing outcomes in 7 patients treated with third‐generation bisphosphonates for otosclerosis‐related sensorineural hearing loss. We provide interval follow‐up of 7 out of 10 patients from our groups' original report.
MATERIALS AND METHODS
A retrospective review was performed on all patients seen at our institution with demonstrated progressive SNHL and otosclerosis who were treated with third‐generation bisphosphonates. This included all 10 patients previously reported in Quesnel et al.7 in addition to 5 patients treated in the interim, spanning January 2008 through March 2017. Institutional review board approval from the human subjects committee was obtained (IRB Protocol #14–206H).
Inclusion criteria were identical to those previously described7 and are as follows: progression of sensorineural component of hearing loss in at least one ear, surgical confirmation of the diagnosis of otosclerosis in one or both ears, and evidence of retro‐fenestral otosclerosis on computed tomography (CT). We added the following inclusion criteria: audiometric follow‐up of at least 60 months (5 years). Based on the aforementioned criteria, 3 patients included in our previous report7 were excluded as they did not follow up, and an additional 5 patients that were treated with bisphosphonates did not have audiometric evidence of progressive SNHL in our system.
Candidates for bisphosphonate treatment were referred to rheumatology (M.S.) for evaluation and treatment using parenteral or enteric third‐generation bisphosphonates. Zoledronate was administered intravenously to 12 out of 15 patients while three patients opted for oral treatment using risedronate. Dosing was carried out using osteoporosis regimens: a single dose of 5 mg of zoledronate or 35 mg of weekly risedronate.
Audiometric data were evaluated before and after treatment. Specifically, we examined the most recent pretreatment audiogram and the last available audiogram post‐treatment. Follow‐up time is recorded as the difference between treatment date and most recent available post‐treatment audiogram. Word recognition scores (WRS) are reported as the percent of words correctly repeated and were based on four sets of 50 word monosyllable sets (CID W‐22).18 Meaningful changes in WRS depend on the starting value and were considered significant based on a binomial model of variance previously described by Thornton and Raffin.19
Bone conduction pure tone thresholds at five frequencies were analyzed (250, 500, 1000, 2000, and 4000 Hz). We further calculated bone conduction pure tone averages (BC‐PTA, “bone curves”) of four frequencies (500, 1000, 2000, and 4000 Hz). Based on previously described methods20, we added 5 dB to each value where the bone conduction testing reached upper limits of testing.7 A bone conduction PTA change of greater than an absolute value of 10 dB was determined to be significant.
Data are presented in raw format by each individual ear with WRS and BC‐PTA at each of the frequencies. We further stratified the data in two formats, based on previously published results from our group7 and using standardized methods for reporting collective audiometric data as agreed upon by the Hearing Committee of the American Academy of Otolaryngology–Head and Neck Surgery.21 Statistical analyses were carried out using Microsoft Excel (Redmond, Washington).
All patients underwent temporal bone CT with evidence of otosclerotic foci. A senior neuroradiologist further reviewed temporal bone CT scans on three patients (numbers 1, 2, and 3) who had both pre‐ and post‐ treatment images. All scans were non‐contrast CT scans obtained at our institution using thin cuts of the temporal bone. High resolution CT or cone beam CT protocols were utilized.
RESULTS
A total of 7 out of 15 treated patients met inclusion criteria for this study, all of which were previously reported on short‐term follow‐up by our group.7 All 14 ears met inclusion criteria and were analyzed here.
Table 1 demonstrates the distribution of patients, including age, sex, status of stapedectomy, treatment type and quantity, and audiometric data prior to treatment. The average age of our cohort is 48.3 ± 10.3 years with 4 males and 3 females. All except one patients had at least one stapedectomy. Patient 6 had an exploratory tympanotomy revealing mobile ossicular chain with round window obliteration. On serial audiograms, all 7 patients were found to have a progressive decline in sensorineural hearing loss prior to bisphosphonate treatment, data shown in our previous report7 and replicated in the Supplementary section as Table S1. Two or more pretreatment audiograms were used to establish progression of SNHL with patient 1's audiograms documented by the otologist in notes but only the immediate pretreatment audiogram was available for analysis. Patients 2–7 were followed for an average of 77.45 months (range 6.8 to 153.3 months) prior to treatment. Within this time period, all patients demonstrated progressive SNHL in one or both ears, at one or more frequencies, or by WRS with significance criteria as delineated in Methods. Five patients that were treated with bisphosphonates were excluded for clinically declining hearing and evidence of SNHL but without serial audiogram data demonstrating progressive SNHL and for not meeting the length of follow‐up period. All patients in our study were treated with third‐generation bisphosphonates as detailed in Table 1.
Table 1.
Patient Demographics and Pre‐Treatment Audiometric Data.
| Patient # | Side | Age/Sex | % WR | BC‐PTA | Stapedectomy | Treatment Post Stapedectomy | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | AD | 56 M | 98 | 30 | Yes | Yes | Risedronate (x4wk), |
| AS | 46 | 46.25 | Yes | Yes | Zoledronate | ||
| 2 | AD | 55 F | 54 | 68.75 | Yes (x2) | Yes | Zoledronate (x3) |
| AS | 72 | 60 | No | ‐ | |||
| 3 | AD | 54 F | 76 | 65 | Yes | Yes | Zoledronate |
| AS | 90 | 73.75 | Yes | No | |||
| 4 | AD | 36 M | 76 | 51.25 | Yes | Yes | Risedronate |
| AS | 90 | 36.25 | No | ‐ | |||
| 5a | AD | 31 F | 96 | 43.75 | No | ‐ | Risedronate |
| AS | 98 | 46.25 | No | ‐ | |||
| 6 | AD | 54 M | 38 | 68.75 | Yes | Yes | Zoledronate |
| AS | 28 | 66.25 | No | ‐ | |||
| 7 | AD | 52 M | 94 | 41.25 | Yes (x2) | Yes | Zoledronate |
| AS | 82 | 36.25 | Yes | Yes |
AD exploratory tympanotomy revealed mobile ossicles with obliterated round window
Wk = weeks, listed audiometric data are bisphosphonate pretreatment values
Table 2 details raw audiometric data from the first available pre‐treatment audiogram and the most recent post‐treatment audiogram. Bone conduction pure tone thresholds are presented at 250, 500, 1000, 2000, and 4000 Hz. Word recognition scores are similarly reported.
Table 2.
Patient Audiometric Data.
| Before Treatment | After Treatment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # | Side | 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | % WR | 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | % WR |
| 1 | AD | 15 | 20 | 30 | 25 | 45 | 98 | 20 | 30 | 25 | 25 | 50 | 98 |
| AS | 25 | 25 | 50 | 55 | 55 | 46 | 20 | 20 | 20 | 50 | 55 | 94 | |
| 2 | AD | 35 | 70 | 75 | 65 | 65 | 54 | 45 | 70 | 70 | 70 | 65 | 58 |
| AS | 30 | 55 | 60 | 60 | 65 | 72 | 40 | 50 | 70 | 60 | 60 | 66 | |
| 3 | AD | 20 | 70 | 55 | 55 | 80 | 76 | 25 | 45 | 55 | 60 | 65 | 80 |
| AS | 50 | 70 | 75 | 75 | 75 | 90 | 45 | 65 | 75 | 75 | 70 | 48 | |
| 4 | AD | 15 | 15 | 35 | 80 | 75 | 76 | 10 | 25 | 30 | 75 | 110 | 88 |
| AS | 5 | 10 | 25 | 55 | 55 | 90 | 5 | 15 | 25 | 75 | 85 | 88 | |
| 5 | AD | 25 | 35 | 45 | 45 | 50 | 96 | 40 | 55 | 50 | 60 | 40 | 100 |
| AS | 30 | 45 | 45 | 45 | 50 | 98 | 40 | 60 | 50 | 50 | 30 | 98 | |
| 6 | AD | 25 | 50 | 70 | 80 | 75 | 38 | 20 | 40 | 60 | 65 | 75 | 60 |
| AS | 15 | 35 | 65 | 80 | 85 | 28 | 25 | 40 | 60 | 80 | 85 | 34 | |
| 7 | AD | 5 | 15 | 35 | 55 | 60 | 94 | 10 | 20 | 10 | 35 | 50 | 94 |
| AS | 10 | 20 | 20 | 50 | 55 | 82 | 10 | 10 | 5 | 30 | 55 | 86 | |
Hz = Hertz, WR = word recognition.
Given variability of data at the pre‐treatment level, we plotted bone conduction pure tone threshold averages from each individual ear and compared this with post‐treatment averages as seen in Figure 1A. The dotted lines indicate the set thresholds for significance as determined by ± 10 dB. Similarly, the word recognition scores as modeled by the binomial distribution for significant change between pre‐ and post‐ treatment values are demonstrated in Figure 1B.
Figure 1.
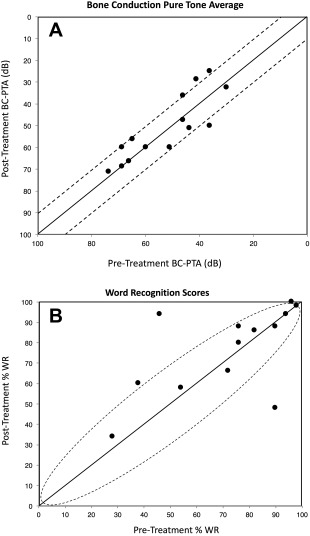
Panel 1A plots bone conduction pure tone threshold averages (BC‐PTA) from each individual ear and compares this with post‐treatment BC‐PTA. The dotted lines represent ±10 dB, which we consider a significant difference. The majority of ears analyzed had stable BC‐PTAs. Panel 1B shows Word Recognition Scores (WRS) as modeled by the binomial distribution for change between pre‐ and post‐treatment, where significant changes depend on pre‐ and post‐treatment values. Black circles within the dotted oval represent no significant change in WRS, where the majority of ears remain. (n = 14, # of ears)
A more recent, standardized method of presenting hearing thresholds and WRS was utilized to numerically represent our data.21 In Figure 2A, we show the full distribution of our patients' bone conduction PTA in relation to their WRS in the pretreatment condition. Figure 2B represents a similar graphic with change from pre‐treatment to post‐treatment, again in both bone conduction PTA and WRS. The majority of patients remain with stable hearing with few outliers.
Figure 2.
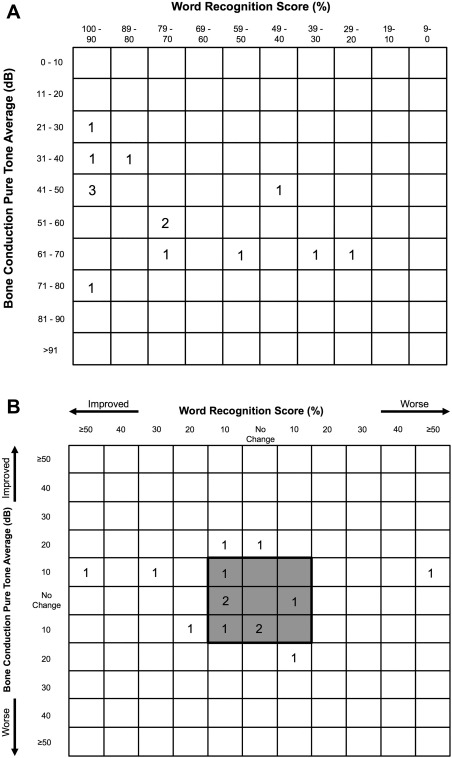
Panel 2A plots a pre‐treatment compilation of audiometric data for each ear in the format recommended by Gurgel et al.21 Each box represents a range of WRS and BC‐PTA with the number of ears in that range within the box. Panel 2B represents the change in WRS and BC‐PTA from pre‐ to post‐treatment. The shaded box diagrammatically indicates the patients with stable hearing over the follow up period, but does not utilize the binomial model of variance.
Table 3 delineates how the vast majority of patients experienced stable or improved hearing as measured by WRS and BC‐PTA in long‐term follow‐up. WRS was stable in 11 ears and improved in 2, while only 1 ear demonstrated a decrement in hearing clarity. For BC‐PTA, again 11 ears demonstrated stable thresholds while 2 were improved and only 1 was worse. The average follow‐up time for patients in this study was 87.55 ± 18.26 months with a range of 61.63 to 109.13 months. Of note, the 5 most recently treated patients that did not meet stated inclusion criteria above had an average of 28.4 months of follow‐up (range 5.5 to 45.4 months) with 4 out of 10 ears showing improved WRS (5 ears stable, 1 without available WRS), and 8 out of 10 ears showing stable BC‐PTA (1 improved, 1 worse).
Table 3.
Patient Hearing Changes Following Bisphosphonate Therapy.
| Patient/Side | WR | BC‐PTA | Follow‐up (months) |
|---|---|---|---|
| 1 AD | Stable | Stable | 109.1 |
| 1 AS | Improved | Stable | |
| 2 AD | Stable | Stable | 104.5 |
| 2 AS | Stable | Stable | |
| 3 AD | Stable | Stable | 89.7 |
| 3 AS | Worse | Stable | |
| 4 AD | Stable | Stable | 97.7 |
| 4 AS | Stable | Worse | |
| 5 AD | Stable | Stable | 61.6 |
| 5 AS | Stable | Stable | |
| 6 AD | Improved | Stable | 84.7 |
| 6 AS | Stable | Stable | |
| 7 AD | Stable | Improved | 66.0 |
| 7 AS | Stable | Improved |
WR = word recognition, BC‐PTA = bone conduction‐pure tone average.
All patients underwent computed tomography imaging of the temporal bone without contrast. In all patients, this demonstrated lucencies in at least one of the following areas of the otic capsule: fenestral, retrofenestral, or cochlear. Table 4 details imaging findings of our patient cohort. One patient had a CT in October 2002 (patient 2), which demonstrated normal findings, however, subsequent CT findings were consistent with otosclerosis (Table 4). Three patients (patients 1–3) had CT scans both before and after treatment. Two cases demonstrated stable findings and one (patient 2) showed increased mineralization at the cochlear capsule following bisphosphonate treatment was identified. Figure 3 displays the pre‐ and post‐treatment CT scans from a patient with stability of the otic capsule (patient 3, Figure 3A,B) and that of patient 2, where improved mineralization was seen after bisphosphonate treatment (Figure 3C,D).
Table 4.
Summary of Patient CT Scan Findings.
| Patient # | CT | First CT Date | Otosclerotic Foci (AU) | Subsequent CT | Post Treatment | Read |
|---|---|---|---|---|---|---|
| 1 | Yes | Nov. 2007 | Fenestral, retrofenstral, RW | Yes | Yes | Stable |
| 2 | Yes | Aug. 2005 | Cochlear capsule | Yes | Yes | Improved |
| 3 | Yes | June 2007 | Fenestral, retrofenstral | Yes | Yes | Stable |
| 4 | Yes | Aug. 2007 | Fenestral, retrofenstral, RW | No | ‐ | ‐ |
| 5 | Yes | Dec. 2004 | Fenestral, RW | No | ‐ | ‐ |
| 6 | Yes | May 2009 | Fenestral, retrofenstral, RW | No | ‐ | ‐ |
| 7 | Yes | Sep. 2009 | Cochlear capsule | No | ‐ | ‐ |
RW = round window.
Figure 3.
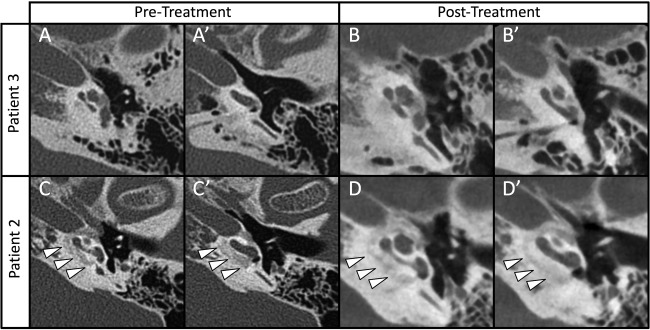
Imaging of temporal bones from two patients with pre‐ and post‐treatment scans. Pre‐treatment images were obtained with traditional thin cut CT and subsequent imaging was obtained with cone‐beam imaging. Panels A and B demonstrate stable findings over time in Patient #3. While panels C and D show axial cuts demonstrating lucencies around the cochlea in 2005 (white arrow heads), these same areas appear more mineralized post treatment (D and D') in 2012. A similar effect was reported recently in a case by Jung et al.33
DISCUSSION
This study demonstrates long‐term (5‐ to 9‐year) stability in hearing for a cohort of patients with progressive otosclerosis and sensorineural hearing loss treated with third‐generation bisphosphonates. Twelve out of 14 ears analyzed and treated showed stable‐to‐improved hearing. This durability in hearing for treated patients is encouraging and suggests that third‐generation bisphosphonates have an important role in the treatment of patients with progressive SNHL and cochlear otosclerosis. While we do not present direct placebo controls here, our group recently showed11 that the average long‐term (10‐year) SNHL due to otosclerosis was significantly more than SNHL due to age alone at each tested frequency. Overall, about one‐third of patients with otosclerosis showed a clinically significant progressive SNHL with average bone conduction thresholds above expected age‐related changes. These data suggest that SNHL does progress more in patients with otosclerosis than in the average age‐related hearing loss.
Bisphosphonate use is a well‐established and accepted treatment modality for patients with metabolic bone disorders, including such common conditions as osteoporosis. The mechanism of action involves the reduction of bone resorption via osteoclast inhibition.22 A one‐time dose of 5 mg intravenous zoledronic acid, the bisphosphonate with the highest affinity for bone, decreases resorption for up to 5 years.23 Such infrequent dosing is convenient for the patient and should provide long‐term stability in bone turnover. In this study, the majority of patients received a single dose of intravenous zoledronate without complications.
While bisphosphonates are known to work through the inactivation of osteoclasts, their specific mechanism of action in otosclerosis and hearing preservation is not well defined. Otosclerosis related sensorineural hearing loss is thought to result from involvement of the endosteal layer of the otic capsule, leading to hyalinization of the spiral ligament.24, 25, 26 Systemic bisphosphonates may halt or slow the process of hyalinization and thereby terminate the progression of SNHL in these patients. This in turn may reduce the amount of released TNF‐alpha, which is known to be toxic to hair cells.27, 28, 29 Decreasing perilymph TNF‐alpha concentrations should have a stabilizing effect on hair cell populations and hearing17, although this has not been scientifically validated. Alternatively, bisphosphonates may directly affect spiral ganglion cells, working to improve their survival.30
While bisphosphonates are widely used for the treatment of many bone disorders, they carry with them the risk of adverse side effects, some of which are serious. Patients may commonly complain of mild esophageal irritation, dysphagia, or reflux with oral administration, while intravenous delivery carries with it a risk of a mild acute phase response with inflammatory symptoms such as myalgia, arthralgia, and fever. Rare but reported adverse effects include hypocalcemia, nephrotoxicity, osteonecrosis of the mandible, atypical femur fractures, suppression of bone formation/resorption, orbital inflammatory syndromes, and musculoskeletal issues.22 For these aforementioned reasons, we strongly encourage our patients be evaluated by a rheumatologist that routinely uses bisphosphonates. This helps to assess each patient's risk profile and aid in their optimal dose and treatment regimen. We encourage a dialogue between rheumatology and otology to assess the risks and benefits of repeated dosing with bisphosphonates.
Among patients with cochlear otosclerosis treated with third‐generation bisphosphonates at our institution, we have not observed any of the severe side effects detailed above. However, given these potential risks, we have focused research efforts on establishing a local delivery mechanism for bisphosphonates to limit systemic side effects. Recently, Kang and colleagues31 describe non‐ototoxic local delivery of bisphosphonates in guinea pigs using alginate beads at the round window membrane. A second study32 used fresh human temporal bone specimens for delivery of bisphosphonates using a micropipette at the oval window. Further investigation is still needed to determine the optimal dose and vehicle for local delivery of bisphosphonates into the human inner ear.
Computed tomography provides one mechanism for monitoring patient response of medical therapy for cochlear otosclerosis. While this is a retrospective study, we did observe stable imaging findings of otosclerotic foci in two patients who underwent CT scan of the temporal bone before and after treatment (Table 4). We additionally report one patient with improved imaging findings where there is evidence of remineralization at the otic capsule. While the two sequential scans were performed using different techniques (high‐resolution CT vs. cone beam CT) the post‐treatment images were judged to represent a clear change in bone density by senior neuroradiology staff. These scans confirm the findings by Jung and colleagues who recently reported a similar case of radiologic improvement in mineralization in a patient with otosclerosis treated with bisphosphonates.33
The shortcomings of this study are multifold, including the limited number of patients treated, its retrospective nature, and lack of true controls. It does, however, build upon results from our previous publication7 and extends the follow‐up period to up to 9 years. This study further demonstrates the need for thoughtful consideration of bisphosphonates for the treatment of cochlear otosclerosis and broaches new questions, such as should these patients routinely have CT scans for surveillance in addition to audiograms? When do the effects of pulse bisphosphonate therapy wear off? Additional analysis and longer term follow‐up of a larger cohort is certainly needed.
CONCLUSION
Patients with cochlear otosclerosis treated with third‐generation bisphosphonates demonstrate stability in sensorineural hearing in long‐term follow‐up. These data suggest that third generation bisphosphonates have an important role in the treatment of patients with progressive SNHL and cochlear otosclerosis.
Supporting information
Supporting Information
Funding Source: None
Financial Disclosures: None
Conflicts of Interest: None
This work was partially presented as a poster at the American Otological Society (AOS) meeting as part of Combined Otolaryngology Spring Meetings (COSM) in Chicago, IL, May 20‐21, 2016.
BIBLIOGRAPHY
- 1. Declau F, Van Spaendonck M, Timmermans JP, et al. Prevalence of otosclerosis in an unselected series of temporal bones. Otol Neurotol 2001;22:596–602. [DOI] [PubMed] [Google Scholar]
- 2. Politzer A. Uber primare erkrankung del knochernen labyrinthkapsel. Ztschr Ohrenh 1893;25(309). [Google Scholar]
- 3. Rudic M, Keogh I, Wagner R, et al. The pathophysiology of otosclerosis: review of current research. Hear Res 2015;330(Pt A):51–56. [DOI] [PubMed] [Google Scholar]
- 4. Arnold W, Friedmann I. [Detection of measles and rubella‐ specific antigens in the endochondral ossification zone in otosclerosis]. Laryngol Rhinol Otol (Stuttg). 1987;66:167–171. [PubMed] [Google Scholar]
- 5. Berrettini S, Ravecca F, Volterrani D, Neri E, Forli F. Imaging evaluation in otosclerosis: single photon emission computed tomography and computed tomography. Ann Otol Rhinol Laryngol 2010;119:215–224. [DOI] [PubMed] [Google Scholar]
- 6. Mafee MF, Valvassori GE, Deitch RL, et al. Use of CT in the evaluation of cochlear otosclerosis. Radiology 1985;156:703–708. [DOI] [PubMed] [Google Scholar]
- 7. Quesnel AM, Seton M, Merchant SN, Halpin C, McKenna MJ. Third‐generation bisphosphonates for treatment of sensorineural hearing loss in otosclerosis. Otol Neurotol 2012;33:1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sakai O, Curtin HD, Fujita A, Kakoi H, Kitamura K. Otosclerosis: computed tomography and magnetic resonance findings. Am J Otolaryngol 2000;21:116–118. [DOI] [PubMed] [Google Scholar]
- 9. Arnold W. Some remarks on the histopathology of otosclerosis. Adv Otorhinolaryngol 2007;65:25–30. [DOI] [PubMed] [Google Scholar]
- 10. Glorig A, Gallo R. Comments on sensorineural hearing loss in otosclerosis In: Schuknecht HF, ed. Schuknecht's Pathology of the Ear. Boston, MA: Little, Brown & Company; 1962:63–78. [Google Scholar]
- 11. Ishai R, Halpin C, Shin JJ, McKenna MJ, Quesnel AM. Long‐term incidence and degree of sensorineural hearing loss in otosclerosis. Otol Neurotol 2016;37:1489–1496. [DOI] [PubMed] [Google Scholar]
- 12. Quaranta N, Bartoli R, Lopriore A, et al. Cochlear implantation in otosclerosis. Otol Neurotol 2005;26:983–987. [DOI] [PubMed] [Google Scholar]
- 13. Cruise AS, Singh A, Quiney RE. Sodium fluoride in otosclerosis treatment: review. J Laryngol Otol 2010;124:583–586. [DOI] [PubMed] [Google Scholar]
- 14. Kennedy DW, Hoffer ME, Holliday M. The effects of etidronate disodium on progressive hearing loss from otosclerosis. Otolaryngol Head Neck Surg 1993;109(3 Pt 1):461–467. [DOI] [PubMed] [Google Scholar]
- 15. Russell RGG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 2008;19:733–759. [DOI] [PubMed] [Google Scholar]
- 16. Brookler K. Medical treatment of otosclerosis: rationale for use of bisphosphonates. Int Tinnitus J 2008;14:92–96. [PubMed] [Google Scholar]
- 17. Brookler KH, Gilston N. Re: Third‐generation bisphosphonates for treatment of sensorineural hearing loss in otosclerosis, Otology & Neurotology 33:1308–1314, 2012. Otol Neurotol 2013;34:778–779. [DOI] [PubMed] [Google Scholar]
- 18. Hirsh IJ, Davis H, Silverman SR, Reynolds EG, Eldert E, Benson RW. Development of materials for speech audiometry. J Speech Hear Disord 1952;17:321–337. [DOI] [PubMed] [Google Scholar]
- 19. Thornton AR, Raffin MJ. Speech‐discrimination scores modeled as a binomial variable. J Speech Hear Res 1978;21:507–518. [DOI] [PubMed] [Google Scholar]
- 20. Halpin C. Measuring audiometric outcomes In: Evidence Based Otolaryngology. New York, NY: Springer; 2008:227–238. [Google Scholar]
- 21. Gurgel RK, Jackler RK, Dobie RA, Popelka GR. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg 2012;147:803–807. [DOI] [PubMed] [Google Scholar]
- 22. Maraka S, Kennel KA. Bisphosphonates for the prevention and treatment of osteoporosis. BMJ 2015;351:h3783. [DOI] [PubMed] [Google Scholar]
- 23. Grey A, Bolland MJ, Horne A, et al. Five years of anti‐resorptive activity after a single dose of zoledronate–results from a randomized double‐blind placebo‐controlled trial. Bone 2012;50:1389–1393. [DOI] [PubMed] [Google Scholar]
- 24. Kelemen G, Linthicum FH. Labyrinthine otosclerosis. Acta Oto‐Laryngol Suppl 1969;253:1–68. [PubMed] [Google Scholar]
- 25. Kwok OT, Nadol JB. Correlation of otosclerotic foci and degenerative changes in the organ of Corti and spiral ganglion. Am J Otolaryngol 1989;10:1–12. [DOI] [PubMed] [Google Scholar]
- 26. McKenna MJ, Merchant SN. Otosclerosis: Sensorineural Hearing Loss In: Merchant SN, Nadol JB, Jr., eds. Schuknecht's Pathology of the Ear. 3rd edn.; Shelton, CT: PMPH‐USA (People's Medical Publishing House), 2010:728. [Google Scholar]
- 27. Haake SM, Dinh CT, Chen S, Eshraghi AA, Van De Water TR. Dexamethasone protects auditory hair cells against TNFalpha‐initiated apoptosis via activation of PI3K/Akt and NFkappaB signaling. Hear Res 2009;255:22–32. [DOI] [PubMed] [Google Scholar]
- 28. Dinh CT, Haake S, Chen S, et al. Dexamethasone protects organ of corti explants against tumor necrosis factor‐alpha‐induced loss of auditory hair cells and alters the expression levels of apoptosis‐related genes. Neuroscience 2008;157:405–413. [DOI] [PubMed] [Google Scholar]
- 29. Aminpour S, Tinling SP, Brodie HA. Role of tumor necrosis factor‐alpha in sensorineural hearing loss after bacterial meningitis. Otol Neurotol 2005;26:602–609. [DOI] [PubMed] [Google Scholar]
- 30. Kao S‐Y, Kempfle JS, Jensen JB, et al. Loss of osteoprotegerin expression in the inner ear causes degeneration of the cochlear nerve and sensorineural hearing loss. Neurobiol Dis 2013;56:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang WS, Sun S, Nguyen K, et al. Non‐ototoxic local delivery of bisphosphonate to the mammalian cochlea. Otol Neurotol 2015;36:953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang WS, Nguyen K, McKenna CE, Sewell WF, McKenna MJ, Jung DH. Intracochlear drug delivery through the oval window in fresh cadaveric human temporal bones. Otol Neurotol 2016;37:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jung ME, WippoldII FJ, Goebel JA. Can high‐resolution computed tomography detect a therapeutic response to medical treatment in a patient with otosclerosis? Otol Neurotol 2016;37:e7–e8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


