Abstract
Background
Despite the increase in sensitivity and specificity of immunoassay technique over years, analytical interference remains to be major area of concern.
The interfering substances are endogenous substances that are natural, polyreactive antibodies as heterophilic or auto antibodies, or human anti-animal antibodies together with other unsuspected binding proteins that are unique to the individual. Interfering substances can interfere with the reaction between analyte and reagent antibodies in immunoassay resulting in false positive or negative values. This ultimately results in misinterpretation of patients reports and finally to wrong course of treatment.
Objective
In our study, we used a retrospective approach to find out the extent of interferences and type of interferences in some cases during our routine practice.
Method
The immunoassay reports which were clinically not correlating were retrospectively evaluated after discussion with the clinician. Over a period of six month a total of 42 samples were evaluated for interference for different immunoassay parameters such as Beta HCG, Estradiol, CA 125, AFP, prolactin, Hepatitis B Surface antigen (HbSAg) and troponin I. The samples were treated with commercially available antibody blocking agents and were reanalyzed. Commercially available diluents were used in some cases to evaluate high dose hook effect. Different platform, methodology and reagents were used for re -analysis.
Results
Out of 42 samples, 19 were found to be affected by interferences The data obtained for interferences was as follows beta HCG - 6 samples (2 positive and 4 negative interference); estradiol - 3 samples (2 positive and 1 negative interference); CA-125-3 samples (2 positive and 1 negative interference), Alfa Feto Protein - 2 samples (2 positive interference); prolactin - 1 sample (positive interference); Hepatitis B Surface antigen - 1 samples (negative interference); troponin I - 2 samples (positive interference).
Conclusion
Despite the use of state of the art laboratory equipments, chances of interference in immunoassay analysis resulting from endogenous substances could not be ruled out. In conclusion, thorough evaluation of all immunoassay reports should be carried out in cases of suspected interference.
Key words: heterophilic- antibody, immunoassay, interferences, cross reactive
INTRODUCTION
All laboratory assays are subject to interferences. The effects of hemolysis, lipemia, and bilirubinemia (i.e., icterus) on laboratory methods are well documented. Each of these may affect the analytical measurement. Despite the analytical sensitivity of immunoassays and measurements often being made without the need for prior extraction, immunoassays may lack adequate specificity and accuracy (1).
Developing immunoassays for the quantification of an analyte in a buffer solution has its own challenges, nonetheless quantification of the same analyte in a biological matrix (usually serum or plasma) bears additional complexities. The challenges include background assay signal changes, biological variability (between matrix samples) exceeding analytical imprecision and recovery of the spiked reference standard can be challenging. Despite the increase in sensitivity and specificity of the immunoassay techniques over years, analytical interference remains to be a major area of concern.
The interfering substances change the measurable concentration of the analyte or the altered antibody binding can potentially result in immunoassay interference. These are endogenous substances that are natural, polyreactive antibodies with other unsuspected binding proteins that are unique to the individual. These substances can interfere with the reaction between analytes and reagent antibodies in immunoassay resulting in false positive or false negative values (2,3,4,5,6). This ultimately results in misinterpretation of patients reports and finally to wrong course of treatment.
Heterophile antibodies accounts for large amount of interference in immunoassay. The presence of a heterophile antibody is characterized by broad reactivity with antibodies of other animal species (which are often the source of the assay antibodies). Such antibodies are commonly referred to as human anti-animal antibodies (HAAA). Human anti-mouse antibodies (HAMA) belong to this category. These can result in both false positive and false negative results (7). These are endogenous antibodies produced against poorly defined antigens. Both IgG and IgM heterophilic antibodies have been reported (8). These antibodies react with various antigens and the variable region of other antibodies (anti-idiotypic antibodies). In most of the cases there is no history of medical treatment with animal immunoglobulin or other well-defined immunogens, these are characteristically multi-specific (reacts with immunoglobulin from two or more species) or exhibit rheumatoid activity. So-called 'sandwich' immunoassays are particularly susceptible to this interference.
High dose hook effect is one of the cause of analytical interference in immunoassay. The hook effect or the prozone effect is a type of interference which plagues certain immunoassays and nephelometric assays, resulting in false negatives or inaccurately low results (9). The effect can also occur because of antigen excess, when both the capture and detection antibodies become saturated by the high analyte concentration. In most case no sandwich can be formed by the capturing antibody, the antigen and the detection antibody (10).
Analytical interference may also be due to cross reactivity. It is the most common interference - mostly in competitive assay. Cross reacting substance compete for binding site of antibody, resulting in over - or underestimation of analyte concentration (11). Cross reactivity is a major problem in diagnostic immunoassays. Cross reactivity can occur where endogenous molecules with a similar epitopes to the measured analyte exist in the sample, these may be metabolites of the analyte, or structurally similar pharmaceutical agents. (12)
Considering these interferences in immunoassay we have done follow up of non clinically correlating results. After excluding the pre analytical and post analytical factors root cause analysis of analytical interferences is done and presented here.
OBJECTIVE
In our study, we have used a retrospective approach to evaluate the extent and type of interferences in immunoassays during our routine practice laboratory.
METHOD
All the immunoassay reports which were clinically non correlating were retrospectively evaluated following consultation with the clinicians. Over a period of six month nearly 87,780 immunoassays were performed of which 42 samples were evaluated for interference - Beta HCG, Estradiol, CA 125, AFP, prolactin, HbSAg and troponin I results were scrutinized. Thorough patient history was collected to evaluate exposure to animal antibody and recent immunoglobulin inoculation. The samples were treated with commercially available antibody blocking agents and were reanalyzed (Table No. 2). Commercially available diluents were used to evaluate high dose hook effect (Table No. 3). Different platform, methodology, reagents were used for re-analysis (Table No. 1). Strict quality control measures were followed throughout the analysis process.
Table 2.
Details of antibody blocking agent used in study
| Sr. No. | Test parameter | Antibody blocking agent | Specification |
|---|---|---|---|
| 1. | Estradiol | True Block | Active HA/HAMA blocker - goat anti-human IgG (GAH IgG) Fc fragment |
| 2. | CA 125 | True Block | Active HA/HAMA blocker - goat anti-human IgG (GAH IgG) Fc fragment |
| 3. | AFP | True Block | Active HA/HAMA blocker - goat anti-human IgG (GAH IgG) Fc fragment |
| 4. | Prolactin | True Block | Active HA/HAMA blocker - goat anti-human IgG (GAH IgG) Fc fragment |
| 5. | Troponin I | True Block | Active HA/HAMA blocker - goat anti-human IgG (GAH IgG) Fc fragment |
Table 3.
Details of diluents used in study
| Sr. No. | Test parameter | Antibody blocking agent | Specification |
|---|---|---|---|
| 1. | Beta HCG | Access Total β hCG Calibrator S0 (zero) | S0 calibrator with beta HCG concentration 0 mIU/mL. |
| 2. | Ca 125 | Access Sample Diluent A | On board sample diluents available ready to use from |
| 3. | AFP | Access AFP sample Diluent | Ready to use |
| 4. | Troponin I | Access Sample Diluent A | On board sample diluent available ready to use from |
| 5. | Estradiol | VITROS High Sample Diluent A Reagent | On board sample diluent available ready to use from |
Table 1.
Details of various platforms, methodologies, reagents used in study
| Sr. No. | Equipment model & make | Methodology | Reagent used |
|---|---|---|---|
| 1. | Access 2 Beckman Coulter | Chemiluminescence Immunoassay | Dedicated reagent |
| 2. | D×1600 Beckman Coulter | Chemiluminescence Immunoassay | Dedicated reagent |
| 3. | Vitros ECi Ortho Clinical Diagnostic | Particle Enhanced Chemiluminescence Immunoassay | Dedicated reagent |
| 4. | Vitros 3600 Ortho Clinical Diagnostic | Particle Enhanced Chemiluminescence Immunoassay | Dedicated reagent |
| 5. | Mindray ELISA Reader | Enzyme Linked Immuno absorbent Assay | DRG Diagnostics ELISA kits for beta HCG, estradiol, Ca 125, AFP, Prolactin |
RESULTS
Out of 42 samples 19 were found to be affected by analytical interferences. The pre-analytical interferences was found in 20 cases and were attributed to wrong time of collection, wrong patient identity and wrong dilution protocols. Post analytical errors were found in 3 cases and were mainly due to wrong transcription in manual entry and wrong calculation in case of manual dilutions.
The present study focused on analytical interference. The data obtained for analytical interferences was as follows: beta HCG - 6 samples (2 positive and 4 negative interference); estradiol - 3 samples (2 positive and 1 negative interference); CA-125-3 samples (2 positive and 1 negative interference), Alfa Feto Protein - 2 samples (2 positive interference); prolactin - 1 sample (positive interference); HBsAg - 1 samples (negative interference); troponin I - 2 samples (positive interference).
In our study, the cases wherever cross reactivity or antibody interference were suspected an alternate platform was used, alternately commercial antibody blocking agents were used. Two different chemiluminescence (CLIA and CMIA) platforms were used. Cases where high dose hook effect was suspected serial dilutions were performed with commercially available diluents.
The serial dilutions were performed in the following order; 1:1, 1:2, 1:4, 1;10, 1:20, 1:50, 1:100, 1:500. The final results obtained in specific dilutions are shown in Table No. 4.
Table 4.
Result before and after evaluation with probable interference
| Sample number | First report | Report after evaluation | Interference reason |
|---|---|---|---|
| Beta HCG | |||
| 1. | 12586 mIU/mL | 6875 mIU/mL (Alternate Platform) | Antibody Interference/Cross Reactivity |
| 2. | 1856 mIU/mL | 15 mIU/mL (Antibody Blocking) | Antibody Interference |
| 3. | 23 mIU/mL | 7584 mIU/mL(1:10 Dilution) | High Dose Hook Effect |
| 4. | 115 mIU/mL | 23584mIU/mL (1:20 Dilution) | High Dose Hook Effect |
| 5. | 85 mIU/mL | 2846 mIU/mL (1:5 Dilution) | High Dose Hook Effect |
| 6. | 8 mIU/mL | 3589 mIU/mL (1:5 Dilution) | High Dose Hook Effect |
| Estradiol | |||
| 1. | 4285 pg/mL | 2865 pg/mL (Alternate Platform) | Antibody Interference/Cross Reactivity |
| 2. | 58 pg/mL | 3421 pg/mL (Antibody Blocking) | Antibody Interference |
| 3. | 1884 pg/mL | 758 pg/mL (Alternate Platform) | Antibody Interference/Cross Reactivity |
| CA 125 | |||
| 1. | 58 U/ml | 87564 U/ml (1:100 Dilution) | High Dose Hook Effect |
| 2. | 13 U/ml | 158964 U/ml (1:500 Dilution) | High Dose Hook Effect |
| 3. | 1852 U/ml | 58 U/ml (Antibody Blocking) | Antibody Interference |
| AFP | |||
| 1. | 86 ng/mL | 86945 ng/mL (1:50 Dilution) | High Dose Hook Effect |
| 2. | 25 ng/mL | 148697 ng/mL (Antibody Blocking) | Antibody Interference |
| Prolactin | |||
| 1. | 984 ng/mL | 28 ng/mL (Antibody Blocking) | Antibody Interference |
| Hbs Ag | |||
| 1. | Negative | Positive (Alternate Platform) | Antibody Interference/Cross Reactivity |
| Troponin I | |||
| 1. | 0.02 ng/ml | >150 ng/ml (1:3 Dilution) | High Dose Hook Effect |
| 2. | 0.02 ng/ml | 87 ng/ml (Antibody Blocking) | Antibody Interference |
DISCUSSION
Analytical errors arising from the presence of antibodies to mouse (monoclonal) immunoglobulins in the patient's plasma or serum have received the most attention but are just one of the many causes of interference in immunoassays. Reproducibility within a laboratory or among laboratories using the same or different analytical systems is no guarantee of the validity or correctness of the results (13). Possibly the most important of the idiosyncratic interfering substances found in patient samples are those that are either autoantibodies against the analyte itself (e.g., insulin autoantibodies) or heterophilic (including antianimal) antibodies that react with one or more of the assay reagents. Both types of antibodied can produce false high or false low results (13).
Specificity of an immunoassay depend on the binding property of the antibody, composition of the antigen and the matrix. Substances that alter the measurable concentration of an analyte in the sample or alter antibody binding can potentially result in assay interference (14, 15,16). These interfering substances may be unique to individuals and their concentration may changes over a period of time. The interfering substances may have low or high affinity and its concentration determines the extent of interference and may affect one or more analytes. Antibody blocking agents may not be sufficient to overcome all types of interferences (14, 15,16).
In immunoassays the heterophile antibody (or any other cross reacting substance) bridges the capture and detection antibodies to mimic analyte binding as such resulting in false high values. In contrast even in the presence of the analyte, heterophile antibody (or any other cross reacting substance) bind to the capture antibody preventing the analyte binding with the capture antibody resulting in falsely low values (negative interference). This is the basic mechanism for false high or low values resulting from the presence of heterophile antibody (Figure 1).
Figure 1.
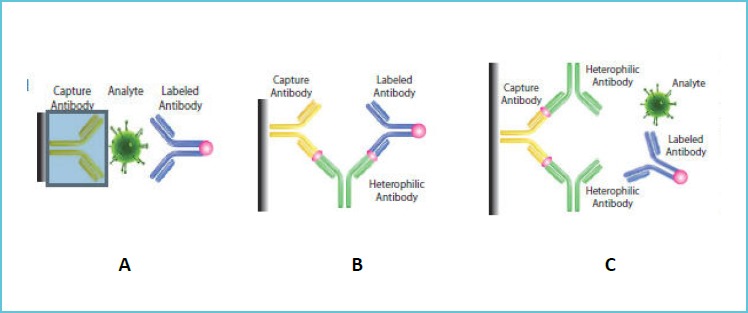
Showing mechanism for: (A) Intended Antigen - Antibody Bridge falsely; (B) high; and (C) low values in presence of heterophile (or any other cross reacting substance) antibody
Heterophile antibodies may be present in all patients (17). The frequency of immunoassay interferences resulting from these antibodies depends on the magnitude of bias in the analytical method that contributes to significant interference (13). The prevalence of potentially interfering antibodies has been reported to be as high as 40%, the incidence of immunoassay interference is estimated to be less than 2%.
They probably arise from mundane activities such as keeping pets, ingesting animal antigens, vaccination, infection, or even blood transfusion. High concentration of interfering proteins, which may be measurable in grams per litre, or proteins with high binding affinity can, however, overwhelm the analytical system, leading to assay interference and erroneous results (17).
The interference from heterophile antibodies can be avoided by combining different assay antibodies from different species with little cross reactivity (18) or by using antibody blocking agent which contains IgM and IgG antibodies with high affinity for the anti-animal antibody.
In high dose hook effect, all the available binding site from capture as well as detection antibodies are occupied by the analyte creating a stage of hyper saturation preventing formation of true antigen-antibody bridge. This can be prevented by diluting the sample as such decreasing analyte concentration and allowing improved antigen - antibody complex formation, eventually and final concentration is obtained by multiplying with the dilution fraction (Figure 2).
Figure 2.
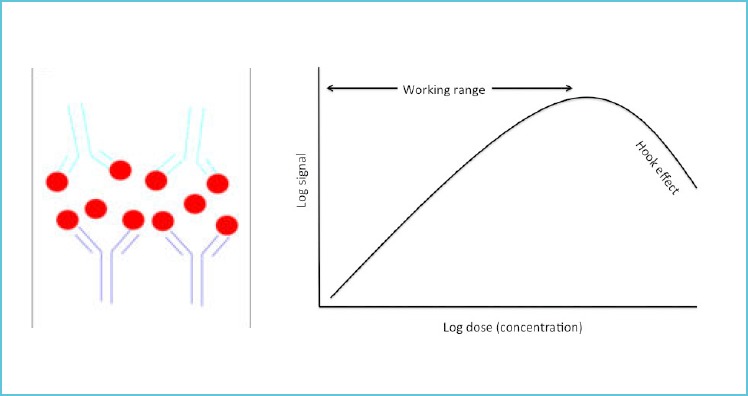
Analyte is binding to both capture as well as detection antibody leading to a reduction in formation of antibody-antigen-antibody complexes and a decrease in signal at higher concentrations of analyte
Limitation of our study is our retrospective approach, where evaluation was indicated only following feedback from clinicians. The screening for interfering antibody was done only after suspecting interference and was done only for the related subjects. The screening was not done actively for all the patients visiting the laboratory. As such, some of the interferences may have been missed during this period.
CONCLUSION
Despite use of state of the art laboratory equipment, chances of interference in immunoassay analysis resulting from endogenous substances cannot be ruled out. In conclusion, thorough evaluation of all non clinically correlating immunoassay results is advised.
REFERENCES
- 1.Eleftherios P. Diamandis. Immunoassay interference: a relatively rare but still important problem. Clinical Biochemistry 37 (2004) 331–332 [DOI] [PubMed] [Google Scholar]
- 2.Jane F. Emerson, MD, PhD, Keane K.Y. Lai, MD. Endogenous Antibody Interferences in Immunoassays. Winter 2013. | Volume 44, Number 1 Lab Medicine 69-74 [Google Scholar]
- 3.M P Brugts, J G L M Luermans, E G W M Lentjes, N J van Trooyen-van Vrouwerff, F A L van der Horst3, P H Th J Slee1, S W J Lamberts and J A M L Janssen Heterophilic antibodies may be a cause of falsely low total IGF1 levels European Journal of Endocrinology (2009) 161 561–565. [DOI] [PubMed] [Google Scholar]
- 4.Selby C. Interference in immunoassay. Ann Clin Biochem 1999;36:704–721. [DOI] [PubMed] [Google Scholar]
- 5.Kricka LJ. Human anti-animal antibody interference in immunological assays. Clin Chem 1999;45:942–956. [PubMed] [Google Scholar]
- 6.Preiser W, Brink NS, Hayman A, Waite J, Balfe P, Tedder RS. False-negative HIV antibody test results. J Med Virol 2000;600:43–47. [PubMed] [Google Scholar]
- 7.Bjerner J., Bormer O.P, Nustad K: The war on heterophilic interference. Clin Chem 2005; 51: 9–11 [DOI] [PubMed] [Google Scholar]
- 8.The Ghost in the assay tube: heterophil antibody interferences in immunoassays - an ever-recurring but often forgotten problem. Communiqué: A Mayo Reference Services Publication. 2003; 28(3):1–4. [Google Scholar]
- 9.Dasgupta A, Wells A, Datta P. Effect of digoxin Fab antibody on the measurement of total and free digitoxin by fluorescence polarization and a new chemiluminescent immunoassay. Ther Drug Monit 1999;21:251–255. [DOI] [PubMed] [Google Scholar]
- 10.CLSI. Interference Testing in Clinical Chemistry; Approved Guideline - Second Edition. CLSI document EP07-A2. Wayne, PA, Clinical and Laboratory Standard Institiute; 2005. [Google Scholar]
- 11.Matthew D Krasowski, Denny Drees, Cory S Morris, Jon Maakestad, John L. Blau and Sean Ekins Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction Krasowski et al. BMC Clinical Pathology. 2014, 14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JJ, Valdes R., Jr Approaches to minimizing interference by cross-reacting molecules in immunoassays. Clin Chem 1991;37:144–153. [PubMed] [Google Scholar]
- 13.Adel AA. Ismail Interference in immunoassays is insidious and could adversely affect patient care BMJ 2001;323:705–70611576963 [Google Scholar]
- 14.Hennig C, Rink L, Fagin U, Jabs WJ, Kirchner H. The influence of naturally occurring heterophilic anti-immunoglobulin antibodies on direct measurement of serum proteins using sandwich ELISAs. J Immunol Methods 2000;235:71–80. [DOI] [PubMed] [Google Scholar]
- 15.Revelen R, Bordron A, Dueymes M, Youinou P, Arvieux J. False positivity in a cyto-ELISA for anti-endothelial cell antibodies caused by heterophile antibodies to bovine serum proteins. Clin Chem 2000;46:273–278. [PubMed] [Google Scholar]
- 16.Lichtenwalner MR, Mencken T, Tully R, Petosa M. False-positive immunochemical screen for methadone attributable to metabolites of verapamil. Clin Chem 1998;44:1039–1041. [PubMed] [Google Scholar]
- 17.Hennig C, Rink L, Fagin U, Jabs WJ, Kirchner H. The influence of naturally occurring heterophilic anti-immunoglobulin antibodies on direct measurement of serum proteins using sandwich ELISAs. J Immunol Methods 2000; 235:71–80. [DOI] [PubMed] [Google Scholar]
- 18.Revelen R, Bordron A, Dueymes M, Youinou P, Arvieux J. False positivity in a cyto-ELISA for anti-endothelial cell antibodies caused by heterophile antibodies to bovine serum proteins. Clin Chem 2000; 46:273–278. [PubMed] [Google Scholar]


