Abstract
We report a case of acute onset of a biliary pancreatitis with cholangitis presented in our emergency department. The patient was under anticoagulant therapy with dabigatran due to persistent atrial fibrillation. Pancreatic enzymes including lipase were elevated above the linear measuring range and bilirubin together with cholestasis enzymes was also highly elevated. An ERCP with papillotomy was urgently indicated because postponing could lead to further deterioration of the patient's condition. Coagulation testing showed a prolonged thrombin time above 160sec which was followed by a diluted thrombin time (Haemoclot Test) resulted in a peak-level of dabigatran thus confirming full anticoagulation. Therefore, idarucizumab (Praxbind®) was administered pre-procedural of ERCP, the patient underwent uneventful ERCP without any bleeding complications, a full recovery was achieved and the patient was scheduled for elective cholecystectomy.
Key words: biliary pancreatitis, cholangitis, anticoagulant therapy, bleeding
INTRODUCTION
Non-VKA oral anticoagulants (NOACs) have been approved for various thromboembolic indications. In the past, the biggest disadvantage of these drugs has been the lack of specific antidotes to reverse the anticoagulant effect in emergency situations. Idarucizumab represents the first novel antidote against the direct thrombin inhibitor dabigatran and was approved by US FDA in October 2015(1).
In our case report we used idarucizumab for dabigatran reversal in a patient with mechanical cholestasis and cholangitis needing urgent endoscopic retrograde cholangiopancreatography (ERCP).
CLINICAL-DIAGNOSTIC CASE
We report the case of a 75 year old man who was admitted to the emergency department because of right sided upper abdominal pain with a duration of a few hours. Besides he described a clay-coloured stool and brown urine in the last days. He denied vomiting, fever or chills.
About 6 weeks before, he was hospitalized in another clinic because of an acute cholecystitis with multiple gallstones. He was taking dabigatran 150mg twice a day for non-valvular atrial fibrillation (A-fib).
He had a CHA2DS2-VASc score of 3 and no history of bleeding. He complained about pressure pain in the epigastrium and right upper quadrant, with no peritoneal signs on examination.
Laboratory testing showed a white cell count of 17.5 × 106/L, with 88% neutrophils, 6.2 U/L bilirubin, (normal range: 0.1-1.2), 131 U/L ALT (0-35), 230 U/L AST (0-45), 164 U/L ALP (40-130), 410 U/L GGT (0-55), 22.000 U/L lipase (0-60) and 37 CRP (0-5). Calculated creatinine clearance (Cockcroft and Gault) was 61 mL/min.
The abdominal ultrasound revealed dilated intrahepatic bile ducts in both liver lobes. The common bile duct (CBD) and pancreas were not visible due to intestinal gas overlay. Indication of urgent ERCP due to acute cholangitis was made. As the patient has taken dagibatran only a few hours before, laboratory testing of coagulation parameters were ordered including thrombin time (TT) and dabigatran drug level (Table 1). During the ERCP a small sphincterotomy was performed with an oozing bleeding (Figure 1). Consequently, 2.5 g idarucizumab (Praxbind®) was injected intravenously and the bleeding stopped immediately. Afterwards one impacted concrement was removed from the distal CBD with the aid of a dormia basket. After the intervention the patient received the second dose of idarucizumab (2.5g) and no further bleeding occurred throughout hospital stay.
Table 1.
Laboratory testing of coagulation parameters
| Prothrombin time (PT) | 10 sec | [9-12 sec] |
| Activated partial thromboplastin time (aPTT) | 54 sec | [26-36 sec] |
| Thrombin time (TT) | 150 sec | [-22 sec] |
| Drug level dabigatran (diluted TT; Haemoclot test) | 54 ng/ml | [50-300 ng/ml] Peak level |
Figure 1.
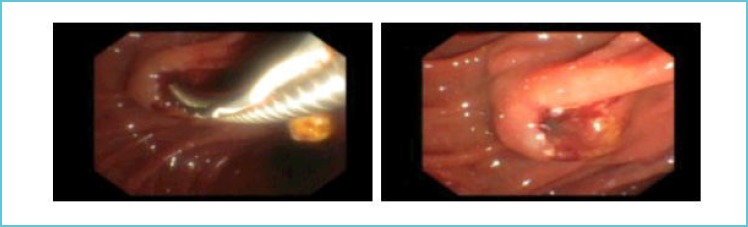
Sphincterotomy during the ERCP showing an oozing bleeding.
DISCUSSION
Bleeding is a rare complication of Non-VKA oral anticoagulants potentially associated with high mortality rates (2). The humanized monoclonal antibody fragment idarucizumab is the first available specific antidote for the NOAC dabigatran. It binds dabigatran with 350-fold higher affinity compared to dabigatran for thrombin (3). The application of idarucizumab completely reversed the anticoagulant effect of dabigatran within minutes and this effect is maintained for up to 24 hours (4).
The recently published ISTH (International Society on Thrombosis and Haemostasis) guideline (3) recommends to use this antidote in the case of life-threatening bleeding, bleeding into a critical organ or closed space, prolonged bleeding despite local hemostatic measures, high risk of recurrent bleeding because of overdose or delayed clearance of NOACs, and need for an urgent intervention associated with a high risk of bleeding (3).
In our case report, the mechanical cholestasis with cholangitis represents an urgent intervention. ERCP with sphincterotomy is considered as higher risk procedure for bleeding, and according to the European Society of Gastrointestinal Endoscopy (ESGE) guidelines the last dose of NOACs should be taken at least 48 hours before the intervention (5).
The thrombin time (TT) and dabigatran drug level represents useful parameters to determine the anticoagulation effect and bleeding risk of dabigatran (3). The TT determines the conversion of fibrinogen to fibrin, which is the final step in the coagulation cascade. It is prolonged in the presence of thrombin inhibitors such as dabigatran and this prolongation is directly proportional to the dabigatran concentration (6).
When analysing dabigatran drug concentration, it is important to consider when the last dose of the NOAC was taken to determine whether the levels are likely to raise or decline over time (3).
According to the ISTH recommendations, antidote administration should be considered if the drug concentration exceeds 30 ng/mL and the patient requires an urgent intervention associated with a high risk of bleeding (3).
In conclusion, idarucizumab can safely normalize clotting times within a few minutes. According to the ISTH guidelines, this antidote is indicated in patients with life-threatening bleeding and/or urgent interventions with a high bleeding risk (3).
At the moment, several antidotes for NOACs are under various stages of development: for example andexanet alfa, an antidote for the oral factor Xa inhibitors or ciraparantag, an antidote of all NOACs (3). Further studies are however needed to refine the use of antidotes for in different situations and patient groups.
TAKE HOME MESSAGES/LEARNING POINTS
With the use of idarucizumab urgent, vital interventions can be performed safely and quickly despite patients are on fully effective therapy with dabigatran.
To determine a substantial bleeding risk in patients with urgent interventions in patients on dabigatran, determination of thrombin time (TT) and dabigatran drug level using the Haemoclot® test are useful to verify dabigatran effectiveness. This is even more indicated, if clear history of medication intake cannot be obtained.
REFERENCES
- 1.Tummala R, Kavtaradze A, Gupta A, Ghosh RK. Specific antidotes against direct oral anticoagulants: A comprehensive review of clinical trials data. International journal of cardiology. 2016;214:292-298. [DOI] [PubMed] [Google Scholar]
- 2.Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al. Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: Executive summary. European heart journal. 2017;38(27):2137-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI, et al. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. Journal of thrombosis and haemostasis : JTH. 2016;14(3):623-627. [DOI] [PubMed] [Google Scholar]
- 4.Pollack CV, Jr., Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, et al. Idarucizumab for Dabigatran Reversal. The New England journal of medicine. 2015;373(6):511-520. [DOI] [PubMed] [Google Scholar]
- 5.Veitch AM, Vanbiervliet G, Gershlick AH, Boustiere C, Baglin TP, Smith LA, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016;48(4):c1. [DOI] [PubMed] [Google Scholar]
- 6.Kyrle PA, Binder K, Eichinger S, Fugger R, Gollackner B, Hiesmayr JM, et al. Dabigatran: patient management in specific clinical settings. Wiener klinische Wochenschrift. 2014;126(17-18):503-508. [DOI] [PMC free article] [PubMed] [Google Scholar]


