Abstract
Objective:
To assess the prevalence of gluten-free diet (GFD) among school-age children in Olmsted County, Minnesota, and compare it with the prevalence of celiac disease (CD) in the same age group.
Methods:
We performed a population-based study in Olmsted County using a survey to collect information from the six school districts in the county for the academic year 2014–2015. The survey contained questions to (1) assess the prevalence of GFD among school-age children in the public schools of Olmsted County; (2) assess the prevalence of CD among school-age children in Olmsted County; and (3) determine the indications for GFD in these children. We used the infrastructure of the Rochester Epidemiology Project (REP) to calculate the prevalence of CD in children aged 4–18 years in December 2014.
Results:
Using the REP data, we identified sixty patients with CD in the county aged 4–18 years; the prevalence of CD among school students in 2014 was 193.6/100,000. The prevalence of GFD in Olmsted County children, however, was higher, at 265/100,000 according to the survey from the school districts. The prevalence of GFD was highest in Rochester, the largest city. GFD was more common among children in secondary schools.
Conclusion:
According to our study, there are more children on GFD than the actual cases of CD in Olmsted County during the study period. This finding could be related to an increased number of children without CD who are following GFD for other indications.
Key words: Celiac disease, children, gluten-free diet, prevalence
INTRODUCTION
Wheat was introduced to the human diet about 10,000 years ago and has remained one of the most important crops thereafter. Currently, wheat is the most consumed food grain in the Western diet and globally.[1] Wheat grain is composed of carbohydrate and protein; the main protein component of wheat is gluten.[2] Gluten is also found in barley and rye. The clinical significance of eliminating gluten came from observations by Willem-Karel Dicke of the correlation between wheat consumption and malabsorptive symptoms in children[3] that was followed by multiple studies identifying gluten as the immunogenic trigger for celiac disease (CD).[3] CD is a common immune-mediated enteropathy precipitated by exposure to dietary gluten in those who have a genetic predisposition.[4] CD affects mainly the small intestines and results in inflammatory changes and villous atrophy that resolve with complete exclusion of gluten products from the diet.[5,6] The worldwide increase in CD occurrence created an increased awareness of CD and of a gluten-free diet (GFD) as the only treatment option.[7]
CD is not the only reason for following GFD. Other wheat/gluten-related medical conditions require following a wheat-free diet or GFD, including wheat allergy, dermatitis herpetiformis, and nonceliac gluten sensitivity (NCGS). Wheat allergy is an IgE-mediated sensitization to different components of wheat[8] and is seen in about 0.4%–1% of children; it is usually outgrown in 80% of children by the age of 5 years.[9,10,11,12] NCGS is a poorly understood entity that has neither an autoimmune nor allergic pathogenesis. NCGS is defined as having adverse symptoms after consuming gluten, without small intestinal injury or long-term complications.[13]
In recent years, the proportion of the American population who want to follow GFD has markedly increased.[14] More people without CD are avoiding gluten, termed persons without CD following GFD people without CD avoiding gluten (PWAG), for other indications such as weight loss, healthier diet, and behavioral concerns.[15]
The prevalence of GFD in patients without CD is not clear. A recent report using National Health and Nutrition Examination Survey (NHANES) data between 2009 and 2012 suggested that the prevalence of PWAG was 0.8% in the United States. The same study suggested that the prevalence of PWAG was higher in females and with increasing age in all racial groups. In contrast to CD, the prevalence of PWAG was higher in non-Hispanic African American persons than in Caucasian and Hispanics groups.[15]
In children, the prevalence of GFD that is followed for reasons other than CD is not known. Older children and adolescents may follow GFD for similar reasons as adults. Despite the lack of strong medical evidence to support GFD in children with autism spectrum disorders, many families elect to eliminate gluten because of autism or behavioral concerns.[16,17]
The aims of the current study were to (1) assess the prevalence of GFD among school-age children in the public schools of Olmsted County, Minnesota; (2) assess the prevalence of CD among school-age children in Olmsted County; and (3) determine the indications for GFD in these children.
METHODS
This study was conducted in Olmsted County, Minnesota, USA, which has a similar population to the US white population, except that a large proportion of the population is employed in the healthcare field.[18,19] The Olmsted County population has the same age, sex, and ethnic characteristics as Minnesota in general, with a majority of non-Hispanic whites (82%) and small minorities of African American (6%), Asian (6%), Hispanic (5%), and mixed ethnicities (1%).[20] Olmsted County provides an exceptional location to perform population-based studies such as this because almost all Olmsted County residents, along with all their inpatient and outpatient medical events and health-care providers, are linked and retrieved for research under the auspices of the Rochester Epidemiology Project (REP), which started in 1966. The REP reflects the residents who gave permission for their records to be used for research purposes (95% grant access to their medical records). The REP has been continuously funded by the National Institutes of Health.[21,22,23] To assess the prevalence of CD in school-age children, we used the REP medical records to identify all school and preschool students who have CD in Olmsted County.
The following inclusion criteria were used to identify children with a CD diagnosis based on the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines: (1) positive serologic markers such as antitissue transglutaminase IgA, (2) confirmatory small bowel biopsy that shows the characteristic histologic findings (increased intraepithelial lymphocytes, villous atrophy, and crypt hyperplasia), (3) Olmsted County residency on December 31, 2014, and (4) authorization for use of medical record for research. The data from the REP along with population data from the US Census Bureau were used to determine the number of students at risk for CD and to calculate the prevalence of CD and GFD in children between 4 and 18 years in Olmsted County on December 31, 2014, assuming that all children were at risk. Descriptive statistics analysis was performed. The prevalence of CD was calculated by using the number of children who met our inclusion criteria as the numerator and the number of children at risk as the dominator. The prevalence of GFD used the survey results as numerator and the number of children at risk as dominator. The 95% confidence intervals (CIs) were calculated assuming the number of cases followed a Poisson distribution.
This study was approved by the Institutional Review Board at Mayo Clinic and Olmsted Medical Center and the public school boards. Public schools in Olmsted County are supervised by six school districts: Rochester, Byron, Dover-Eyota, Chatfield, Pine Island, and Stewartville. To assess the prevalence of GFD among the school-age children in Olmsted County, we used a survey to collect information from public schools participating in the National School Lunch Program (NSLP) in Olmsted County school districts. The NSLP is a nationwide program that provides lunch meals for students in public schools at free or reduced price.[24] The survey was completed by the nutritional service directors and nurses of the school districts for the 2014–2015 academic year. The survey contained the following questions: (1) total number of students at the primary and secondary schools; (2) number of students who get their lunch daily from schools through the NSLP; (3) number of students who eat GFD and have CD; (4) number of students who eat GFD without having CD (PWAG); and (5) reported reasons for avoiding gluten in those without CD (nonceliac group).
RESULTS
Using the REP data, we identified 60 school-age children (37 girls, 23 boys) with CD on December 31, 2014, in Olmsted County. The overall prevalence of CD in Olmsted County in 2014 was 193.6 (95% CI, 144.6–242.57) per 100,000 using the population census data. The prevalence was slightly higher for girls (244.1 [95% CI, 165.5–322.8] per 100,000) than boys (145.7 [95% CI, 86.1–205.2] per 100,000).
Using the survey results conducted for the academic year 2014–2015, we identified 82 school-age children in Olmsted County public school systems who were on GFD. Based on that, the sex-adjusted prevalence of GFD for children in Olmsted County was 265/100,000, higher than the prevalence of CD in Olmsted County children. Characteristics of students on GFD are shown in Table 1. GFD was more prevalent in girls (56%) and older children in secondary schools (52%). Of the 82 children on GFD, 33 (40%) were considered PWAG. The school survey identified multiple indications for following GFD other than CD that are summarized in Table 2 and Figure 1.
Table 1.
Characteristics of school-age children on gluten-free diet in Olmsted county

Table 2.
Reported indications for gluten-free diet in persons who avoid gluten (n=33)

Figure 1.
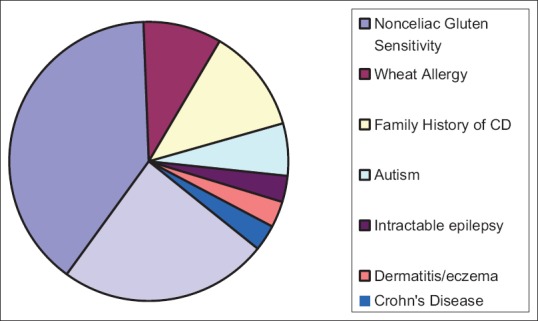
Reported indications for gluten-free diet in persons who avoid gluten
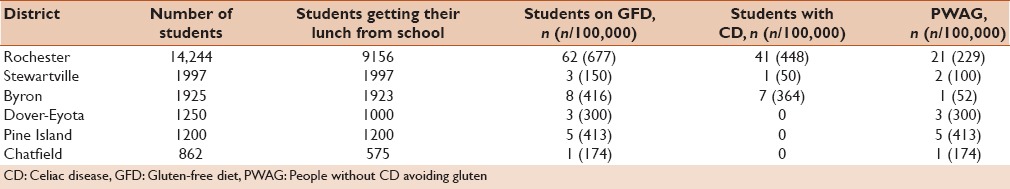
The prevalence of GFD, CD, and PWAG in the individual school districts are shown in Table 3. The highest prevalence of GFD and CD was in Rochester (677 and 448/100,000, respectively), which is the largest city in Olmsted County. The prevalence of PWAG was the highest in Pine Island (population 3263) at 413/100,000.
Table 3.
Results of school survey
DISCUSSION
Results of the recent NHANES have shown that half of those who eat GFD do not have CD and that the prevalence of PWAG is higher in females and increases with age.[25] Results of our study were consistent with the national trend. Approximately, 40% of school-age children in Olmsted County who are following GFD are PWAG, and more than 50% of PWAG eat GFD without any clinical indications. Some students in our study were eating GFD because they have a family history of CD but without being tested for or having a diagnosis of CD. Others are following GFD because they have Crohn's disease, epilepsy, dermatitis/eczema, or autism. Moreover, more females eat GFD than males.
These results raise concerns about the popularity of GFD, especially among young persons. The high number of PWAG in secondary school students could be due to a fashionable trend among the younger generations driven by the notion that GFD is healthier and can help them lose weight. On the basis of the survey results, 49 of the children following GFD had CD, which is lower than the number of patients with CD identified by reviewing the records (n = 60), which suggests that many children with CD bring their own lunch to school and do not use the NSLP despite the offering of a gluten-free menu. This may be explained by the fear of cross-contamination or the fear of being treated differently because of their restricted diet. The most common reason for following GFD in children without CD was NCGS. NCGS is characterized by a wide spectrum of intestinal and extraintestinal symptoms secondary to gluten ingestion.[26] The real problem, however, is that no consensus exists about the definition of NCGS in terms of symptoms and laboratory biomarkers. In Olmsted County, about 16% of students who eat GFD indicated NCGS as a reason for following GFD.
The high prevalence of GFD in children without a clear medical indication is concerning because many reports have suggested that GFD is not better or healthier, as advertised.[27,28] GFD is hard to follow, which was reflected in lower scores on quality of life questionnaires in children with CD.[29] Even though the gluten-free market has grown substantially in the United States, it is still not available everywhere and might be a challenge for many families. Gluten-free products are substantially more expensive and lack many nutrients that are important for all patients with CD and especially for children who are still growing, especially B vitamins, fiber, and iron.[30,31,32] Packaged gluten-free foods frequently contain more fat and sugar than their gluten-containing counterparts, which will result in increased fat and calorie intake in those following GFD. Hence, overweight and new-onset insulin resistance and metabolic syndrome have been identified in persons after initiation of a GFD.[33,34,35]
To the best of our knowledge, our study is one of the first to investigate the reasons for adhering to GFD in children who do not have CD. With the help of the REP and school districts in Olmsted County, we were able to study the prevalence of CD and GFD in our community. Our study highlights the need for more effort to educate the young population and their families about the advantages and disadvantages of GFD. We, as health-care providers, have the responsibility to counsel patients on the concerns about following GFD when it is not medically indicated and to advocate for healthier diets.
Our study has some limitations including its retrospective nature and the racial demographics of Olmsted County, which is more than 80% white. Another limitation is that we were able to collect data from public schools, but not the private schools, in Olmsted County. Moreover, about 26% of school-age children in Olmsted County public schools pack their lunch from home, some of whom may be following GFD; this may result in an underestimation of the prevalence of GFD in Olmsted County.
This is a preliminary study that highlights the trend of following GFD in children without CD in the communities of Olmsted County. There is a need for more studies in the future at the national level.
CONCLUSION
There are more children on GFD than the actual cases of CD in Olmsted County during the study period. This finding could be related to an increased number of children without CD who are following GFD for other indications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Heun M, Schafer-Pregl R, Klawan D, Castagna R, Accerbi M, Borghi B, et al. Site of Einkorn wheat domestication identified by DNA fingerprinting. Science. 1997;278:1312–4. [Google Scholar]
- 2.Shewry PR, Halford NG, Belton PS, Tatham AS. The structure and properties of gluten: An elastic protein from wheat grain. Philos Trans R Soc Lond B Biol Sci. 2002;357:133–42. doi: 10.1098/rstb.2001.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Berge-Henegouwen GP, Mulder CJ. Pioneer in the gluten free diet: Willem-Karel Dicke 1905-1962, over 50 years of gluten free diet. Gut. 1993;34:1473–5. doi: 10.1136/gut.34.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, et al. The oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA, et al. American College of Gastroenterology. ACG clinical guidelines: Diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–76. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leffler DA, Edwards-George J, Dennis M, Schuppan D, Cook F, Franko DL, et al. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Dig Dis Sci. 2008;53:1573–81. doi: 10.1007/s10620-007-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang JY, Kang AH, Green A, Gwee KA, Ho KY. Systematic review: Worldwide variation in the frequency of coeliac disease and changes over time. Aliment Pharmacol Ther. 2013;38:226–45. doi: 10.1111/apt.12373. [DOI] [PubMed] [Google Scholar]
- 8.Sotkovský P, Sklenář J, Halada P, Cinová J, Setinová I, Kainarová A, et al. A new approach to the isolation and characterization of wheat flour allergens. Clin Exp Allergy. 2011;41:1031–43. doi: 10.1111/j.1365-2222.2011.03766.x. [DOI] [PubMed] [Google Scholar]
- 9.Keet CA, Matsui EC, Dhillon G, Lenehan P, Paterakis M, Wood RA, et al. The natural history of wheat allergy. Ann Allergy Asthma Immunol. 2009;102:410–5. doi: 10.1016/S1081-1206(10)60513-3. [DOI] [PubMed] [Google Scholar]
- 10.Poole JA, Barriga K, Leung DY, Hoffman M, Eisenbarth GS, Rewers M, et al. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics. 2006;117:2175–82. doi: 10.1542/peds.2005-1803. [DOI] [PubMed] [Google Scholar]
- 11.Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, et al. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006;117:1118–24. doi: 10.1016/j.jaci.2005.12.1352. [DOI] [PubMed] [Google Scholar]
- 12.Lack G. Clinical practice. food allergy. N Engl J Med. 2008;359:1252–60. doi: 10.1056/NEJMcp0800871. [DOI] [PubMed] [Google Scholar]
- 13.Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012;10:13. doi: 10.1186/1741-7015-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mintel Gluten-Free Foods – US – September 2014. 2014. Available from: http://www.store.mintel.com/gluten-free-foods-us-september-2014 .
- 15.Choung RS, Ditah IC, Nadeau AM, Rubio-Tapia A, Marietta EV, Brantner TL, et al. Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: Findings from the national health and nutrition examination surveys from 1988 to 2012. Am J Gastroenterol. 2015;110:455–61. doi: 10.1038/ajg.2015.8. [DOI] [PubMed] [Google Scholar]
- 16.Marí-Bauset S, Zazpe I, Mari-Sanchis A, Llopis-González A, Morales-Suárez-Varela M. Evidence of the gluten-free and casein-free diet in autism spectrum disorders: A systematic review. J Child Neurol. 2014;29:1718–27. doi: 10.1177/0883073814531330. [DOI] [PubMed] [Google Scholar]
- 17.Hyman SL, Stewart PA, Foley J, Cain U, Peck R, Morris DD, et al. The gluten-free/casein-free diet: A double-blind challenge trial in children with autism. J Autism Dev Disord. 2016;46:205–20. doi: 10.1007/s10803-015-2564-9. [DOI] [PubMed] [Google Scholar]
- 18.Yunginger JW, Reed CE, O'Connell EJ, Melton LJ, 3rd, O'Fallon WM, Silverstein MD, et al. A community-based study of the epidemiology of asthma. incidence rates, 1964-1983. Am Rev Respir Dis. 1992;146:888–94. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 19.Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Potential influence of migration bias in birth cohort studies. Mayo Clin Proc. 1998;73:1053–61. doi: 10.4065/73.11.1053. [DOI] [PubMed] [Google Scholar]
- 20.St. Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA, et al. Generalizability of epidemiological findings and public health decisions: An illustration from the rochester epidemiology project. Mayo Clin Proc. 2012;87:151–60. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St. Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: The rochester epidemiology project. Am J Epidemiol. 2011;173:1059–68. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melton LJ., 3rd History of the rochester epidemiology project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 23.Rocca WA, Yawn BP, St. Sauver JL, Grossardt BR, Melton LJ., 3rd History of the rochester epidemiology project: Half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–13. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Food and Nutrition Service, USDA. National school lunch program and school breakfast program: Nutrition standards for all foods sold in school as required by the healthy, hunger-free kids act of 2010.final rule and interim final rule. Fed Regist. 2016;81:50131–51. [PubMed] [Google Scholar]
- 25.Choung RS, Ditah IC, Nadeau AM, Rubio-Tapia A, Marietta EV, Brantner TL, et al. Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: Findings from the national health and nutrition examination surveys from 1988 to 2012. Am J Gastroenterol. 2015;110:455–61. doi: 10.1038/ajg.2015.8. [DOI] [PubMed] [Google Scholar]
- 26.Catassi C, Elli L, Bonaz B, Bouma G, Carroccio A, Castillejo G, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): The salerno experts' criteria. Nutrients. 2015;7:4966–77. doi: 10.3390/nu7064966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Palma G, Nadal I, Collado MC, Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr. 2009;102:1154–60. doi: 10.1017/S0007114509371767. [DOI] [PubMed] [Google Scholar]
- 28.Gaesser GA, Angadi SS. Gluten-free diet: Imprudent dietary advice for the general population? J Acad Nutr Diet. 2012;112:1330–3. doi: 10.1016/j.jand.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Mustalahti K, Lohiniemi S, Collin P, Vuolteenaho N, Laippala P, Mäki M, et al. Gluten-free diet and quality of life in patients with screen-detected celiac disease. Eff Clin Pract. 2002;5:105–13. [PubMed] [Google Scholar]
- 30.Missbach B, Schwingshackl L, Billmann A, Mystek A, Hickelsberger M, Bauer G, et al. Gluten-free food database: The nutritional quality and cost of packaged gluten-free foods. PeerJ. 2015;3:e1337. doi: 10.7717/peerj.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens L, Rashid M. Gluten-free and regular foods: A cost comparison. Can J Diet Pract Res. 2008;69:147–50. doi: 10.3148/69.3.2008.147. [DOI] [PubMed] [Google Scholar]
- 32.Hallert C, Grant C, Grehn S, Grännö C, Hultén S, Midhagen G, et al. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment Pharmacol Ther. 2002;16:1333–9. doi: 10.1046/j.1365-2036.2002.01283.x. [DOI] [PubMed] [Google Scholar]
- 33.Kulai T, Rashid M. Assessment of nutritional adequacy of packaged gluten-free food products. Can J Diet Pract Res. 2014;75:186–90. doi: 10.3148/cjdpr-2014-022. [DOI] [PubMed] [Google Scholar]
- 34.Zuccotti G, Fabiano V, Dilillo D, Picca M, Cravidi C, Brambilla P, et al. Intakes of nutrients in italian children with celiac disease and the role of commercially available gluten-free products. J Hum Nutr Diet. 2013;26:436–44. doi: 10.1111/jhn.12026. [DOI] [PubMed] [Google Scholar]
- 35.Miranda J, Lasa A, Bustamante MA, Churruca I, Simon E. Nutritional differences between a gluten-free diet and a diet containing equivalent products with gluten. Plant Foods Hum Nutr. 2014;69:182–7. doi: 10.1007/s11130-014-0410-4. [DOI] [PubMed] [Google Scholar]


