Abstract
Background:
Infection is a common serious complication postpediatric cardiac surgery. Diagnosis of infection after cardiopulmonary bypass (CPB) is difficult in the presence of surgical stress, hemodynamic instability, and inflammatory reaction.
Aim:
The purpose of this study is to investigate the value of available inflammatory biomarkers and its validity to differentiate infection from inflammation postpediatric cardiac surgery and to find the trend and the change in the level of these biomarkers shortly after cardiac surgery.
Methods:
We conducted a prospective study that included all children who underwent cardiac surgery in Prince Sultan Cardiac Centre-Qassim from November 2013 to October 2015. C-reactive protein, erythrocyte sedimentation rate, white blood cell count, and neutrophil count were measured for all patients presurgery, 4 consecutive days postsurgery, and predischarge. Patients were divided into two groups (the infected and the noninfected group). We compared the level of biomarkers between both groups. Then, we further analyzed the effects of CPB and preoperative steroid on postoperative inflammatory biomarker levels. Collected data were then reviewed and analyzed.
Results:
There were 134 pediatric cardiac patients included during the study period. Group 1 (bacterial negative culture group) had 125 cases and Group 2 (bacterial positive culture group) had nine cases. We found no statistically significant difference in inflammatory biomarker elevation between both groups. Only Group 2 had higher (RACHS) Risk adjustment for congenital heart surgery score, more ventilator days, and more drop in platelet count on the 2nd and 3rd postoperative days in comparison with the noninfected group 1. Both groups of patients who were in on and off CPB had the same level of inflammatory biomarkers with no significant differences. Giving corticosteroid preoperatively did not affect the trend of biomarker elevation and made no difference when it was compared to the group of patients who did not receive corticosteroid before surgery.
Conclusion:
Common inflammatory biomarkers cannot differentiate between infection and inflammation within the first 5 days postpediatric cardiac surgery as these reflect the inflammatory process rather than infection. Trend is more important than single reading.
Key words: Cardiopulmonary bypass, C-reactive protein, infection, inflammation, pediatric cardiac surgery
INTRODUCTION
Sepsis accounts for 60%–80% of lost lives per year in children.[1] Incidence of sepsis in cardiac intensive care ranges between 13% and 30.8%.[2]
The term “systemic inflammatory response syndrome” (SIRS) describes nonspecific, generalized inflammatory processes that affect the body. The classification of severity of SIRS into uncomplicated SIRS, sepsis, and severe sepsis depends on the existence of documented positive culture or source of infection and the body's response to sepsis. Sepsis, however, is not always confirmed by positive cultures. Negative culture sepsis represents a well-known recognized entity in critically ill patients. It has been reported that only 40%–60% of patients with severe sepsis or shock have a microbiologically documented infection.[3]
Following any major surgery, trauma, burn, or significant insult, the nervous system activates the stress response and other active mediators leading to inflammation.[4] The body's response to tissue injury is rapid and highly amplified. Similar response may arise in the presence of infection. Cardiac surgery provokes a vigorous inflammatory response because of surgical trauma in addition to endothelium damage induced by extracorporeal bypass circulation leading to inflammatory mediators' release in the blood.[5] In the report from the Society of Thoracic Surgeons National Database, 20% (22,000 patients) of “low-risk” patients developed postoperative complications, the incidence of multiple organ dysfunction syndrome following cardiopulmonary bypass (CPB) was 11%, with a mortality rate of 41% in these patients as reported in various studies.[6]
Postpediatric cardiac surgery, some patients will have high fever with evidence of SIRS that mimics sepsis. Compromised cardiac function and low cardiac output state following cardiac surgery make the differentiation between sepsis and inflammatory reaction even more difficult. Applying SIRS criteria may not be enough to prove or to rule out infection in a child who developed inflammatory reaction postcardiac surgery. To be on the safe side, most of the physicians will choose to start broad-spectrum antibiotics and wait for culture results to prove or to exclude sepsis-induced SIRS. The decision to stop, de-escalate, or extend antibiotics coverage in a child who developed SIRS after cardiac surgery can be challenging when the patient has negative cultures but yet still demonstrating significant clinical evidence of SIRS or high level of inflammatory biomarkers. On the other hand, the extended use of antibiotics in an intensive care setup may lead to development of new multidrug-resistant strains of bacteria, increasing the cost, and resulting in unnecessarily prolonged Intensive Care Unit (ICU) stay.
Since fever is common postoperatively, identifying which of the many febrile children postcardiac surgery is actually septic may be more difficult task since neither clinical findings nor routine laboratory markers, except for positive cultures results, are accurate or reliable enough to make a definitive diagnosis of infection.[7]
C-reactive protein (CRP) levels from septic patients were reported according to the American College of Chest Physicians and Society of Critical Care Medicine (ACCP/SCCM) Consensus Conference classification in 1991 as mean values of 70 mg/L in SIRS patients, 98 mg/L in sepsis, 145 mg/L in severe sepsis, and 173 mg/L in septic shock.[8] Subsequently in 2001, they revealed that previously described diagnostic criteria for SIRS published in 1992 were overly sensitive and nonspecific.[9] A limited number of studies tried to investigate the values and levels of inflammatory biomarkers in inflammation and infection trying to identify clearer cutoff points of their levels in infection versus inflammation. The information available in children to answer this subject is scarce and limited.
The aim of our study is to find trend and level of common inflammatory biomarkers postpediatric cardiac surgery and compare these levels in inflammation and infection status trying to reach a cutoff point, which can help differentiating between these two entities in children undergoing cardiac surgery.
METHODS
This prospective noninterventional observational study was undertaken in Cardiac Surgical ICU at Prince Sultan Cardiac Center-Qassim between November 2013 and October 2015 after obtaining Institutional Review Board's approval. We included all children with cardiac disease who underwent corrective or palliative surgery. Routine white blood cell (WBC) counts with neutrophil counts, platelet counts, CRP, and erythrocyte sedimentation rate (ESR) were collected from all patients presurgery, then on 4 consecutive days postsurgery, and then predischarge from ICU between day 5 and day 7 postsurgery. Although procalcitonin (PCT) was found to be better marker for sepsis evaluation in some studies,[10,11] it was not included in our study due to unavailability in our center. Intravenous cefuroxime postcardiac surgery was used routinely and continued until removal of chest drains usually between days 2 and 3 postoperatively. If infection is suspected based on clinical or serological finding, then sepsis workup that included blood, urine, and tracheal aspirate cultures was taken before changing antibiotics to broad-spectrum antibiotic coverage. After collecting data, cases were divided based on positive culture results into two groups, the bacterial positive culture group who had organism isolated from cultures and the bacterial negative culture group who had negative cultures or they did not need to have any cultures as they were asymptomatic. Inflammatory markers were analyzed and compared between the two groups and P < 0.05 was considered statistically significant. Furthermore, we compared between inflammatory markers in cases who needed CPB and those who did not need bypass. In addition, cases on bypass were divided into those who received hydrocortisone 12 h presurgery and those who did not receive hydrocortisone and we compared biomarkers between both subgroups to see whether preoperative hydrocortisone alters postoperative level of biomarkers.
Continuous data were analyzed between groups and in between subgroups using unpaired Student's t- test. Data are presented, as mean ± standard error of the mean, and P < 0.05 was considered statistically significant.
RESULTS
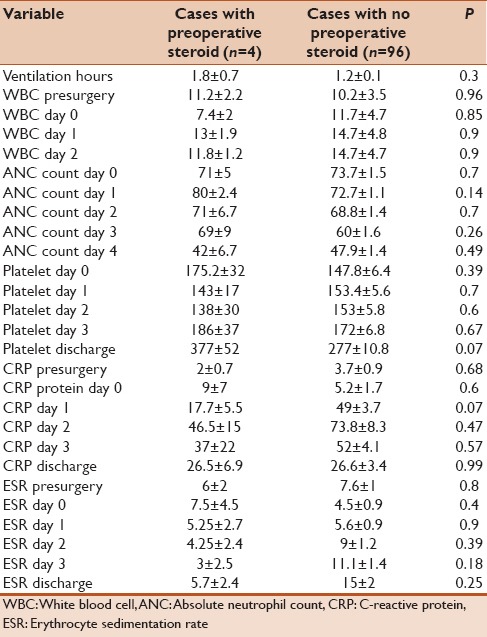
One hundred and thirty-four children were included and had complete data during the period of study. Of them, 84 patients were asymptomatic postsx2urgery and demonstrated no signs of SIRS and no clinical or serological evidence of infection. Fifty out of134 patients developed some evidence of SIRS after cardiac surgery that cannot be differentiated from possible infection and therefore underwent sepsis work. Nine out of 134 cases had positive cultures (6.7%) and were labeled as bacterial-positive culture group. Table 1 summarizes the site of infection and isolated organisms in each case. The rest of the patients including asymptomatic cases in addition to cases with signs of SIRS but negative cultures (125/134) were labeled as bacterial-negative culture group. Demographic data, RASCH score, and ventilation days for both groups were summarized in Table 2. We found that bacterial-positive culture group had higher RASCH score and ventilator hours in comparison to the bacterial-negative culture group. When we compared the inflammatory biomarkers between both groups, we found that the bacterial-positive culture group had more drop in their platelet count in the 2nd and 3rd days in comparison with the noninfected group. No other significant differences in other biomarkers were noted [Table 3]. When comparing on-pump cases (100/134) and off-pump cases (34), on-pump group had more notable drop in platelet count while other markers showed no statistically significant differences in comparison to off-pump cases as shown in Table 4. Similarly, we did not find any statistically significant difference in inflammatory biomarker levels between four cases that received hydrocortisone and 96 cases who did not receive hydrocortisone presurgery as shown in Table 5.
Table 1.
Isolated organism in positive culture group
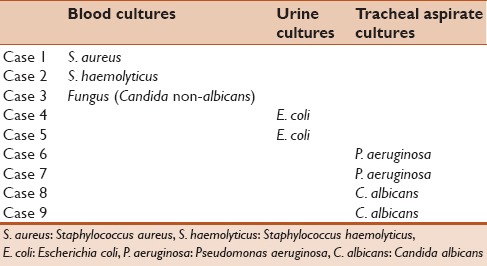
Table 2.
Demographic data of positive and negative culture groups

Table 3.
Inflammatory biomarkers between positive and negative culture groups
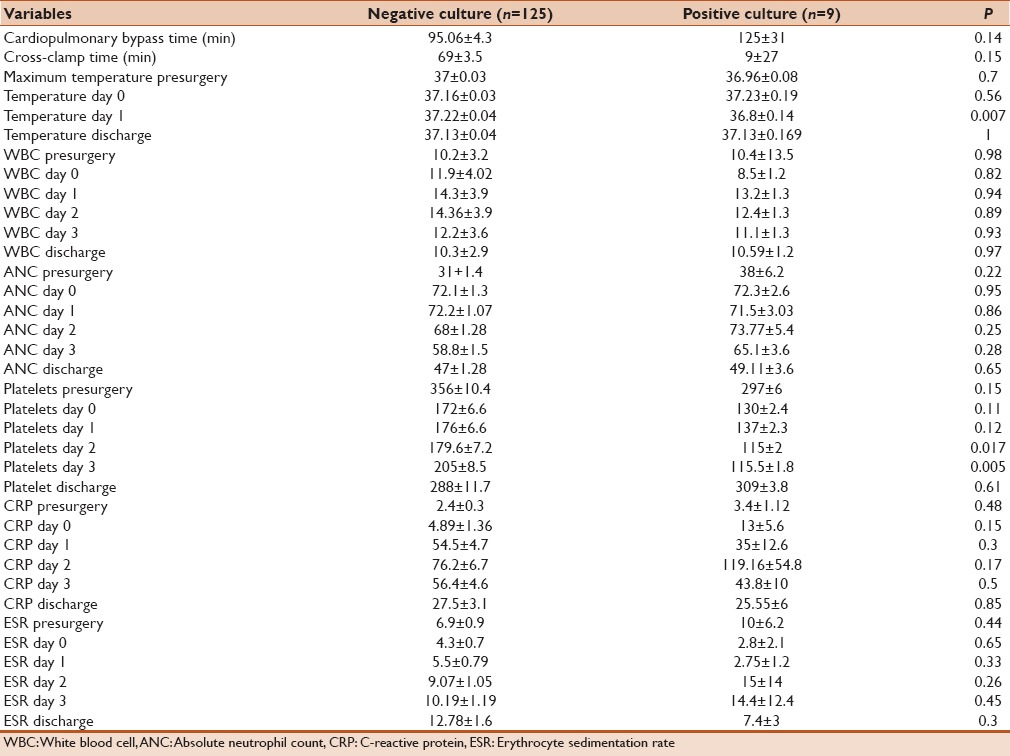
Table 4.
Data comparing between on- and off-pump cases
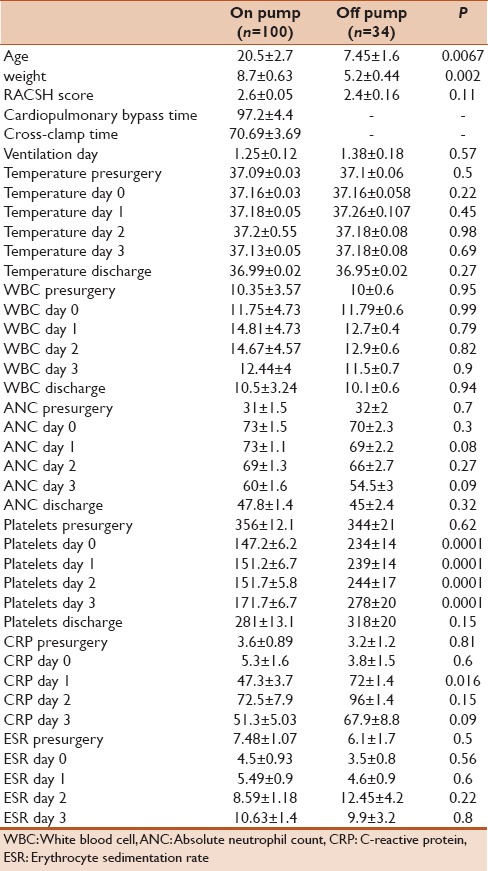
Table 5.
Difference and trend of inflammatory biomarkers in cases who received hydrocortisone presurgery and those who did not receive
We studied the trend of some inflammatory markers postsurgery (ESR, CRP, and WBCs). We found that WBC count and ESR slightly increased from baseline but they did not reach high level (10.6 mm/h) [Figure 1], while CRP had significant increase from day 1 postsurgery and kept increasing to reach its peak of 71 mg/L at day 2 after surgery, then it started to drop slowly from day 3 onward, but it did not reach baseline level even by the 5th day postsurgery [Figure 1]. Absolute neutrophil count had significant increment as it increased immediately postoperatively and stayed at high level 2 days postsurgery and then started to drop gradually until the 5th day postsurgery.
Figure 1.
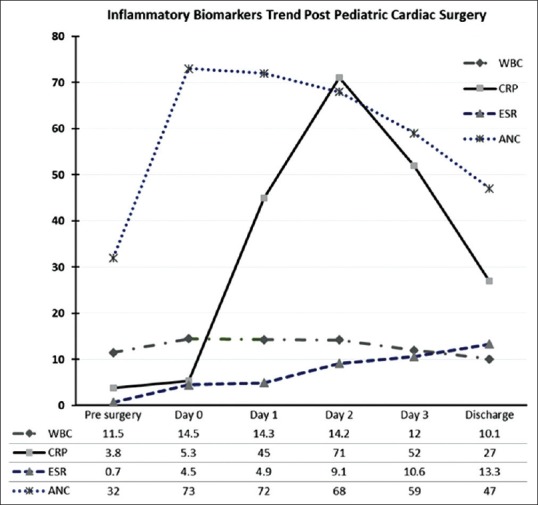
Trend of inflammatory biomarkers' postpediatric cardiac surgery. WBC: white blood cell, ESR: Erythrocyte sedimentation rate; CRP: c-reactive protein, ANC: absolute neutrophil count
DISCUSSION
Fever postcardiac surgery is a common comorbidity, particularly in postbypass cases. Some studies reported the incidence of postoperative fever in as high as 46% of patients.[12] However, real infection rate among pediatric patients undergoing cardiac surgery is between 13% and 30.8%.[2]
Diagnosis of sepsis based on clinical evaluation of infection without any other supportive biological test is difficult, especially in Cardiac ICUs. Use of broad-spectrum antibiotics frequently may save a life in definite infective cases, but it will increase multidrug-resistant strain organisms if it is used injudiciously with no clear indication.[12]
Vijarnsorn et al. studied the incidence of infection and fever in 230 patients after pediatric cardiac surgery and they reported 99 patients (43%) who developed fever while major infection rate was found in 13.5% of cases.[2] In our study, 50% of our patients had fever during the first 5 days postsurgery while positive cultures were found in only 6.7% of cases.
When infection is suspected, applying SIRS criteria is insufficient to give a clear diagnosis of sepsis since many patients might have significant inflammatory process following bypass circulation and the stress associated with surgery.
Available biomarkers such as CRP, ESR, and WBC counts are often inconclusive for distinguishing infection from SIRS in the postoperative period. Some reports investigated few additional biomarkers to check their level in infection and inflammation.
Vijarnsorn et al.[2] found that, beyond the first 3 postoperative days, an elevated level of ESR (>23 mm/h) was associated with the presence of major infection in children undergoing cardiac surgery who developed postoperative fever. Conversely, the pattern of fever, amount of WBC, and absolute neutrophil count (ANC) were not shown to be useful diagnostically. Their data were lacking the CRP level, but they commented that serum CRP values are not specific and they increase in post-CPB patients even in the absence of infection.[2]
Beghetti et al. studied the kinetics of some biomarkers in children postcardiac surgery and they found that levels of interleukin-6 and CRP increased significantly after surgery and remained elevated for up to 5 days postsurgery.[13] A recent study in 2014 by Jaworski et al.[14] Found that CPR use post cardiac surgery may over diagnosis the infection and led to antibiotic abuse.
In our study, we found that CRP had transient increment postcardiac surgery with a peak at day 3, with a mean of 71 mg/L. The value started to decline, but it did not reach baseline level till the end of the 5th day after surgery while WBC (14.4 × 109 cell/L) and ESR (13.3 mm/h) had only slight increment postcardiac surgery, although our negative culture group was small which may be considered as a limitation of the study.
Some published reports tried to compare between the level of these biomarkers in real infection and inflammation postcardiac surgery aiming to find specific cutoff level for each of these markers, but results have been limited. Vijarnsorn et al.[2] in their paper studied CPR, WBC, and ANC in febrile postcardiac surgery patients and compared two groups, one with proven infection and the other with no infection, and they found no statistically significant difference between the levels in both groups. Mare et al.[15] found that the number of WBCs and the concentration of CRP were similar in patients with definite sepsis, those with possible sepsis, and those with noninfected SIRS. Cicarelli et al.[16] concluded in their study that CRP is not a useful indicator for infection in surgical ICUs. Arkader and Casella studied CRP in 97 adult patients postcardiac surgery with CPB. They concluded that CRP level of 150 mg/L had 84% specificity and it could be used as a cutoff level of infection.[17]
In their conference at the 2002 SCCM/ESICM/ACCP/ATS/SIS international sepsis consensus, definitions pointed to genetic premorbid factors, which can influence the outcome of sepsis, modifying both the disease process and the approach taken to therapy. They emphasized that genetic factors play a greater role in determining the risk of premature mortality due to sepsis. Genetic factors may result in a more aggressive inflammatory response to an invading organism in different patients[9] that makes finding cutoff point for CRP more difficult as it may differ in each individual after each insult. Hence, some authors recommended serial CRP measurements rather than a single-level measurement as it may be more useful in diagnosing sepsis and infection as well as in monitoring the response to therapy.
Although there is a large body of literature dealing with clinical applications and the discriminative value of a single biomarker level, only limited published reports investigated the value of studying trend of biomarkers rather than solo reading. In 1991, Matson et al. demonstrated in their study of critically ill patients that a 25% increase in plasma CRP over the previous day's level was highly suggestive of sepsis.[18] Neumaier and Scherer[19] stressed the point that just using a single CRP value following the 4th postoperative day is unreliable for detecting infection. They recommended a minimum of a preoperative and a 2nd-day postsurgery CRP level as necessary to follow the complications postorthopedic surgery. In their study, they recommended that CRP concentrations above 96 mg/L after the 4th postoperative day might aid in early detection of surgical complications with a sensitivity of 92% and a specificity of 93%. Chapman et al.[20] following operatively managed neck of femur fractures suggested that CRP value in excess of the threshold-defined level, by the formula 500/day postoperatively, might indicate the presence of a postoperative infection.
ANC is used specially in neonates to diagnose sepsis. Some studies suggested cutoff point of ANC >5400/mm3 with sensitivity and specificity of 89.47% and 80.95%, respectively.[21] However, this value may not be applicable in postcardiac surgery patients, as it will be elevated as part of inflammatory process. Wilcox et al. studied the kinetics of neutrophil count postsurgery in 41 adult patients and they found that oral and blood neutrophil counts increased immediately after CPB, however oral rinse to examine the neutrophil and blood neutrophil counts returned to baseline by day 3 whereas peripheral circulating neutrophil counts remained persistently elevated up to the 7th day postsurgery.[22] Similarly, we found a significant increment in the neutrophil count postsurgery from day 1 and then it remained high for another 2 days before it starts to decline though remains predominant in deferential count till 5th day postsurgery.
PCT had shorter half-life (18–24 h). The elevation of PCT related to CPB is usually observed 1st day after surgery. Several studies showed that PCT is valuable for diagnosing infection.[23] Other contradicting studies found that PCT could not discriminate between infection and noninfection, but PCT is useful as a prognostic marker.[24] Due to logistic reasons and unavailability of PCT in our institute, we could not evaluate this important biomarker in children undergoing cardiac surgery.
Although off-pump cardiac surgical cases are expected to have less inflammatory response as compared to on-pump cases, it appears that cardiac surgery by itself triggers a significant stress response associated with the release of various cytokines and stress hormones.[25] Parolari et al. studied the systemic inflammation response after on-pump and off-pump coronary bypass surgery. In a 1-month follow-up, they found that there is a protracted postoperative activation of inflammation persisting several days after surgery.[26] This postoperative activation is not affected by the surgical strategy (on pump or off pump). We found similar findings in our study with no differences in the biomarker level between on- and off-pump cases apart from thrombocytopenia.
New trend in neonatal cardiac surgery is to give single or multiple corticosteroid doses presurgery, assuming that it may decrease the inflammatory response.[27] Our protocol is to give one dose of hydrocortisone 3 mg/kg 8 h before surgery to all neonate patients with open-heart surgery. Graham et al. found that preoperative dose of methylprednisolone does not improve markers of inflammation following neonatal cardiac surgery.[27] We noted similar results when we compared the effect of steroid on biomarkers in our neonates who received or did not receive preoperative steroid [Table 5].
CONCLUSION
Infection is a serious postpediatric cardiac surgery complication. Common biomarkers lack the accuracy of diagnosis in patients who had early infection versus severe inflammation. Serial levels rather than a single reading may be helpful in early diagnosis of infection in pediatric cardiac patients after cardiac surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kissoon N, Carcillo JA, Espinosa V, Argent A, Devictor D, Madden M, et al. World federation of pediatric intensive care and critical care societies: Global sepsis initiative. Pediatr Crit Care Med. 2011;12:494–503. doi: 10.1097/PCC.0b013e318207096c. [DOI] [PubMed] [Google Scholar]
- 2.Vijarnsorn C, Winijkul G, Laohaprasitiporn D, Chungsomprasong P, Chanthong P, Durongpisitkul K, et al. Postoperative fever and major infections after pediatric cardiac surgery. J Med Assoc Thai. 2012;95:761–70. [PubMed] [Google Scholar]
- 3.de Prost N, Razazi K, Brun-Buisson C. Unrevealing culture-negative severe sepsis. Crit Care. 2013;17:1001. doi: 10.1186/cc13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr. 2013;37:21S–9S. doi: 10.1177/0148607113496117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: Implications for the anesthesiologist. Anesthesiology. 2002;97:215–52. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Kollef MH, Wragge T, Pasque C. Determinants of mortality and multiorgan dysfunction in cardiac surgery patients requiring prolonged mechanical ventilation. Chest. 1995;107:1395–401. doi: 10.1378/chest.107.5.1395. [DOI] [PubMed] [Google Scholar]
- 7.Daiza T, Shinya U, Harrison T. Procalcitonin guided antibiotic management in postoperative cardiac surgery patients. J Surg Sci. 2014;2:2333–4711. [Google Scholar]
- 8.American College of Chest Physicians/Society of Critical Care Medicine consensus conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 9.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med. 2003;29:530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 10.Michalik DE, Duncan BW, Mee RB, Worley S, Goldfarb J, Danziger-Isakov LA, et al. Quantitative analysis of procalcitonin after pediatric cardiothoracic surgery. Cardiol Young. 2006;16:48–53. doi: 10.1017/S1047951105002088. [DOI] [PubMed] [Google Scholar]
- 11.Garcia IJ, Gargallo MB, Torné EE, Lasaosa FJ, Viñas AT, Tolosa CV, et al. Procalcitonin: A useful biomarker to discriminate infection after cardiopulmonary bypass in children. Pediatr Crit Care Med. 2012;13:441–5. doi: 10.1097/PCC.0b013e31823890de. [DOI] [PubMed] [Google Scholar]
- 12.Villasís-Keever MA, Zapata-Arenas DM, Penagos-Paniagua MJ. Frequency of postoperative fever in children with congenital heart disease undergoing cardiovascular surgery and associated risk factors. Rev Esp Cardiol. 2002;55:1063–9. doi: 10.1016/s0300-8932(02)76757-2. [DOI] [PubMed] [Google Scholar]
- 13.Beghetti M, Rimensberger PC, Kalangos A, Habre W, Gervaix A. Kinetics of procalcitonin, interleukin 6 and C-reactive protein after cardiopulmonary-bypass in children. Cardiol Young. 2003;13:161–7. doi: 10.1017/s1047951103000301. [DOI] [PubMed] [Google Scholar]
- 14.Jaworski R, Haponiuk I, Irga-Jaworska N, Chojnicki M, Steffens M, Szofer-Sendrowska A, et al. Kinetics of C-reactive protein in children with congenital heart diseases in the early period after cardiosurgical treatment with extracorporeal circulation. Adv Med Sci. 2014;59:19–22. doi: 10.1016/j.advms.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Mare TA, Treacher DF, Shankar-Hari M, Beale R, Lewis SM, Chambers DJ, et al. The diagnostic and prognostic significance of monitoring blood levels of immature neutrophils in patients with systemic inflammation. Crit Care. 2015;19:57. doi: 10.1186/s13054-015-0778-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cicarelli DD, Vieira JE, Benseñor FE. C-reactive protein is not a useful indicator for infection in surgical Intensive Care Units. Sao Paulo Med J. 2009;127:350–4. doi: 10.1590/S1516-31802009000600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arkader R, Casella V. The value of procalcitonin in diagnosis of bacterial infection patients submitted to cardiac surgery with cardiopulmonary bypass. Einstein. 2004;2:120–2. [Google Scholar]
- 18.Matson A, Soni N, Sheldon J. C-reactive protein as a diagnostic test of sepsis in the critically ill. Anaesth Intensive Care. 1991;19:182–6. doi: 10.1177/0310057X9101900204. [DOI] [PubMed] [Google Scholar]
- 19.Neumaier M, Scherer MA. C-reactive protein levels for early detection of postoperative infection after fracture surgery in 787 patients. Acta Orthop. 2008;79:428–32. doi: 10.1080/17453670710015355. [DOI] [PubMed] [Google Scholar]
- 20.Chapman G, Holton J, Chapman A. A threshold for concern? C-reactive protein levels following operatively managed neck of femur fractures can detect infectious complications with a simple formula. Clin Biochem. 2016;49:219–24. doi: 10.1016/j.clinbiochem.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Harmansyah H, Alasiry E, Daud D. Absolute neutrophil count as predictor of early onset sepsis. Clin Med Res. 2015;4:87–91. [Google Scholar]
- 22.Wilcox ME, Charbonney E, d'Empaire PP, Duggal A, Pinto R, Javid A, et al. Oral neutrophils are an independent marker of the systemic inflammatory response after cardiac bypass. J Inflamm (Lond) 2014;11:32. doi: 10.1186/s12950-014-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jebali MA, Hausfater P, Abbes Z, Aouni Z, Riou B, Ferjani M, et al. Assessment of the accuracy of procalcitonin to diagnose postoperative infection after cardiac surgery. Anesthesiology. 2007;107:232–8. doi: 10.1097/01.anes.0000271871.07395.ad. [DOI] [PubMed] [Google Scholar]
- 24.Schröder J, Staubach KH, Zabel P, Stüber F, Kremer B. Procalcitonin as a marker of severity in septic shock. Langenbecks Arch Surg. 1999;384:33–8. doi: 10.1007/s004230050170. [DOI] [PubMed] [Google Scholar]
- 25.Suleiman MS, Zacharowski K, Angelini GD. Inflammatory response and cardioprotection during open-heart surgery: The importance of anaesthetics. Br J Pharmacol. 2008;153:21–33. doi: 10.1038/sj.bjp.0707526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parolari A, Camera M, Alamanni F, Naliato M, Polvani GL, Agrifoglio M, et al. Systemic inflammation after on-pump and off-pump coronary bypass surgery: A one-month follow-up. Ann Thorac Surg. 2007;84:823–8. doi: 10.1016/j.athoracsur.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 27.Graham EM, Atz AM, McHugh KE, Butts RJ, Baker NL, Stroud RE, et al. Preoperative steroid treatment does not improve markers of inflammation after cardiac surgery in neonates: Results from a randomized trial. J Thorac Cardiovasc Surg. 2014;147:902–8. doi: 10.1016/j.jtcvs.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


