Abstract
Introduction:
Cytologic features of papillary thyroid carcinoma (PTC) have been extensively documented in literature. However, PTC variants can prove to be diagnostically challenging on fine needle aspiration cytology (FNAC).
Aims:
To study the FNAC features of PTC and its variants and explore the causes for misdiagnosis.
Materials and Methods:
This is a retrospective study. All cases of histopathologically (HP) confirmed cases of PTC during a 2-year period (January 2012 to December 2013) with presurgical FNAC were included. The cytologic findings and FNAC diagnosis of each case were documented and compared with the HP report. The misdiagnosed cases were reviewed to look for any cytological clues and reasons for misdiagnosis.
Results:
A total of 58 cases were included. The overall diagnostic accuracy was 55.6% which improved to 64.8% on including suspicious for PTC cases. Follicular variant was the most misdiagnosed variant; 41.2% of the cases were called follicular neoplasm. Oncocytic variant showed cells with abundant eosinophilic cytoplasm along with bizarre giant cells. Warthin tumor-like variant showed cells with moderate eosinophilic cytoplasm with close apposition of lymphocytes in a background of reactive lymphocytes and lymphoid tangles. Cystic variant was paucicellular. Columnar cell variant showed tall columnar cells with nuclear stratification. Cribriform–morular variant showed syncytial sheets of cells and hyaline globules.
Conclusions:
PTC variants have distinct cytomorphological features. In some variants (follicular, columnar cell), nuclear grooves and inclusions may not be apparent, contributing to the diagnostic confusion. Benign nodule adjacent to the tumor can dominate the FNAC smear and lead to misdiagnosis.
Keywords: Fine needle aspiration cytology, papillary thyroid carcinoma, pitfalls, variants
INTRODUCTION
Papillary thyroid carcinoma (PTC) is the most common type of thyroid malignancy with a generally better prognosis than that of other carcinomas. Fine needle aspiration cytology (FNAC) is a well-accepted method for diagnosing PTC with an estimated accuracy of approximately 94%.[1,2] Since its first description in the 1970s,[3] FNAC diagnosis of PTC has made immense progress and has evolved significantly to encompass the newly described variants.
The various cytologic features of classic PTC are well-documented in literature and include high cellularity, papillary fronds with anatomical edges, enlarged oval nucleus with longitudinal intranuclear grooves, nuclear crowding and overlapping, cellular swirls, and chewing gum colloid.[4,5,6] Several histologic variants of PTC described in the literature include follicular, oncocytic, tall cell, columnar cell, diffuse sclerosing, cribriform–morular, etc. Cytologic diagnosis of these variants is clinically significant as some of them are associated with poor prognosis such as tall cell, columnar cell, and diffuse sclerosing variants.[7]
In this study, an attempt is made to: (1) to study the morphological features of PTC and its variants on FNAC, (2) to explore the feasibility of FNAC in diagnosing PTC variants, and (3) to investigate the causes for misdiagnosis.
MATERIALS AND METHODS
This is a retrospective study. All cases of histopathologically confirmed cases of PTC from our institute with a prior FNAC diagnosis, during a 2-year period from January 2012 to December 2013 were included.
Inclusion criteria: All cases of biopsy proven PTC with a preoperative FNA.
Exclusion criteria: Cases of PTC with no FNAC.
The FNA smears were reviewed by two pathologists with minimal patient details – age, sex, and site of aspiration. They were blinded regarding the clinical diagnosis and nature of the prior FNA diagnosis. Their findings were then compared with the initial diagnosis and any deviation from the initial report was noted. The cytologic findings and FNAC diagnosis of each case was documented and compared with the HP report. The misdiagnosed cases were reviewed to look for any cytological clues and reasons for misdiagnosis.
Statistical analysis
As this study emphasizes on the cytomorphological features of PTC variants, it is limited by the small number of cases, and hence, statistical analysis is restricted to diagnostic accuracy (ability of FNAC to diagnose cases of PTC variants as malignant). An attempt is also made to compare the FNAC positive predictive value (PPV) among the different variants.
RESULTS
The search of HP records in our department revealed 80 cases of PTC during the study period. Fifty-eight of the cases had undergone FNAC prior to surgery. The cytological diagnosis offered in the different HP variants of PTC is highlighted in Table 1.
Table 1.
FNAC diagnosis in the various histological variants of PTC
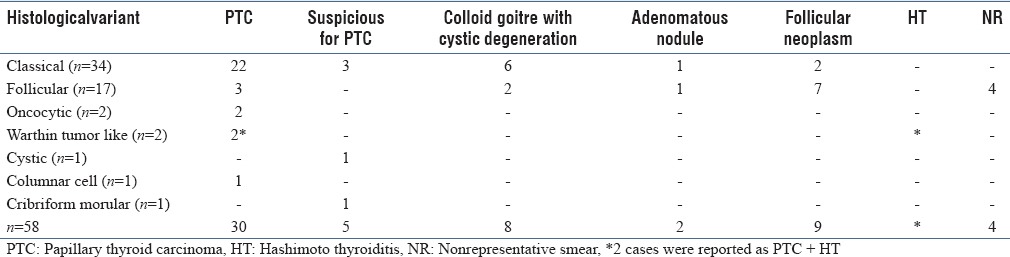
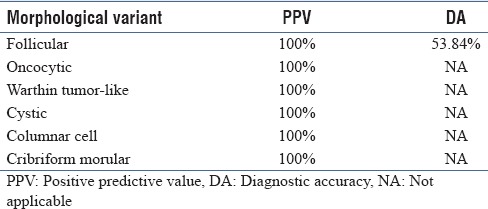
As depicted in Table 1, in 4/58 cases, the FNAC smears were inadequate. A correct diagnosis of PTC was offered in 30/54 (55.6%) cases. The diagnostic accuracy of PTC improved to 64.8% on including suspicious for PTC cases. Positive predictive value and diagnostic accuracy for the different variants of PTC are depicted in Table 2. Diagnostic accuracy could be calculated only for the follicular variant.
Table 2.
Statistical values of different PTC variants
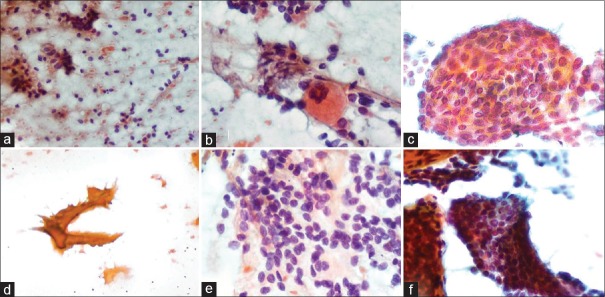
Classical PTC: Majority of the cases (20/34, i.e., 58.8%) showed cellular smears with papillary fronds composed of cells with enlarged oval nuclei with intranuclear grooves, inclusions with nuclear crowding and overlapping, and background “chewing gum” colloid [Figure 1]. The cases reported as suspicious were paucicellular and showed few cell clusters with nuclear overlapping and crowding. Six cases showed cyst macrophages and cell clusters showing degenerative changes; nuclear changes of PTC were not apparent. Even in the 2 cases reported as follicular neoplasm, the nuclear features were not appreciable. The 1 case reported as adenomatous nodule showed background abundant colloid; HP showed PTC in a background of nodular colloid goitre.
Figure 1.
Cytologic features observed in classic PTC: (a) Atypical bare nuclei (Pap stain ×200), (b) Histocytic giant cells (Pap stain ×200), (c) Swirling sheets (Pap stain ×400), (d) Chewing gum colloid, (Pap stain ×200), (e) Oval enlarged nuclei, with overlapping and intranuclear grooves (Pap stain ×400), (f) Cell clusters with anatomical edges (Pap stain ×200)
Follicular variant of PTC: Majority of the cases (7/17, 41.2%) were reported as follicular neoplasm. Upon review, all these cases showed a repetitive follicular pattern with cells showing enlarged oval nuclei along with nuclear crowding and overlapping [Figure 2a]. In 3 cases, intranuclear grooves were noted prompting the diagnosis as PTC. Background was hemorrhagic and showed atypical bare nuclei in 10 cases. “Chewing gum” colloid was noted in 1 case reported as PTC. Two cases also showed giant cells. Similar to the classical variant, 2 cases showed cyst macrophages and follicular cells showing degenerative changes. One case reported as adenomatous nodule showed PTC coexisting with nodular colloid goitre on tissue sections.
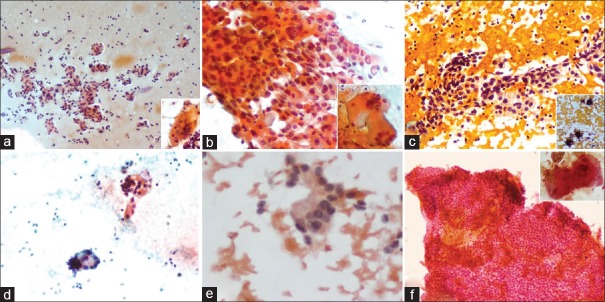
Figure 2.
(a) Repetitive microfollicular clusters background atypical bare nuclei (Pap stain ×200), Inset: Histiocytic giant cell, (b) Clusters of pleomorphic oncocytic cells (Pap stain ×400), Inset: Pleomorphic multinucleate giant cells, (c) Papillary fronds of eosinophilic cells with background lymphocytes (Pap stain ×200), Inset: psammoma bodies, (d) Occasional clusters of atypical cells, (Pap stain ×200), (e) Tall columnar cells with nuclear crowding, stratification and attempted rosette formation (Pap stain ×400), (f) Syncytial sheets of cells with focal acellular area (Left top) (Pap stain ×200), Inset: Hyaline globules
Oncocytic variant of PTC: Both cases showed papillary fronds and follicles with cells showing abundant eosinophilic cytoplasm and nuclear features of PTC. Significant nuclear pleomorphism and large giant cells were noted [Figure 2b].
Warthin tumor-like variant of PTC: Both cases showed large papillary aggregates of enlarged columnar cells with abundant eosinophilic cytoplasm with nuclear features of PTC and close apposition of lymphocytes in a background of mature lymphocytes, occasional plasma cells, lymphoid tangles, giant cells, cyst macrophages, and atypical bare nuclei. Numerous psammoma bodies were seen in one case [Figure 2c]. Both cases were reported as PTC in a background of Hashimoto's thyroiditis.
Cystic variant of PTC: Smears showed occasional clusters of pleomorphic cells, with few showing intranuclear grooves in a background of cyst macrophages [Figure 2d].
Columnar cell variant of PTC: Smears showed follicles and clusters composed of tall columnar cells with nuclear pseudo-stratification, hyperchromasia, and moderate vacuolated cytoplasm with occasional cells showing longitudinal intranuclear grooves. Atypical bare nuclei and attempted rosette-like arrangement of cells were seen [Figure 2e].
Cribriform-morular variant: In addition to clusters of cells showing nuclear grooves, the smears also showed hyaline-like globules and large syncytial sheets of cells [Figure 2f].
DISCUSSION
FNA for cytologic evaluation of thyroid cancer was originally used by Martin and Ellis in 1930. However, it gained popularity as a useful diagnostic tool after the report by Crockford and Bain (1974)[8] and another paper by Miller and Hamburger (1979).[9] Today it is widely accepted as a screening and diagnostic tool that has therapeutic implications. The sensitivity and specificity for FNAC of thyroid lesions shows a wide range of values, i.e., 65–98% and 72–100%, respectively.[10]
The conventional PTC on FNA shows thick or thin papillary fronds with fibrovascular cores, nuclear crowding and overlapping, irregular nuclear contours, intranuclear cytoplasmic inclusions, and nuclear grooves. Psammoma bodies, “chewing gum” colloid, and metaplastic squamous cells may also be present.[4,5] Cellular swirls have been recently described as a useful diagnostic clue for PTC.[6]
The follicular variant of PTC poses a diagnostic challenge on FNA due to its morphologic overlap with other follicular lesions. This problem is further compounded by the lack of papillary fronds or nuclear features of PTC. Leung et al.[11] and Das et al.[12] observed papillary fronds in 53.8% and 50% cases of follicular variant PTC, respectively. Lin et al.[13] observed a low sensitivity of 25% for FNAC diagnosis of follicular variant PTC in comparison to 74% for the classical PTC. More recently, Shih et al.[14] reported a sensitivity and specificity of 42% and 83% respectively. Diagnosis is difficult in the absence of typical nuclear features of PTC. In this study, nuclear features of PTC were seen only in 3 (17.6%) cases.
Oncocytic variant can be differentiated from other oncocytic tumors by the presence of nuclear features of PTC. Pleomorphic cells with abundant granular eosinophilic cytoplasm are important diagnostic clues.[15] Other tumors with oncocytic cells such as hurthle cell adenoma, hyalinizing trabecular adenoma, and oncocytic variant of medullary carcinoma need to be excluded. The cytomorphology of hyalinizing trabecular adenoma (HTA) has many overlapping features with PTC and medullary carcinoma. Goellner et al.[16] observed that the arrangement of oval-to-spindle shaped cells in curvilinear parallel array radiating from a central acellular hyaline zone and the absence of papillary fronds are features that favor a diagnosis of HTA over PTC.
Warthin tumor-like variant shows tall columnar cells with eosinophilic cytoplasm (Hurthle-like cells) with a close association of mature lymphoplasma cells along with giant cells, thus mimicking HT.[17,18] We had two cases of this variant that were diagnosed as PTC in a background of HT. Also to be kept in mind is the fact that partial nuclear features of PTC can be seen in cases of Hashimoto thyroiditis to avoid overdiagnosing this variant, particularly in cases with cellular aspirates.[19]
Cystic PTC, unlike usual PTCs shows low cellularity in smears. Nuclear features of PTC, nucleomegaly, and background thin colloid are observed.[20]
Pseudostratification of nuclei is a prominent feature of columnar cell variant of PTC. Tall cell variant on the other hand shows a single layer of columnar cells with deeply eosinophilic cytoplasm with high N:C ratio and nuclear features of PTC lining the papillary fronds.[21,22] Arrangement of cell in singles, small groups, and in rosette-like pattern was observed by Sen et al.[21]
Cribriform-morular variant of PTC is a rare variant usually associated with familial adenomatous polyposis colon. The cytologic features of this variant, as described in literature, includes hypercellularity, cribriform and papillary fronds of tall columnar cells, morules, spindle cells, ground-glass nuclei, hyaline material, hemosiderin-laden macrophages, and absence of colloid in the background.[23] We observed distinct hyaline-like globules similar to the ones seen in salivary gland tumors.
Diagnostic limitations of PTC on FNA has been debated as follows: (1) overlap of cytological patterns between neoplastic and non-neoplastic lesions, (2) overlap of cytological features between various neoplasms, (3) coexistence of non-neoplastic and neoplastic processes and multiple malignancies in the same gland.[24] In this study, we encountered similar difficulties, with follicular variant PTC being the most misdiagnosed entity. The cytomorphological overlap with follicular neoplasm and the absence/indiscernible nuclear features contributed to the misdiagnosis. The authors also observed that a dominant colloid nodule adjacent to a small tumor may reduce the chances of sampling the tumor during FNAC, as was observed in 2 of our cases. Ultrasound-guided FNAC may prove useful in this regard.
Use of additional diagnostic tools such as immunocytochemistry (CK19 and P63)[25] or the detection of RAS mutations in thyroid FNA specimens[26] can minimize the diagnostic errors in suspicious cases.
To conclude, cytological features of PTC have been extensively discussed in literature, with FNAC having admirable diagnostic accuracy in experienced hands. However, the PTC variants can prove to be a diagnostic challenge, particularly the ones that do not show characteristic nuclear features. As some of these variants are associated with poor prognosis, awareness of their cytomorphological features may facilitate early diagnosis and more aggressive management with better patient outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kini SR, Miller JM, Hamburger JI, Smith MJ. Cytopathology of papillary carcinoma of thyroid by fine needle aspiration. Acta Cytol. 1980;24:511–21. [PubMed] [Google Scholar]
- 2.Akhtar M, Ali MA, Huq M, Bakry M. Fine needle aspiration biopsy of papillary thyroid carcinoma: Cytologic, histologic and ultrastructural correlations. Diagn Cytopathol. 1991;7:373–9. doi: 10.1002/dc.2840070410. [DOI] [PubMed] [Google Scholar]
- 3.Zajicek J. Introduction to aspiration biopsy. Monogr Clin Cytol. 1974;4:1–211. [PubMed] [Google Scholar]
- 4.Jayaram G, Orell SR. Thyroid. In: Orell SR, Sterrett GF, editors. Orell and Sterrett's Fine needle aspiration cytology. 5th ed. India: Churchill Livingstone; 2012. pp. 134–42. [Google Scholar]
- 5.Kini SR. Guide to clinical aspiration biopsy: Thyroid. New York: Igaku-Shoin; 1987. Techniques of fine needle aspiration biopsy; pp. 5–12. [Google Scholar]
- 6.Kumar S, Singh N, Siddaraju N. “Cellular swirls” and similar structures on fine needle aspiration cytology as diagnostic clues to papillary thyroid carcinoma: A report of 4 cases. Acta Cytol. 2010;54:939–42. [PubMed] [Google Scholar]
- 7.Rosai J, Tallini G. Thyroid gland. In: Rosai J, editor. Rosai and Ackerman's Surgical Pathology. 10th ed. China: Mosby, Elsevier; 2011. pp. 502–13. [Google Scholar]
- 8.Crockford PM, Bain GO. Fine needle aspiration biopsy of the thyroid. Can Med Assoc J. 1974;110:1029–32. [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JM, Hamburger JI, Kini SR. Diagnosis of thyroid nodules by fine needle aspiration and needle biopsy. JAMA. 1979;241:481–6. [PubMed] [Google Scholar]
- 10.Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: An appraisal. Ann Intern Med. 1993;118:282–9. doi: 10.7326/0003-4819-118-4-199302150-00007. [DOI] [PubMed] [Google Scholar]
- 11.Leung CS, Hartwick RW, Bédard YC. Correlation of cytologic and histologic features in variants of papillary carcinoma of the thyroid. Acta Cytol. 1993;37:645–50. [PubMed] [Google Scholar]
- 12.Das KD, Khanna CM, Tripathi RP, Pant CS, Mandal AK, Chandra S, et al. Solitary nodular goiter: Review of cytomorphologic features in 441 cases. Acta Cytol. 1999;43:563–74. doi: 10.1159/000331147. [DOI] [PubMed] [Google Scholar]
- 13.Lin HS, Komisar A, Opher E, Blaugrund SM. Follicular Variant of Papillary Carcinoma: The Diagnostic Limitations of Preoperative Fine-Needle Aspiration and Intraoperative Frozen Section Evaluation. Laryngoscope. 2000;110:1431–6. doi: 10.1097/00005537-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Shih SR, Shun CT, Su DH, Hsiao YL, Chang TC. Follicular variant of papillary thyroid carcinoma. Diagnostic limitations of fine needle aspiration cytology. Acta Cytol. 2005;49:383–6. doi: 10.1159/000326170. [DOI] [PubMed] [Google Scholar]
- 15.Mishra A, Jayalakshmi V, Chaturvedi UP, Chikhale NP, Patel RD, Cherian S. Oncocytic variant of Papillary thyroid carcinoma and associated lymphocytic thyroiditis. A case report with review of literature. Int J Head Neck Surg. 2013;4:89–91. [Google Scholar]
- 16.Goellner JR, Carney JA. Cytologic features of fine-needle aspirates of Hyalinizing trabecular adenoma of the thyroid. Am J Clin Pathol. 1989;91:115–9. doi: 10.1093/ajcp/91.2.115. [DOI] [PubMed] [Google Scholar]
- 17.Paliogiannis P, Attene F, Trogu F, Trignano M. Warthin-Like Papillary Carcinoma of the Thyroid Gland: Case Report and Review of the Literature. Case Rep Oncol Med. 2012;2012:689291. doi: 10.1155/2012/689291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasei M, Kumar PV, Malekhoseini SA, Kadivar M. Papillary Hürthle Cell Carcinoma (Warthin-like Tumor) of the Thyroid. Report of a Case with Fine Needle Aspiration Findings. Acta Cytol. 1998;42:1437–40. doi: 10.1159/000332181. [DOI] [PubMed] [Google Scholar]
- 19.Haberal AN, Toru S, Ozen O, Arat Z, Bilezikci B. Diagnostic pitfalls in the evaluation of fine needle aspiration cytology of the thyroid: Correlation with histopathology in 260 cases. Cytopathology. 2009;20:103–8. doi: 10.1111/j.1365-2303.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang GC, Stern CM, Messina AV. Cystic papillary thyroid carcinoma in fine needle aspiration may represent a subset of the encapsulated variant in WHO classification. Diagn Cytopathol. 2010;38:721–6. doi: 10.1002/dc.21282. [DOI] [PubMed] [Google Scholar]
- 21.Sen A, Nalwa A, Mathur SR, Jain D, Iyer VK. Cytomorphology of columnar cell variant of papillary carcinoma thyroid: A case report and review of the literature. Cytojournal. 2014;11:27. doi: 10.4103/1742-6413.143303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dina R, Capitanio A, Damiani S. A morphometric analysis of cytological features of tall cell variant and classical papillary carcinoma of the thyroid. Cytopathology. 2000;11:124–8. doi: 10.1046/j.1365-2303.2000.00232.x. [DOI] [PubMed] [Google Scholar]
- 23.Pradhan D, Sharma A, Mohanty SK. Cribriform-morularvariant of papillary thyroid carcinoma. Pathol Res Pract. 2015;211:712–6. doi: 10.1016/j.prp.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen GK, Lee MW, Ginsberg J, Wragg T, Bilodeau D. Fine-needle aspiration of the thyroid: An overview. Cytojournal. 2005;2:12. doi: 10.1186/1742-6413-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonzanini M, Amadori PL, Sagramoso C, Dalla Palma P. Expression of cytokeratin 19 and protein p63 in fine needle aspiration biopsy of papillary thyroid carcinoma. Acta Cytol. 2008;52:541–8. doi: 10.1159/000325595. [DOI] [PubMed] [Google Scholar]
- 26.Gupta N, Dasyam AK, Carty SE, Nikiforova MN, Ohori NP, Armstrong M, et al. RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J Clin Endocrinol Metab. 2013;98:E914–22. doi: 10.1210/jc.2012-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]