Abstract
Giant magnetocaloric materials are highly promising for technological applications in magnetic refrigeration. Although giant magnetocaloric effects were discovered in first-order magnetic transition materials, it is accompanied by some non-desirable drawbacks, such as important hysteretic phenomena, irreversibility of the effect, or poor mechanical stability, which limits their use in applications. Here, we report the discovery of a giant magnetocaloric effect in commercialized Ho2O3 oxide at low temperature (around 2 K) without hysteresis losses. Ho2O3 is found to exhibit a second-order antiferromagnetic transition with a Néel temperature of 2 K. At an applied magnetic field change of 5 T and below 3.5 K, the maximum value of magnetic entropy change , the refrigerant capacity (RC) were found to be 31.9 J.K−1.kg−1 and 180 J.K−1, respectively.
Introduction
Cryocoolers able of cooling at low temperature (1.8–20 K) are widely utilized in different applications. As examples, they are used in hydrogen and helium liquefactions, superconducting quantum interference device (SQUID), medical instrumentation and diverse scientific research technologies. Superconducting magnet materials producing strong magnetic fields are broadly utilized in medicine and laboratories for scientific aims. Generally, liquid helium is used for cooling them, due to the fact that superconducting magnets exhibit low superconducting order temperature transition. However, liquid helium is expensive and scarcer which is not convenient from the economic point of view. Consequently, low-energy consumption cryocoolers are required. Magnetic refrigeration based on magnetocaloric effect (MCE) is a promising solution for refrigeration at low temperature1–7. Recently, considerable efforts were devoted to rare-earth based intermetallic compounds for low temperature magnetic refrigeration and some of them exhibit good MCE properties8–16. One of the main challenges in developing magnetic cryocoolers is to find a suitable working temperature range, large reversible magnetocaloric effect with low magnetic hysteresis losses. To date, the common used materials for cryogenic refrigeration17–19 based on magnetocaloric effect20–22 are hydrated salts. Such materials are used in low temperature cooling systems for detectors in space mission or laboratory facilities. The performance of an adiabatic demagnetization refrigerator (ADR)23 is critically dependent on the design and construction of these salt pills that produce cooling. However, the only available salts refrigerants present some drawbacks because they are hydrated, which requires to be encapsulated in a hermetic container to prevent dehydration. Furthermore, hydrated salts are fabricated by growth which is not appropriate with industrial processes because it is very time consuming.
One can notice that for room temperature applications giant magnetocaloric effects (MCE) were reported in materials with a first-order magnetic transition (FOMT) such as LaFe13−xSix 24–29 Gd5(Si,Ge)4 6 and others30–32. However, FOMTs occur in a narrow temperature window and are often accompanied with some non-desirable drawbacks such as the irreversibility of the MCE, very large thermal and magnetic-field hysteresis losses33 and their high material cost. We note that very recently, the irreversibility of the MCE has been overcome in FOMT FeRh thin films34 using dual-stimulus multicaloric cycle. It is known that magnetic materials with a second-order magnetic transition (SOMT) lack a very large (−ΔSM),35–40 but they do present some advantages such as low magnetic hysteresis and tunable order temperature by varying composition. In this work, we found that commercialized Ho2O3 powders presents a giant magnetocaloric effect without magnetic hysteresis losses at low temperature.
Experimental Details
Holmium (II) oxide Ho2O3 polycrystalline powders (≥99.9% purity) was provided by Sigma Aldrich factory and heated at 1200 K for 48 hours. The crystalline structure was checked by x-ray diffraction (XRD) using D5000 Siemens diffractometer with Co-Kα1 radiation (λ = 1.5406 Å). Vibrating sample magnetometer (VSM)-quantum design was used to perform magnetic measurements at temperature ranging from 1.8 to 50 K, under an external applied magnetic field up to 5 T.
Results and Discussion
The XRD pattern of Ho2O3 powders is displayed in Fig. 1. Different diffraction peaks can be observed, indicating a polycrystalline character of the sample. All the diffraction peaks can be indexed according to the bixbyite structure, which is in agreement with the data found in the literature41. The lattice parameters were determined using Rietveld refinement and were found to be a = b = c = 10.6186 Å with RWP = 10.1%, RP = 12.18% and χ 2 = 4.82. The absence of any additional peak in the XRD pattern demonstrates that there are no spurious phases in the detection limit of XRD experiments. Figure 2 displays the temperature dependence of the magnetic susceptibility recorded at an applied magnetic field of 0.05 T for the Ho2O3 compound. As can be observed, the sample shows a decrease in magnetic susceptibility with increasing temperature which is associated with the antiferromagnetic interaction in Ho2O3 in agreement with previous reports41. In order to determine the Néel temperature (TN), we display in the inset of Fig. 1(a) the versus T plot. The TN is defined as the inflection point of derivative and it is around 2 K. Inset of Fig. 2 shows the change of magnetic susceptibility (χ −1) as a function of temperature. In the paramagnetic region, χ −1(T) was fitted using the classical Curie-Weiss law:
| 1 |
where C is the Curie constant and θ p is the Curie-Weiss temperature.
Figure 1.
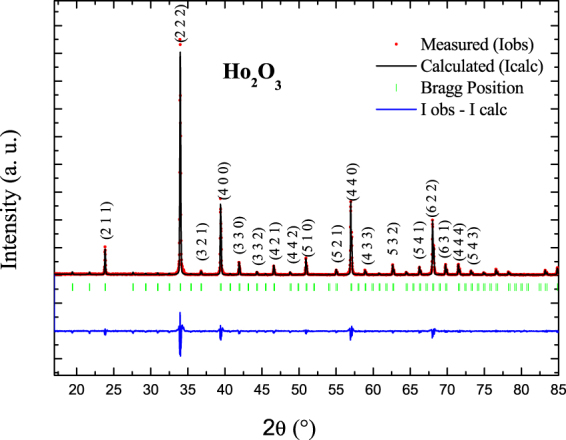
Rietveld refined powder XRD patterns of the Ho2O3 compound.
Figure 2.

Change of the magnetic susceptibility and dχ/dT as a function of temperature recorded at an applied magnetic field of 0.05 T for Ho2O3 compound. Inset shows the inverse susceptibility versus temperature, symbol and solid line represent the experimental and linear fit obtained using Curie Weiss law.
From the linear fit, the C and θ p parameters were obtained. The negative (θ p = −7 K) value confirms the presence of an antiferromagnetic interaction. The C constant is related to the effective paramagnetic moment by the following relation ; where NA = 6.023 1023 mol−1 is the Avogadro number, µB = 9.274 10−24 (A/m2) is the Bohr magneton and kB is the Boltzmann constant. From the determined C parameter, we have deduced the real effective moment of Ho value which was found to be = 11.8 µB. The experimental effective paramagnetic moment is higher than the theoretical value ( = 10.6 µB), which could be attributed to the crystal field effects which favors a high spin configuration41.
Figure 3 shows the temperature dependence of the magnetization at different applied magnetic fields. We display in the inset of Fig. 3 the magnetic field dependence of transition temperature. As shown, the magnetic transition is sensitive to the high magnetic field. Sharp change of the M(T) curve can be observed with increasing temperature at low magnetic field, while the increase of the applied field leads to a broader distribution of the M (T) curve. With increasing applied magnetic field more magnetic moments are forced to follow the direction of the applied field, which induced a broader distribution of the M(T) curve.
Figure 3.
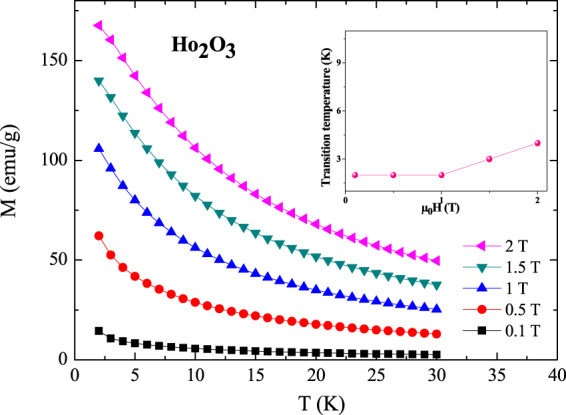
Temperature dependence of magnetization obtained at different magnetic fields (μ0H) up to 2 T of the Ho2O3 compound. Inset shows the magnetic field dependence of transition temperature.
Isothermal magnetization curves were measured at various temperatures (Fig. 4). The gradual evolution of these curves to linear behavior characterizes an increase of the paramagnetic contribution above TN. Figure 4(b) presents the magnetic hysteresis loop of the Ho2O3 powder recorded at 2 K. The hysteresis loop is closed and completely reversible. These properties are highly suitable for magnetic refrigeration42. In order to investigate the nature of the magnetic phase transition, Arrott plots (H/M versus M2) were studied (Fig. 5). According to the Banerjee criterion43, a magnetic transition is the first-order when the slope of Arrott curves is negative, whereas it will be second-order when the slope is positive. As can be observed, positive slopes are observed for all temperatures which show that the H2O3 compound undergoes a SOMT.
Figure 4.

(a) Isothermal magnetization curves obtained at different temperatures from 2 to 30 K with an increment of 1 K of the Ho2O3 compound. (b) The full magnetization curve measured at transition temperature.
Figure 5.
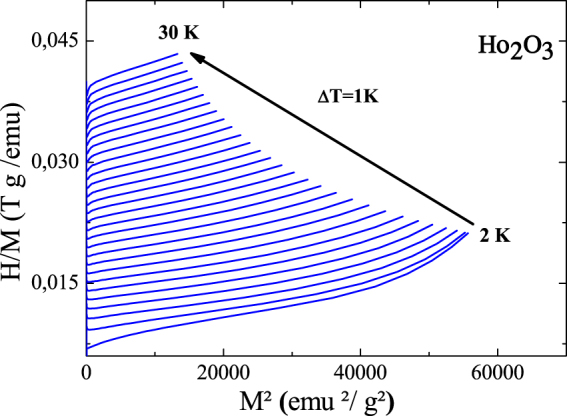
Arrott plots of the Ho2O3 compound.
The magnetocaloric effect can be related to the magnetic properties of the material through the thermodynamics Maxwell’s relation. It has been calculated in terms of isothermal magnetic entropy change using isothermal magnetization obtained at various temperatures (Fig. 5). According to the thermodynamically theory6, the isothermal magnetic entropy changes associated with a magnetic field change is given by:
| 2 |
From the Maxwell’s thermodynamic relation
| 3 |
One can obtain the following expression
| 4 |
Where µ0Hmax is the maximum external field.
Figure 6 displays the temperature dependence of the magnetic entropy change of Ho2O3 powders obtained at different applied magnetic field changes (1, 2, 3, 4, and 5 T). For all fields, the (−ΔSM) curves show a maximum at around 3 K, which it is close to TN. We note that for a second-order phase transition the (−ΔSM)(T) should show a peak with a maximum around TN, however, since the TN of the sample is too low (2 K), we only observe half peak of (−ΔSM)(T). The peak magnitude increases when ΔH increases, from 8.2 to 31.9 J/kgK with increasing applied magnetic field change from 1 to 5 T, respectively. The large magnetocaloric effect in H2O3 can be understood by its high magnetization associated with its sharp magnetization change at the antiferromagnetic-paramagnetic and the presence of crystal field effects above the transition.
Figure 6.
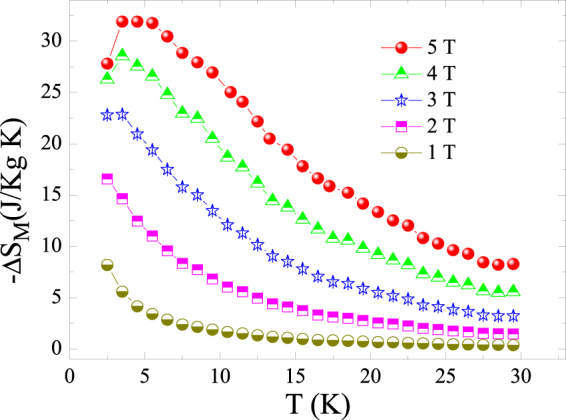
Temperature dependence of magnetic entropy change (−ΔSM) under different magnetic field changes 1, 2, 3, 4, and 5 T of the Ho2O3 compound.
Other important parameters of refrigerant materials are the refrigerant capacity RC and the adiabatic temperature change ΔTad. According to Wood and Potter44 the RC of a reversible refrigeration cycle operating between Th and Tc (temperatures of the hot and cold reservoirs, respectively) is defined as RC = (− Δ S M) × ΔT, where (− Δ S M), is the magnetic entropy change at the hot and cold ends of the cycle and ΔT = Th − Tc. For magnetic field changes of 0–4 T and 0–5 T, the values of RC were estimated to be 165 and 180 J/kg, respectively.
ΔTad can be calculated from magnetization and heat capacity measurements Cp 6
| 5 |
The temperature dependence of ΔTad and heat capacity for magnetic field changes of 1 T is shown in Fig. 7. It can be seen that ΔTad increases with decreasing temperature. The maximum values of adiabatic temperature change reaches 1.08 K for a magnetic field change of 1 T.
Figure 7.

Temperature dependence of magnetic entropy change (−ΔSM), heat capacity and adiabatic temperature change ΔTad under magnetic field changes 1 T of the Ho2O3 compound.
In order to examine the usefulness of the Ho2O3 compound reported in this work, we made a comparative study of T N,C, , RC with other magnetocaloric materials found in literature with low temperature magnetic transitions. The comparison is summarized in Table 1. It can be concluded that is larger or comparable to those of reported potential magnetic refrigerant materials,13–15,45–51. However, the ordering temperature of Ho2O3 compound is smaller than the other materials. We note that the RC factor is comparable or smaller than those of the materials reported in Table 1. The giant values of (−∆SM), the low-cost and fast way of preparation suggest that this compound is one suitable candidate as a magnetic refrigerant in low temperature range (around 2 K).
Table 1.
Magnetic ordering temperature (TN,C), maximum values of (−ΔSM max) and refrigerant capacity (RC) under the magnetic field change of 5 T of the Ho2O3 compound and some potential magnetic refrigerant materials.
Conclusion
In this paper, we have studied the magnetic and magnetocaloric properties of Ho2O3 powders. Magnetic measurements have shown the presence of an antiferromagnetic–paramagnetic transition around 2 K and a giant magnetic entropy change with second-order magnetic transition. Strong influence of crystal field effect is also observed in magnetic properties as well as magnetocaloric effect. Our study demonstrates that the Ho2O3 material could be considered as a potential candidate for magnetic refrigeration applications at low temperature (around 2 K).
Acknowledgements
This work is mainly supported by the PHC Maghreb 15MAG07.
Author Contributions
A.B. and E.K.H. performed experiments and wrote the manuscript. R.M., H.L. and E.L. guided for writing the manuscript and provided financial aids through their projects.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Debye P. Einige Bemerkungen zur Magnetisierung bei tiefer Temperatur. Ann. Phys. 1926;386:1154–1160. doi: 10.1002/andp.19263862517. [DOI] [Google Scholar]
- 2.Giauque WF. A thermodynamic treatment of certain magnetic effects. A proposed method of producing temperatures considerably below 1° absolute. J. Am. Chem. Soc. 1927;49:1864–1870. doi: 10.1021/ja01407a003. [DOI] [Google Scholar]
- 3.Giauque WF, MacDougall DP. Attainment of temperatures below 1° absolute by demagnetization of Gd2(SO4)3 8H2O. Phys. Rev. 1933;43:768. doi: 10.1103/PhysRev.43.768. [DOI] [Google Scholar]
- 4.Li LW. Review of magnetic properties and magnetocaloric effect in the intermetallic compounds of rare earth with low boiling point metals. Chin. Phys. B. 2016;25:037502. doi: 10.1088/1674-1056/25/3/037502. [DOI] [Google Scholar]
- 5.Li LW, Hu G, Qi Y, Umehara I. Hydrostatic pressure effect on magnetic phase transition and magnetocaloric effect of metamagnetic TmZn compound. Sci. Rep. 2017;7:42908. doi: 10.1038/srep42908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, et al. Excellent magnetocaloric properties in RE2Cu2Cd (RE = Dy and Tm) compounds and its composite materials. Sci. Rep. 2016;6:34192. doi: 10.1038/srep34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li LW, et al. low field magnetocaloric effect and field-induced metamagnetic transition in TmZn. Appl. Phys. Lett. 2014;104:092416. doi: 10.1063/1.4867882. [DOI] [Google Scholar]
- 8.Yang Y, et al. Magnetic and magnetocaloric properties of the ternary cadmium based intermetallic compounds of Gd2Cu2Cd and Er2Cu2Cd. J. Alloys Compd. 2017;692:665–669. doi: 10.1016/j.jallcom.2016.09.104. [DOI] [Google Scholar]
- 9.Yalin Y, Li LW, Kunpeng S, Yang Q, Dexuan H. Large magnetocaloric effect in a wide temperature range induced by two successive magnetic phase transitions in Ho2Cu2Cd compound. Intermetallics. 2017;80:22. doi: 10.1016/j.intermet.2016.10.005. [DOI] [Google Scholar]
- 10.Zhang Y, et al. Large reversible magnetocaloric effect in RE2Cu2In (RE = Er and Tm) and enhanced refrigerant capacity in its composite materials. J. Phys. D: Appl. Phys. 2016;49:145002. doi: 10.1088/0022-3727/49/14/145002. [DOI] [Google Scholar]
- 11.Zhang Y, et al. Study of the magnetic phase transitions and magnetocaloric effect in Dy2Cu2 In compound. J. Alloys Compd. 2016;667:130–133. doi: 10.1016/j.jallcom.2016.01.157. [DOI] [Google Scholar]
- 12.Li LW, Namiki T, Huo D, Qian Z, Nishimura K. Two successive magnetic transitions induced large refrigerant capacity in HoPd In compound. Appl. Phys. Lett. 2013;103:222405. doi: 10.1063/1.4834815. [DOI] [Google Scholar]
- 13.Li LW, et al. Magnetic properties and magnetocaloric effect in metamagnetic RE2Cu2O5 (RE = Dy and Ho) cuprates. J. Alloy Comp. 2016;658:500. doi: 10.1016/j.jallcom.2015.10.289. [DOI] [Google Scholar]
- 14.Zhang Y, et al. Magnetic properties and magnetocaloric effect in the aluminide RENiAl2 (RE = Ho and Er) compounds. Intermetallics. 2017;88:61. doi: 10.1016/j.intermet.2017.05.011. [DOI] [Google Scholar]
- 15.Zhang Y, et al. Magnetic properties and magnetocaloric effect in TmZnAl and TmAgAl compounds. J. Alloys. Comp. 2016;656:635. doi: 10.1016/j.jallcom.2015.10.026. [DOI] [Google Scholar]
- 16.Boutahar A, et al. Magnetocaloric effect in CoEr2 intermetallic compound. J. Magn. Magn. Mater. 2017;444:106. doi: 10.1016/j.jmmm.2017.08.015. [DOI] [Google Scholar]
- 17.Evangelisti M, et al. Cryogenic Magnetocaloric Effect in a Ferromagnetic Molecular Dimer. Ang. Chem. Int. Ed. 2011;50:29. doi: 10.1002/anie.201102640. [DOI] [PubMed] [Google Scholar]
- 18.Li J, et al. Enhanced Cryogenic Magnetocaloric Effect Induced by Small Size GdNi5 Nanoparticles. J. Mater. Sci. Technol. 2014;30:973. doi: 10.1016/j.jmst.2014.01.009. [DOI] [Google Scholar]
- 19.Barclay A, Steyert WA. Materials for magnetic refrigeration between 2 K and 20 K. Cryogenics. 1982;22:73. doi: 10.1016/0011-2275(82)90098-4. [DOI] [Google Scholar]
- 20.Gschneidner KA, Pecharsky VK, Tsoko AO. Recent developments in magnetocaloric materials. Rep. Prog. Phys. 2005;68:1479. doi: 10.1088/0034-4885/68/6/R04. [DOI] [Google Scholar]
- 21.Pecharsky VK, Gschneidner K. Magnetocaloric effect from indirect measurements: Magnetization and heat capacity. J. Appl. Phys. 1999;86:565. doi: 10.1063/1.370767. [DOI] [Google Scholar]
- 22.Pecharsky VK, Gschneidner KA. Giant Magnetocaloric Effect in Gd5(Si2Ge2) Phys. Rev. Lett. 1997;78:4494. doi: 10.1103/PhysRevLett.78.4494. [DOI] [PubMed] [Google Scholar]
- 23.Jang D, et al. Large magnetocaloric effect and adiabatic demagnetization refrigeration with YbPt2Sn. Nat. Commu. 2015;6:8680. doi: 10.1038/ncomms9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boutahar A, Phejar M, Boncour MV, Bessais L, Lassri H. Theoretical Work in Magnetocaloric Effect of LaFe13−xSix Compounds. J. Supr. Cond. Novel Magn. 2014;27:5. doi: 10.1007/s10948-013-2442-7. [DOI] [Google Scholar]
- 25.Rosca M, et al. Neutron diffraction study of LaFe11.31Si1.69 and LaFe11.31Si1.69H1.45 compounds. J. Alloys Comp. 2010;490:50. doi: 10.1016/j.jallcom.2009.10.093. [DOI] [Google Scholar]
- 26.Fujita A, Fujieda S, Fukamichi SK, Mutamira H, Goto T. Itinerant-electron metamagnetic transition and large magnetovolume effects in La(FexSi1−x)13 compounds. Phys. Rev. B. 2001;65:014410. doi: 10.1103/PhysRevB.65.014410. [DOI] [Google Scholar]
- 27.Hu F-X, Shen B-G, Sun J-R, Cheng Z-H. Influence of negative lattice expansion and metamagnetic transition on magnetic entropy change in the compound LaFe11.4Si1.6. Appl. Phys. Lett. 2001;78:3675. doi: 10.1063/1.1375836. [DOI] [Google Scholar]
- 28.Balli M, Rosca M, Fruchart D, Ginoux D. Effect of interstitial nitrogen on magnetism and entropy change of LaFe11.7Si1.3 compound. J. Magn. Magn. Mater. 2009;321:123. doi: 10.1016/j.jmmm.2008.08.081. [DOI] [Google Scholar]
- 29.Boutahar A, Zehani K, Bessais L, Lassri H, Hlil EK. Influence of bismuth on magnetism and magnetocaloric properties of LaFe11.6Si1.4 intermetallic compound. J. Rare Earth. 2015;33:740. doi: 10.1016/S1002-0721(14)60479-8. [DOI] [Google Scholar]
- 30.Gschneidner KA, Pecharsky VK. Magnetocaloric Materials. Annu. Rev. Mater. Sci. 2000;30:387. doi: 10.1146/annurev.matsci.30.1.387. [DOI] [Google Scholar]
- 31.Tegus. O, Brück. E, Buschow KH, Boer. FR. Transition-metal-based magnetic refrigerants for room-temperature applications. Nature (London) 2002;415:150. doi: 10.1038/415150a. [DOI] [PubMed] [Google Scholar]
- 32.Franco. V, Blazquez JS, Ingale B, Conde A. The Magnetocaloric Effect and Magnetic Refrigeration Near Room Temperature: Materials and Models. Annu. Rev. Mater. Res. 2012;42:305. doi: 10.1146/annurev-matsci-062910-100356. [DOI] [Google Scholar]
- 33.Provenzano V, Shapiro AJ, Shull RD. Reduction of hysteresis losses in the magnetic refrigerant Gd5Ge2Si2 by the addition of iron. Nature (London) 2004;429:853. doi: 10.1038/nature02657. [DOI] [PubMed] [Google Scholar]
- 34.Liu, Y., et al. Large reversible caloric effect in FeRh thin films via a dual-stimulus multicaloric cycle. Nature Commun, 10.1038/ncomms11614 (2016). [DOI] [PMC free article] [PubMed]
- 35.Boutahar. A, et al. The Influence of Vanadium on Magnetism and Magnetocaloric Properties of Fe80−xVxB12Si8 (x = 8, 10, and 13.7) Amorphous Alloys, J. Suprcond. Novel Magn. 2014;27:2401. doi: 10.1007/s10948-014-2619-8. [DOI] [Google Scholar]
- 36.Chen. J, Shen BG, Dong QY, Hu FX, Sun JR. Giant reversible magnetocaloric effect in metamagnetic HoCuSi compound. Appl. Phys. Lett. 2010;96:152501. doi: 10.1063/1.3386536. [DOI] [Google Scholar]
- 37.Bonilla CM, et al. Universal behavior for magnetic entropy change in magnetocaloric materials: An analysis on the nature of phase transitions. Phys. Rev. B. 2010;81:224424. doi: 10.1103/PhysRevB.81.224424. [DOI] [Google Scholar]
- 38.Boutahar A, Lassri H, Hlil EK. Low Temperature Giant Magnetocaloric Effect and Critical Behavior in Amorphous Co100−xErx (x = 55, 65) Alloys. J. Supr. Cond. Novel Magn. 2014;27:2865. doi: 10.1007/s10948-014-2773-z. [DOI] [Google Scholar]
- 39.Plaza. EJR, Sousa. VSR, Reis. MS, Von Ranke. P. J., A comparative study of the magnetocaloric effect in RNi2 (R = Dy, Ho, Er) intermetallic compounds. J. Alloys Comp. 2010;505:357. doi: 10.1016/j.jallcom.2010.05.182. [DOI] [Google Scholar]
- 40.Moubah R, et al. Enhanced magnetocaloric properties of FeZr amorphous films by C ion implantation. Mater. Lett. 2016;175:5. doi: 10.1016/j.matlet.2016.03.124. [DOI] [Google Scholar]
- 41.Heiba ZK, Bakr, Mohamed M. Structural and magnetic properties of Mn doped Ho2O3 nanocrystalline. J. Mol. Str. 2015;1102:140. doi: 10.1016/j.molstruc.2015.08.048. [DOI] [Google Scholar]
- 42.Boutahar. A, Lassri. H, Hlil. EK. Magnetic, magnetocaloric properties and phenomenological model in amorphous Fe60Ru20B20 alloy. Solid Stat. Commun. 2015;221:9. [Google Scholar]
- 43.Banerjee BK. On a generalised approach to first and second order magnetic transitions. Phys. Lett. 1964;12:16. doi: 10.1016/0031-9163(64)91158-8. [DOI] [Google Scholar]
- 44.Wood ME, Potter W. H, General analysis of magnetic refrigeration and its optimization using a new concept: maximization of refrigerant capacity. Cryogenics. 1985;25:667. doi: 10.1016/0011-2275(85)90187-0. [DOI] [Google Scholar]
- 45.Li LW, Nishimura K. Giant reversible magnetocaloric effect in antiferromagnetic superconductor Dy0.9Tm0.1Ni2B2CDy0.9Tm0.1Ni2B2C compound. Appl. Phys. Lett. 2009;95:132505. doi: 10.1063/1.3240399. [DOI] [Google Scholar]
- 46.Hermes W, Rodewals UC, Pottgen R. Large reversible magnetocaloric effect due to a rather unstable antiferromagnetic ground state. J. Appl. Phys. 2010;108:113919. doi: 10.1063/1.3518556. [DOI] [Google Scholar]
- 47.Zhang H, et al. Giant magnetic refrigerant capacity in Ho3Al2 compound. Solid State Comm. 2012;152:1127. doi: 10.1016/j.ssc.2012.04.004. [DOI] [Google Scholar]
- 48.Luo Q, Zhao DQ, Pan MX, Wang WH. Magnetocaloric effect of Ho-, Dy and Er-based bulk metallic glasses in helium and hydrogen liquefaction temperature range, App. Phy. Lett. 2007;90:211903. [Google Scholar]
- 49.Li LW, et al. Giant low field magnetocaloric effect and field-induced metamagnetic transition in TmZn. Appl. Phys. Lett. 2015;107:132401. doi: 10.1063/1.4932058. [DOI] [Google Scholar]
- 50.Gupta SB, Suresh KG. Giant low field magnetocaloric effect in soft ferromagnetic ErRuSi. Appl. Phys. Lett. 2013;102:022408. doi: 10.1063/1.4775690. [DOI] [Google Scholar]
- 51.Li L, et al. Giant reversible magnetocaloric effect in ErMn2Si2 compound with a second order magnetic phase transition. Appl. Phys. Lett. 2012;100:152403. doi: 10.1063/1.4704155. [DOI] [Google Scholar]


