Abstract
Background
Glucocorticoids (GCs) measured in neonatal hair might reflect intrauterine as well as postpartum GC regulation. We aimed to identify factors associated with neonatal hair GC levels in early life, and their correlation with maternal hair GCs.
Methods
In a single-center observational study, mother–infant pairs (n = 107) admitted for >72 h at the maternity ward of a general hospital were included. At birth and an outpatient visit (OPV, n = 72, 44 ± 11 days postpartum), maternal and neonatal hair was analyzed for cortisol and cortisone levels by LC–MS/MS. Data were analyzed regarding: (1) neonatal GC levels postpartum and at the OPV, (2) associations of neonatal GC levels with maternal GC levels and (3) with other perinatal factors.
Results
(1) Neonatal GC levels were >5 times higher than maternal levels, with a decrease in ±50% between birth and the OPV for cortisol. (2) Maternal and neonatal cortisol, but not cortisone, levels were correlated both at postpartum and at the OPV. (3) Gestational age was associated with neonatal GC postpartum (log-transformed β (95% CI): cortisol 0.07 (0.04–0.10); cortisone 0.04 (0.01–0.06)) and at the OPV (cortisol 0.08 (0.04–0.12); cortisone 0.00 (−0.04 to 0.04)), while weaker associations were found between neonatal GCs and other perinatal and maternal factors.
Conclusions
Neonatal hair GCs mainly reflect the third trimester increase in cortisol, which might be caused by the positive feedback loop, a placenta-driven phenomenon, represented by the positive association with GA. Between birth and 1.5 months postpartum, neonatal hair cortisol concentrations decrease sharply, but still appear to reflect both intra- and extrauterine periods.
Keywords: hair glucocorticoids, infant, positive feedback loop, HPA axis, cortisol, cortisone
Introduction
Prenatal exposure to excessive glucocorticoids (GCs) has been associated with an increased risk of cardiovascular diseases and depressive disorders (1, 2). This might be due to permanent alterations in the settings of the fetal hypothalamic–pituitary–adrenal (HPA) axis, which are protective in the short term, but might pose a risk in the long term (3).
The development of the fetal HPA axis is, among other factors, influenced by the placental transfer of maternal GCs throughout pregnancy (4). During early gestation, maternal GCs are the main supply. By the second half of gestation, the fetal adrenal starts producing its own steroids, predominantly sex steroids (which serve as a substrate for the placental production of estriol) and precursor GCs, since the adrenocortical enzymes are not fully matured yet (5). Subsequently, during the last 6–8 weeks of pregnancy, the more matured fetal adrenal produces increasing amounts of cortisol and cortisone under the control of corticotropic-releasing hormone (CRH) production in the placenta, which – in contrast to the negative feedback loop between cortisol and CRH under non-pregnant conditions – establishes a positive feedback loop (6). This increase in cortisol concentration promotes maturation of the fetal lungs as well as of other organs (7).
Knowledge on the fetal HPA axis development is mainly based on animal studies (5, 8), as it is difficult to measure fetal HPA axis activity in humans. Up till now, amniotic fluid GC levels and umbilical cord GC levels have been used to assess intrauterine GC regulation. Cortisol in amniotic fluid has previously been correlated with maternal cortisol levels (9) and onset of labor (10). However, the source of amniotic fluid cortisol remains uncertain, although findings point toward fetal production (11, 12). In addition, sampling of amniotic fluid is a stressful occasion and only provides cross-sectional information. Alternatively, umbilical cord blood can be drawn non-invasively, but GC levels are influenced by delivery (13) and might not reflect normal intrauterine HPA axis activity. GCs measured in scalp hair might offer a solution, as it is used as a measure for HPA axis activity over time without the disturbing influence of the circadian rhythm. The hair GC concentrations reflect the exposure in the time frame during which the hair grows (14).
Maternal hair GC levels seem to reflect HPA axis activity during pregnancy (15, 16, 17). Neonatal hair GC levels have also been associated with pre- and perinatal factors. A recent study by Hoffman and coworkers (18) has shown that gestational age as well as birth weight had a positive association with cortisol levels in neonatal hair. Neonatal hair GC levels were also significantly higher than maternal hair GC levels. This study suggests that features of the fetal adrenal development are represented in neonatal hair GC levels, although these findings are limited due to the fact that this has only been described in one study population, cortisone levels were not taken into account and the course followed by GC levels in hair postpartum has not been studied.
Therefore, we aimed to describe cortisol and cortisone concentrations in neonatal hair, obtained directly postpartum, and their relation with maternal hair GC levels and pre- and perinatal factors. Finally, we explored the differences in hair GC concentrations between birth and an outpatient visit (OPV) at approximately 6 weeks postpartum, as well as which factors are of influence on this difference.
Methods
Population
From February 2012 to August 2013, mother–infant pairs were included in the OLVG West Hospital in Amsterdam, The Netherlands. Subjects were informed regarding the study before or within 24 h after delivery. The infant needed to be admitted to the hospital (maternity ward or neonatal care unit) for at least 72 h for a neonatal or maternal reason, as this was an inclusion criterion for a simultaneous study (19). Subjects were excluded for the following reasons: (1) insufficient knowledge of the Dutch or English language, (2) mental retardation of one or both parents, (3) multiple pregnancy, (4) use of illicit drugs or regular (>2 IU/week) alcohol use during the last trimester, (5) use of systemic corticosteroids during pregnancy, (6) if participating in this study would interfere with regular care or (7) use of psychotropic medication.
The study was approved by the medical ethics committees of the OLVG West Hospital and the VU University Medical Center in Amsterdam, The Netherlands. Written informed consent was obtained from all participants.
Determinants
The mother filled in a questionnaire about demographic characteristics.
Information on perinatal characteristics and the reasons for admission to the hospital were obtained from medical records.
Hair cortisol measurements
On the first day, postpartum neonatal hair was cut from the posterior vertex of the scalp, as close as possible to the scalp, as this region shows the least variance between different strands (14). At the OPV around 6 weeks, postpartum neonatal hair was collected again. The total length of hair directly postpartum was analyzed, with the assumption that it is an indication of GC concentrations during fetal life, while at the OPV, only the centimeter of hair closest to the scalp was analyzed, with the assumption that it gives an indication of GC concentrations during the first weeks of life (20, 21).
Maternal hair was also collected on the first day postpartum and at the OPV. Only the centimeter closest to the scalp of maternal hair was analyzed. As, in adults, hair grows approximately 1 cm every month (16, 20, 21), the hair measurement postpartum is indicative for the GC levels during the last month of pregnancy.
GC levels (cortisol and cortisone) were measured in hair as previously described (20). In short, in the presence of deuterium-labeled GCs as the internal standard, cortisol was extracted using LC-grade methanol at 25°C for 18 h. These extracts were subsequently centrifuged and cleaned using solid phase extraction. GC concentrations were quantified by liquid chromatography–tandem mass spectrometry LC–MS/MS (Waters XEVO-TQ-S system, Waters Corporation, Milford, MA, USA). GC concentrations were reported as pg per mg hair, and 1.25 mg was required for a reliable measurement.
Statistics
Analyses were performed with regard to:
Concentrations of GCs in neonatal hair directly postpartum and at the OPV. GC levels were expressed as median (range). Subsequently, GC levels were log-transformed and paired t-tests were performed.
The relation between maternal and neonatal (log-transformed) hair GC levels postpartum and at the OPV was assessed using Pearson correlation coefficients and linear regression.
Factors associated with neonatal hair GCs directly postpartum were assessed using linear regression. Additional analyses were performed to assess the effect of the factors associated with GC levels directly postpartum on the course of GC levels (expressed as delta cortisol and cortisone) and on the GC levels at the OPV, corrected for age at the time of sampling. The following factors, based on literature (15, 16, 17, 18), were taken into consideration: (a) perinatal: gestational age, birth weight (in kg and S.D. score), sex, mode of delivery, perinatal infection, respiratory distress (meconium-containing amniotic fluid, respiratory insufficiency, respiratory support, persistent pulmonary hypertension of the neonate); (b) maternal: age, ethnicity, maternal smoking, parity (primi- vs multipara), hypertensive disorders (pregnancy-induced hypertension, pre-existent hypertension, pre-eclampsia/HELLP syndrome (hemolysis, elevated liver enzymes and low platelet count)).
Results with a P-value <0.05 were considered to be statistically significant, although borderline statistically significant results (0.10 > P > 0.05) when found for both cortisol and cortisone were also further explored.
Results
Population
A total of 107 mother–infant pairs donated hair directly postpartum. At the OPV, 72 mother–infant pairs donated hair. The OPV took place at 44 ± 11 days postpartum (range: 22–87 days). Perinatal and demographic characteristics are presented in Table 1.
Table 1.
Baseline characteristics of the study population (n = 107).
| Mean ± s.d., median (range) or n (%) | |
|---|---|
| Perinatal | |
| Gestational age | 39.5 ± 1.8, 39.5 (33.9–42.1) |
| Birth weight | 3480 ± 629, 3569 (1806–5290) |
| Male sex | 61 (55.5) |
| Vaginal delivery | 40 (32.5) |
| Perinatal infection | 34 (30.9) |
| Respiratory problems | 9 (8.2) |
| Maternal | |
| Age | 33.9 ± 4.8, 34 (21–44) |
| Non-Dutch ethnicity | 52 (47.3) |
| Smoking | 2 (1.8) |
| Nulliparae | 67 (54.5) |
| Hypertensive disorders | 7 (6.4) |
Concentration of GCs in neonatal hair
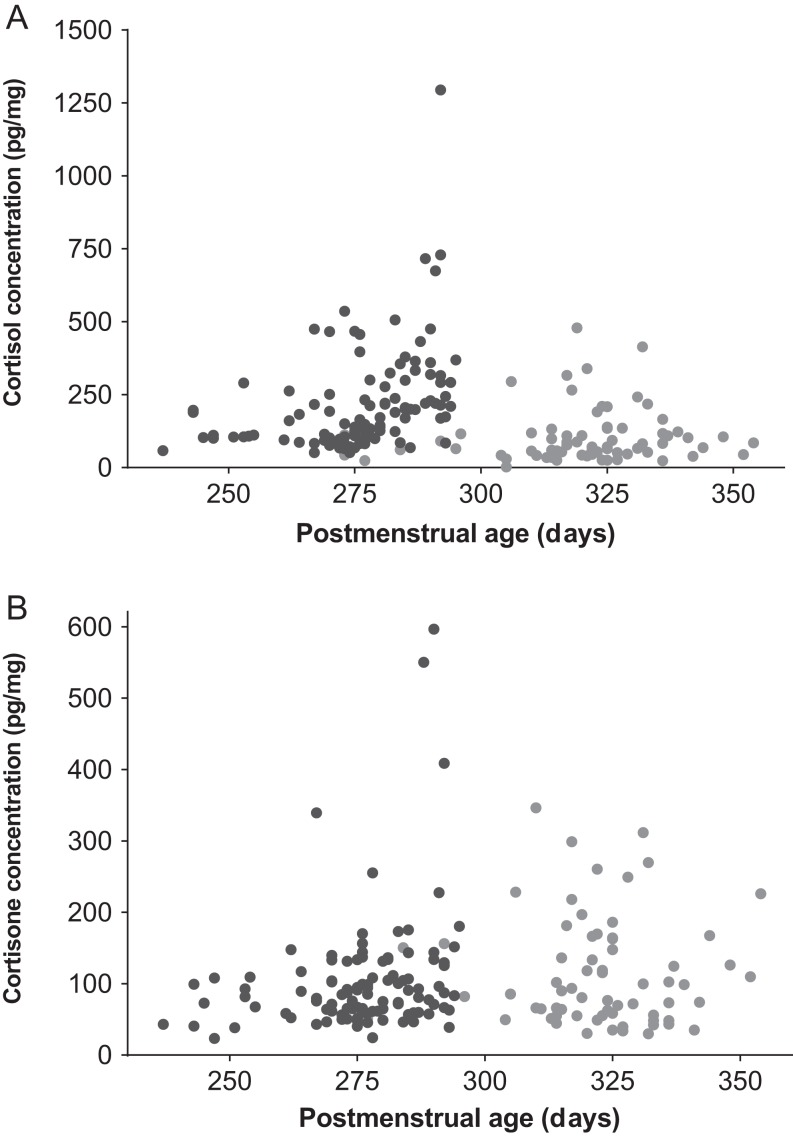
Results are displayed in Fig. 1 and Table 2. Directly postpartum, the median concentration of cortisol was 169 pg/mg (range: 51–1294), while the median concentration of cortisone was 85 pg/mg (range: 23–597). Maternal GC levels were much lower than neonatal levels, with median concentrations of 5 (range: 0–672) and 18 (2–87) pg/mg respectively.
Figure 1.
Neonatal hair cortisol (A) and cortisone (B) levels measured directly postpartum (dark circle) and at the OPV (light circle).
Table 2.
Concentrations of neonatal and maternal hair glucocorticoid concentrations postpartum and at the outpatient visit.
| Postpartum (median, range (pg/mg)) (n = 107) | Outpatient visit (median, range (pg/mg)) (n = 72) | P value* | ||
|---|---|---|---|---|
| Infant | Cortisol | 169, 51–1294 | 71, 2–479 | <0.001 |
| Cortisone | 85, 23–597 | 91, 30–346 | 0.99 | |
| Maternal | Cortisol | 5, 0–672 | 4, 1–79 | 0.001 |
| Cortisone | 18, 2–87 | 18, 8–43 | 0.75 |
Values expressed as median, range in pg/mg.
Analyzed with a paired t-test, performed with log-transformed GC concentrations.
Course of GC levels postpartum
Between birth and the OPV, a steep decrease in cortisol concentrations in infant hair was observed (Fig. 1 and Table 2). Maternal hair cortisol levels showed a subtle decrease between birth and the OPV. In contrast, infant and maternal hair cortisone levels remained stable, although a wide range of values were observed. At the OPV, both infant cortisol and cortisone concentrations were still higher than the GC levels in maternal hair. Age of the neonate at the OPV was negatively associated with hair cortisol, but not with cortisone levels (log-transformed β (95% CI): cortisol −0.01 (−0.02 to −0.001), P = 0.03; cortisone: 0.00 (−0.01 to 0.01), P = 0.70). Age of the neonate at the OPV was not associated with delta cortisol or cortisone.
Correlations with maternal hair GCs
Directly postpartum, maternal and neonatal hair cortisol were positively associated (n = 107, r = 0.336, β 0.23 (95% CI: 0.11–0.36), P < 0.001), while no correlations were found between maternal and infant hair cortisone (P = 0.66). At the OPV, the association between maternal and infant hair cortisol was stronger than directly postpartum (n = 71, r = 0.457, β 0.41 (95% CI: 0.22–0.60), P < 0.001), and no correlation was found for cortisone (P = 0.12).
Factors associated with neonatal hair GCs
The effect of several perinatal and maternal factors on hair GC levels measured directly postpartum was studied (Table 3). Gestational age was strongly associated with both cortisol and cortisone levels, as illustrated in Fig. 1, and this association remained significant when only term-born infants (n = 98) were studied. Additionally, there was a positive association for both cortisol and cortisone levels with perinatal infection (defined as the need for treatment with antibiotics for ≥7 days). Birth weight was associated with both cortisol and cortisone when expressed in kilograms, but this association was lost when birth weight was expressed as the s.d. score. Moreover, delivery via cesarian section was associated with both lower cortisol and cortisone levels, while multiparity was associated with lower cortisol levels.
Table 3.
Associations of neonatal hair glucocorticoid concentrations directly postpartum with perinatal and maternal factors.
| β (95% CI) | P value | ||
|---|---|---|---|
| Perinatal factors | |||
| Gestational age | Cortisol | 0.07 (0.04 to 0.10) | <0.001 |
| Cortisone | 0.04 (0.01 to 0.06) | 0.004 | |
| Gestational age (only term pregnancies) | Cortisol | 0.11 (0.07 to 0.16) | <0.001 |
| Cortisone | 0.04 (−0.001 to 0.08) | 0.06 | |
| Birth weight (kg) | Cortisol | 0.09 (−0.003 to 0.17) | 0.06 |
| Cortisone | 0.10 (0.03–0.17) | 0.008 | |
| Birth weight (s.d.) | Cortisol | 0.01 (−0.05 to 0.06) | 0.79 |
| Cortisone | 0.03 (−0.02 to 0.07) | 0.23 | |
| Male sex | Cortisol | 0.10 (−0.02 to 0.21) | 0.09 |
| Cortisone | 0.08 (−0.01 to 0.18) | 0.07 | |
| Delivery via cesarian section | Cortisol | −0.14 (−0.26 to −0.03) | 0.015 |
| Cortisone | −0.14 (−0.24 to −0.05) | 0.003 | |
| Perinatal infection (≥7 days antibiotics) | Cortisol | 0.17 (0.06–0.29) | 0.003 |
| Cortisone | 0.22 (0.13–0.31) | <0.001 | |
| Respiratory problems | Cortisol | −0.07 (−0.28 to 0.13) | 0.47 |
| Cortisone | −0.06 (−0.22 to 0.11) | 0.52 | |
| Maternal factors | |||
| Age | Cortisol | −0.01 (−0.02 to 0.01) | 0.31 |
| Cortisone | 0.00 (−0.01 to 0.01) | 0.82 | |
| Ethnicity | Cortisol | −0.04 (−0.15 to 0.08) | 0.55 |
| Cortisone | −0.09 (−0.16 to 0.01) | 0.07 | |
| Maternal smoking | Cortisol | −0.09 (−0.18 to 0.003) | 0.06 |
| Cortisone | −0.09 (−0.43 to 0.26) | 0.62 | |
| Parity | Cortisol | −0.26 (−0.36 to −0.16) | <0.001 |
| Cortisone | −0.07 (−0.17 to 0.02) | 0.12 | |
| Hypertensive disorders | Cortisol | −0.02 (−0.25 to 0.21) | 0.85 |
| Cortisone | −0.15 (−0.33 to 0.04) | 0.12 |
Values represent log-transformed β (95% confidence interval) as calculated with linear regression.
Next, the effect of the (borderline) significant factors on the course of GC levels was studied (Table 4). Gestational age was associated with a trend toward a steeper decrease in cortisol and cortisone between birth and the OPV. Additionally, perinatal infection was associated with a steeper decrease in cortisone, while delivery via cesarian section was associated with a smaller decrease in cortisone.
Table 4.
Associations of the course of neonatal hair glucocorticoid concentrations with perinatal and maternal factors.
| Effect on delta | Effect on OPV values | ||||
|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | ||
| Perinatal factors | |||||
| Gestational age | Cortisol | −0.02 (−0.05 to 0.00) | 0.08 | 0.08 (0.04 to 0.12) | <0.001 |
| Cortisone | −0.006 (−0.013 to 0.001) | 0.08 | 0.00 (−0.04 to 0.04) | 0.87 | |
| Birth weight (in kg) | Cortisol | −0.05 (−0.12 to 0.02) | 0.19 | 0.13 (0.003 to 0.26) | 0.05 |
| Cortisone | −0.02 (−0.03 to 0.01) | 0.15 | 0.00 (−0.12 to 0.12) | 0.96 | |
| Male sex | Cortisol | 0.02 (−0.07 to 0.11) | 0.68 | 0.19 (0.02 to 0.36) | 0.03 |
| Cortisone | −0.01 (−0.03 to 0.02) | 0.46 | 0.00 (−0.14 to 0.13) | 0.99 | |
| Delivery via caesarian section | Cortisol | 0.06 (−0.04 to 0.16) | 0.24 | −0.06 (−0.25 to 0.13) | 0.55 |
| Cortisone | 0.03 (0.01 to 0.05) | 0.02 | −0.08 (−0.23 to 0.08) | 0.33 | |
| Perinatal infection (≥7 days antibiotics) | Cortisol | −0.06 (−0.15 to 0.04) | 0.25 | −0.04 (−0.22 to 0.14) | 0.66 |
| Cortisone | −0.03 (−0.05 to −0.004) | 0.02 | 0.12 (−0.03 to 0.27) | 0.11 | |
| Maternal factors | |||||
| Parity | Cortisol | 0.07 (−0.02 to 0.16) | 0.11 | −0.11 (−0.28 to 0.05) | 0.18 |
| Cortisone | 0.01 (−0.01 to 0.03) | 0.32 | 0.01 (−0.13 to 0.14) | 0.91 | |
Values represent log-transformed β (95% confidence interval) as calculated with linear regression. All associations were corrected for age at the OPV.
Finally, the effect of these factors on the GC levels at the OPV was analyzed (Table 4). Gestational age was still positively associated with infant hair cortisol levels, but not with cortisone. Additionally, males had higher cortisol levels in hair at the OPV, but no association was found with cortisone. The other factors were not associated with OPV hair GC levels.
Discussion
In this study, we have described the levels of cortisol and cortisone in neonatal hair, both directly postpartum, as well as at an OPV at 44 ± 11 days postpartum. GC levels in neonatal hair directly postpartum seem to reflect intrauterine GC exposure. GC levels in neonatal hair are much higher than maternal levels and appear to be influenced mainly by gestational age, possibly reflecting the normal prenatal increase in endogenous fetal cortisol. After birth, cortisol levels decrease sharply, although at the OPV, neonatal levels are still much higher compared to maternal levels. This suggests that at that time point, GC levels represent the intra- and extrauterine period, since GC levels in infants are not markedly different from maternal cortisol levels (22, 23). Additionally, at birth, neonatal hair GC levels are associated with other perinatal factors such as perinatal infection, although to a lesser degree than gestational age.
In our study, we could confirm the association described by Hoffmann and coworkers (18) between neonatal hair cortisol levels and both gestational age and birth weight directly postpartum. However, we did not find an association with birth weight SDS. Since birth weight and gestational age are correlated, the association with birth weight probably reflects the effect of gestational age rather than of intrauterine growth. The association with gestational age might be indicative of several mechanisms. First, adrenal maturation occurs throughout pregnancy, resulting in increased cortisol production by the fetal adrenal (5). A higher concentration of GCs in hair might therefore reflect a longer exposure to the maturing HPA axis. However, since the association between gestational age and neonatal hair GCs is also still present in term neonates, another mechanism appears to be present as well. Induction of labor has been suggested to be partly due to an increase in cortisol, which occurs in all species studied to date and which promotes fetal organ maturation (7, 24). This increase in cortisol is thought to be due to a positive feedback loop established between placenta-derived CRH and cortisol originating from the fetal adrenals (6, 25), which can only be broken by the severance of the umbilical cord. Fetal distress may accelerate this feedback loop (8), which might explain the increased hair GC levels in neonates who are treated for a perinatal infection.
It is as of yet unknown whether neonatal hair GC levels fully result from fetal cortisol production or whether the transplacental supply of cortisol might also contribute to neonatal GC levels in hair. Previous research has suggested that cortisol is transferred via the placenta to the fetus, although most cortisol is inactivated to cortisone by placental 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2) (8). However, as maternal serum cortisol levels are 10 times higher than fetal serum levels, even small amounts of cortisol could account for about 40% of the variance in fetal concentrations (4). In our study, we found a positive correlation between maternal and neonatal hair cortisol levels, but not with cortisone. The positive correlation between neonatal and maternal hair cortisol levels might therefore be a reflection of placental transfer. However, this does not explain why the neonatal hair GC levels were much higher compared to maternal levels. We speculate that this may be due to differences in hair growth and structure between the fetus and its mother.
While it is feasible that cortisol in neonatal hair is derived from hair follicles, where it is incorporated after diffusion from blood (14), cortisol in amniotic fluid might contribute to the GC concentrations measured in hair. Moreover, although hair growth in utero is roughly known, the specifics are still unclear. The first stage of hair growth starts during the 15th week of gestation, and by week 18–20, the entire scalp is covered with hair in the primary, anagen stage. Next, between week 24 and 28, the anagen hair converts into telogen hair via a catagen phase (26). Hair growth, as well as the conversion to more mature hair, is region-specific and dependent on several biochemical and individual variations (26, 27, 28). Whether hair in the anagen phase already contains GCs, or whether the accumulation of GCs occurs at a later phase, is unknown. Therefore, although it is thought that neonatal hair reflects at least the third trimester of pregnancy (26), the true time window which is represented by GCs measured in hair is not known. Since perinatal infection and mode of delivery also appear to influence hair GC levels, it is likely that the last stages of pregnancy have a significant contribution to GC levels measured in hair. Future studies should include measurements of growth velocity of neonatal hair.
Our study showed significantly increased GC levels in neonatal hair compared to maternal hair at the OPV (44 ± 11 days postpartum), although a decrease between birth and the OPV was observed in cortisol levels. This suggests that GC levels at the OPV represent a combination of intra- and extrauterine influences, supported by our finding that GC levels at the OPV are still associated with several perinatal factors. However, due to the biochemical and individual variations in hair growth (26, 27, 28), and since hair was only measured twice in this study, the contribution of intrauterine and extrauterine influences on hair GC levels at the OPV is unknown. We recommend to assess at which point of time intrauterine factors are no longer related to hair GC levels, since this might provide a clear view of early life influences on HPA axis development. Since hair GC levels appear to be moderately stable in the second half of the first year of life (29), intrauterine influences on hair GC levels most likely disappear within the first 6 months.
Our study has several strengths and limitations. First, GC analyses were performed using LC–MS/MS, which has high sensitivity. Hoffman and coworkers (18) measured hair cortisol levels with an immunoassay, which might explain the fact that they did not find an association between maternal and neonatal hair cortisol levels, and reported maternal and neonatal cortisol levels which were much higher compared to our results, since immunoassays are more sensitive to cross-reactivity than LC–MS/MS (30, 31). Cross-reactivity is particularly important to take into account when researching newborns, as they have high concentrations of (precursors of) sex steroids and GCs (5), which are partly of maternal origin. Additionally, we measured cortisone as well as cortisol, which provides valuable knowledge due to the conversion of cortisol to cortisone by placental 11βHSD2 (8). Our database also allowed us to analyze a wide range of pre- and perinatal factors. One of the limitations of our study is that the participants might not represent a normal population, since the participants in our study had to be hospitalized >72 h. Additionally, there might be a selection bias at the OPV measurements due to losses to follow-up, although the number of participants was relatively high (67%). Finally, there was a discrepancy between mother and child in the time frame that the hair measurements represented. In mothers, only the last centimeter of hair was analyzed. As adult hair grows approximately 1 cm per month (20, 21), these analyses are representative of only the last month of pregnancy. Neonatal hair was analyzed in its entirety and is therefore representative of the intrauterine period during which cortisol can be incorporated in hair. Correlations between GCs in maternal and neonatal hair should therefore be interpreted in this context.
In conclusion, our findings suggest that infant hair GCs reflect the third trimester increase in cortisol, which might be caused by the positive feedback loop, a placenta-driven phenomenon, represented by a positive association with GA. Between birth and 1.5 months postpartum, cortisol concentrations decrease sharply. At this time point, GC levels appear to reflect both the intra- and extrauterine period, since neonatal levels are significantly higher than maternal GC levels. Perinatal complications and maternal HPA axis activity had minor influences on infant hair GCs.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study was financially supported by Nuts Ohra by a non-restricted grant (SSRI-158115/754). Nuts Ohra was not involved in the design and conduct of the study, collection, management, analysis and interpretation of the data, neither in preparation, review or approval of the manuscript and decision to submit the manuscript for publication. The authors report no financial or other relationship relevant to the subject of this article.
Acknowledgements
Kristien Dorst has been acknowledged for her great contribution to the hair analysis.
References
- 1.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Frontiers in Behavioral Neuroscience 2009. 3 19 ( 10.3389/neuro.08.019.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiong F, Zhang L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Frontiers in Neuroendocrinology 2013. 34 27–46. ( 10.1016/j.yfrne.2012.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiological Reviews 2014. 94 1027–1076. ( 10.1152/physrev.00029.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet 1998. 352 707–708. ( 10.1016/S0140-6736(05)60824-0) [DOI] [PubMed] [Google Scholar]
- 5.Brosnan PG. The hypothalamic pituitary axis in the fetus and newborn. Seminars in Perinatology 2001. 25 371–384. ( 10.1053/sper.2001.29038) [DOI] [PubMed] [Google Scholar]
- 6.McLean M, Smith R. Corticotrophin-releasing hormone and human parturition. Reproduction 2001. 121 493–501. ( 10.1530/rep.0.1210493) [DOI] [PubMed] [Google Scholar]
- 7.Fencl MD, Stillman RJ, Cohen J, Tulchinsky D. Direct evidence of sudden rise in fetal corticoids late in human gestation. Nature 1980. 287 225–226. ( 10.1038/287225a0) [DOI] [PubMed] [Google Scholar]
- 8.Watterberg KL. Adrenocortical function and dysfunction in the fetus and neonate. Seminars in Neonatology 2004. 9 13–21. ( 10.1016/j.siny.2003.08.003) [DOI] [PubMed] [Google Scholar]
- 9.Sarkar P, Bergman K, Fisk NM, O’Connor TG, Glover V. Ontogeny of foetal exposure to maternal cortisol using midtrimester amniotic fluid as a biomarker. Clinical Endocrinology 2007. 66 636–640. ( 10.1111/j.1365-2265.2007.02785.x) [DOI] [PubMed] [Google Scholar]
- 10.Ohana E, Mazor M, Chaim W, Levy J, Sharoni Y, Leiberman JR, Glezerman M. Maternal plasma and amniotic fluid cortisol and progesterone concentrations between women with and without term labor. A comparison. Journal of Reproductive Medicine 1996. 41 80–86. [PubMed] [Google Scholar]
- 11.Fencl MM, Koos B, Tulchinsky D. Origin of corticosteroids in amniotic fluid. Journal of Clinical Endocrinology and Metabolism 1980. 50 431–436. ( 10.1210/jcem-50-3-431) [DOI] [PubMed] [Google Scholar]
- 12.Partsch CJ, Sippell WG, MacKenzie IZ, Aynsley-Green A. The steroid hormonal milieu of the undisturbed human fetus and mother at 16–20 weeks gestation. Journal of Clinical Endocrinology and Metabolism 1991. 73 969–974. ( 10.1210/jcem-73-5-969) [DOI] [PubMed] [Google Scholar]
- 13.Leong MK, Murphy BE. Cortisol levels in maternal venous and umbilical cord arterial and venous serum at vaginal delivery. American Journal of Obstetrics and Gynecology 1976. 124 471–473. ( 10.1016/0002-9378(76)90170-8) [DOI] [PubMed] [Google Scholar]
- 14.Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology 2013. 38 1220–1235. ( 10.1016/j.psyneuen.2012.11.015) [DOI] [PubMed] [Google Scholar]
- 15.Braig S, Grabher F, Ntomchukwu C, Reister F, Stalder T, Kirschbaum C, Genuneit J, Rothenbacher D. Determinants of maternal hair cortisol concentrations at delivery reflecting the last trimester of pregnancy. Psychoneuroendocrinology 2015. 52 289–296. ( 10.1016/j.psyneuen.2014.12.006) [DOI] [PubMed] [Google Scholar]
- 16.D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiology and Behavior 2011. 104 348–353. ( 10.1016/j.physbeh.2011.02.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dettmer AM, Rosenberg KL, Suomi SJ, Meyer JS, Novak MA. Associations between parity, hair hormone profiles during pregnancy and lactation, and infant development in Rhesus monkeys (Macaca mulatta). PLoS ONE 2015. 10 e0131692 ( 10.1371/journal.pone.0131692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman MC, D’Anna-Hernandez K, Benitez P, Ross RG, Laudenslager ML. Cortisol during human fetal life: characterization of a method for processing small quantities of newborn hair from 26 to 42 weeks gestation. Developmental Psychobiology 2017. 59 123–127. ( 10.1002/dev.21433) [DOI] [PubMed] [Google Scholar]
- 19.Kieviet N, van Keulen V, van de Ven PM, Dolman KM, Deckers M, Honig A. Serotonin and poor neonatal adaptation after antidepressant exposure in utero. Acta Neuropsychiatrica 2017. 29 43–53. ( 10.1017/neu.2016.30) [DOI] [PubMed] [Google Scholar]
- 20.Noppe G, de Rijke YB, Dorst K, van den Akker EL, van Rossum EF. LC–MS/MS-based method for long-term steroid profiling in human scalp hair. Clinical Endocrinology 2015. 83 162–166. ( 10.1111/cen.12781) [DOI] [PubMed] [Google Scholar]
- 21.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Science International 2000. 107 5–12. ( 10.1016/S0379-0738(99)00146-2) [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Blanco A, Vento M, Diago V, Chafer-Pericas C. Reference ranges for cortisol and alpha-amylase in mother and newborn saliva samples at different perinatal and postnatal periods. Journal of Chromatography: B, Analytical Technologies in the Biomedical and Life Sciences 2016. 1022 249–255. ( 10.1016/j.jchromb.2016.04.035) [DOI] [PubMed] [Google Scholar]
- 23.Jonetz-Mentzel L, Wiedemann G. Establishment of reference ranges for cortisol in neonates, infants, children and adolescents. European Journal of Clinical Chemistry and Clinical Biochemistry 1993. 31 525–529. [PubMed] [Google Scholar]
- 24.Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proceedings of the Nutrition Society 1998. 57 113–122. ( 10.1079/PNS19980017) [DOI] [PubMed] [Google Scholar]
- 25.Challis JR, Hooper S. Birth: outcome of a positive cascade. Baillière’s Clinical Endocrinology and Metabolism 1989. 3 781–793. ( 10.1016/S0950-351X(89)80053-9) [DOI] [PubMed] [Google Scholar]
- 26.Gareri J, Koren G. Prenatal hair development: implications for drug exposure determination. Forensic Science International 2010. 196 27–31. ( 10.1016/j.forsciint.2009.12.024) [DOI] [PubMed] [Google Scholar]
- 27.Berger HM, King J, Doughty S, Wharton BA. Nutrition, sex, gestational age, and hair growth in babies. Archives of Disease in Childhood 1978. 53 290–294. ( 10.1136/adc.53.4.290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furdon SA, Clark DA. Scalp hair characteristics in the newborn infant. Advances in Neonatal Care 2003. 3 286–296. ( 10.1016/j.adnc.2003.09.005) [DOI] [PubMed] [Google Scholar]
- 29.Liu CH, Snidman N, Leonard A, Meyer J, Tronick E. Intra-individual stability and developmental change in hair cortisol among postpartum mothers and infants: implications for understanding chronic stress. Developmental Psychobiology 2016. 58 509–518. ( 10.1002/dev.21394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackermans MT, Endert E. LC–MS/MS in endocrinology: What is the profit of the last 5 years? Bioanalysis 2014. 6 43–57. ( 10.4155/bio.13.300) [DOI] [PubMed] [Google Scholar]
- 31.Shackleton C. Clinical steroid mass spectrometry: a 45-year history culminating in HPLC–MS/MS becoming an essential tool for patient diagnosis. Journal of Steroid Biochemistry and Molecular Biology 2010. 121 481–490. ( 10.1016/j.jsbmb.2010.02.017) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a