Abstract
Background
The UK abdominal aortic aneurysm (AAA) screening programmes currently invite only men for screening because the benefit in women is uncertain. Perioperative risk is critical in determining the effectiveness of screening, and contemporary estimates of these risks in women are lacking. The aim of this study was to compare mortality following AAA repair between women and men in the UK.
Methods
Anonymized data from the UK National Vascular Registry (NVR) for patients undergoing AAA repair (January 2010 to December 2014) were analysed. Co‐variables were extracted for analysis by sex. The primary outcome measure was in‐hospital mortality. Secondary outcome measures included mortality by 5‐year age groups and duration of hospital stay. Logistic regression was performed to adjust for age, calendar time, AAA diameter and smoking status. NVR‐based outcomes were checked against Hospital Episode Statistics (HES) data.
Results
A total of 23 245 patients were included (13·0 per cent women). Proportionally, more women than men underwent open repair. For elective open AAA repair, the in‐hospital mortality rate was 6·9 per cent in women and 4·0 per cent in men (odds ratio (OR) 1·48, 95 per cent c.i. 1·08 to 2·02; P = 0·014), whereas for elective endovascular AAA repair it was 1·8 per cent in women and 0·7 per cent in men (OR 2·86, 1·72 to 4·74; P < 0·001); the results in HES were similar. For ruptured AAA, there was no sex difference in mortality within the NVR; however, in HES, for ruptured open AAA repair, the in‐hospital mortality rate was higher in women (33·6 versus 27·1 per cent; OR 1·36, 1·16 to 1·59; P < 0·001).
Conclusion
Women have a higher in‐hospital mortality rate than men after elective AAA repair even after adjustment. This higher mortality may have an impact on the benefit offered by any screening programme offered to women.
Short abstract
Mortality double in women
Introduction
Randomized trial evidence has shown that, in men, abdominal aortic aneurysm (AAA) screening reduces AAA‐related mortality (relative risk reduction 42 (95 per cent c.i. 31 to 51) per cent)1, and is highly cost‐effective2. As a result, AAA screening in men aged 65 years was fully implemented across England and Wales in 2013, and is now established across the UK. In historical studies, screening for AAA in women has not been demonstrated to be clinically effective, although the only randomized trial was underpowered3.
Recent reanalysis of economic models of AAA screening in men has demonstrated that it remains highly cost‐effective2 despite reductions in disease prevalence4, 5, and that the prevalence threshold for cost‐effectiveness in men is lower than the historical estimates of disease prevalence in older women6. Approximately one in seven elective AAA repairs in England and Wales are performed on women7, and women account for one‐third of all deaths from ruptured AAA8. Together with a fourfold higher rupture rate in women than men at equivalent aortic diameters9, 10, this suggests that there may be a strong case for the initiation of AAA screening in women, or selected high‐risk groups of women.
An important determinant of the effectiveness of any screening programme is the risk associated with treatment. For AAA screening, it cannot be assumed that this risk (the risk of AAA repair) is the same for women as for men. Sex differences in vascular surgical outcomes have been described11, 12, and case series13, 14 have shown differences after AAA repair, with women exposed to a higher perioperative risk15. However, no large studies have been able to explore the reasons for these differences or adjust for co‐variables. Excess perioperative risk in women16 may offset any advantage gained by preventing rupture, and would also have a negative impact on the cost‐effectiveness of any screening programme to identify women with AAA. Yet, many of the data used to compare the risks of surgery between men and women are dated, and are limited by the modest representation of women in studies of AAA. Furthermore, past studies have contained inadequate detail to determine whether any differences observed are confounded by patient characteristics.
The aim of this study was to quantify differences in in‐hospital mortality between women and men undergoing AAA repair in the UK National Health Service (NHS) using data obtained from the National Vascular Registry (NVR), which allows adjustment for relevant co‐variables, such as smoking and AAA diameter, and the Hospital Episode Statistics (HES) data set.
Methods
National Vascular Registry
The NVR was established to measure the quality and outcomes of care for patients who undergo major vascular surgery in NHS hospitals, and was commissioned by the Healthcare Quality Improvement Partnership as part of the National Clinical Audit and Patient Outcomes Programme17. Data are assessed using consistency and range checks, as well as comparison with HES data. NHS Trusts in England are duty‐bound to provide HES data for financial probity17. Case ascertainment, which is the number of cases input to the NVR compared with that estimated from HES, was 84 per cent in 2012–2014 for elective AAA repair and 75 per cent for ruptured AAA repair7. The NVR is the largest validated registry of admissions to NHS hospitals for AAA repair, and includes cases from Northern Ireland, Scotland and Wales as well as England18.
Anonymized individual patient‐level data from the NVR for patients who underwent AAA repair in the UK between January 2010 and December 2014 were obtained under a data‐sharing agreement with the Healthcare Quality Improvement Partnership. Patients with elective and ruptured AAA were included; symptomatic AAA, duplicate data and patients who presented with alternative diagnoses (acute aortic dissection, chronic aortic dissection or aortic transection) were excluded. Patients who underwent an operation other than endovascular aneurysm repair (EVAR) or open repair (complex EVAR, open revision or EVAR revision) were also excluded. Complex open AAA repairs were not included, nor were any records that did not record the sex of the patient.
Operation type is recorded at the time of operation in the NVR according to the OPCS‐4 codes, in addition to a number of supplementary procedure options to differentiate infrarenal, juxtarenal and suprarenal aneurysms. Any juxtarenal or suprarenal AAA is classified as a complex aneurysm.
National Vascular Registry variables included
Demographic co‐variables available were age and sex. Preoperative clinical co‐variables available were AAA diameter, co‐morbidities (diabetes, hypertension, chronic obstructive pulmonary disease, ischaemic heart disease, heart failure, chronic renal failure and stroke), smoking status and indication for operation. Postoperative co‐variables were level 3 admission (high‐dependency unit or ICU), duration of stay in critical care and in hospital, and death (in‐hospital mortality only) (Appendix S1 S1, supporting information).
Hospital Episode Statistics analysis
HES is the administrative data set for the NHS in England, which contains information regarding patient admission to NHS hospitals. HES data are anonymized by the allocation of a unique anonymous identifier to each patient, so individuals can be tracked through admissions to different hospitals. HES was analysed for all infrarenal AAA repairs (excluding those under cardiac or cardiothoracic surgery specialties) for procedures performed between 1 January 2010 and 31 December 2014 in order to match the NVR analysis.
The algorithm for identifying cases utilized a modified version of a previously published methodology19. It was based on an iterative process in which a group of clinicians looked at the records of a sample of cases with potentially ambiguous codes (such as L188, L189) and, by inspecting the associated diagnostic and specialty codes, determined the most likely categorization, for example AAA. This included the codes for aortoiliac bypass where there is an associated diagnostic code of AAA (I171).
Clinical review of coding showed that the emergency codes (L194 versus L184) were not used accurately, and that HES coding systems do not accurately differentiate between acute admission for symptomatic aneurysms and ruptured aneurysm. Therefore, a set of decision rules was developed to establish a proxy classification by which to identify ruptured aneurysms. Admissions were classified as ruptured AAA, on the basis of the coding of method of admission; those with any EVAR code (L271–L289) were classified as EVAR, and emergency admissions where the date of the first procedure was the day of admission were assumed to represent cases of ruptured AAA. One weakness of the NVR is that it is a self‐reported data set and open to bias. The reason for including a comparable analysis based on HES data within this article was to demonstrate that the differences in the NVR are not unique to this registry and may therefore represent real differences in outcomes. It also allowed the authors to cross‐check that the number of deaths recorded in the NVR and HES were comparable.
Study design
The primary outcome measure was in‐hospital mortality for men and women by indication and operation type calculated from NVR data. NVR‐based outcomes were checked against HES data. Secondary outcome measures included mortality by 5‐year age groups, and duration of stay.
Statistical analysis
Summary statistics are presented by sex using mean(s.d.) for continuous, non‐skewed data and median (i.q.r.) for skewed data. Categorical data are presented as counts and percentages. Differences in characteristics between men and women were assessed using the Mann–Whitney U test for all continuous co‐variables and χ2 test for categorical variables.
The risk of death in hospital was modelled using logistic regression separately for elective open repair, elective EVAR, emergency open repair and emergency EVAR. The odds ratio (OR) for sex was first estimated for each operation type without adjustment for any other characteristics. Potential confounding of the sex effect was then investigated by adjusting for age, calendar year of operation, AAA diameter and smoking status. These co‐variables were chosen because of their excellent data completeness within the NVR, and only individuals with complete data on all the adjustment variables in that model were retained. In a sensitivity analysis, further adjustment was made for the occurrence of each co‐morbidity recorded in the NVR using available data. Individuals with missing information on any co‐variable were excluded from these analyses. All continuous variables were assumed to act linearly on the log odds. The numbers reported are affected by the extent of missing data. Wald tests were used to obtain two‐sided P values for the estimated log ORs. All statistical analyses were completed using R version 3.2.4 (R Project for Statistical Computing, Vienna, Austria) and SPSS® version 22.0 (IBM, Armonk, New York, USA).
Results
National Vascular Registry data
Between January 2010 and December 2014, some 23 245 patients underwent elective or ruptured AAA repair (EVAR or open) in the UK and had outcomes reported to the NVR.
Elective surgical repair
In all, 18 693 patients had elective AAA repair, of whom 16 465 (88·1 per cent) were men. At any age, proportionally fewer women than men underwent EVAR compared with open surgical repair (Fig. 1). Although the use of EVAR increased progressively in older age groups, it decreased with larger aneurysm diameter (Table 1, supporting information). The duration of hospital stay was longer in women than in men after elective EVAR and open AAA repair. The time spent in critical care was longer in women after open AAA repair (Table 1).
Figure 1.
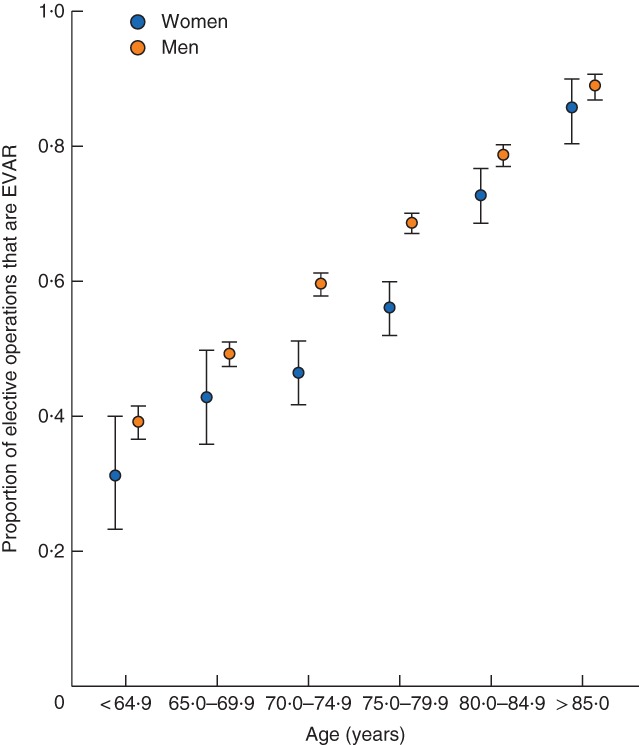
Overall proportion of elective abdominal aortic aneurysm operations that are endovascular aneurysm repair (EVAR), with 95 per cent confidence intervals, by sex and age
Table 1.
Characteristics and outcomes for elective open and endovascular abdominal aortic aneurysm repair
| Open repair | EVAR | |||||
|---|---|---|---|---|---|---|
| Women | Men | P ‡ | Women | Men | P ‡ | |
| UK National Vascular Registry | 922 | 6013 | 1306 | 10 452 | ||
| Age (years)* | 74·1(7·7) | 71·5(7·6) | < 0·001§ | 78·4(7·1) | 76·0(7·6) | < 0·001§ |
| (n = 922) | (n = 6012) | (n = 1306) | (n = 10 452) | |||
| AAA diameter (mm)† | 60 (56–67) | 62 (57–72) | < 0·001§ | 59 (56–65) | 60 (57–67) | < 0·001§ |
| (n = 877) | (n = 5796) | (n = 1227) | (n = 9849) | |||
| Co‐morbidities | ||||||
| Diabetes | 94 of 372 (25·3) | 702 of 2735 (25·7) | 0·921 | 139 of 615 (22·6) | 1636 of 5293 (30·9) | < 0·001 |
| Hypertension | 149 of 372 (40·1) | 1192 of 2735 (43·6) | 0·221 | 307 of 615 (49·9) | 2165 of 5293 (40·9) | < 0·001 |
| COPD | 66 of 372 (17·7) | 347 of 2735 (12·7) | 0·009 | 121 of 615 (19·7) | 810 of 5293 (15·3) | 0·006 |
| IHD | 132 of 372 (35·5) | 1074 of 2735 (39·3) | 0·183 | 232 of 615 (37·7) | 2512 of 5293 (47·5) | < 0·001 |
| Heart failure | 13 of 372 (3·5) | 109 of 2735 (4·0) | 0·752 | 45 of 615 (7·3) | 451 of 5293 (8·5) | 0·351 |
| Renal failure | 32 of 372 (8·6) | 152 of 2735 (5·6) | 0·027 | 82 of 615 (13·3) | 510 of 5293 (9·6) | 0·005 |
| Stroke | 26 of 372 (7·0) | 141 of 2735 (5·2) | 0·181 | 25 of 615 (4·1) | 272 of 5293 (5·1) | 0·293 |
| Current smoker | 285 of 878 (32·5) | 1467 of 5780 (25·4) | < 0·001 | 246 of 1197 (20·6) | 1577 of 9600 (16·4) | < 0·001 |
| Duration of critical care stay (days)† | 3 (2–5) | 2 (1–4) | 0·021§ | 1 (0–1) | 1 (0–1) | 0·005§ |
| (n = 471) | (n = 3188) | (n = 504) | (n = 3677) | |||
| Duration of hospital stay (days)† | 9 (7–13) | 8 (6–11) | < 0·001§ | 4 (2–6) | 3 (2–5) | < 0·001§ |
| (n = 922) | (n = 6013) | (n = 1306) | (n = 10 452) | |||
| In‐hospital death | 64 of 922 (6·9) | 243 of 6013 (4·0) | < 0·001 | 23 of 1306 (1·8) | 74 of 10 452 (0·7) | < 0·001 |
| HES – England | ||||||
| In‐hospital death | 64 of 1066 (6·0) | 213 of 6832 (3·1) | < 0·001 | 27 of 1642 (1·6) | 104 of 13 070 (0·8) | < 0·001 |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.) and
median (i.q.r.).
EVAR, endovascular aneurysm repair; AAA, abdominal aortic aneurysm; COPD, chronic obstructive pulmonary disease; IHD, ischaemic heart disease; HES, Hospital Episode Statistics.
χ2 test, except
Mann–Whitney U test.
Some 6935 patients underwent elective open surgical repair. Women were older (mean 74·1 versus 71·5 years) and more likely to smoke (32·5 versus 25·4 per cent). The in‐hospital mortality rate was 6·9 per cent in women compared with 4·0 per cent in men (OR 1·77, 95 per cent c.i. 1·33 to 2·35; P < 0·001) (Fig. 2 and Table 2). Stratification by age group demonstrated an increase in mortality rate with age (Table S2, supporting information). After adjustment, the sex difference in mortality remained significant (OR 1·48, 1·08 to 2·02; P = 0·014) (Table 2).
Figure 2.
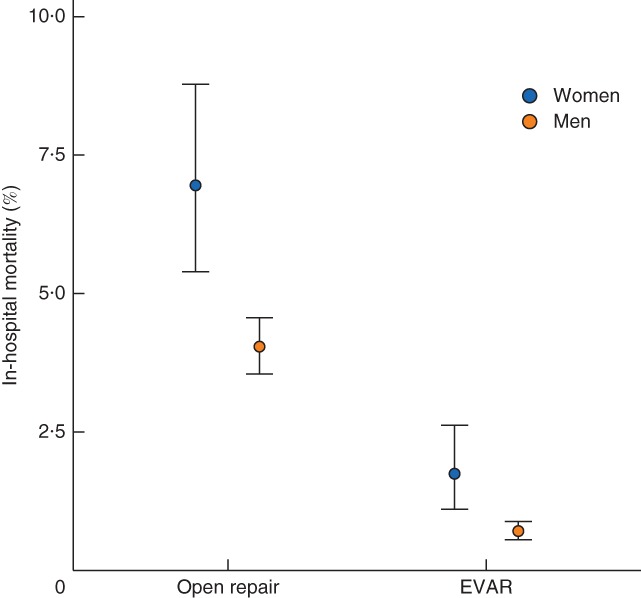
In‐hospital mortality for elective abdominal aortic aneurysm repair, with 95 per cent confidence intervals, by sex. EVAR, endovascular aneurysm repair
Table 2.
Logistic regression analyses of in‐hospital mortality for women versus men after elective abdominal aortic aneurysm repair
| Type of repair | Data source | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|---|
| n | Odds ratio | P | n | Odds ratio | P | ||
| Open repair | NVR | 6935 | 1·77 (1·33, 2·35) | < 0·001 | 6408 | 1·48 (1·08, 2·02) | 0·014 |
| HES | 7898 | 1·98 (1·49, 2·65) | < 0·001 | ||||
| EVAR | NVR | 11 758 | 2·51 (1·57, 4·03) | < 0·001 | 10 165 | 2·86 (1·72, 4·74) | < 0·001 |
| HES | 14 712 | 2·08 (1·36, 3·19) | < 0·001 | ||||
Values in parentheses are 95 per cent confidence intervals. Odds ratios are shown for women versus men.
Adjusted for age, calendar year, abdominal aortic aneurysm diameter and smoking status. NVR, National Vascular Registry; HES, Hospital Episode Statistics; EVAR, endovascular aneurysm repair.
A total of 11 758 patients underwent elective (non‐complex) EVAR. The women were older than the men (mean 78·4 versus 76·0 years). More women were hypertensive (49·9 versus 40·9 per cent) or a smoker (20·6 versus 16·4 per cent), whereas more men were diabetic (30·9 versus 22·6 per cent) or had ischaemic heart disease (47·5 versus 37·7 per cent). The in‐hospital mortality rate was 1·8 per cent in women and 0·7 per cent in men (OR 2·51, 1·57 to 4·03; P < 0·001) (Fig. 2 and Table 2), and increased with age (Table S2, supporting information). The sex difference was similar after adjustment for age, smoking status, AAA diameter and calendar year (OR 2·86, 1·72 to 4·74; P < 0·001) (Table 2). After further adjustment (sensitivity analysis) for co‐morbidities, the estimated sex differences were of the same or greater magnitude, although the confidence intervals were wider owing to considerable missing co‐morbidity data (Table S3, supporting information).
Ruptured aneurysm repair
A total of 4552 patients underwent surgery for a ruptured AAA, of whom 3767 were men (82·8 per cent). The majority (81·1 per cent) underwent open repair (Table 3); among these, women were older (mean 77·7 versus 74·8 years) and had smaller aneurysm diameters (median 72 versus 80 mm), whereas more men had diabetes (26·6 versus 16·4 per cent). There was no difference between men and women in duration of stay in hospital or critical care after rupture. The proportion of women undergoing a ruptured AAA repair was higher than the proportion undergoing elective repair.
Table 3.
Characteristics and outcomes for ruptured open and endovascular abdominal aortic aneurysm repair
| Open repair | EVAR | |||||
|---|---|---|---|---|---|---|
| Women | Men | P ‡ | Women | Men | P ‡ | |
| UK National Vascular Registry | 653 | 3037 | 132 | 730 | ||
| Age (years)* | 77·7(7·1) | 74·8(7·9) | < 0·001§ | 79·8(8·6) | 77·4(8·7) | 0·002§ |
| (n = 653) | (n = 3037) | (n = 132) | (n = 730) | |||
| AAA diameter (mm)† | 72 (60–80) | 80 (70–90) | < 0·001§ | 69 (60–80) | 77 (64–90) | < 0·001§ |
| (n = 286) | (n = 1503) | (n = 122) | (n = 648) | |||
| Co‐morbidities | ||||||
| Diabetes | 44 of 269 (16·4) | 350 of 1318 (26·6) | < 0·001 | 18 of 71 (25) | 79 of 396 (19·9) | 0·872 |
| Hypertension | 131 of 269 (48·7) | 507 of 1318 (38·5) | 0·002 | 35 of 71 (49) | 179 of 396 (45·2) | 0·614 |
| COPD | 46 of 269 (17·1) | 181 of 1318 (13·7) | 0·184 | 16 of 71 (23) | 83 of 396 (21·0) | 0·893 |
| IHD | 93 of 269 (34·6) | 506 of 1318 (38·4) | 0·271 | 29 of 71 (41) | 184 of 396 (46·5) | 0·465 |
| Heart failure | 14 of 269 (5·2) | 67 of 1318 (5·1) | 1·000 | 9 of 71 (13) | 42 of 396 (10·6) | 0·762 |
| Renal failure | 25 of 269 (9·3) | 92 of 1318 (7·0) | 0·231 | 11 of 71 (15) | 55 of 396 (13·9) | 0·861 |
| Stroke | 21 of 269 (7·8) | 103 of 1318 (7·8) | 1·000 | 5 of 71 (7) | 37 of 396 (9·3) | 0·691 |
| Current smoker | 178 of 591 (30·1) | 786 of 2693 (29·2) | 0·693 | 27 of 125 (21·6) | 163 of 662 (24·6) | 0·543 |
| Duration of critical care stay (days)† | 5 (2–8) | 4 (2–10) | 0·952§ | 2 (1–4) | 2 (1–4) | 0·644§ |
| (n = 286) | (n = 1503) | (n = 62) | (n = 394) | |||
| Duration of hospital stay (days)† | 12 (3–23) | 11 (5–21) | 0·861§ | 9 (4–17) | 8 (4–16) | 0·621§ |
| (n = 653) | (n = 3017) | (n = 132) | (n = 730) | |||
| In‐hospital death | 260 of 653 (39·8) | 1120 of 3037 (36·9) | 0·172 | 33 of 132 (25·0) | 151 of 730 (20·7) | 0·321 |
| HES – England | ||||||
| In‐hospital death | 284 of 845 (33·6) | 1109 of 4089 (27·1) | < 0·001 | 31 of 244 (12·7) | 148 of 1276 (11·6) | 0·612 |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.) and
median (i.q.r.).
EVAR, endovascular aneurysm repair; AAA, abdominal aortic aneurysm; COPD, chronic obstructive pulmonary disease; IHD, ischaemic heart disease; HES, Hospital Episode Statistics.
χ2 test, except
Mann–Whitney U test.
The in‐hospital mortality rate after open repair of ruptured AAA was 39·8 per cent in women and 36·9 per cent in men (OR 1·13, 95 per cent c.i. 0·95 to 1·35; P = 0·161) (Fig. 3 and Table 4), and increased with age (Table S4, supporting information). The sex difference remained non‐significant after adjustment (Table 4).
Figure 3.
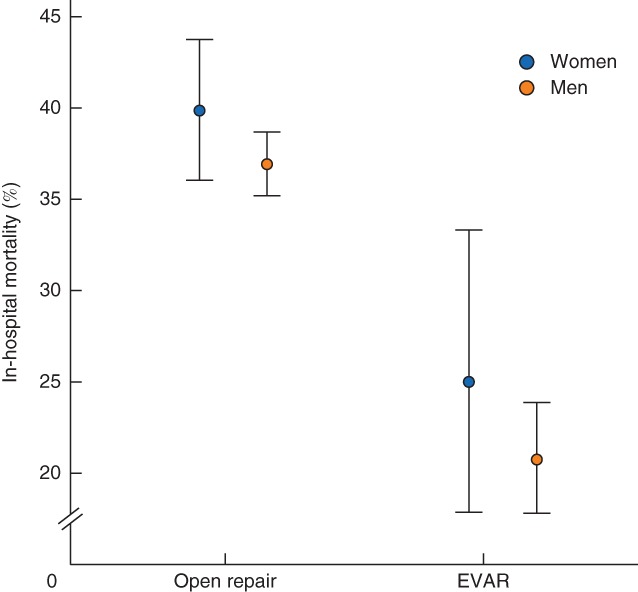
In‐hospital mortality for ruptured abdominal aortic aneurysm repair, with 95 per cent confidence intervals, by sex. EVAR, endovascular aneurysm repair
Table 4.
Logistic regression analyses of in‐hospital mortality for women versus men after ruptured abdominal aortic aneurysm repair
| Type of repair | Data source | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|---|
| n | Odds ratio | P | n | Odds ratio | P | ||
| Open repair | NVR | 3690 | 1·13 (0·95, 1·35) | 0·161 | 2975 | 0·91 (0·75, 1·12) | 0·382 |
| HES | 4934 | 1·36 (1·16, 1·59) | < 0·001 | ||||
| EVAR | NVR | 862 | 1·28 (0·83, 1·97) | 0·272 | 713 | 1·50 (0·92, 2·44) | 0·112 |
| HES | 1520 | 1·11 (0·73, 1·68) | 0·621 | ||||
Values in parentheses are 95 per cent confidence intervals. Odds ratios are shown for women versus men.
Adjusted for age, calendar year, abdominal aortic aneurysm diameter and smoking status. NVR, National Vascular Registry; HES, Hospital Episode Statistics; EVAR, endovascular aneurysm repair.
For ruptured EVAR, the in‐hospital mortality rate was 25·0 per cent in women versus 20·7 per cent in men (OR 1·28, 0·83 to 1·97; P = 0·272) (Tables 3 and 4) and AAA diameter was smaller in women than men. A sex difference in mortality was not evident after adjustment for age, smoking status, AAA diameter and calendar year (Table 4), or after further adjustment (sensitivity analysis) for co‐morbidities (Table S3, supporting information).
Hospital Episode Statistics
HES data for the same calendar period as the NVR analyses also provided evidence of sex differences for outcomes after elective AAA repair. For open AAA repair, the in‐hospital mortality rate in HES was 6·0 per cent for women and 3·1 per cent for men (OR 1·98, 95 per cent c.i. 1·49 to 2·65; P < 0·001) (Tables 1 and 2), similar to NVR data. For elective EVAR, the HES mortality rates were 1·6 per cent in women and 0·8 per cent in men (OR 2·08, 1·36 to 3·19; P < 0·001), again similar to NVR data.
For ruptured AAA, the HES data demonstrated a greater in‐hospital mortality rate in women compared with men (33·6 versus 27·1 per cent; OR 1·36, 1·16 to 1·59; P < 0·001) after open AAA repair, but not for EVAR (OR 1·11, 0·73 to 1·68; P = 0·621) (Tables 3 and 4).
Discussion
This contemporary study suggests that women undergoing elective AAA repair in the UK have an approximately twofold higher in‐hospital mortality rate after either open repair or EVAR in both the NVR and HES data sets. In previous work14, this group identified differences between men and women in elective AAA outcomes, but was unable to explore the reasons for these differences or adjust for co‐variables. In the present study, the excess mortality risk in elective AAA repair was demonstrated to be largely independent of age, aneurysm diameter, smoking status and calendar time. Furthermore, sensitivity analysis, in which further adjustment was made for co‐morbidities, continued to support the sex differences seen, mostly after elective EVAR. Although the sex differences identified may have a negative impact on any future screening in women, further studies are needed.
Female sex has previously been associated with increased mortality risk after AAA repair. For example, in an analysis of data from the American College of Surgeons National Surgical Improvement Program (2011–2014), Deery and co‐workers20 similarly demonstrated that women undergoing elective AAA repair were older, had smaller aneurysms, and had a higher mortality rate (30 day) after EVAR (3·2 versus 1·2 per cent) and open AAA repair (8·0 versus 4·0 per cent). Egorova and colleagues21 analysed the Medicare population (1995–2006), noting perioperative mortality rates after elective open repair of 6·4 per cent for women (compared with 6·9 per cent in the present cohort) and 3·0 per cent for women undergoing elective EVAR (compared with 1·8 per cent here).
Mehta and colleagues22 also demonstrated that elective EVAR carries a greater risk of death in women than men (3·2 versus 1·0 per cent; P < 0·005) although in their series no sex difference was found among those undergoing open repair. Similarly, Abedi et al.23 described higher mortality rates in women following elective EVAR (3·4 versus 2·1 per cent; P = 0·01), in addition to higher morbidity rates (17·8 versus 10·6 per cent; P < 0·001), which included a composite of 21 postoperative adverse events24. Taken together, these findings highlight a higher postoperative risk for women after elective AAA repair.
There is currently no consensus on the role of screening for AAA in women and, to date, there has been only one small randomized trial of screening in women3; this did not show any mortality benefit, although it was underpowered. The excess elective mortality in women identified in the present study in addition to an increased duration of stay may erode some of the potential benefit from screening in women, although one‐third of all deaths from AAA rupture in England are now in women8, and the poor outcomes identified in this study after emergency AAA repair (which are similar for women and men) may still favour AAA screening in women. In an evaluation of the cost‐effectiveness of screening 65‐year‐old women, Wanhainen and colleagues25 found that the incremental cost–effectiveness ratio was similar to that for screening men; however, contemporary data on this are required.
Other parameters that may relate to an increased mortality risk in women undergoing AAA repair include more complex arterial anatomy and occult cardiovascular disease. Aneurysm morphology has been shown to differ between the sexes in several small cohort studies; women are more likely to have shorter, more angulated infrarenal necks26 and small iliac arteries27, 28, 29, 30, making EVAR more challenging. For ruptured AAA, the aneurysm necks are often shorter and more angulated in women, rendering most unsuitable for EVAR31. These morphological factors may explain the lower utilization of EVAR in women in the present study and may also partially explain the worse outcomes in women. The Canadian Society for Vascular Surgery Aneurysm Study Group32 identified that women were more likely to be older, have a positive family history of AAA, and have significant aortoiliac occlusive disease. In the era of EVAR, the diameter of the iliac axis and possible aortoiliac occlusive disease play a major role in the technical success of the procedure33. A detailed analysis of a population undergoing AAA repair, with known coexisting co‐morbid factors and well characterized anatomy before surgery, is required to assess the precise interactions of sex and other parameters on subsequent mortality after AAA repair.
It is difficult to suggest specific strategies to improve mortality in women undergoing AAA repair, given that the exact reasons for their poorer outcome remain unknown. Similar findings have been demonstrated in the USA, suggesting that this is not a problem specific to the NHS. It may be that a quality improvement project is required to improve outcomes in women after elective AAA repair. One suggestion may be the routine use of adjuncts such as cardiopulmonary exercise testing (CPET) to quantify risk in women undergoing AAA repair and guide aggressive preoperative optimization. CPET may be performed safely in high‐risk populations and is a recognized reliable measure of cardiovascular reserve in patients undergoing major surgery, such as open AAA repair34. Patients classified as high risk based on CPET can then be optimized before operation by means of a variety of strategies, in order to improve their cardiovascular reserve, including commencement of high‐dose statin therapy, antiplatelet treatment, better control of hypertension by adding extra agents, lifestyle advice including smoking cessation, weight loss, correction of anaemia, and improved control of diabetes. Another suggestion might be the design of endografts more suitable for women.
For ruptured open AAA repair, which includes the majority of ruptures, the NVR data suggested no difference in hospital mortality (P = 0·161) whereas the HES data suggested a higher mortality rate among women (OR 1·36, 95 per cent c.i. 1·16 to 1·59; P < 0·001), although these data were unadjusted. This difference remains unexplained, although it may reflect bias in reporting ruptured AAA repairs to the NVR as the case ascertainment for such cases is only 75 per cent7. It is also interesting to note that the median AAA diameter reported at the time of repair of ruptured AAA is about 1 cm smaller in women than men. One unknown in the NVR and HES cohorts is the turn‐down rate for elective or ruptured AAA repair, which would influence the true mortality rate. In a retrospective cohort study from England35, the turn‐down rate was 13 per cent (mostly secondary to medical co‐morbidity), with women being more likely to be turned down (OR 11·26, 95 per cent c.i. 3·96 to 31·99; P < 0·001). Similarly, Ulug and co‐workers36 demonstrated that turn‐down rates were higher in women than men (34 versus 19 per cent; OR 2·27, 1·21 to 4·23), compared with 9·2 per cent within the first 5 years of the NHS Abdominal Aortic Aneurysm Screening Programme (men only)37.
Although these data suggest that differences exist between women and men undergoing AAA repair in the UK, it does not suggest that AAA screening in women would not be beneficial. The ongoing National Institute for Health Research (NIHR) trial of AAA screening of women in Leicestershire and Northamptonshire (Female Aneurysm Screening Study) and the ongoing Health Technology Assessment (HTA)‐funded modelling study to examine the potential clinical benefit and cost‐effectiveness of AAA screening in women (Screening Women for abdominal aortic Aneurysms; SWAN study) will add the much‐needed context of these findings to any possible AAA screening in women. Although the NVR is the largest validated registry of AAA repairs performed in the UK, some patients are not entered in the database, which raises the possibility of bias. By analysing the HES data set separately, the authors have demonstrated that the in‐hospital mortality rate differs between men and women for elective repairs in both the NVR and HES, suggesting that reporting bias does not explain the differences seen. One limitation of using the HES data set to assess rupture is the difficulty in separating ruptured aneurysms from symptomatic AAAs requiring urgent surgery. However, a modified version of a previously published algorithm19 for analysing HES data was used here, which relies on admission method and day of surgery to separate these different pathologies with different inherent risks.
Not all fields within the NVR are mandatory and some data fields were missing; for example, co‐morbidity data were missing in approximately half of the NVR data set. This deficiency of the NVR has now been addressed in that co‐morbidity data are better captured. The sensitivity analysis demonstrated similar ORs after adjustment for co‐morbidities, but with wider confidence intervals, which is to be expected owing to the amount of missing data. Only a well designed trial of matched women and men undergoing elective AAA repair would truly explain the differences seen. However, even after further adjustment, the present study continues to support the finding of sex differences in mortality.
In this study, patients were excluded from specific analyses where data were not available. The NVR was established as part of the National Clinical Audit and Patient Outcomes Programme17 and HES is the administrative data set for the NHS in England. These data sets are not primarily designed for research, and therefore the quality of the data within this observational study may be poor.
Supporting information.
Additional supporting information may be found online in the supporting information tab for this article.
Supporting information
Appendix S1 National Vascular Registry codes
Fig. S1 Flow chart of patients included from National Vascular Registry database
Table S1 Overall proportion of elective open and endovascular aneurysm repairs by sex and abdominal aortic aneurysm diameter
Table S2 In‐hospital deaths for elective open and endovascular abdominal aneurysm repair by sex and age
Table S3 Sensitivity analysis
Table S4 In‐hospital deaths for ruptured open and endovascular abdominal aortic aneurysm repair by sex and age
Acknowledgements
D.A.S. and A.S. are funded by the UK NIHR. This paper was written in collaboration with members of the SWAN project, a modelling study to examine the potential clinical benefit and cost‐effectiveness of AAA screening in women (www.nets.nihr.ac.uk/projects/hta/1417901). The SWAN project was funded by the UK NIHR HTA programme (project number 14/179/01). The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS or the Department of Health. The aspect of this paper relating to the analysis of HES presents independent research funded by the NIHR under the Programme Grants for Applied Research programme (RP‐PG‐1210‐12009). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. This publication is based on data collected by or on behalf of the Healthcare Quality Improvement Partnership, who have no responsibility or liability for the accuracy, currency, reliability and/or correctness of this publication. [Correction added on 18th August 2017, after first online publication: the final sentence of the Acknowledgements section has been added].
Disclosure: The authors declare no conflict of interest.
References
- 1. Thompson SG, Ashton HA, Gao L, Buxton MJ, Scott RA; Multicentre Aneurysm Screening Study (MASS) Group . Final follow‐up of the multicentre aneurysm screening study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg 2012; 99: 1649–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glover MJ, Kim LG, Sweeting MJ, Thompson SG, Buxton MJ. Cost‐effectiveness of the National Health Service Abdominal Aortic Aneurysm Screening Programme in England. Br J Surg 2014; 101: 976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002; 89: 283–285. [DOI] [PubMed] [Google Scholar]
- 4. Benson RA, Poole R, Murray S, Moxey P, Loftus IM. Screening results from a large United Kingdom abdominal aortic aneurysm screening center in the context of optimizing United Kingdom National Abdominal Aortic Aneurysm Screening Programme protocols. J Vasc Surg 2016; 63: 301–304. [DOI] [PubMed] [Google Scholar]
- 5. Sidloff D, Stather P, Dattani N, Bown M, Thompson J, Sayers R et al Aneurysm global epidemiology study: public health measures can further reduce abdominal aortic aneurysm mortality. Circulation 2014; 129: 747–753. [DOI] [PubMed] [Google Scholar]
- 6. Ulug P, Powell JT, Sweeting MJ, Bown MJ, Thompson SG; SWAN Collaborative Group . Meta‐analysis of the current prevalence of screen‐detected abdominal aortic aneurysm in women. Br J Surg 2016; 103: 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vascular Society of Great Britain and Ireland. National Vascular Registry : 2015 Annual Report. https://www.vsqip.org.uk/content/uploads/2015/12/NVR‐2015‐annual‐report.pdf [accessed 30 August 2016]. [Google Scholar]
- 8. Office for National Statistics . The 21st Century Mortality Files – Deaths Dataset, England and Wales (updated 2015). https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/the21stcenturymortalityfilesdeathsdataset [accessed 5 November 2016].
- 9. Powell JT, Brown LC. The natural history of abdominal aortic aneurysms and their risk of rupture. Acta Chir Belg 2001; 101: 11–16. [PubMed] [Google Scholar]
- 10. Sweeting MJ, Thompson SG, Brown LC, Powell JT; Collaborators RESCAN. Meta‐analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg 2012; 99: 655–665. [DOI] [PubMed] [Google Scholar]
- 11. Enzler MA, Ruoss M, Seifert B, Berger M. The influence of gender on the outcome of arterial procedures in the lower extremity. Eur J Vasc Endovasc Surg 1996; 11: 446–452. [DOI] [PubMed] [Google Scholar]
- 12. Hedayati N, Humphries MD, Zhou W. Gender and outcomes of carotid artery interventions. Vasc Endovascular Surg 2014; 48: 99–105. [DOI] [PubMed] [Google Scholar]
- 13. Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation 2007; 115: 2865–2869. [DOI] [PubMed] [Google Scholar]
- 14. Desai M, Choke E, Sayers RD, Nath M, Bown MJ. Sex‐related trends in mortality after elective abdominal aortic aneurysm surgery between 2002 and 2013 at National Health Service Hospitals in England: less benefit for women compared with men. Eur Heart J 2016; 37: 3452–3460. [DOI] [PubMed] [Google Scholar]
- 15. Lo RC, Bensley RP, Hamdan AD, Wyers M, Adams JE, Schermerhorn ML; Vascular Study Group of New England . Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg 2013; 57: 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dillavou ED, Muluk SC, Makaroun MS. A decade of change in abdominal aortic aneurysm repair in the United States: have we improved outcomes equally between men and women? J Vasc Surg 2006; 43: 230–238. [DOI] [PubMed] [Google Scholar]
- 17. Vascular Society of Great Britain and Ireland. National Vascular Registry : 2014. Progress Report. https://www.vsqip.org.uk/reports/nvr‐progress‐report/ [accessed 1 March 2015]. [Google Scholar]
- 18. Sidloff DA, Gokani VJ, Stather PW, Choke E, Bown MJ, Sayers RD. National Vascular Registry report on surgical outcomes and implications for vascular centres. Br J Surg 2014; 101: 637–642. [DOI] [PubMed] [Google Scholar]
- 19. Michaels JA. Use of mortality rate after aortic surgery as a performance indicator. Br J Surg 2003; 90: 827–831. [DOI] [PubMed] [Google Scholar]
- 20. Deery SE, Soden PA, Zettervall SL, Shean KE, Bodewes TC, Pothof AB et al Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J Vasc Surg 2017; 65: 1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Egorova NN, Vouyouka AG, McKinsey JF, Faries PL, Kent KC, Moskowitz AJ et al Effect of gender on long‐term survival after abdominal aortic aneurysm repair based on results from the Medicare National Database. J Vasc Surg 2011; 54: 1–12.e6. [DOI] [PubMed] [Google Scholar]
- 22. Mehta M, Byrne WJ, Robinson H, Roddy SP, Paty PS, Kreienberg PB et al Women derive less benefit from elective endovascular aneurysm repair than men. J Vasc Surg 2012; 55: 906–913. [DOI] [PubMed] [Google Scholar]
- 23. Abedi NN, Davenport DL, Xenos E, Sorial E, Minion DJ, Endean ED. Gender and 30‐day outcome in patients undergoing endovascular aneurysm repair (EVAR): an analysis using the ACS NSQIP dataset. J Vasc Surg 2009; 50: 486–491, 491.e1–4. [DOI] [PubMed] [Google Scholar]
- 24. American College of Surgeons . ACS National Surgival Quality Improvement Program® (ASC NSQIP)®. https://www.facs.org/quality‐programs/acs‐nsqip [accessed 1 September 2016].
- 25. Wanhainen A, Lundkvist J, Bergqvist D, Björck M. Cost‐effectiveness of screening women for abdominal aortic aneurysm. J Vasc Surg 2006; 43: 908–914. [DOI] [PubMed] [Google Scholar]
- 26. Hultgren R, Vishnevskaya L, Wahlgren CM. Women with abdominal aortic aneurysms have more extensive aortic neck pathology. Ann Vasc Surg 2013; 27: 547–552. [DOI] [PubMed] [Google Scholar]
- 27. Biebl M, Hakaim AG, Hugl B, Oldenburg WA, Paz‐Fumagalli R, McKinney JM et al; Zenith Investigators. Endovascular aortic aneurysm repair with the Zenith AAA endovascular graft: does gender affect procedural success, postoperative morbidity, or early survival? Am Surg 2005; 71: 1001–1008. [PubMed] [Google Scholar]
- 28. Sampaio SM, Panneton JM, Mozes GI, Andrews JC, Noel AA, Karla M et al Endovascular abdominal aortic aneurysm repair: does gender matter? Ann Vasc Surg 2004; 18: 653–660. [DOI] [PubMed] [Google Scholar]
- 29. Abbruzzese TA, Kwolek CJ, Brewster DC, Chung TK, Kang J, Conrad MF et al Outcomes following endovascular abdominal aortic aneurysm repair (EVAR): an anatomic and device‐specific analysis. J Vasc Surg 2008; 48: 19–28. [DOI] [PubMed] [Google Scholar]
- 30. Dillavou ED, Muluk SC, Rhee RY, Tzeng E, Woody JD, Gupta N et al Does hostile neck anatomy preclude successful endovascular aortic aneurysm repair? J Vasc Surg 2003; 38: 657–663. [DOI] [PubMed] [Google Scholar]
- 31. Trial Investigators IMPROVE. Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one‐year outcomes from the IMPROVE randomized trial. Eur Heart J 2015; 36: 2061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnston KW. Influence of sex on the results of abdominal aortic aneurysm repair. Canadian Society for Vascular Surgery Aneurysm Study Group. J Vasc Surg 1994; 20: 914–923. [DOI] [PubMed] [Google Scholar]
- 33. Kaladji A, Daoudal A, Duménil A, Göksu C, Cardon A, Clochard E et al Predictive models of complications following endovascular aortic aneurysm repair. Ann Vasc Surg 2017; 40: 19–27. [DOI] [PubMed] [Google Scholar]
- 34. Goodyear SJ, Yow H, Saedon M, Shakespeare J, Hill CE, Watson D et al Risk stratification by pre‐operative cardiopulmonary exercise testing improves outcomes following elective abdominal aortic aneurysm surgery: a cohort study. Perioper Med (Lond) 2013; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karthikesalingam A, Nicoli TK, Holt PJ, Hinchliffe RJ, Pasha N, Loftus IM et al The fate of patients referred to a specialist vascular unit with large infra‐renal abdominal aortic aneurysms over a two‐year period. Eur J Vasc Endovasc Surg 2011; 42: 295–301. [DOI] [PubMed] [Google Scholar]
- 36. Ulug P, Sweeting MJ, von Allmen RS, Thompson SG, Powell JT. Women assessed for intact abdominal aortic aneurysm repair fare worse than men: systematic reviews of morphological suitability for endovascular repair, non‐intervention rates and operative mortality. Lancet 2017; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jacomelli J, Summers L, Stevenson A, Lees T, Earnshaw JJ. Impact of the first 5 years of a national abdominal aortic aneurysm screening programme. Br J Surg 2016; 103: 1125–1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 National Vascular Registry codes
Fig. S1 Flow chart of patients included from National Vascular Registry database
Table S1 Overall proportion of elective open and endovascular aneurysm repairs by sex and abdominal aortic aneurysm diameter
Table S2 In‐hospital deaths for elective open and endovascular abdominal aneurysm repair by sex and age
Table S3 Sensitivity analysis
Table S4 In‐hospital deaths for ruptured open and endovascular abdominal aortic aneurysm repair by sex and age


