Abstract
Background
The partial edentulous posterior mandible is often a challenge area that requires a bone reconstructive surgery for implants placement.
Purpose
This RCT was aimed to evaluate complications rate and vertical bone gain after Guided Bone Regeneration (GBR) with dense non‐resorbable d‐PTFE titanium‐reinforced membranes (Group A) versus titanium meshes covered by cross‐linked collagen membranes (Group B).
Material and Methods
40 partially edentulous patients with atrophic posterior mandible, were randomly divided into two study group: 20 patients were treated with one stage GBR by means of non‐resorbable d‐PTFE titanium‐reinforced membranes (Group A); and 20 patients, by means of titanium mesh covered by cross‐linked collagen membranes (Group B). All complications were recorded, distinguishing between “surgical” and “healing” and between “minor” or “major.”. Primary implants stability and vertical bone gain were also evaluated.
Results
In the group A, surgical and healing complication rates were 5.0% and 15.0%, respectively. In the group B, surgical and healing complication rates were 15.8% and 21.1%, respectively. No significant differences between two study group were observed regarding complications rate implant stability and vertical bone gain.
Conclusions
Both GBR approaches for the restoration of atrophic posterior mandible achieved similar results regarding complications, vertical bone gain and implant stability.
Keywords: alveolar ridge reconstruction, atrophy, bone augmentation, bone defects, bone regeneration, edentulous mandible, guided bone regeneration
1. INTRODUCTION
Alveolar atrophy is a pathological condition characterized by moderate or severe resorption of alveolar bone due to tooth loss.1, 2 The loss of teeth determines the loss of the functional stimulus for the alveolar bone. Consequently, the bone undergoes constant and predictable resorption, which differs depending on location: in the mandible, it is primarily horizontal; in interforaminal regions, it is centripetal; however, it is vertical and centrifugal in retroforaminal areas.3, 4 Alveolar bone resorption and the presence of the inferior alveolar nerve (IAN) make the posterior regions of the mandible the most challenging to treat using osseointegrated implants.5 Bone‐augmentation techniques proposed to increase bone volume in the posterior regions of the mandible include bone grafts (inlay and onlay), osseodistraction, transposition of the IAN, titanium mesh, and guided bone regeneration (GBR).6
In the last 10 years, short‐ and long‐term studies have demonstrated that GBR is a successful and reliable technique for vertical and horizontal ridge augmentation.5, 7, 8, 9, 10, 11 GBR can be achieved with two different approaches: application of either a polytetrafluoroethylene (PTFE) titanium‐reinforced membrane (ie, a non‐resorbable membrane) or a collagen membrane (ie, a resorbable membrane).7, 12, 13, 14, 15 To achieve vertical bone augmentation, a resorbable membrane must be supported by a space‐making device, such as a titanium mesh (Ti mesh) or a titanium osteosynthesis plate.14, 15
However, the use of a barrier device is a technique‐sensitive procedure that is not free of complications. The main cause of GBR failure is related to early or late exposure of a barrier device, leading to contamination and infection of the biomaterial, irreversibly compromising bone regeneration.16, 17, 18, 19
In this randomized clinical trial, we sought to evaluate complication rates and vertical bone gain (VBG) after GBR with dense PTFE titanium‐reinforced membranes versus with titanium mesh covered with cross‐linked collagen membranes.
2. MATERIAL AND METHODS
This study was designed as a pilot, parallel‐group, double‐blind, randomized, comparative clinical trial. The study was conducted in accordance with the principles of the Declaration of Helsinki. The study was approved by the Ethical Committee of the Sant'Orsola‐Malpighi Hospital (Prot. CMF 01/2013; number 30/2013/O/Disp).
2.1. Study design and patient selection
The study included 40 patients with partial edentulism, associated with alveolar atrophy in the posterior regions of the mandible, who were referred to the Unit of Oral and Maxillofacial Surgery, Alma Mater Studiorum, University of Bologna, Italy. The inclusion criteria were as follows: (1) edentulism in posterior regions of the mandible with vertical and horizontal bone resorption of the alveolar ridge requiring three‐dimensional bone regeneration and implant‐supported rehabilitation; (2) a vertical peri‐implant bone defect of ≥ 2 mm in the alveolar ridge that must be regenerated after placement of implants in a three‐dimensional “ideal” position; (3) capacity to understand and accept the conditions of the study; and 4 continuing participation in the study for at least 1 year of follow up.
The exclusion criteria were as follows: (1) residual bone height < 5 mm; (2) insufficient oral hygiene; (3) a smoking habit of > 10 cigarettes/day; (4) abuse of alcohol or drugs; (5) pregnancy; (6) acute local or systemic infection; (7) uncontrolled diabetes or other metabolic disease; (8) severe hepatic or renal dysfunction; (9) HIV, HBV, or HCV; (10) chemotherapy or radiotherapy within the last 5 years; (11) immunosuppression therapy; (12) autoimmune disorders; or (13) bisphosphonate therapy.
We planned to treat 40 patients; they were randomized into two study groups, depending on a previous computer‐generated randomization sequence. Group A included 20 patients treated by means of a dense PTFE (d‐PTFE) titanium‐reinforced membrane, and Group B included 20 patients treated by means of a titanium mesh (Ti mesh) and cross‐linked collagen membrane.
Patients who met the inclusion criteria were informed about the objectives and conditions of the study. Each patient received written information and provided written informed consent before participation in any study‐related procedure. After enrollment, each patient received a unique identification number, according to which all data were recorded. Patient was blinded and did not know the assigned study group.
2.2. Clinical procedures
Following selection, all patient data (ie, personal, medical, and diagnostic data) were recorded on a specific data collection form (CRF), which was completed on each day of monitoring. Prior to surgery, all patients were evaluated and treated for periodontal and dental health and received oral hygiene instructions. All patients received prophylactic antibiotic therapy with 2 g of amoxicillin and clavulanic acid (or 600 mg of clindamycin in cases of penicillin allergies) and anti‐inflammatory therapy with 100 mg of nimesulide 1 h before treatment (or 600 mg of Ibuprofen in cases of nimesulide allergies).
Surgical and prosthetic protocol
On the day of the implant and reconstructive surgery (T0), local anesthesia was administered: articaine 4% with epinephrine 1:100.000. A mid‐crestal horizontal incision within the keratinized tissue of the edentulous ridge was extended distally to the external oblique ridge of the mandibular ramus. To preserve the lingual nerve when approaching the third molar area, the incision was inclined at ∼45° in the buccal direction, using the external oblique ridge as a reference line. The mid‐crestal horizontal incision was extended mesially on the gingival sulcus of the two adjacent teeth, forming two vertical incisions on the buccal and lingual sides. Taking care to avoid flap laceration or perforation, we elevated a full‐thickness surgical flap buccally and lingually; the mental nerve was gently isolated, and the mental foramen was exposed. Because careful management of the soft tissue was important for obtaining and maintaining primary closure of the surgical flap above the area of bone regeneration, particular care was taken to passivate and release the buccal and lingual flaps in the order that each would advance coronally, as suggested by Ronda et al. in 2011 and 2015; Todisco et al in 2010 and Maiorana et al. in 2001and 2005.12, 20, 21, 22, 23
One or more tapered implants with double‐variable thread designs and a double‐acid‐etched (DAE) surface (BT SAFE; Biotec srl, Vicenza, Italy) were placed in the three‐dimensional “ideal” position to stabilize the barrier device and to maintain a three‐dimensional space under it. Implant sites were prepared according to the manufacturer's protocol, using twisted drills and/or piezoelectric inserts to complete implant sites, depending on proximity to the IAN. Subsequently, tapered implants were inserted in the corresponding sites until achieving the optimal position (ie, the implant platform was ∼3 mm apical to the gingival margin of the two adjacent teeth, independent of alveolar ridge levels). Thus, the most coronal portion of the implants was left to protrude from the alveolar ridge, showing the amount of planned vertical bone regeneration. Because the implants protruded coronally over the alveolar ridge level, the vertical bone peri‐implant defect could be measured accurately due to vertical and horizontal bone resorption.
After implant placement, the cortical bone of the mandible was perforated repeatedly to reach the bone marrow to promote the migration of osteogenic cells and osteoprogenitor stem cells and the formation of coagulum under the barrier device.
Additionally, ∼0.5–1.0 g of autogenous bone was harvested from the external oblique ridge of the mandibular ramus using a bone scraper (Safescraper, Meta, RE, Italy); grafting material for bone regeneration was prepared by mixing 50% autogenous bone and 50% bone allograft (EnCore, Osteogenics Biomedical, Lubbock, Texas).
At this point, the randomization envelope was opened, and the assigned treatment was revealed to the surgeon: a d‐PTFE titanium‐reinforced membrane (Cytoplast Ti‐250XL; Osteogenics Biomedical) was used in Group A, whereas a Ti mesh (Trinon Titanium; Karlsruhe, Germany) and cross‐linked collagen membrane (Osseoguard, Zimmer Biomet, Warsaw, Indiana) were used in Group B.
The d‐PTFE membrane or Ti mesh was modeled and adapted to maintain a three‐dimensional space for bone regeneration. First, it was fixed on the lingual side using two or more titanium mini‐screws. Then, the grafting material, consisting of a 50:50 mixture of bone allograft and autogenous bone, was carefully positioned around the implants to restore, vertically and horizontally, the peri‐implant bone defect. Finally, the d‐PTFE membrane or Ti mesh was fixed definitively on the buccal side using two or more titanium mini‐screws. The collagen membrane was applied over the Ti mesh, according to the principles of guided bone regeneration (GBR).
After evidence that the surgical flaps could advance coronally without tension to cover the augmented area, a double suture (Cytoplast PTFE suture; Osteogenics Biomedical) was used to ensure primary closure of the surgical wound. Horizontal mattress sutures were used for flap overlapping, whereas multiple interrupted sutures were used for hermetic closure of the flaps.12, 13, 20, 24, 25
Antibiotic therapy was prescribed to reduce the risk of infections: amoxicillin plus clavulanic acid (Augmentin; GlaxoSmithKline, Verona, Italy) at 3 g/day for 7 days or clindamycin (Dalacin; Pfizer srl, Italy) at 600 mg/day for 6 days (in penicillin‐allergic patients). Anti‐inflammatory therapy with NSAIDs was also recommended: nimesulide (Aulin; Helsinn Birex Pharmaceuticals Ltd., Dublin, Ireland) at 200 mg/day for 3 days and 100 mg/day for 3 more days or ibuprofen (Brufen; Gekofar, Milan, Italy) at 1800 mg/day for 3 days and 1200 mg/day for 3 more days (in nimesulide‐allergic patients).
For the first 15 days, patients were instructed to consume a fluid diet, whereas patients were instructed to consume a soft diet and to exercise good oral hygiene for the next 15 days. We also recommended three 2‐min treatments of chlorhexidine 0.2% (Curasept ADS; Curaden Healthcare srl, Varese, Italy) each day. Sutures were removed 15 days after surgery.
On the day of the reopening surgery (T1), after 9 months of submerged healing, a mid‐crestal horizontal incision within the keratinized tissue was performed without vertical incisions to remove the barrier devices and the mini‐screws, to measure the vertical peri‐implant bone gain around each implant, and to expose the submerged implants using healing screws. Moreover, a connective tissue graft was performed on the buccal side to increase the thickness of the peri‐implant soft tissues.
On the day of functional loading (T2), ∼2–3 months after reopening surgery, the implant‐supported fixed metal‐ceramic restorations were delivered to the patients. All definitive restorations were placed in occlusion, where the occlusal surface was thoroughly modeled so that it was in contact with reduced areas during laterality and protrusion excursions, to reduce the dislocating vectorial components; more contacts were maintained in maximum intercuspidation.
Figures 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 showed a clinical case of Group A. Instead Figures 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 showed a clinical case of Group B.
Figure 1.
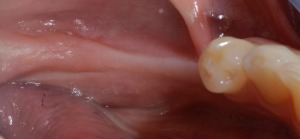
Preoperative clinical view
Figure 2.
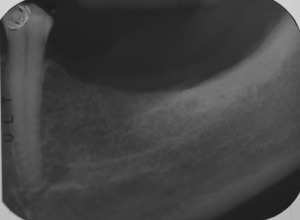
Preoperative peri‐apical x‐rays
Figure 3.
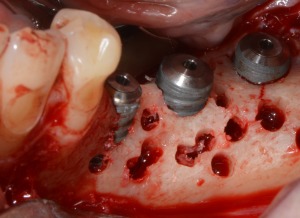
Cortical perforations and implants insertion
Figure 4.
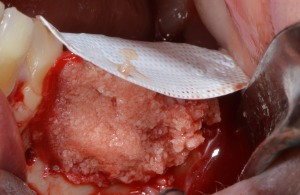
D‐PTFE membrane fixation and bone grafting with autogenous bone and bone allograft
Figure 5.
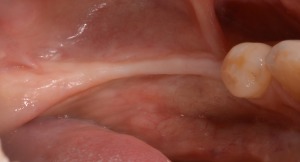
Clinical healing at 9 months
Figure 6.
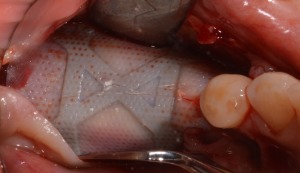
D‐PTFE membrane after 9 months
Figure 7.
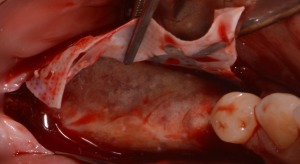
D‐PTFE membrane removal at the reopening surgery
Figure 8.
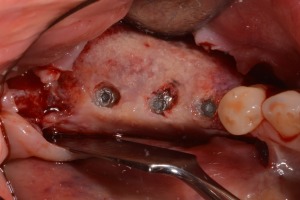
Three‐dimensional bone regeneration around implants occlusal view
Figure 9.
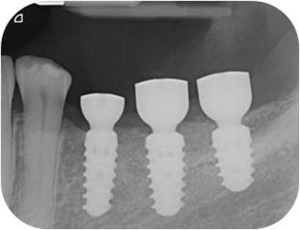
Peri‐apical x‐rays after healing screws placement
Figure 10.
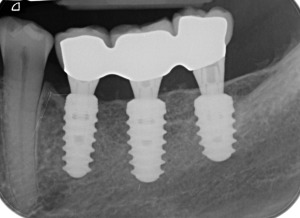
Peri‐apical x‐rays after definitive restoration
Figure 11.
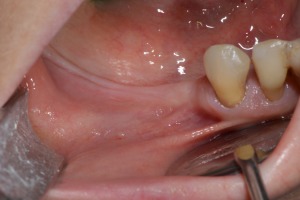
Preoperative clinical view
Figure 12.

Preoperative peri‐apical x‐rays
Figure 13.
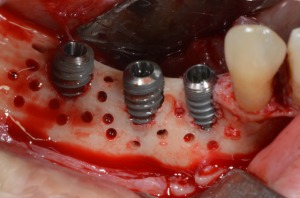
Cortical perforations and implants insertion
Figure 14.
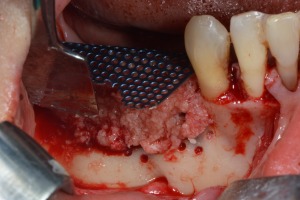
Ti‐mesh fixation and bone grafting with autogenous bone and bone allograft
Figure 15.
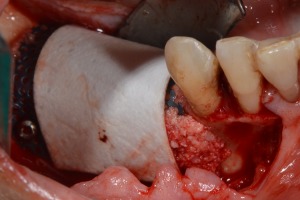
Application of collagen membrane over the Ti‐mesh
Figure 16.
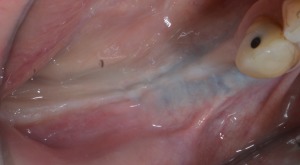
Clinical healing 9 months after surgery
Figure 17.
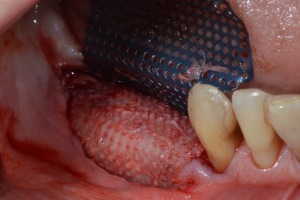
Ti‐Mesh removal at the re‐opening surgery
Figure 18.
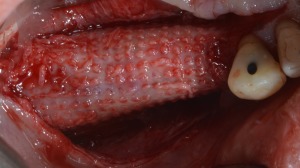
Three‐dimensional bone regeneration around implants
Figure 19.
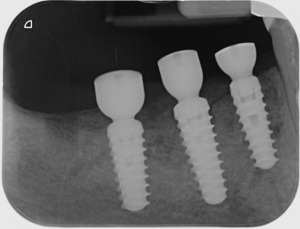
Peri‐apical x‐rays after healing screws placement
Figure 20.
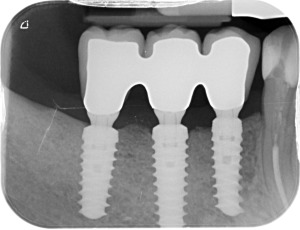
Peri‐apical x‐rays after definitive restoration
2.3. Data collection
During surgery and postoperative visits, all clinical and healing data were recorded on a specific data collection form (CRF), which was completed on each day of intervention or monitoring. All data were collected by a blinded examiner.
Implant stability
Implant stability was evaluated with two different methods: insertion torque (IT, expressed in Ncm) and resonance frequency analysis (RFA, expressed in ISQ). IT was measured with a manual dynamometric torque wrench, able to measure torques up to 100 Ncm. RFA was used to measure the implant stability quotient (ISQ) (Osstell AB, Göteborg, Sweden). These variables were evaluated for each implant in Groups A and B at T0 and T1.
Surgical and healing complications
Complications were evaluated for Groups A and B from T0 to T2. They were divided into surgical complications and healing complications, as suggested by Fontana et al. 2011.19 The former complications were classified as:
Class A, including flap damage (soft tissue perforation or laceration),
Class B, including neurological damage (paresthesia or disesthesia), and
Class C, including vascular damage (hemorrhage).
The latter complications were divided into four classes, according to the presence and extent of exposure, as well as the presence of a purulent exudate.
Class I: membrane exposure ≤ 3 mm, no purulent exudate.
Class II: membrane exposure ≥ 3 mm, no purulent exudate.
Class III: membrane exposure, with purulent exudate.
Class IV: abscess, without membrane exposure.
Healing complications were also divided into major or minor, depending on the influence on newly formed bone, according to Merli et al 2007.14
Peri‐implant bone defects and vertical bone gain
A peri‐implant bone defect was defined as the vertical distance between the top of the implant shoulder and the first visible bone‐implant contact. It was recorded during T0 to assess the initial peri‐implant bone defect (IBD) and during T1 to assess the “final” peri‐implant bone defect (FBD). These variables were assessed at four sites (mesial, distal, buccal, and lingual) around each implant using a University of North Carolina (UNC)‐15 periodontal probe with a 1‐mm graduated scale, rounding values to the nearest 0.5 mm. Implants were used as reference point for direct measurements of bone augmentation before and after surgery.
The vertical bone gain (VBG) was calculated as the difference between the FBD and IBD at the four sites (mesial, distal, buccal, and lingual) for each implant. It was expressed in millimeters (mm).
2.4. Data management and statistical analysis
Data management
We used a data collection form and data management system using Excel (Microsoft Excel 2011; Windows, ver. 14.0.0). Data were entered by a blinded operator. Before entry, data were evaluated for accuracy and completeness; logical consistency was verified, and the ranges of quantitative data were computed. Data were analyzed using the SPSS software (ver. 8.11.5; SPSS, Inc, Chicago, Illinois).
Statistical analysis
The power‐analysis showed that with a minimum of 17 patients per group (total of 34 patients), it will be possible to detect a 35% difference in complications and a difference of 1 mm in VBG between the two groups with a standard deviation σ =1 and a significance level of a = 0.05 with a power of 80%. To protect from possible drop‐outs, the sample size was increased by to 20 patients per group (total of 40 patients).
The results obtained in the two study groups (Groups A and B) were subjected to statistical description and analyses using specific tests to determine statistically significant differences between them. Both the intent‐to‐treat and per‐protocol populations were analyzed. The patient was regarded as the statistical unit of analysis for all analyses, except that of implant stability, which was carried out considering the implant as the statistical unit. Statistical differences in complication rates were investigated using Fisher's exact test. Differences in implant stability, peri‐implant bone defects, and VBG at T0 and T1 were investigated using t‐tests for unpaired data. Statistical significance was set at α = 0.05. The statistician was blinded and external to working group.
3. RESULTS
3.1. Intent‐to‐treat population
In total, 40 patients (13 males, 27 females) with a mean age of 52 years were treated according to the protocol described previously. All patients underwent bone augmentation surgery and received 108 implants to restore posterior regions of the mandible. All implants were placed simultaneously with guided bone regeneration (GBR) procedures: implant diameters were 3.7, 4.1, and 4.8 mm and implant lengths were 8, 10, and 12 mm. All implants were inserted in Type I or II bone, according to Lekholm and Zarb's classification.26
Of the 40 patients, 18 (45.0%) were classified as ASA I and 22 (50%) as ASA II. No patient with ASA III or IV status was treated. Moreover, 29 (72.5%) were non‐smokers, and 11 (27.5%) smoked fewer than 10 cigarettes per day.
Of the 40 patients, 23 (57.5%) had no periodontal disease, 12 (30.0%) had chronic periodontitis, and 5 (12.5%) had aggressive periodontitis. All periodontal patients were treated with periodontal therapy before augmentation and implant surgery.
3.2. Per‐protocol population
All 40 patients underwent implant and bone augmentation surgery (at T0).
During the healing period (T0‐T1), one patient with three implants dropped out immediately after the GBR procedure due to a car crash involving maxilla‐facial trauma. After the reopening surgery (T1‐T2), one patient with three implants dropped out and did not undergo implant restoration with a definitive prosthesis for logistical and economic reasons. Additionally, three patients with seven implants did not undergo reopening surgery because major complications occurred, and the removal of barrier devices and implants was required before T1. Consequently, 40 patients with 108 implants (20 in Group A and 20 in Group B) were considered in the statistical analyses of variables recorded at T0.
In total, 39 patients with 106 implants (20 in Group A and 19 in Group B) were considered for the analysis of those variables recorded between T0 and T1, and 36 patients with 99 implants (19 in Group A and 17 in Group B) were considered for the variables recorded at T1. Finally, 35 patients with 96 implants (18 in Group A and 17 in Group B) completed the entire study protocol with definitive restoration with implants (T2).
3.3. Primary implant stability
All implants (n = 108, 100%) were placed according to the initial implant‐prosthetic treatment planning. Of the 108 implants, 106 (97.2%) showed optimal primary stability, with insertion torque values > 35 Ncm and ISQ values > 60. The mean insertion torque was 80 ± 10 Ncm, whereas the mean RFA value was 86.5 ± 5.0 ISQ. Bone density was Type I and II at all sites.
In Group A, at T0, the mean IT was 80.0 ± 10.0 Ncm, and the mean RFA value was 87.0 ± 5.0 ISQ. In Group B, at T0, the mean IT was 79.0 ± 10.0 Ncm, and the mean RFA value was 84.5 ± 6.0 ISQ. There was no statistically significant difference between Groups A and B regarding primary implant stability (P > .05).
At reopening surgery (T1), all implants (n = 99, 100%) showed successful osseointegration after the submerged healing period. Reverse torque at 25 Ncm was used to assess adequate osseointegration. Mean RFA values at reopening surgery were 71.0 ± 8.0 ISQ and 66.5 ± 10.0 ISQ in Groups A and B, respectively.
3.4. Surgical complications
After the implant and bone augmentation surgeries (T0), four surgical complications occurred in four different patients. All these complications belonged to Class B: that is, neurological complications. No flap or vascular damage was recorded during T0. Moreover, no complication occurred in either group during T1.
In Group A, one case of temporary paresthesia of the mental nerve was observed, yielding a surgical complications rate of 5% (1/20). In Group B, the surgical complications rate was 15.8% (3/19), because three cases of temporary paresthesia of the mental nerve occurred. All neurological injuries showed spontaneous recovery during the first month after surgery.
The difference in the surgical complication rates between Groups A and B was not statistically significant (P = .34).
3.5. Healing complications
In total, 7 complications occurred in 39 patients during the healing period (T0‐T1). In Group A, three healing complications were observed, yielding an overall complication rate of 15.0% (n = 3/20). Of these, two complications (Classes III and IV) affected the amount of newly formed bone or the success of the bone augmentation surgery and were, therefore, classified as major complications. One complication (Class II) did not affect the amount of newly formed bone and was, therefore, classified as a minor complication. In Group A, the rates of major and minor healing complications were 10.0% and 5%, respectively. Table 1 provides a description of the healing complications in Group A.
Table 1.
Complication rate and type in group A
| Timing | N. patients | N. complications | Complications features | Complication class | Complications type |
|---|---|---|---|---|---|
| 0–1 month | 20 | 1 | Abscess without exposition | Class IV | Major |
| 1–3 months | 20 | 1 | Early membrane exposure with infection | Class III | Major |
| 3–6 months | 20 | 1 | Late membrane exposure without infection | Class II | Minor |
| 6–9 months | 20 | 0 | ‐ | ‐ | ‐ |
In Group B, four cases of healing complications were observed, yielding an overall complication rate of 21.1% (n = 4/19). Of these, three (Class III and IV) were classified as major complications, whereas one (Class II) was classified as a minor complication. In Group B, the rates of major and minor healing complication were 15.8% and 5.3%, respectively. Table 2 provides a description of the healing complications in Group B.
Table 2.
Complication rate and type in group B
| Timing | N. patients | N. complications | Complications features | Complication class | Complications type |
|---|---|---|---|---|---|
| 0–1 month | 19 | 2 |
‐Abscess without exposition ‐Early exposure with infection |
Class IV Class III |
Major Major |
| 1–3 months | 19 | 1 | Late exposure with infection | Class III | Major |
| 3–6 months | 19 | 1 | Late exposure without infection | Class II | Minor |
| 6–9 months | 19 | 0 | ‐ | . | . |
No statistically significant difference was observed between Groups A and B regarding the overall healing complication rates (P = .69) or the major or minor healing complication rate (P = .99).
3.6. Peri‐implant bone defect and vertical bone gain
Mean values of peri‐implant bone defects (IBD and FDB) and vertical bone gain (VBG) in the two groups are presented in Tables 3 and 4. In Group A, IBD and FBD were 3.8 ± 0.7 (range, 2.7–4.9) mm and −0.5 ± 0.6 (range, −1.2–0.4) mm, respectively. Consequently, in Group A, the VBG was 4.2 ± 1.0 (range, 2.7–5.8) mm. Table 3 reports the values of IBD, FDB, and VBG in Group A according to implant site. In Group A, 63.0% (n = 34) of the implants showed supra‐implant bone overgrowth during T1. In these cases, an osteotomy was necessary to place healing screws. Only 25.9% (n = 14) showed partial bone regeneration with the presence of a residual bone defect, which was always observed at buccal sites.
Table 3.
Peri‐implant bone defect and vertical bone gain in Group A
| Site | Peri‐implant bone defect T0 (mm) | Peri‐implant bone defect T1 (mm) | Vertical bone gain T1 (mm) |
|---|---|---|---|
| Mesial | 3.2 ± 0.9 | −0.5 ± 0.6 | 3.6 ± 1.2 |
| Lingual | 3.6 ± 0.9 | −0.6 ± 0.6 | 4.2 ± 1.2 |
| Distal | 3.5 ± 0.8 | −0.7 ± 0.6 | 4.1 ± 1.0 |
| Buccal | 4.8 ± 0.7 | −0.3 ± 1.0 | 5.0 ± 1.0 |
| Mean | 3.8 ± 0.7 | −0.5 ± 0.6 | 4.2 ± 1.0 |
Table 4.
Peri‐implant bone defect and vertical bone gain in Group B
| Site | Peri‐implant bone defect T0 (mm) | Peri‐implant bone defect T1 (mm) | Vertical bone gain T1 (mm) |
|---|---|---|---|
| Mesial | 3.1 ± 0.9 | −0.4 ± 0.7 | 3.3 ± 1.0 |
| Lingual | 3.4 ± 0.8 | −0.4 ± 0.6 | 3.8 ± 0.8 |
| Distal | 3.8 ± 1.0 | −0.4 ± 0.6 | 4.0 ± 1.0 |
| Buccal | 5.7 ± 1.6 | −0.4 ± 1.5 | 5.1 ± 1.4 |
| Mean | 4.0 ± 0.8 | −0.2 ± 0.7 | 4.1 ± 1.0 |
In Group B, the mean values of IBD (T0) and FBD (T1) were 4.0 ± 0.8 (range, 2.9–5.4) mm and −0.2 ± 0.7 (range, −1.0–1.3) mm, respectively. Consequently, in Group B, VBG was 4.1 ± 1.0 (range, 2.6–6.3) mm. Table 4 presents the values of IBD, FDB, and VBG in Group B according to implant site. In Group B, 61.4% (n = 27) of the implants showed supra‐implant bone overgrowth. Osteotomies were performed in these individuals to place healing screws. Only 22.7% (n = 10) showed partial bone regeneration with the presence of a residual bone defect at the buccal site. No statistically significant difference was observed between the groups in IBD, FBD, or VBG parameters (P = .29, .20, and .58, respectively).
4. DISCUSSION
Many studies have demonstrated high success rates with dental implants in terms of both function and esthetics.27, 28, 29, 30, 31 However, edentulous areas are often characterized by alveolar bone atrophy due to the continued bone resorption that occurs after tooth loss.1, 2, 4
Although many studies have confirmed the reliability of short and ultrashort implants in atrophic mandibles and maxillae,32, 33 a three‐dimensional reconstruction of alveolar bone is often required to provide an esthetic and functional restoration.34, 35 It was reported that the mean vertical dimensions of a partially edentulous posterior mandible from the IAN to the edge of the bone crest were 8.7 ± 5.6 mm and 9.2 ± 3.8 mm for the first and second molar areas, respectively.36. The horizontal dimensions were 6.6 ± 3.0 mm and 6.9 ± 3.2 for the first and second molar areas, respectively.37 Because the presence of the inferior alveolar nerve requires a “safety zone” of at least 1–2 mm38 and the resorption pattern in the retroforaminal area is vertical and centrifugal,3, 4 most long‐term edentulous patients do not have adequate bone volumes to place standard or short implants.36, 37, 39 Consequently, the posterior mandible is a challenging area that typically requires bone augmentation during or before implant surgery.35
In this study, guided bone regeneration (GBR) using d‐PTFE Ti‐reinforced membranes or Ti mesh covered with collagen membranes was used to achieve an adequate implant‐prosthetic restoration. Because this procedure is still considered to be highly technique‐sensitive and not free of complications that can affect the amount of newly formed bone and the success rate of the treatment, Fontana et al. in 201119 suggested a classification of surgical and healing complications and recommended that these be managed differently according to class.
To date, there have been many reports regarding complication rates, vertical bone gain (VBG), and success rates after bone augmentation with PTFE membranes and Ti mesh.40, 41 However, there have not been randomized clinical studies comparing the clinical outcomes of these two approaches.
If a membrane or mesh remains submerged completely for at least 6–9 months of uneventful healing, it is possible to achieve complete bone formation under the barrier device. However, if the membrane or mesh suffers early or late exposure, the amount of newly formed bone under the barrier could be affected negatively. Consequences of barrier exposure range from incomplete bone growth to failure of the entire regenerative surgery.19
The main cause of GBR failure is related to early or late exposure of the barrier device, leading to contamination and infection of the biomaterials, irreversibly compromising bone regeneration.16, 17, 18, 42, 43, 44 Other complications, such as the onset of an abscess with purulent exudate, can also lead to a complete failure of GBR even without exposure of the membrane.19
In this study, healing complications in Group A were limited to one case of early membrane exposure with infection, one abscess without exposure, and one case of late membrane exposure without infection. The first two were considered major complications because they resulted in the failure of the entire regenerative and implant surgery: all implants, all graft biomaterials, and PFTE membranes were removed to resolve the infection. No loss of pre‐existing bone occurred. The latter involved no infection, and the newly formed bone was not affected. As reported by others, even major complications in GBR procedures, unlike other bone augmentation techniques, typically do not affect the initial bone volume 48–49. In Group B, one case of abscess without exposure, one early exposure with infection, one late exposure with infection, and one late exposure without infection were observed. Only the latter complication was considered a minor complication because the others markedly affected the newly formed bone. In two cases, all implants, all graft biomaterials, and the PFTE membranes were removed; however, in the case of late exposure with infection, only the Ti mesh was removed, which maintained all implants in situ, and the restoration was completed with a definitive prosthesis.
Considering the types of complications, major complications were seen in 10% and 15.8% for d‐PTFE membranes and Ti mesh, respectively; no statistically significant difference was observed between the groups. The complications that occurred in this study are similar to those reported in the literature describing barrier device exposure and infection as frequent problems associated with this technique.5, 40, 41, 43, 44, 45
In particular, there have been reports of a range of barrier device exposures: between 0% and 45%5 and between 12% and 50%41, 45 for PTFE membranes and Ti mesh, respectively. The results of this study are close to those reported by Simion et al. and Roccuzzo et al.,40, 41 who reported rates of exposure of 12% and 22% for PTFE and Ti mesh, respectively.
The significance of membrane exposure for the successful outcome of GBR procedures has been much debated. Several studies have shown that the onset of exposure is important for the success of the procedure. In fact, some authors have demonstrated that “premature” exposure (during the first 4 postoperative weeks) of the barrier device can affect new bone formation more than can late exposure.16, 46, 47 In this study, device exposure during the first 4 postoperative weeks in both groups resulted in incomplete bone regeneration or total failure of the regenerative procedures. In contrast, late exposure of the barrier devices did not influence bone regeneration in either group.
In terms of Ti mesh, a positive association of time and extent of device exposure with newly formed bone resorption has been established.44 In the case of Ti mesh exposure, the lack of bone volume was significantly positively correlated with the area of mesh exposed, with a 16.3% deficit in bone volume for every cm2 of mesh exposed. Additionally, there were positive associations of the lack of bone volume and early exposure with planned bone volume.44
In this study, the surgical complications involved one case of temporary paresthesia for d‐PTFE membranes and three for Ti mesh plus collagen membranes, yielding rates of 5% and 15.8%, respectively. This difference was not statistically significant. All surgical complications belonged to Class B19 and recovered within 1 month after GBR surgery. These nerve injuries were probably caused by stretching of the mental nerve during flap passivation. Moreover, this class of complication is often related to the need for extensive flap management to achieve a tension‐free closure over the barrier device.12, 13, 19 Another cause of nerve injuries involves management of the barrier device: during bone augmentation surgery, d‐PTFE membranes were shaped, adapted, and stabilized more readily and quickly than were the Ti mesh devices. Additionally, during the reopening surgery, Ti mesh required more time and more effort than did the non‐resorbable membranes. Thus, the latter showed better manageability and ease of use and required less dexterity and fewer manual skills.
Regarding VBG, the effectiveness of GBR for the resolution of vertical and horizontal bone defects has been demonstrated in short‐ and long‐term studies.5, 19, 43, 48 We showed mean bone gains of 4.2 and 4.1 mm for d‐PTFE membranes and Ti mesh plus collagen membranes, respectively. There were no significant statistically differences, confirming the effectiveness of both devices for bone regeneration in the posterior atrophic mandible.
Additionally, in both groups, a low percentage (< 20%) of partial bone regeneration with a residual peri‐implant bone defect was recorded. The findings presented here are similar to reports in the literature. Simion et al. in 2007,25 reported a mean VBG with PTFE membranes of 3.85 mm. These results were confirmed by the systematic review performed by Milinkovic e Cordaro in 2014,49 which reported an average VBG of 3.04 mm with a one‐stage GBR approach with non‐resorbable membranes. The mean complication rate was 13.1%, and these cases were usually related to membrane exposure.49 Similarly, in 2004, Roccuzzo et al.50 reported a mean VBG of 4.8 (range, 4–7) mm after Ti mesh reconstructive surgery. Ricci et al.,51 reported a mean VBG of 4.16 ± 0.59 mm, with a healing complication rate of 22.8% for the Ti mesh approach.51 In this study, a one‐stage reconstructive approach was used in both groups.
Regarding the timing of implant placement, there is evidence to support the use of GBR at the time of implant placement and before implant placement when a peri‐implant bone defect is present.7, 12, 13, 20, 25, 41, 51 Additionally, some reports comparing one‐stage and two‐stage approaches did not detect any statistically significant differences in VBG, with values of 4.5 mm in both.43, 51, 52 When a one‐stage procedure is performed, high primary implant stability is required. In this study, implants in both groups showed high IT and RFA torque values, probably due to the implant characteristics: specifically, the double‐variable thread design. In atrophic posterior mandibles, the stabilization of implants can be difficult because of the reduced bone volume available above the mandibular canal.
Implant placement occurred simultaneously with bone augmentation, whereas and functional loading was delayed until 3–4 months after reopening surgery.
Loading was scheduled to occur after the soft tissues had completely healed following the connective tissue graft53 and was timed to progressively modify the occlusal and lateral contacts of the definitive prosthesis.12, 13, 20 Some authors have recommended delayed implant placement to allow better osseointegration and bone maturation.25 Others have suggested delaying implant placement and then immediately loading the implants after bone augmentation.21 Importantly, all “lost” implants were removed because of GBR failures. No implant placed using a simultaneous approach failed to osseointegrate.
Formation of an abscess without barrier device exposure in the surgical area generally leads to the total failure of a GBR procedure and the consequent failure of the implants. Removal of the barrier device with complete curettage of the area and systemic antibiotic therapy is immediately required.19 Consistent with the literature,19 in this study, abscess formation in both groups occurred during the first postoperative month, probably due to bacterial contamination of the barrier device during membrane handling or bacterial contamination of the graft. Regarding vertical bone augmentation procedures, the stability of the regenerated bone has been debated. Some authors have demonstrated that regenerated bone responds favorably under functional loading and shows stability over time.7, 12, 46, 54, 55, 56, 57 The preliminary results of this study do not permit us to draw a conclusion in this regard. A longer follow‐up period after the definitive loading is needed to observe any significant difference between d‐PTFE membranes and Ti mesh plus collagen membranes with regard to peri‐implant bone stability and crestal bone loss.
5. CONCLUSIONS
The preliminary results of this randomized controlled trial showed that d‐PTFE membranes and titanium mesh plus collagen membranes produced similar results in terms of healing complication types and rates. In contrast, d‐PTFE membranes showed a lower rate of surgical complications. In both groups, similar vertical bone gain (VBG) and bone formation were achieved, with no complications occurring, confirming the reliability and effectiveness of guided bone regeneration (GBR) for restoring the atrophic posterior mandible. Finally, the use of double‐variable tapered implants seemed to be reliable in both immediate GBR approaches.
ACKNOWLEDGMENTS
The authors are grateful to Clarisciences for the descriptive and analytical statistical analyses. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/DtZXwp
CONFLICT OF INTEREST
None
Cucchi A, Vignudelli E, Napolitano A, Marchetti C, Corinaldesi G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non‐resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin Implant Dent Relat Res. 2017;19:821–832. https://doi.org/10.1111/cid.12520
The copyright line in this article was changed on 3 October 2017 after online publication.
REFERENCES
- 1. Atwood DA. Reduction of residual ridges: a major oral disease entità. J Prosthet Dent. 1971;26:266–279. [DOI] [PubMed] [Google Scholar]
- 2. Atwood DA. Bone loss of edentulous alveolar ridges. J Periodontol. 1979;50:11–21. [DOI] [PubMed] [Google Scholar]
- 3. Misch CE, Judy KW. Classification of partially edentulous arches for implant dentistry. Int J Oral Implantol. 1987;4:7–12. [PubMed] [Google Scholar]
- 4. Cawood J, Howell RA. classification of the edentulous jaw. Int J Oral Maxillofac Surg. 1988;17:232–236. [DOI] [PubMed] [Google Scholar]
- 5. Rocchietta I, Fontana F, Simion M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: a systematic review. J Clin Periodontol. 2008;35:203–215. [DOI] [PubMed] [Google Scholar]
- 6. Corbella S, Taschieri S, Del Fabbro M. Long‐term outcomes for the treatment of atrophic posterior mandible: a systematic review of literature. Clin Implant Dent Relat Res. 2015;17:120–132. [DOI] [PubMed] [Google Scholar]
- 7. Simion M, Jovanovic SA, Tinti C, Benfenati SP. Long‐term evaluation of osseointegrated implants inserted at the time or after vertical ridge augmentation. A retrospective study on 123 implants with 1–5 year follow‐up. Clin Oral Implants Res. 2001;12:35–45. [DOI] [PubMed] [Google Scholar]
- 8. Canullo L, Malagnino VA. Vertical ridge augmentation around implants by e‐PTFE titanium‐reinforced membrane and bovine bone matrix: a 24‐ to 54‐month study of 10 consecutive cases. Int J Oral Maxillofac Implants. 2008;23:858–866. [PubMed] [Google Scholar]
- 9. Fontana F, Santoro F, Maiorana C, Iezzi G, Piattelli A, Simion M. Clinical and histological evaluation of allogeneic bone matrix versus autogenous bone chips associated with titanium‐reinforced e‐PTFE membrane for vertical ridge augmentation: a prospective pilot study. Int J Oral Maxillofac Implants. 2008;23:1003–1012. [PubMed] [Google Scholar]
- 10. Canullo L, Sisti A. Early implant loading after vertical ridge augmentation (VRA) using e‐PTFE titanium‐reinforced membrane and nano‐structured hydroxyapatite: 2‐year prospective study. Eur J Oral Implantol. 2010;S3:59–69. [PubMed] [Google Scholar]
- 11. Clementini M, Morlupi A, Canullo L, Agrestini C, Barlattani A. Success rate of dental implants inserted in horizontal and vertical guided bone regenerated areas: a systematic review. Int J Oral Maxillofac Surg. 2012;41:847–852. [DOI] [PubMed] [Google Scholar]
- 12. Ronda M, Stacchi C. Management of a coronally advanced lingual flap in regenerative osseous surgery: a case series introducing a novel technique. Int J Periodontics Restorative Dent. 2011;31:505–513. [PubMed] [Google Scholar]
- 13. Ronda M, Rebaudi A, Torelli L, Stacchi C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: a prospective randomized controlled clinical trial. Clin Oral Implants Res. 2014;25:859–866. [DOI] [PubMed] [Google Scholar]
- 14. Merli M, Migani M, Esposito M. Vertical ridge augmentation with autogenous bone grafts: resorbable barriers supported by ostheosynthesis plates versus titanium‐reinforced barriers. A preliminary report of a blinded, randomized controlled clinical trial. Int J Oral Maxillofac Implants. 2007;22:373–382. [PubMed] [Google Scholar]
- 15. Merli M, Moscatelli M, Mariotti G, Rotundo R, Bernardelli F, Nieri M. Bone level variation after vertical ridge augmentation: resorbable barriers versus titanium‐reinforced barriers. A 6‐year double‐blind randomized clinical trial. Int J Oral Maxillofac Implants. 2014;29:905–913. [DOI] [PubMed] [Google Scholar]
- 16. Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta‐analysis. J Periodontol. 2001;72:512–516. [DOI] [PubMed] [Google Scholar]
- 17. Verardi S, Simion M. Management of the exposure of e‐PTFE membranes in guided bone regeneration. Pract Proced Aesthet Dent. 2007;19:111–117. [PubMed] [Google Scholar]
- 18. Esposito M, Grusovin MG, Kwan S, Worthington HV, Coulthard P. Interventions for replacing missing teeth: bone augmentation techniques for dental implant treatment. Cochrane Database Syst Rev. 2008;16:CD003607 [DOI] [PubMed] [Google Scholar]
- 19. Fontana F, Maschera E, Rocchietta I, Simion M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int J Periodontics Restorative Dent. 2011;31:265–273. [PubMed] [Google Scholar]
- 20. Ronda M, Stacchi C. A novel approach for the coronal advancement of the buccal flap. Int J Periodontics Restorative Dent. 2015;35:795–801. [DOI] [PubMed] [Google Scholar]
- 21. Todisco M. Early loading of implants in vertically augmented bone with non‐resorbable membranes and deproteinised anorganic bovine bone. An uncontrolled prospective cohort study. Eur J Oral Implantol. 2010;3:47–58. [PubMed] [Google Scholar]
- 22. Maiorana C, Santoro F, Rabagliati M, Salina S. Evaluation of the use of iliac cancellous bone and anorganic bovine bone in the reconstruction of the atrophic maxilla with titanium mesh: a clinical and histologic investigation. Int J Oral Maxillofac Implants. 2001;16(3):427–432. [PubMed] [Google Scholar]
- 23. Maiorana C, Beretta M, Salina S, Santoro F. Reduction of autogenous bone graft resorption by means of bio‐oss coverage: a prospective study. Int J Periodontics Restorative Dent. 2005;25:19–25. [PubMed] [Google Scholar]
- 24. Tinti C, Parma‐Benfenati S. Vertical ridge augmentation: surgical protocol and retrospective evaluation of 48 consecutively inserted implants. Int J Periodontics Restorative Dent. 1998;18:434–443. [PubMed] [Google Scholar]
- 25. Simion M, Fontana F, Rasperini G, Maiorana C. Vertical ridge augmentation by expanded‐polytetrafluoroethylene membrane and a combination of intraoral autogenous bone graft and deproteinized anorganic bovine bone (Bio Oss). Clin Oral Implants Res. 2007;18:620–629. [DOI] [PubMed] [Google Scholar]
- 26. Lekholm U, Zarb GA. Patient selection and preparation In: Brånemark PI, Zarb GA, Albrektsson T, eds. Tissue‐Integrated Prostheses: Osseointegration in Clinical Dentistry. Chicago: Quintessence; 1985:199–209. [Google Scholar]
- 27. Adell R, Eriksson B, Leckholm U. A long‐term follow‐up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990;5:347–359. [PubMed] [Google Scholar]
- 28. Buser D, Ingimarsson S, Dula K, Lussi A, Hirt HP, Belser UC. Long‐term stability of osseointegrated implants in augmented bone: a 5‐year prospective study in partially edentulous patients. Int J Periodont Restor Dent. 2002;22:109–117. [PubMed] [Google Scholar]
- 29. Buser D, Janner SF, Wittneben JG, Brägger U, Ramseier CA, Salvi GE. 10‐year survival and success rates of 511 titanium implants with a sandblasted and acid‐etched surface: a retrospective study in 303 partially edentulous patients. Clin Implant Dent Relat Res. 2012;14:839–851. [DOI] [PubMed] [Google Scholar]
- 30. Albrektsson T, Zarb G, Worthington PMD, Eriksson AR. The long term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11–25. [PubMed] [Google Scholar]
- 31. Lekholm U, Gunne J, Henry P, et al. Survival of the Brånemark implant in partially edentulous jaws: a 10‐year prospective multi center study. J Oral Maxillofac Implants. 1999;14:639–645. [PubMed] [Google Scholar]
- 32. De Santis D, Cucchi A, Rigoni G, Longhi C. Short implants with oxidized surface in posterior areas of atrophic jaws: 3‐ to 5‐year results of a multicenter study. Clin Implant Dent Relat Res. 2015;17:442–452. [DOI] [PubMed] [Google Scholar]
- 33. Malchiodi L, Cucchi A, Ghensi P, Consonni D, Nocini PF. Influence of crown‐implant ratio on implant success rates and crestal bone levels: a 36‐month follow‐up prospective study. Clin Oral Implants Res. 2014;25:240–251. [DOI] [PubMed] [Google Scholar]
- 34. Belser U, Buser D, Higginbottom F. Consensus statements and recommended clinical procedures regarding esthetics in implant dentistry. Int J Oral Maxillofac Implants. 2004;19:73–74. [PubMed] [Google Scholar]
- 35. Merli M, Moscatelli M, Mariotti G, Pagliaro U, Raffaelli E, Nieri M. Comparing membranes and bone substitutes in a one‐stage procedure for horizontal bone augmentation. A double‐blind randomised controlled trial. Eur J Oral Implantol. 2015;8:271–281. [PubMed] [Google Scholar]
- 36. Guler AU, Sumer M, Sumer P, Bicer I. The evaluation of vertical heights of maxillary and mandibular bones and the location of anatomic landmarks in panoramic radiographs of edentulous patients for implant dentistry. J Oral Rehabil. 2005;32:741–746. [DOI] [PubMed] [Google Scholar]
- 37. Juodzbalys G, Raustia AM. Accuracy of clinical and radiological classification of the jawbone anatomy for implantation—a survey of 374 patients. J Oral Implantol. 2004;30:30–39. [DOI] [PubMed] [Google Scholar]
- 38. Greenstein G, Tarnow D. The mental foramen and nerve: clinical and anatomical factors related to dental implant placement: a literature review. J Periodontol. 2006;77:1933–1943. [DOI] [PubMed] [Google Scholar]
- 39. Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res. 2006;17:136–159. [DOI] [PubMed] [Google Scholar]
- 40. Roccuzzo M, Ramieri G, Bunino M, Berrone S. Autogenous bone graft alone or associated with titanium mesh for vertical alveolar ridge augmentation: a controlled clinical trial. Clin Oral Implants Res. 2007;18:286–294. [DOI] [PubMed] [Google Scholar]
- 41. Simion M, Fontana F, Rasperini G, Maiorana C. Long‐term evaluation of osseointegrated implants placed in sites augmented with sinus floor elevation associated with vertical ridge augmentation: a retrospective study of 38 consecutive implants with 1‐ to 7‐year follow‐up. Int J Periodont Restor Dent. 2004;24:208–221. [PubMed] [Google Scholar]
- 42. Merli M, Moscatelli M, Mazzoni A, et al. Fence technique: guided bone regeneration for extensive three‐dimensional augmentation. Int J Periodont Restor Dent. 2013;33:129–136. [DOI] [PubMed] [Google Scholar]
- 43. Corinaldesi G, Pieri F, Sapigni L, Marchetti C. Evaluation of survival and success rates of dental implants placed at the time of or after alveolar ridge augmentation with an autogenous mandibular bone graft and titanium mesh: a 3‐ to 8‐year retrospective study. Int J Oral Maxillofac Implants. 2009;24:1119–1128. [PubMed] [Google Scholar]
- 44. Lizio G, Corinaldesi G, Marchetti C. Alveolar ridge reconstruction with titanium mesh: a three‐dimensional evaluation of factors affecting bone augmentation. Int J Oral Maxillofac Implants. 2014;29:1354–1363. [DOI] [PubMed] [Google Scholar]
- 45. von Arx T, Hardt N, Wallkamm B. The TIME technique: a new method for localized alveolar ridge augmentation prior to placement of dental implants. Int J Oral Maxillofac Implants. 1996;11:387–394. [PubMed] [Google Scholar]
- 46. Simion M, Baldoni M, Rossi P, Zaffe D. A comparative study of the effectiveness of e‐PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent. 1994;14:166–180. [PubMed] [Google Scholar]
- 47. Moses O, Pitaru S, Artzi Z, Nemcovsky CE. Healing of dehiscence‐ type defects in implants placed together with different barrier membranes: a comparative clinical study. Clin Oral Implants Res. 2005;16:210–219. [DOI] [PubMed] [Google Scholar]
- 48. Merli M, Merli I, Raffaelli E, Pagliaro U, Nastri L, Nieri M. Bone augmentation at implant dehiscences and fenestrations. A systematic review of randomised controlled trials. Eur J Oral Implantol. 2016;9:11–32. [PubMed] [Google Scholar]
- 49. Milinkovic I, Cordaro L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int J Oral Maxillofac Surg. 2014;43:606–625. [DOI] [PubMed] [Google Scholar]
- 50. Roccuzzo M, Ramieri G, Spada MC, Bianchi SD, Berrone S. Vertical alveolar ridge augmentation by means of a titanium mesh and autogenous bone grafts. Clin Oral Implants Res. 2004;15:73–81. [DOI] [PubMed] [Google Scholar]
- 51. Ricci L, Perrotti V, Ravera L, Scarano A, Piattelli A, Iezzi G. Rehabilitation of deficient alveolar ridges using titanium grids before and simultaneously with implant placement: a systematic review. J Periodontol. 2013;84:1234–1242. [DOI] [PubMed] [Google Scholar]
- 52. Pieri F, Corinaldesi G, Fini M, Aldini NN, Giardino R, Marchetti C. Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: a 2‐year prospective study. J Periodontol. 2008;79:2093–2103. [DOI] [PubMed] [Google Scholar]
- 53. Zucchelli G, Mazzotti C, Mounssif I, Mele M, Stefanini M, Montebugnoli L. A novel surgical–prosthetic approach for soft tissue dehiscence coverage around single implant. Clin Oral Impl Res. 2013;24:957–962. [DOI] [PubMed] [Google Scholar]
- 54. Buser D, Dula K, Belser U, Hirt HP, Berthold H. Localized ridge augmentation using guided bone regeneration. 1. Surgical procedure in the maxilla. Int J Periodontics Restorative Dent. 1993;13:29–45. [PubMed] [Google Scholar]
- 55. Buser D, Dula K, Hirt HP, Schenk RK. Lateral ridge augmentation using autografts and barrier membranes: A clinical study with 40 partially edentulous patients. J Oral Maxillofac Surg. 1996;54:420–432. [DOI] [PubMed] [Google Scholar]
- 56. Jovanovic SA, Spiekermann H, Richter EJ. Bone regeneration around titanium dental implants in dehisced defect sites: a clinical study. Int J Oral Maxillofac Implants. 1992;7:233–245. [PubMed] [Google Scholar]
- 57. Scarano A, Carinci F, Assenza B, Piattelli M, Murmura G, Piattelli A. Vertical ridge augmentation of atrophic posterior mandible using an inlay technique with a xenograft without miniscrews and miniplates: case series. Clin Oral Implant Res. 2011;22:1125–1130. [DOI] [PubMed] [Google Scholar]


