Abstract
Several different strategies are effective for medical treatment of motor problems in Parkinson's disease (PD). Many guidelines and evidence‐based reviews are available, but there is no documentation or consensus in favor of just one treatment strategy. This review presents two algorithms that may be helpful when deciding how to treat a PD patient at various stages of the disease. The first algorithm suggests one way to treat PD from the first onset of motor symptoms. It is largely based on treatment recommendations from the Scandinavian countries and Germany. The other algorithm is meant as assistance for choosing among the different device‐aided treatments for advanced PD. There is not sufficient comparative data to recommend one particular line of treatment, neither in early PD nor in advanced disease with motor complications. Individualized treatment is needed for each patient. The current algorithms only represent an alternative for aiding treatment decisions.
Keywords: apomorphine, deep brain stimulation, levodopa‐carbidopa intestinal gel, motor complications, Parkinson's disease, treatment
1. INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative disorder causing cell death in dopaminergic neurons in the Substantia nigra, but also in neurons in several other brain stem nuclei and in the cerebral cortex.1 Typically, the patients may have a combination of motor and non‐motor symptoms even when PD is first diagnosed.2 Typical motor problems used to make a clinical diagnosis are hypokinesia/bradykinesia with resting tremor and/or rigidity. With time, postural changes and especially gait‐related problems may often be troublesome.3 Dopaminergic treatment can alleviate motor symptoms for some time, but motor fluctuations may appear even at early stages of the disease.4 Therefore, most treatment strategies also aim at delaying the evolvement of motor fluctuations as long as possible. Various treatment recommendations and guidelines are available, from individual authors and from national and international associations and specialist groups.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Some of these are evidence based, like the recently published national guidelines from Germany and Sweden,18, 19 while many others are based on consensus in expert groups. The guidelines differ on various points, and many questions are left open. Especially, for many treatment options there are not sufficient comparative data to allow for evidence‐based recommendations of only one alternative.
This review presents one possible strategy for medical treatment of motor problems in PD. It is written with special reference to the treatment recommendations from the Scandinavian countries (Denmark,14 Norway,16 Sweden 19) and Germany,18 but represent the personal view of the authors. Also non‐motor symptoms represent a major health problem for many PD patients.20 Some of these symptoms may respond to standard PD therapies, while more specific treatments are needed for many others. We have not included non‐motor treatments in this review because such therapies are under investigation, but so far less documented in PD patients.21
2. INITIAL TREATMENT
Unfortunately, no effective neuroprotective treatment for PD is available today. The DATATOP study22 found that the use of selegiline delays the need for levodopa in early PD, but most investigators have interpreted this as a symptomatic effect caused by MAO‐B inhibition that delays dopaminergic elimination from dopaminergic synapses. However, two randomized double‐blind long‐term Scandinavian studies comparing selegiline to levodopa in early PD found that patients receiving placebo needed progressively higher doses of levodopa than patients receiving selegiline.23, 24 This progressive increase in difference between the groups is difficult to explain only by symptomatic mechanisms, and a disease‐modifying effect was proposed in both studies, but has not been widely accepted internationally. Furthermore, the results of the ADAGIO study, which was a double‐blind delayed‐start trial of rasagiline in early PD, indicated a disease‐modifying effect of a dose of 1 mg per day, but not of 2 mg.25 Like selegiline, rasagiline is a MAO‐B inhibitor with symptomatic effect in PD. The interpretation of the ADAGIO results has been questioned,26, 27 and there is no international consensus to use rasagiline as a neuroprotective drug. The open‐label 3‐year ADAGIO follow‐up study failed to demonstrate long‐term benefits of early‐start rasagiline treatment.28 In spite of this, because these MAO‐B inhibitors have a definite, but small symptomatic effect, and because a potential disease‐modifying effect has not been entirely excluded, we recommend starting treatment with either selegiline 10 mg per day or rasagiline 1 mg per day once the diagnosis has been made.16 However, for older patients (eg above 75 years of age) MAO‐B inhibitors may not be indicated because of a shorter life expectancy (not time enough time to benefit from a slight, possible effect on disease progression) and a potentially increased risk of adverse effects. Both the new German and Swedish guidelines give MAO‐B inhibitor treatment high priority as one of several options for initial treatment,18, 19 but the German guidelines stress that a possible disease‐modifying effect of MAO‐B inhibitors is still unclear. They recommend that these medications should not be introduced if possible disease modification is the sole indication.18
3. SYMPTOMATIC TREATMENT
Dopaminergic drugs (levodopa and dopamine agonists) are the most effective symptomatic treatments. We have no reason to believe that withholding dopaminergic treatment is beneficial. Therefore, we recommend that such treatment should be started when the patient experiences bothersome symptoms.14, 16, 18, 19 However, how the symptomatic treatment should be started is less clear. The evidence‐based German and Swedish guidelines have given high priority to three different options of early symptomatic treatment—levodopa, dopamine agonist and MAO‐B inhibitor,18, 19 with a somewhat lower priority to early combination treatment with MAO‐B inhibitor and levodopa.19 There is no doubt that levodopa is the most potent oral drug, but most patients on levodopa develop motor fluctuations over time, at least with daily doses of 600 mg and above.29 Motor fluctuations are much less common on dopamine agonist monotherapy, and compared to levodopa monotherapy, the occurrence of motor fluctuations seems to be delayed when treatment is started with a dopamine agonist and levodopa is added only when more potent treatment is needed.30, 31 However, no studies have assessed whether delayed addition of levodopa is better than an early combination of dopamine agonist and low doses of levodopa. It is also unclear whether initiation of the therapy with a dopamine agonist gives a long‐term benefit concerning development of dyskinesias, lasting also after levodopa has been added to the therapy. A recent long‐term open‐label study found very small, but persistent benefits in patient‐rated mobility scores when PD treatment was initiated with levodopa compared with dopamine agonist or MAO‐B inhibitor.32
The use of dopamine agonists may also be somewhat limited by the side effects. Hallucinations, somnolence and leg edema are more common with some agonists than with levodopa,30, 31 and ergot derivative dopamine agonists may cause retroperitoneal, pleuropulmonary and heart valve fibrosis.33 In addition, there has been increasing concern about impulse control disorder (ICD), such as gambling, compulsive sexual behavior, compulsive buying and/or binge‐eating. In a large cross‐sectional study, Weintraub et al.34 identified ICD in 17.9% of PD patients taking a dopamine agonist, but only in 6.9% of other PD patients. Other investigators reported that as much as 39% of PD patients using agonists fulfilled clinical criteria for ICD,35 while a recent study found that, compared to controls, the odds ratios of having impulsive or compulsive behaviors were 7.4 in PD patients taking dopamine agonist without levodopa, 4.6 in those treated with both dopamine agonist and levodopa, and 1.2 those taking levodopa alone.36 Even though most of these patients and caregivers do not consider ICD as a serious problem, the risk of ICD must be considered each time when deciding how to start dopaminergic treatment. It appears that ICD is somewhat more common on oral pramipexole and ropinirole than on transdermal rotigotine.35, 37
Dopamine agonists should be used with some caution, but delaying motor complications is considered so important that the treatment recommendations suggest starting symptomatic treatment with a non‐ergot dopamine agonist, at least in “younger” patients (eg age <70‐75 years, ie patients with a life expectancy long enough to have a high risk of developing motor fluctuations on levodopa).14, 16 However, because levodopa is the most potent option for symptomatic treatment, it is possible that an early combination of a non‐ergot dopamine agonist and a low dose of levodopa (up to 3‐400 mg/day) will prove to be an even better strategy. Levodopa is the only drug with highest priority for initial treatment in the new Swedish guidelines,19 but the German recommendation is to use the lowest possible effective dose.18
If the initial symptomatic therapy over time shows to be insufficient, it is recommended to increase the dose, whether treatment was started with a dopamine agonist or with levodopa. If satisfactory effect is still not achieved with one dopaminergic drug, levodopa‐agonist combination therapy is recommended.14, 15, 16 Based on the discussion above, an earlier introduction of combination therapy may be beneficial. An algorithm showing a possible strategy for treating PD is shown in Figure 1.
Figure 1.
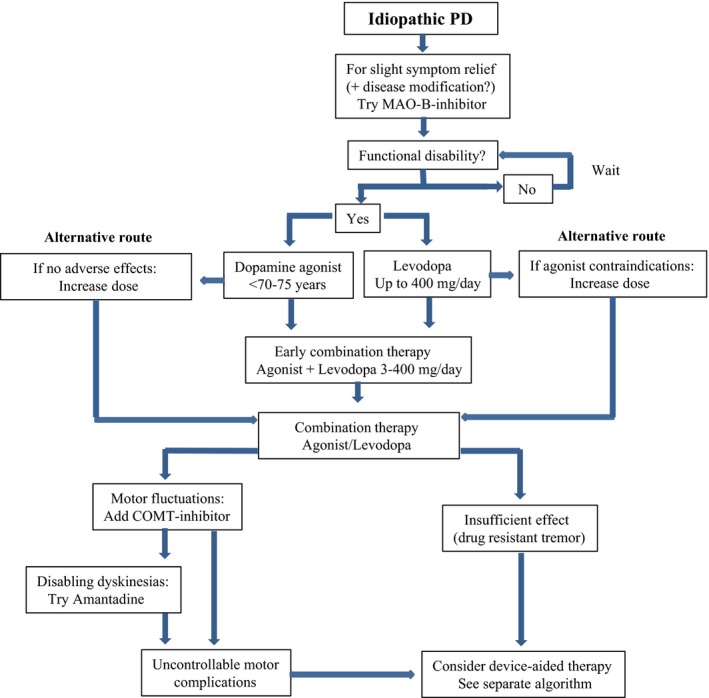
Algorithm for medical treatment of PD. Originally based on the Norwegian treatment recommendations,16 but with several modifications made by the authors. [Correction added on 19 April 2017, after initial online publication: In the bottom right frame of Figure 1, the name of the therapy mentioned was previously incorrect and it has been corrected in this version.]
4. TREATING MOTOR COMPLICATIONS
After some years of dopaminergic treatment, and especially levodopa, many patients develop motor complications like wearing‐off and dyskinesias.38 Motor fluctuations may have different pathophysiological causes, but treatment strategies aiming at less pulsatile dopaminergic stimulation may be beneficial.39 The use of smaller and more frequent doses of levodopa may help. MAO‐B inhibitors prevent the breakdown of dopamine, and catechol‐O‐methyltransferase (COMT) inhibitors reduce the metabolism of levodopa, extending its plasma half‐life. Both treatments may thus prolong the action of levodopa and may be used against wearing‐off and other motor fluctuations. If the patient is already using a MAO‐B inhibitor when motor complications evolve, addition of a COMT inhibitor is recommended.16, 19
Bothersome peak‐dose dyskinesias may improve with dose reduction, but with a risk of impaired symptom control and increased off‐time. Amantadine treatment may be an alternative. The anti‐dyskinetic effect of amantadine is well documented,40, 41 and amantadine has been recommended against motor fluctuations and dyskinesias.14, 18 However, this drug is not readily available in all countries and was therefore not included in the Norwegian guidelines.16
Safinamide, a MAO‐B inhibitor that also has other pharmacological actions, has recently been introduced as a new option for treating motor fluctuations in mid‐ and late‐stage PD.42 Safinamide reduces off‐time in a similar way like the other MAO‐B inhibitors. An anti‐dyskinetic effect of safinamide has been suggested, but so far not demonstrated. The Swedish guidelines gives amantadine and safinamide a lower priority, but lists them as possible add‐on therapies for patients that experience motor complications in spite of optimized dopaminergic therapy.19
5. DEVICE‐AIDED TREATMENT OPTIONS
Device‐aided interventions should be considered if the above‐mentioned strategies are not sufficient for controlling motor complications, or if the patient has motor symptoms that do not respond sufficiently to standard oral treatments. The current device‐aided options are continuous subcutaneous apomorphine infusion, continuous jejunal infusion of levodopa‐carbidopa intestinal gel (LCIG), and deep brain stimulation (DBS). Additionally, apomorphine injections sc with portable pens can be considered as a demand treatment for patients with troublesome off‐periods.18, 19
DBS is best documented, with highest priority in the German and Swedish guidelines.18, 19 DBS is safe and effective, also for long‐term treatment.43, 44, 45, 46 In most centers, the subthalamic nucleus (STN) is the preferred target for treating motor complications in PD. STN‐DBS may reduce all cardinal motor symptoms of PD, as well as motor fluctuations and dyskinesias.47 One study has claimed that DBS of the internal segment of globus pallidus (GPi) is equally effective,48 but the effect of STN‐DBS reported in that study was clearly inferior to what is usually seen.47, 49 Another recent randomized study has confirmed that motor symptoms and function improve more after STN‐DBS than after GPi‐DBS and that there are no differences in psychiatric and social outcome between the two targets.50, 51 Adverse effects may be related to the surgical procedure, to the implanted hardware, or to the stimulation.47 Implant infection is most common, seen after 5.6% of the procedures in our material from Oslo.52 In many of these patients, parts of the implanted system have to be temporarily removed and DBS treatment stopped for some months, while the infection is treated. However, severe side effects are rare, and we have found a 3‐year survival of 97%.53 Pre‐ or post‐operative symptomatic intracerebral hematomas have been reported in 0%‐1.3% of cases from different studies.54 Less severe side effects include balance and gait problems, which have been reported in 8.2%‐13.7% of patients in randomized controlled trials and dysarthria in 5.8%‐10.3%.54
There has been special concern about psychiatric and cognitive side effects. Especially, a higher frequency of suicide ideation and suicides has been reported after DBS, but is still not entirely clear.55, 56 Pooled data show increased scores for depression and hypomania, but at varying degrees.54, 57 As concerns cognitive decline, a recent meta‐analysis of controlled studies concluded that STN‐DBS is relatively safe, but that slight cognitive changes may occur, especially in executive function and verbal fluency.58 In a broad neuropsychological test program, we only found that STN‐DBS may be associated with slight personality changes in the direction of increased impulsivity.59
Levodopa‐carbidopa intestinal gel infusion is also safe and well documented, with a good long‐term effect on PD motor symptoms and reduced risk of motor fluctuations.60, 61, 62, 63, 64 Most adverse effects are device‐related. Some patients may experience increasing dyskinesias,64 but optimized LCIG infusion may also be used to treat troublesome dyskinesias.65 Safety data from four prospective studies were recently integrated to assess the safety of LCIG infusion after median 911‐day treatment.66 The most frequently reported procedure/device adverse events were complications of device insertion and abdominal pain, considered serious in 17% of the patients. Most of the non‐procedure/device events were typical for levodopa treatment and an elderly population. However, severe acute or subacute polyneuropathy has been reported in some patients.63, 67 Adverse events led to discontinuation in 17% of the patients, most frequently because of complication of device insertion. The authors concluded that LCIG infusion is a treatment with high efficacy and relatively low discontinuation rate in patients with advanced PD.66
Apomorphine pump is the least documented of the device‐aided treatment options, but is also the least invasive method. Long‐term data are sparse, and it appears that the dropout rate is often higher than for the other advanced therapies.68 However, also this method is safe and effective in patients with bothersome motor complications,69, 70, 71 reducing off‐time and improving dyskinesias. Skin complications at the infusion site are among the most common adverse reactions, as are dopamine agonist side effects like neuropsychiatric changes, somnolence and orthostatic hypotension. Data after 6 months treatment from a recent prospective study showed a significant increase in health‐related quality of life, but only 100 from 142 patients remained on apomorphine treatment.72 Also a recent retrospective study of long‐term efficacy showed that continuous apomorphine infusion has a good effect on motor fluctuations and dyskinesias, but three quarters of the patients discontinued within the first four years, with decreasing therapeutic effect as the main reason for discontinuation.73
6. SELECTION OF ADVANCED TREATMENT
A few observational studies have tried to compare two different advanced therapies.68, 74 However, at present no direct comparative data support the use of one device‐aided therapy over another. An evidence‐based review has been published, but this only provides recommendations as to which patients could be candidates for each of the device‐aided treatments, and it can only to some extent help choosing one single therapy for a particular patient.75 Both indications and contraindications may limit the available options. It is also important to remember that non‐motor symptoms may play a major role for each patient, and these must be taken into consideration before choosing a device‐aided treatment. However, because few studies have assessed the effects on non‐motor symptoms, such symptoms should not be a decisive reason for recommending one therapy.75
Good clinical response to levodopa is a prerequisite for all therapies, except in patients with drug‐resistant tremor who may respond well to DBS. High age (>70‐75 years) is a relative contraindication, while cognitive impairment and previously severe or ongoing psychiatric symptoms are absolute contraindications against DBS because of the risk of worsening these conditions. Cerebrovascular and other brain and systemic diseases may also represent contraindications against brain surgery. LCIG and apomorphine pump treatment can be performed on patients with mild cognitive impairment, but severe dementia is considered a contraindication against all device‐aided therapies. Patients on pump treatments will usually also need regular social support, and absence of such support might constitute a relative contraindication for these therapies. Drug‐induced psychiatric side effects may be a contraindication against apomorphine, while intestinal disease and contraindications for abdominal surgery count against LCIG. A more detailed review of frequent questions and contraindications related to device‐aided treatments has recently been published.54
Availability and reimbursement issues may be a problem in some countries. However, in patients where two or all three device‐aided therapies are both eligible and available, a risk‐benefit analysis must be performed. The choice of advanced therapy is probably best performed by a team of healthcare professionals with experience of all three advanced therapy options.19 It is important to decide which treatment is most likely to relieve the patient's most bothersome problems and restore daily functions. The patient should be well informed about all available options, to play a major part in decision making. A crude algorithm that may help choosing device‐aided treatments is shown in Figure 2.
Figure 2.
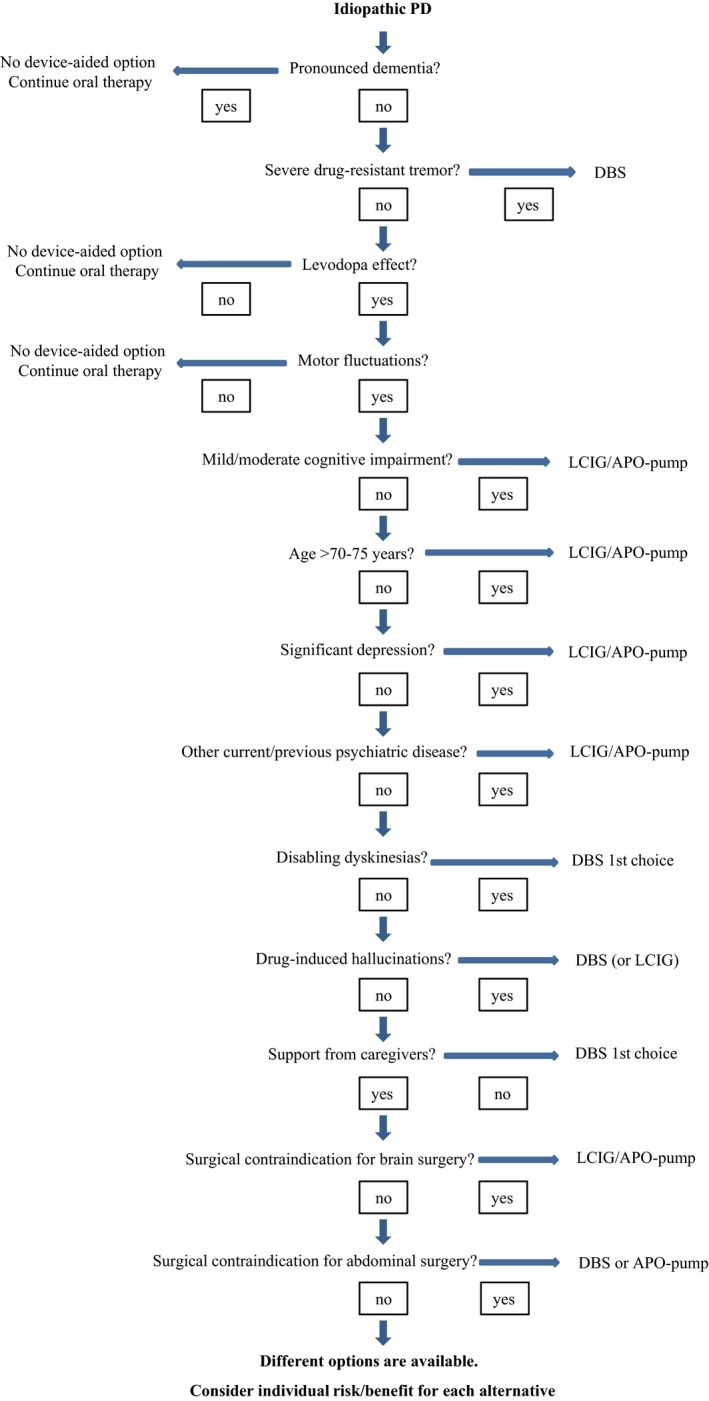
Algorithm for the choice of device‐aided treatment. Originally based on Odin and Nyholm,76 but with modifications made by the authors. [Correction added on 19 April 2017, after initial online publication: In the upper left frame of Figure 2, the name of the therapies mentioned were previously incorrect and these have been corrected in this version.]
7. DISCUSSION
Almost fifty years after levodopa came in widespread clinical use, this drug is still a cornerstone in our treatment of motor symptoms in PD. However, there is still no consensus on exactly how such treatment should be administered. Evidence‐based reviews conclude that there are different well‐documented treatment strategies, but there is no evidence that one strategy should be chosen over another.7, 8, 9, 10, 11, 12, 75 Therefore, it is important to treat each patient individually. Focus should be put on which symptoms—motor as well as non‐motor—that are most bothersome to the patient in each stage of the disease. The algorithms presented here are only meant as support, listing just one alternative decision process for treating motor problems, and they should therefore be applied with caution.
It is also important to remember that medication is only one part of PD treatment. Information, various forms of social and practical support, physical exercise and balance training may be important. Besides, a multidisciplinary approach is recommended,19 with various kinds of health professionals collaborating with patient, family and other caregivers to obtain the best, individualized approach. Team‐based rehabilitation should be considered for the patients.19
CONFLICTS OF INTEREST
ED has received honoraria as a consultant from AbbVie, Britannia and Global Kinetics, and for lectures from AbbVie, Allergan, Desitin, Global Kinetics, GSK, Lundbeck, Medtronic, NordicInfu Care, Orion, UCB and Zambon. PO has received honoraria as a consultant from AbbVie, Bial, Britannia, Global Kinetics, Gruenenthal, and for lectures from AbbVie, Bial, Britannia, Desitin, Global Kinetics, Gruenenthal, NordicInfu Care and Zambon.
ACKNOWLEDGMENTS
We have no acknowledgments to declare.
Dietrichs E, Odin P. Algorithms for the treatment of motor problems in Parkinson's disease. Acta Neurol Scand. 2017;136: 378–385. https://doi.org/10.1111/ane.12733
REFERENCES
- 1. Ferrer I, Martinez A, Blanco R, Dalfó E, Carmona M. Neuropathology of sporadic Parkinson disease before the appearance of parkinsonism: preclinical Parkinson disease. J Neural Transm. 2011;118:821–839. [DOI] [PubMed] [Google Scholar]
- 2. Müller B, Larsen JP, Wentzel‐Larsen T, Skeie GO, Tysnes OB; Parkwest Study Group . Autonomic and sensory symptoms and signs in incident, untreated Parkinson's disease: frequent but mild. Mov Disord. 2011;26:65–72. [DOI] [PubMed] [Google Scholar]
- 3. Ellis TD, Cavanaugh JT, Earhart GM, et al. Identifying clinical measures that most accurately reflect the progression of disability in Parkinson disease. Parkinsonism Relat Disord. 2016;25:65–71. [DOI] [PubMed] [Google Scholar]
- 4. Stocchi F, Jenner P, Obeso JA. When do levodopa motor fluctuations first appear in Parkinson's disease? Eur Neurol. 2010;63:257–266. [DOI] [PubMed] [Google Scholar]
- 5. Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson's disease (2001): treatment guidelines. Neurology. 2001;56(11 Suppl 5):S1–S88. [DOI] [PubMed] [Google Scholar]
- 6. Miyasaki JM, Martin W, Suchowersky O, Weiner WJ, Lang AE. Practice parameter: initiation of treatment for Parkinson's disease: an evidence‐based review. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11–17. [DOI] [PubMed] [Google Scholar]
- 7. Pahwa R, Factor SA, Lyons KE, et al. Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence‐based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:983–995. [DOI] [PubMed] [Google Scholar]
- 8. National Collaborating Centre for Chronic Conditions (UK) . Parkinson's Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK); 2006. [PubMed] [Google Scholar]
- 9. Oertel WH, Berardelli A, Bloem BR, et al. Chapter 14: early (uncomplicated) Parkinson's disease In: Gilhus NE, Barnes MR, Brainin M, eds. European Handbook of Neurological Management: Vol 1. 2nd edition. Chichester: Blackwell; 2010:217–236. [Google Scholar]
- 10. Oertel WH, Berardelli A, Bloem BR, et al. Chapter 15: late (complicated) Parkinson's disease In: Gilhus NE, Barnes MR, Brainin M, eds. European Handbook of Neurological Management. Vol 1, 2nd edition. Chichester: Blackwell; 2010:237–267. [Google Scholar]
- 11. Ferreira JJ, Katzenschlager R, Bloem BR, et al. Summary of the recommendations of the EFNS/MDS‐ES review on therapeutic management of Parkinson's disease. Eur J Neurol. 2013;20:5–15. [DOI] [PubMed] [Google Scholar]
- 12. Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311:1670–1683. [DOI] [PubMed] [Google Scholar]
- 13. Grimes D, Gordon J, Snelgrove B, et al. Canadian Neurological Sciences Federation. Canadian guidelines on Parkinson's disease. Can J Neurol Sci. 2012;39(4 Suppl 4):S1–S30. [DOI] [PubMed] [Google Scholar]
- 14. DANMODIS . Parkinsons sygdom. Klinisk vejledning. 2nd Edition, 2011. http://neuro.dk/wordpress/wp-content/uploads/2012/09/Parkinsons_sygdom_Klinisk_Vejledning_2011.pdf. Accessed December 7, 2016.
- 15. SWEMODIS . Svenska riktlinjer för utredning och behandling av Parkinsons sjukdom. 7th Edition, 2014. http://www.swemodis.se/images/Dokument/Terapird%20Parkinsons%20sjukdom%20version%207%202014.pdf. Accessed December 7 2016.
- 16. Referansegruppen for Nasjonal kompetansetjeneste for bevegelsesforstyrrelser . Revidert terapianbefaling ved Parkinsons sykdom. 6th Edition, 2014. https://helse-stavanger.no/seksjon/NKB/Documents/Nyhetsbulletin/Nyhetsbulletin%202014%20-%20nr.%202.pdf. Accessed December 7, 2016.
- 17. Reichmann H. Modern treatment in Parkinson's disease, a personal approach. J Neural Transm. 2016;123:73–80. [DOI] [PubMed] [Google Scholar]
- 18. Deutsche Gesellschaft für Neurologie . Idiopathisches Parkinson‐Syndrom. Leitlinien für Diagnostik und Therapie in der Neurologie. 2016. www.dgn.org/images/red_leitlinien/LL_2016/PDFs_Download/030010_LL_langfassung_ips_2016.pdf. Accessed October 17, 2016.
- 19. Socialstyrelsen . Nationella riktlinjer för vård vid multipel skleros och Parkinsons sjukdom. Stöd för styrning och ledning. Stockholm, 2016. http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/20392/2016-12-1.pdf. Accessed December 6, 2016.
- 20. Chaudhuri KR, Odin P, Antonini A, Martinez‐Martin P. Parkinson's disease: the non‐motor issues. Parkinsonism Relat Disord. 2011;17:717–723. [DOI] [PubMed] [Google Scholar]
- 21. Schrag A, Sauerbier A, Chaudhuri KR. New clinical trials for nonmotor manifestations of Parkinson's disease. Mov Disord. 2015;30:1490–1504. [DOI] [PubMed] [Google Scholar]
- 22. Parkinson Study Group . Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med. 1993;328:176–183. [DOI] [PubMed] [Google Scholar]
- 23. Larsen JP, Boas J, Erdal JE. Does selegiline modify the progression of early Parkinson's disease? Results from a five‐year study. The Norwegian‐Danish Study Group. Eur J Neurol. 1999;6:539–547. [DOI] [PubMed] [Google Scholar]
- 24. Pålhagen S, Heinonen E, Hägglund J, Kaugesaar T, Mäki‐Ikola O, Palm R; Swedish Parkinson Study Group . Selegiline slows the progression of the symptoms of Parkinson disease. Neurology. 2006;66:1200–1206. [DOI] [PubMed] [Google Scholar]
- 25. Olanow CW, Rascol O, Hauser R, et al. A double‐blind, delayed‐start trial of rasagiline in Parkinson's disease. N Engl J Med. 2009;361:1268–1278. [DOI] [PubMed] [Google Scholar]
- 26. Ahlskog JE, Uitti RJ. Rasagiline, Parkinson neuroprotection, and delayed‐start trials: still no satisfaction? Neurology. 2010;74:1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clarke CE, Patel S, Ives N, Rick C, Wheatley K, Gray R. Should treatment for Parkinson's disease start immediately on diagnosis or delayed until functional disability develops? Mov Disord. 2011;26:1187–1193. [DOI] [PubMed] [Google Scholar]
- 28. Rascol O, Hauser RA, Stocchi F, et al. Long‐term effects of rasagaline and the natural history of treated Parkinson's disease. Mov Disord. 2016;31:1489–1496. [DOI] [PubMed] [Google Scholar]
- 29. Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–2508. [DOI] [PubMed] [Google Scholar]
- 30. Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five‐year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. N Engl J Med. 2000;342:1484–1491. [DOI] [PubMed] [Google Scholar]
- 31. Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4‐year randomized controlled trial. Arch Neurol. 2004;61:1044–1053. [DOI] [PubMed] [Google Scholar]
- 32. PD Med Collaborative Group , Gray R, Ives N, et al. Long‐term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson's disease (PD MED): a large, open‐label, pragmatic randomised trial. Lancet. 2014;384:1196–1205. [DOI] [PubMed] [Google Scholar]
- 33. Antonini A, Poewe W. Fibrotic heart‐valve reactions to dopamine‐agonist treatment in Parkinson's disease. Lancet Neurol. 2007;6:826–829. [DOI] [PubMed] [Google Scholar]
- 34. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross‐sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. [DOI] [PubMed] [Google Scholar]
- 35. Garcia‐Ruiz PJ, Martinez Castrillo JC, Alonso‐Canovas A, et al. Impulse control disorder in patients with Parkinson's disease under dopamine agonist therapy: a multicentre study. J Neurol Neurosurg Psychiatry. 2014;85:840–844. [DOI] [PubMed] [Google Scholar]
- 36. Erga AH, Alves G, Larsen JP, Tysnes OB, Pedersen KF. Impulsive and compulsive behaviors in Parkinson disease: the Norwegian ParkWest Study. J Parkinsons Dis. 2016. doi: 10.3233/JPD‐160977.[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rizos A, Sauerbier A, Antonini A, et al. A European multicentre survey of impulse control behaviours in Parkinson's disease patients treated with short‐ and long‐acting dopamine agonists. Eur J Neurol. 2016;23:1255–1261. [DOI] [PubMed] [Google Scholar]
- 38. Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology. 2009;72(21 Suppl 4):S1–S136. [DOI] [PubMed] [Google Scholar]
- 39. Chaudhuri KR, Rizos A, Sethi KD. Motor and nonmotor complications in Parkinson's disease: an argument for continuous drug delivery? J Neural Transm. 2013;120:1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sawada H, Oeda T, Kuno S, et al. Amantadine for dyskinesias in Parkinson's disease: a randomized controlled trial. PLoS One. 2010;5:e15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ory‐Magne F, Corvol JC, Azulay JP, et al. Withdrawing amantadine in dyskinetic patients with Parkinson disease: the AMANDYSK trial. Neurology. 2014;82:300–307. [DOI] [PubMed] [Google Scholar]
- 42. Fabbri M, Rosa MM, Abreu D, Ferreira JJ. Clinical pharmacology review of safinamide for the treatment of Parkinson's disease. Neurodegener Dis Manag. 2015;5:481–496. [DOI] [PubMed] [Google Scholar]
- 43. Deuschl G, Schade‐Brittinger C, Krack P, et al. A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 44. Krack P, Batir A, Van Blercom N, et al. Five‐year follow‐up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349:1925–1934. [DOI] [PubMed] [Google Scholar]
- 45. Schüpbach WM, Chastan N, Welter ML, et al. Stimulation of the subthalamic nucleus in Parkinson's disease: a 5 year follow up. J Neurol Neurosurg Psychiatry. 2005;76:1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013;368:610–622. [DOI] [PubMed] [Google Scholar]
- 47. Kleiner‐Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta‐analysis of outcomes. Mov Disord. 2006;21(Suppl 14):S290–S304. [DOI] [PubMed] [Google Scholar]
- 48. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Toft M, Dietrichs E. Medication costs following subthalamic nucleus deep brain stimulation for Parkinson's disease. Mov Disord. 2014;29:275–276. [DOI] [PubMed] [Google Scholar]
- 50. Odekerken VJ, Boel JA, Schmand BA, et al. GPi vs STN deep brain stimulation for Parkinson disease: three‐year follow‐up. Neurology. 2016;86:755–761. [DOI] [PubMed] [Google Scholar]
- 51. Boel JA, Odekerken VJ, Geurtsen GJ, et al. Psychiatric and social outcome after deep brain stimulation for advanced Parkinson's disease. Mov Disord. 2016;31:409–413. [DOI] [PubMed] [Google Scholar]
- 52. Bjerknes S, Skogseid IM, Sæhle T, Dietrichs E, Toft M. Surgical site infections after deep brain stimulation surgery: frequency, characteristics and management in a 10‐year period. PLoS One. 2014;9:e105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Toft M, Lilleeng B, Ramm‐Pettersen J, et al. Long‐term efficacy and mortality in Parkinson's disease patients treated with subthalamic stimulation. Mov Disord. 2011;26:1931–1934. [DOI] [PubMed] [Google Scholar]
- 54. Odin P, Ray Chaudhuri K, Slevin JT, et al. Collective physician perspectives on non‐oral medication approaches for the management of clinically relevant unresolved issues in Parkinson's disease: consensus from an international survey and discussion program. Parkinsonism Relat Disord. 2015;21:1133–1144. [DOI] [PubMed] [Google Scholar]
- 55. Soulas T, Gurruchaga JM, Palfi S, Cesaro P, Nguyen JP, Fénelon G. Attempted and completed suicides after subthalamic nucleus stimulation for Parkinson's disease. J Neurol Neurosurg Psychiatry. 2008;79:952–954. [DOI] [PubMed] [Google Scholar]
- 56. Weintraub D, Duda JE, Carlson K, et al. Suicide ideation and behaviours after STN and GPi DBS surgery for Parkinson's disease: results from a randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2013;84:1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser‐Vandewalle V. Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism Relat Disord. 2006;12:265–272. [DOI] [PubMed] [Google Scholar]
- 58. Xie Y, Meng X, Xiao J, Zhang J, Zhang J. Cognitive changes following bilateral deep brain stimulation of subthalamic nucleus in Parkinson's disease: a meta‐analysis. Biomed Res Int. 2016;2016:3596415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pham U, Solbakk AK, Skogseid IM, et al. Personality changes after deep brain stimulation in Parkinson's disease. Parkinsons Dis. 2015;2015:490507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nyholm D, Nilsson Remahl AI, Dizdar N, et al. Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology. 2005;64:216–223. [DOI] [PubMed] [Google Scholar]
- 61. Nyholm D, Klangemo K, Johansson A. Levodopa/carbidopa intestinal gel infusion long‐term therapy in advanced Parkinson's disease. Eur J Neurol. 2012;19:1079–1085. [DOI] [PubMed] [Google Scholar]
- 62. Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa‐carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double‐blind, double‐dummy study. Lancet Neurol. 2014;13:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fernandez HH, Standaert DG, Hauser RA, et al. Levodopa‐carbidopa intestinal gel in advanced Parkinson's disease: final 12‐month, open‐label results. Mov Disord. 2015;30:500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Buongiorno M, Antonelli F, Cámara A, et al. Long‐term response to continuous duodenal infusion of levodopa/carbidopa gel in patients with advanced Parkinson disease: the Barcelona registry. Parkinsonism Relat Disord. 2015;21:871–876. [DOI] [PubMed] [Google Scholar]
- 65. Antonini A, Fung VS, Boyd JT, et al. Effect of levodopa‐carbidopa intestinal gel on dyskinesia in advanced Parkinson's disease patients. Mov Disord. 2016;31:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lang AE, Rodriguez RL, Boyd JT, et al. Integrated safety of levodopa‐carbidopa intestinal gel from prospective clinical trials. Mov Disord. 2016;31:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Uncini A, Eleopra R, Onofrj M. Polyneuropathy associated with duodenal infusion of levodopa in Parkinson's disease: features, pathogenesis and management. J Neurol Neurosurg Psychiatry. 2015;86:490–495. [DOI] [PubMed] [Google Scholar]
- 68. Antonini A, Isaias IU, Rodolfi G, et al. A 5‐year prospective assessment of advanced Parkinson disease patients treated with subcutaneous apomorphine infusion or deep brain stimulation. J Neurol. 2011;258:579–585. [DOI] [PubMed] [Google Scholar]
- 69. Manson AJ, Turner K, Lees AJ. Apomorphine monotherapy in the treatment of refractory motor complications of Parkinson's disease: long‐term follow‐up study of 64 patients. Mov Disord. 2002;17:1235–1241. [DOI] [PubMed] [Google Scholar]
- 70. Katzenschlager R, Hughes A, Evans A, et al. Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson's disease: a prospective study using single‐dose challenges. Mov Disord. 2005;20:151–157. [DOI] [PubMed] [Google Scholar]
- 71. Drapier S, Gillioz AS, Leray E, et al. Apomorphine infusion in advanced Parkinson's patients with subthalamic stimulation contraindications. Parkinsonism Relat Disord. 2012;18:40–44. [DOI] [PubMed] [Google Scholar]
- 72. Drapier S, Eusebio A, Degos B, et al. Quality of life in Parkinson's disease improved by apomorphine pump: the OPTIPUMP cohort study. J Neurol. 2016;263:1111–1119. [DOI] [PubMed] [Google Scholar]
- 73. Borgemeester RW, Drent M, van Laar T. Motor and non‐motor outcomes of continuous apomorphine infusion in 125 Parkinson's disease patients. Parkinsonism Relat Disord. 2016;23:17–22. [DOI] [PubMed] [Google Scholar]
- 74. Martinez‐Martin P, Reddy P, Katzenschlager R, et al. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson's disease. Mov Disord. 2015;30:510–516. [DOI] [PubMed] [Google Scholar]
- 75. Volkmann J, Albanese A, Antonini A, et al. Selecting deep brain stimulation or infusion therapies in advanced Parkinson's disease: an evidence‐based review. J Neurol. 2013;260:2701–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Odin P, Nyholm D. Patient selection for continuous dopaminergic stimulation therapy In: Aquilonius S‐M, Mouradian MM, eds. Parkinson's Disease. Role of Continuous Dopaminergic Stimulation. Crowthorne: ESP Bioscience; 2012:162–172. [Google Scholar]


