Abstract
Aim
To describe the natural progression and the rates of arthroplasty of a cohort of hip and knee osteoarthritis (OA) patients.
Methods
An observational study of 247 consecutive patients who attended an OA clinic between May 2008 and August 2009. Follow‐up survey was conducted from July 2014 to December 2014, with the primary end point being joint replacement surgery.
Results
One hundred and sixty‐seven patients had knee OA and 80 patients had hip OA. When adjusted for other variables (age, gender, body mass index, Kellgren‐Lawrence stage, symptom duration, presence of OA elsewhere and pain score), patients with hip OA demonstrated 86% increased hazard of surgery compared to knee OA patients (95% CI increase of 19% to 193%). At 6 years after initial consultation, 67% of patients with knee OA did not require a knee replacement surgery, while 40% (30, 51) of hip OA patients did not undergo surgery (95% CI: 59–74%). Overall at 6 years, 58% of patients (95% CI: 51–64%) did not undergo joint replacement surgery.
Conclusion
Knee and hip OA patients appear to behave differently, with hip OA patients more likely to undergo arthroplasty. There is a significant number of both hip OA and knee OA patients who did not require arthroplasty at the end of 6 years, suggesting a major role for conservative therapy.
Keywords: arthroplasty, hip, knee, osteoarthritis
Introduction
Osteoarthritis (OA) of the hip and knee is a major cause of disability globally.1, 2 There are significant direct and indirect arthritis‐attributable costs to individuals with disabling hip and/or knee OA.3 In Australia, OA is the second leading reason for patient visits to a rheumatologist.4
Osteoarthritis is a condition that is characterized by pain, mobility impairment, decreased function and reduced quality of life. Clinical guidelines share broad agreement in recommending a combined pharmacological and non‐pharmacological approach to the management of hip and knee OA, with timely access to total joint replacement (TJR) surgery/arthroplasty for those with advanced stage disease.5, 6, 7, 8 There is good evidence that arthroplasty results in improved function and decrease pain in this population9, 10 and the number performed in Australia continues to rise each year. According to the National Joint Replacement Registry annual report, in 2014, 78 000 arthroplasties were performed in Australia, costing over AUD$1.2 billion dollars. Despite the demonstrated effectiveness, there is a significant proportion (20–40%) of patients who had undergone arthroplasty who do not have an optimal outcome and report dissatisfaction with the outcome.11 There are wide variations in the timing and use of this valuable resource and studies have highlighted differences in age and preoperative status of patients who undergo arthroplasty, indicating substantial differences in the timing of joint replacement surgery.12, 13, 14, 15, 16 Due to the spiraling costs of Joint replacements and unsatisfactory outcomes for some, arthroplasty should only be undertaken when all other non‐operative management strategies have been exhausted and where there is a good probability of surgical success.17, 18, 19
What is the value in knowing how patients with OA hip and knee present and progress over time? Previous studies such as the ROAD study in Japan looking at radiological progression and functional status in OA suggest discrepancy between radiological OA and symptomatic OA in the knee.20 In a meta‐analysis of functional status progression, there was limited evidence for worsening of pain and function after 3 years in both knee and hip OA groups.21 Therefore, in addition to symptom severity and radiological severity, other factors such as comorbidities and an individual's capacity to benefit from surgery should be taken into consideration and attempts to optimize conditions prior to surgical selection is recommended.22, 23, 24, 25 Further evaluation of differences in presentation, progression and rates of arthroplasty between hip and knee OA will shed light into the most effective way of managing this chronic condition. This in turn will enable changes to be made in healthcare systems to ensure better access and timely health care for all, starting at the primary care level with transition in to tertiary care at the most appropriate time.
Results from other chronic disease models of care indicate that management of OA should be integrated within a multidiscipline team.18 OA management clinics have been established across Australia to help coordinate conservative care and help prioritize patients suitable for arthroplasty, but there is no standard policy as yet. The two well described examples of new chronic disease management service models are the Osteoarthritis Hip and Knee Service (OAHKS) in Victoria and the Osteoarthritis Chronic Care Program (OACCP) in New South Wales.26, 27 There are some data to show benefits in terms of efficiency and effectiveness of patient flow, coupled with the financial advantages to individual healthcare networks.28, 29, 30
Over the past 10 years within our OAHKS clinic at St Vincent Hospital, Melbourne, Australia, we have observed differences between patients presenting from primary care to the tertiary institution with hip or knee OA. It appeared that patients with hip OA at the time of initial presentation had more severe radiological disease, shorter duration of symptoms and required joint replacement surgery earlier than their knee counterparts.31
This study was conducted to further analyze observed differences between hip and knee OA patients. The objectives of this study were to analyze the types of patients with hip and knee OA that were referred to a dedicated OA clinic at a large tertiary hospital, and to determine if there was a disparity in rates of arthroplasty between hip and knee OA patients. We also looked at how the rates of arthroplasty changed over a 6‐year period, and presenting factors that would predict need for arthroplasty. This information will assist to better understand OA as a chronic disease and provide decision support for rheumatologists in surgical selection as every patient with knee and hip OA may not need surgery. The ultimate goal is providing the most cost‐effective and efficient use of resources.
Materials and Methods
Study design and setting
The data used in this study was collected as a part of a larger international multicenter prospective observational cohort study.32 Only the cohort from a dedicated tertiary OA clinic at St Vincent's Hospital, Melbourne (SVHM), Australia is reported in this publication. We describe the characteristics and rates of arthroplasty in hip and knee OA in a nested cohort in a fixed time period from May 2008 to August 2009 based at OAHKS clinic. The study protocol was reviewed and approved by the Human Research Ethics committee of SVHM (HREC 120/07). The procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2013.
The OAHKS at SVHM was established in 2006 as part of a Victorian Government initiative to improve access to elective arthroplasty across the state of Victoria. The model employed at SVHM was unique from the other established sites as it utilized rheumatology staff and an advanced musculoskeletal physiotherapist (AMP) to provide the management and coordination of care to patients with hip and knee OA, with established pathways to orthopedic surgery when required. This model of care allowed patients to be monitored over an extended period of time, with patterns and progression of the disease to be observed and recorded.
Patient care was provided by two rheumatologists, one rheumatology registrar, one AMP, a dietitian, one rheumatology nurse and a research assistant. Patients were managed by a set of standard guidelines based on European League Against Rheumatism recommendations.33, 34 Interventions included education on pain self‐management and medication safety, physiotherapy and an exercise program, and dietetic interventions for all patients. Joint aspiration and intra‐articular steroid injections were given when appropriate as part of routine care when there was a significant joint effusion or if they had a poor response to analgesics and physiotherapy and if the patient consented. Not more than three injections per year were given to the same joint. Exercise involved a combination of lower limb strength training and aerobic, neuromuscular and a range of motion exercise. Dietetic interventions were mainly in the form of advice focused on getting a full dietary assessment and then healthy eating recommendations. In some instances a very low calorie diet (VLCD) such as Optifast was recommended depending upon the age (under 65) and in the absence of some comorbidities such as insulin‐dependent diabetes mellitus and renal failure. Patients were seen at the OAHKS clinic every 3 months. If conservative management was successful after a 3–6 month trial, it was continued indefinitely until the patient developed worsening symptoms. Patients were referred back to the general practitioners for continuation of care whenever possible. Conservative therapy was considered failed if there was no improvement after 3–6 months of conservative care, when the patient reported they were still suffering and had not improved. This was measured with patient and physician global assessment and a visual analog pain scale showing an increase in pain. But these tests do not supersede what the patient says and clinical judgement was used. A referral for consideration of joint replacement was made when conservative therapy failed, provided the patient was of the right age, preferably more than 60 years, X‐ray grading was advanced with a grading of 3–4, the patient was fit for surgery depending on other comorbidities and if the patient was agreeable to surgery.
Patient consent: informed consent was obtained from each patient included in the study.
Study objectives
Primary objective
To test the observation that patients with hip and knee OA patients behave differently with regard to progression to arthroplasty. The hypothesis was that patients with hip OA require surgery earlier from presentation to the OAHKS clinic compared to those with knee OA.
Secondary objectives
To test if there is a predictable pattern for progression leading to joint replacement and to describe demographic details of patients, including comorbidities, radiological grading, body mass index (BMI) and disability in hip and knee OA. For the latter, we included the baseline Multi‐Attribute Arthritis Prioritization Tool (MAPT) score, Hip disability and Osteoarthritis Outcome Score (HOOS) and Knee injury and Osteoarthritis Outcome Score (KOOS). KOOS was collected when the primary joint was the knee and HOOS was collected when the worst joint to be managed was the hip.
The MAPT score can be seen as an instrument that measures the level of ‘distress’. It is calculated by entering the hip and knee questionnaire responses into the purpose‐built database which applies a mathematical algorithm based on the weightings of the various questions. The resultant MAPT score is a number between 0 and 100. Previous studies have suggested that the higher the score, the greater impact of the disease, especially if sustained.23, 28, 35
The KOOS is a 42‐item self‐administered questionnaire proven valid for management of knee OA.36, 37, 38 The KOOS assesses five separate dimensions: pain, symptoms, activities of daily living, sports and recreation function and knee‐related quality of life. The inclusion of the subscale sport and recreation function may be considered an advantage, as although sport and recreation function is not relevant to all patients, the subscale improves validity by assessing functions considered extremely or very important by some patients. A score from 0 to 100 is calculated for each dimension, with 0 indicating extreme knee problems and 100 representing best possible score with no knee problems. It should be noted that this is a population‐specific score. KOOS demonstrates adequate content validity, internal consistency, test‐retest reliability, construct validity and responsiveness for our study population with early and late OA.38
The HOOS is an adaptation of the KOOS intended to evaluate symptoms and functional limitations related to hip OA. It consists of 40 items assessing five separate patient‐relevant dimensions: pain, symptoms, activity limitations‐daily living, sports recreation function and hip‐related quality of life with 100 representing best possible score. HOOS is a valid scale to assess hip OA outcomes and the added subscales sport and recreation function and hip‐related quality of life were an added benefit. HOOS demonstrates adequate content validity, internal consistency, test‐retest reliability, construct validity and responsiveness for our study population with early and late OA.39
Sample
The subjects are part of a large longitudinal cohort of patients with OA of the hip and knee at the OAHKS clinic. They were followed prospectively as part of a longitudinal study started in 2007. Signed informed consent has been obtained from each patient at the initial visit.
We looked at consecutive patients who attended the clinic between May 2008 and August 2009. Two hundred and forty‐seven consecutive patients who gave consent during the study period were included. There was no sample bias or any specific inherent bias in our receipt of referrals or referral for surgery.
Inclusion criteria
Exclusion criteria
Inability to complete the questionnaire because of language barriers.
Patients who did not fulfil the criteria for OA.
Information was collected from the initial patient questionnaires and clinician's questionnaire. Patients completed two questionnaires (HOOS or KOOS and MAPT) concerning pain and function for the target joint. The clinician collected patient data on a clinical questionnaire during the initial consultation, including demographics such as age, sex, weight, comorbidities and X‐ray of the relevant joint. Comorbidities were collected using the standardized questionnaire used by Osteoarthritis Research Society International (OARSI) Outcome Measures in Rheumatology (OMERACT) task force publication on the OARSI/OMERACT initiative to define states of severity and indication for joint replacement in hip and knee OA.32 This was a dichotomous questionnaire with 14 questions. Presence of OA elsewhere was incorporated in this and it was a dichotomous question. BMI was calculated using the equation weight in kilograms/[height in meters × height in meters]. The X‐ray examination used the Kellgren‐Lawrence (KL) grading system:42 grade o = normal joint; grade 1 = small osteophyte of doubtful significance; grade 2 = definite osteophyte; grade 3 = osteophyte and joint space narrowing; grade 4 = severe joint space narrowing. Patients with KL grades 1 and 2 were classified as mild OA and grades 3 and 4 as severe OA.
The follow‐up survey was conducted from July 2014 to December 2014 using hospital medical records, contacting general practitioners and contacting patients. The primary end point was arthroplasty.
Statistical methods
There was no formal sample size calculation performed a priori mainly due to the observational nature of the study. The aim was to recruit at least 100 patients. However, regardless of this aim, the recruitment period was determined to be at least 12 months to avoid any seasonal patterns and variations. Post hoc sample size and power was not calculated as this is against recommendations. As the study shows a positive result (e.g., there is a statistically significant difference in surgery requirement between knee and hip OA which persists after adjustments), the power is adequate. However, there may be other factors that are driving the observed difference which were not measured. This has been added to study limitations.
Baseline characteristics are presented as frequency and percentage (categorical variables) or median and interquartile range (IQR) (continuous variables). Comparisons between knee and hip OA groups were performed using non‐parametric tests (rank sum test for continuous variables and Fisher's exact test for categorical variables).
Time to joint replacement surgery was estimated using Kaplan–Meier estimates, log rank test and Cox regression. Variables that were either associated with time to surgery or were unevenly distributed between hip and knee OA groups were entered into multivariate Cox regression. Subscales of HOOS and KOOS showed high positive correlations among individual scores (from 0.5 to 0.9) therefore to avoid estimation issues caused by multi‐collinearity, only pain score was entered into multivariate regression as it showed the strongest association with the outcome. Continuous variables were adequately transformed and examined with Martingale residuals. Variables with large proportion of missing observations (such as baseline MAPT score) were excluded from the final model. Proportional hazard assumptions were checked using Schoenfeld residuals; overall model fit was evaluated using Cox Snell residuals and it showed a good fit.
All analyses were performed using Stata version 13.2 (StataCorp LP, College Station, TX, USA).
Results
A total of 247 patients were included in the cohort. There were 167 patients with knee OA and 80 patients with hip OA. Seven patients with missing data for BMI at initial presentation were excluded from the analysis.
Table 1 shows demographic data of the patients.
Table 1.
Comparison of baseline characteristic between patients with hip and knee osteoarthritis. Continuous variables were compared using rank sum test, while Fisher's exact test was used for categorical variables
| Baseline characteristic | Missing data n | Knee osteoarthritis n (%)/median (IQR) | Hip osteoarthritis n (%)/median (IQR) | P‐value |
|---|---|---|---|---|
| Total | 0 | 167 (100%) | 80 (100%) | — |
| Age (years) | 0 | 68.00 (60.00, 76.00) | 67.00 (59.00, 75.00) | 0.317 |
| Gender | 0 | — | — | 0.890 |
| Female | 0 | 100 (59.9) | 49 (61.3) | — |
| Male | 0 | 67 (40.1) | 31 (38.8) | — |
| BMI (kg/m2) | 7 | 31.32 (27.40, 36.00) | 27.54 (23.36, 31.97) | < 0.001 |
| K/L grading scale | 21 | — | — | 0.324 |
| Stage 1 | 0 | 5 (3.0) | 2 (2.5) | — |
| Stage 2 | 0 | 19 (11.4) | 9 (11.3) | — |
| Stage 3 | 0 | 59 (35.3) | 22 (27.5) | — |
| Stage 4 | 0 | 68 (40.7) | 45 (56.3) | — |
| Symptom duration (years) | 2 | 3.90 (2.00, 8.00) | 2.70 (1.60, 5.60) | 0.073 |
| Number of comorbid conditions | 0 | 2.00 (1.00, 3.00) | 2.00 (1.00, 3.00) | 0.977 |
| Comorbidities | ||||
| Hypertension | 0 | 96 (57.5) | 42 (52.5) | 0.495 |
| Osteoarthritis elsewhere | 0 | 93 (55.7) | 41 (51.2) | 0.585 |
| Diabetes | 0 | 39 (23.4) | 7 (8.7) | 0.005 |
| Ischaemic heart disease | 0 | 27 (16.2) | 7 (8.7) | 0.166 |
| Back pain | 0 | 15 (9.0) | 10 (12.5) | 0.249 |
| Baseline KOOS and HOOS scales | ||||
| QOL | 5 | 25.00 (12.50, 43.75) | 31.25 (12.50, 43.75) | 0.922 |
| Sport/ Recreation | 6 | 0.00 (0.00, 10.00) | 12.50 (3.13, 25.00) | < 0.001 |
| ADL | 4 | 42.65 (27.94, 58.82) | 38.24 (22.06, 57.35) | 0.226 |
| Symptom | 4 | 44.64 (32.14, 64.29) | 45.00 (31.25, 65.00) | 0.656 |
| Pain | 4 | 44.44 (30.56, 58.33) | 37.50 (25.00, 52.50) | 0.118 |
| Baseline MAPT | 33 | 22.78 (4.45, 52.53) | 39.91 (16.28, 83.57) | 0.002 |
ADL, adjusted daily living; BMI, body‐mass index; HOOS, hip injury and osteoarthritis outcome score (0 = worst, 100 = best); IQR, interquartiles range; K/L, Kellgren Lawrence radiological score; KOOS, knee injury and osteoarthritis outcome score (0=worst, 100=best); MAPT, multi‐attribute prioritization tool; N, number; QOL, quality of life.
The median age was 68 years (IQR: 60–75) with 40% being male. Half of the cohort had a BMI > 30 kg/m2 and 50% were classified as K/L grade 4. Median duration of symptoms was 3.6 years (IQR: 1.7–6.7). Out of 19 measured comorbidities, only 14% of patients did not report any comorbidities, the commonest reported comorbidity being hypertension (56%) and OA at other joints (55%). Seventy percent of patients had knee OA, 30% with hip OA. Patients with hip OA had lower BMIs, reported less incidence of diabetes but had higher KOOS and HOOS scores at the sport and recreation scale. They also tend to have shorter duration of symptoms; however, this difference was not statistically significant. Baseline MAPT scores were considerably higher in patients with hip OA; however, this score was not captured in slightly over 10% of them.
Time to joint replacement surgery since the first consultation at the clinic was examined using Kaplan–Meier estimates as shown in Fig. 1.
Figure 1.
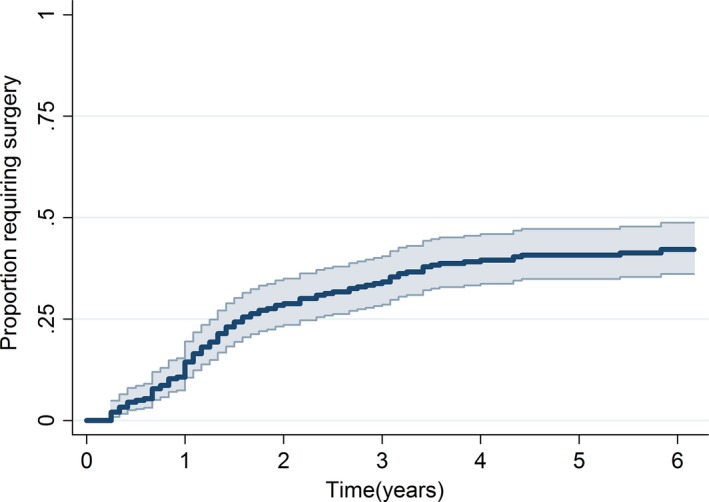
Kaplan–Meier estimate of time to joint replacement surgery with 95% confidence intervals for overall cohort.
At 6 years, an overall 58% of patients (95% confidence interval [CI]: 51–64%) had not undergone joint replacement surgery.
Figure 2 shows time to joint replacement surgery in hip and knee OA separately.
Figure 2.
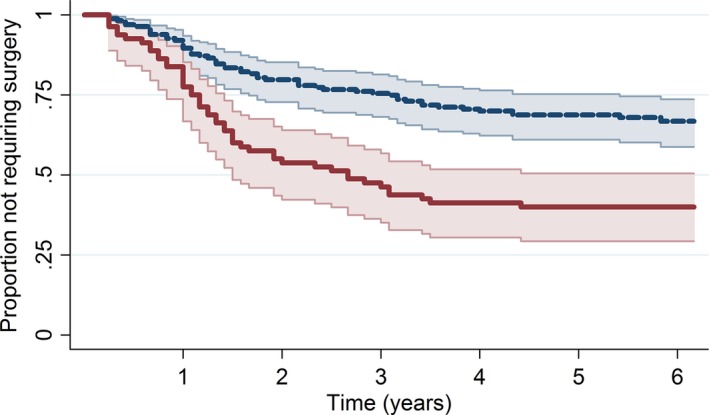
Kaplan–Meier estimate of time to joint replacement surgery with 95% confidence intervals stratified by joint involvement.  , 95% CI;
, 95% CI;  , Knee joint;
, Knee joint;  , 95% CI;
, 95% CI;  , Hip joint.
, Hip joint.
Patients with hip OA had a 138% higher hazard of surgery compared to patients with knee OA. At 6 years after initial consultation, 67% of patients with knee OA had not required knee replacement surgery, while only 40% of patients with hip OA had not undergone surgery (95% CI: 59–74%). The median time to surgery for patients with hip OA was 2.5 years.
Strong association with the time to surgery was also shown by K/L grading scale as shown in Fig. 3.
Figure 3.
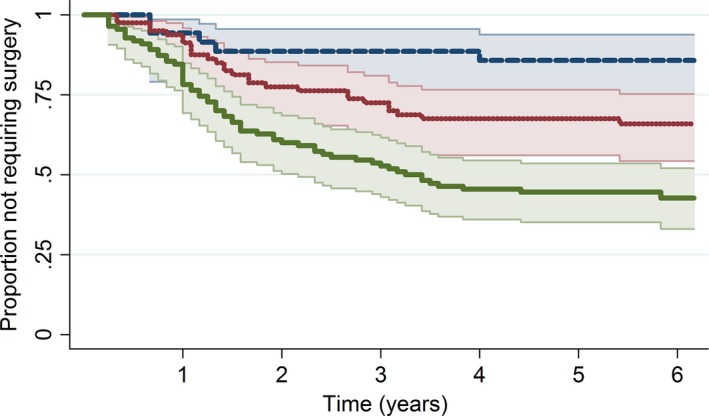
Kaplan–Meier estimate of time to joint replacement surgery with 95% confidence intervals stratified by Kellgren–Lawrence (K/L) grading scale.  , 95% CI;
, 95% CI;  , Stage 1/2;
, Stage 1/2;  , 95% CI;
, 95% CI;  , Stage 3;
, Stage 3;  , 95% CI;
, 95% CI;  , Stage 4.
, Stage 4.
Patients with grade 4 had 5.3 times higher hazard of surgery (95% CI: 2.1–13.0) compared to grades 1–2 and those in grade 3 had 2.6 times higher hazard (95% CI: 1.00–6.77). Surgery requirements within 6 years post‐initial consultations were 15% (95% CI: 6–31%) for grades 1–2, 34% (95% CI: 25–46) for grade 3 and 57% (95% CI: 48–67%) for grade 4. Median time to surgery in the grade 4 subgroup was 3.25 years.
Table 2 shows univariate and multivariate analysis of effect of each variable on time to arthroplasty.
Table 2.
Relationship between baseline variables and time to joint‐replacement surgery before and after adjustment for other variables
| Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|
| HR [95% CI] | P‐value | HR [95% CI] | P‐value | |
| Index joint | ||||
| Knee | Ref | — | — | — |
| Hip | 2.38 [1.61, 3.53] | <0.001 | 1.86 [1.19, 2.93] | 0.007 |
| Age (years) | 1.01 [0.99, 1.03] | 0.181 | Quadratic | 0.036 |
| Gender | ||||
| Female | Ref | — | — | — |
| Male | 0.70 [0.46, 1.06] | 0.089 | 0.85 [0.54, 1.34] | 0.494 |
| BMI | 0.99 [0.96, 1.03] | 0.706 | 0.97 [0.94, 1.00] | 0.083 |
| K/L grading scale | ||||
| Stage 1–2 | Ref | — | — | — |
| Stage 3 | 2.61 [1.00, 6.77] | 0.049 | 2.62 [1.00, 6.92] | 0.051 |
| Stage 4 | 5.23 [2.10, 13.03] | <0.001 | 4.96 [1.95, 12.58] | 0.001 |
| Symptom duration (years) | 0.98 [0.95, 1.01] | 0.209 | 0.98 [0.94, 1.02] | 0.302 |
| Number of comorbidities | 0.92 [0.79, 1.06] | 0.255 | n/a | n/a |
| Comorbidities | ||||
| Hypertension | ||||
| No disease | Ref | — | — | — |
| Disease | 1.18 [0.79, 1.75] | 0.421 | n/a | n/a |
| Osteoarthritis elsewhere | ||||
| No disease | Ref | — | — | — |
| Disease | 0.59 [0.40. 0.88] | 0.009 | 0.63 [0.41, 0.96] | 0.033 |
| Diabetes | ||||
| No disease | Ref | — | — | — |
| Disease | 0.79 [0.46, 1.34] | 0.376 | n/a | n/a |
| Ischaemic heart disease | ||||
| No disease | Ref | — | — | — |
| Disease | 0.89 [0.49, 1.63] | 0.709 | n/a | n/a |
| Back pain | ||||
| No disease | Ref | — | — | — |
| Disease | 1.22 [0.71, 2.08] | 0.467 | n/a | n/a |
| Baseline KOOS and HOOS scales | ||||
| Pain score | 0.97 [0.96, 0.98] | <0.001 | 0.97 [0.96, 0.99] | <0.001 |
| Symptom score | 0.98 [0.97, 0.99] | <0.001 | n/a | n/a |
| ADL score | 0.97 [0.96, 0.98] | <0.001 | n/a | n/a |
| Sport rec score | 0.99 [0.97, 1.00] | 0.042 | n/a | n/a |
| QOL score | 0.98 [0.97, 0.99] | <0.001 | n/a | n/a |
| Baseline MAPT | 1.01 [1.01, 1.02] | <0.001 | n/a | n/a |
ADL, adjusted daily living; BMI, body‐mass index; CI, confidence interval; HR, hazard ratio; HOOS, hip injury and osteoarthritis outcome score (0=worst, 100=best); K/L, Kellgren Lawrence radiological score; KOOS, knee injury and osteoarthritis outcome score (0=worst, 100=best); MAPT, multi‐attribute prioritization tool; n/a, the variable was not included into multivariate model either due to no association; collinearity or large proportion of missing observations; QOL, quality of life.
When adjusted for other variables (age, gender, BMI, K/L grade, symptom duration, presence of OA elsewhere and pain score), patients with hip OA demonstrated an 86% increased hazard of surgery compared to knee patients (95% CI increase of 19–193%). The relationship between K/L grading score and time to surgery remained almost the same after adjusting for other variables, suggesting that none of the variables measured confounded this relationship.
Other factors that show independent association with increased hazard of surgery were also absence of OA at other joints, increased baseline MAPT score and lower scores on baseline HOOS and KOOS scales, which is shown in Table 2.
After adjustment for other variables, age showed a quadratic relationship with time to surgery, with the largest hazard of surgery at a mean age (67 years) and decreasing hazard with younger and older age.
Figure 4 shows remaining patients without arthroplasty.
Figure 4.
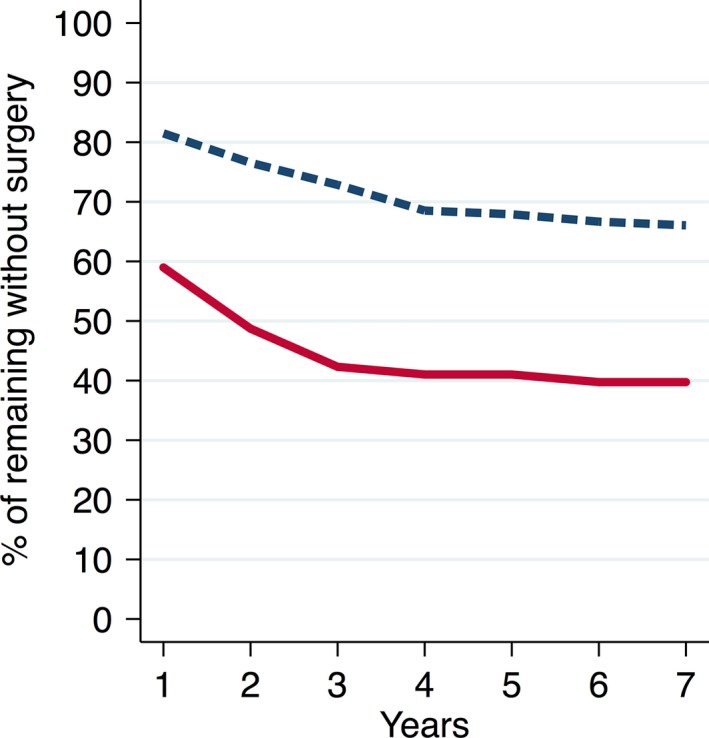
Remaining patients without arthroplasty.  , Knee = 162;
, Knee = 162;  , Hips = 78.
, Hips = 78.
There were 134 patients who did not have arthroplasty, out of which 103 were knee OA patients and 31 were hip OA patients. Eighty (77%) of the knee OA patients and 18 (58%) of the hip OA patients reported improvement with conservative treatment as the reason for not having arthroplasty. One patient from each group had severe comorbidities making them unsuitable for surgery. Flour knee OA patients and one hip OA patient were reported as deceased. There were 29 patients with no records about arthroplasty and they were excluded.
Discussion
Our study highlights two important observations. The first observation is that a larger proportion of patients with hip OA present with more severe symptoms and need earlier arthroplasty than those with knee OA (hazards ratio 1.86, 95% CI: 1.19–2.93; P < 0.001). The latter group appears to present earlier and can be managed conservatively for longer.
The second observation is that the rate of progression to surgery slows down significantly in both groups after 3 years as shown in Fig. 2. This indicates that there is a major role for conservative management of both hip and knee OA patients in the long term. An important message is that we should persist with conservative management no matter what the stage of disease at first presentation. Those who need surgery will declare themselves, but by the same token, there will be a significant population where urgent surgery is not required despite radiographic changes and can be managed with non‐operative means. This will have enormous impact on the health budget.
It is interesting to consider reasons why hip OA patients present with more advanced disease than knee OA patients. One reason may be to do with structural anatomy. The hip joint is a more proximal joint surrounded by muscle groups, making it more stable to withstand injury for a longer time before developing symptoms. The knee joint is a hinge joint not surrounded by many muscles and may be less able to withstand the same impact of injury compared with the hip joint and may present early.43 Other possible factors are the peripheral location of the knee joint which makes it more prone to injury and the effect of obesity. Forces transmitted by the knee joint are of great clinical significance.44 Obesity, which increases the overall magnitude of loads across the knee, is associated with an increased incidence of OA as well as accelerated progression of the disease.45 The knee joint may have enhanced pain perception and sensory proprioception which prompts early symptomatic presentation.46 The triggers for symptomatic presentation in hip OA are less understood. One explanation is the occurrence of microfractures in a degenerative joint.
Studies did not support a specific level of pain or function that defines an indication for joint replacement in hip and knee OA.32 However, there are a number of patient‐related factors associated with response, including obesity, mental health, radiographic severity, baseline pain and function, index joint (knee vs. hip), sex and high level of comorbidity.17, 23 Previous studies have shown that the lifetime risk of undergoing arthroplasty is estimated to be substantially less than the risk of developing symptomatic hip or knee OA. For the knee, the difference between these risk estimates was particularly wide. The reasons for the size of these differences were not clear.47 Some of the possible reasons were disparity between radiographic and symptomatic OA and recent data suggesting that OA does not necessarily progress over time, such that many patients may never require surgery.48 A systematic review showed that the pain and functional status in hip and knee OA seem to deteriorate slowly, with limited evidence for worsening after 3 years of follow‐up. In specific subgroups, prognosis in the first 3 years of follow‐up was either worse or better. Prognostic factors included biomechanical factors, psychological factors, clinical factors and treatment modalities.17
Our findings show that for a large proportion of patients presenting with knee and hip OA, arthroplasty can be delayed. Whether this is a function of the patient or the clinician is difficult to determine. The reason for this could be multi‐factorial. Our patients were managed in a dedicated OA clinic with a multidisciplinary team including rheumatologists, physiotherapists and a dietitian. Patients referred for surgical management were triaged from the orthopedic outpatient wait list clinic. They underwent a fixed protocol management at least for 6 months. This was explained to the patients so that they are willing initially to try non‐operative treatment. Patients who do not respond to a fixed spell of conservative management and were fit and willing to have surgery, were referred for joint replacement. Criteria for referral were significant pain, functional impairment and structural damage balanced against the expected benefit. Patients who improved were followed up by the clinic or they were referred back to the primary care provider. They were all enrolled in a fixed protocol multidisciplinary conservative management model of care and this may have worked.28 Other possible reasons are patients being too ill for surgery, refusal of surgery, fear of surgery (do we put them off?), regression to the mean, response shift with change in patient's and carer's perspectives (they feel less distressed as their perception changes with good medical care and interventions), contextual healing (healing resulting from the clinical encounter consisting of a causal connection between clinician–patient interaction or a particular component of the interaction and improvement in the condition of the patient,49 management of co‐morbidities made them better, and a combination of other factors.16, 50 The tapering off effect with arthroplasties can be a result of patients who can be operated on are done earlier and those who have multiple comorbidities are conservatively managed.
This study is not without limitations. The nature of our clinic being a tertiary referral center may have caused center and selection bias where the patients were referred for the reason that they are not ready for surgery. It is an observational study. About 30% of the patients were not followed up by the OAHKS clinic in later years as they were referred back to primary care physicians and this may have affected the decision regarding joint replacement surgery if it was not initiated by the OA clinic. Some patients were lost to follow up and this may have affected the data. In some patients the decision for arthroplasty was modified due to comorbidities. This model of care is not the usual one in most public hospitals, as we have access to quick surgery after a spell of standard conservative management due to our arrangement with the orthopedics unit. As mentioned earlier, there may be a selection bias, but this study and cohort reflect real‐life clinical practice. The unique findings demonstrate what actually happens in a longitudinal OA cohort followed up over 6 years.
In conclusion, our study suggests knee and hip OA progress differently, with hip OA patients more likely to undergo arthroplasty earlier than knee OA patients from the time of presentation. We found that arthroplasty rates were different, higher early and tapering off in later years in both groups.
It appears that structured conservative management works well for both groups. There is a significant number of both hip OA and knee OA patients who did not require arthroplasty at the end of 6 years, suggesting a major role for conservative therapy.
Funding sources
There are no external funding sources.
Conflicts of interest
The authors declare no competing interests.
Author Contributions
CD and KL: Conception and design, drafting the article, final approval of the article and responsible for the integrity of the article = 20%. CP: Acquisition of data, drafting the article, final approval of the article and responsible for the integrity of the article = 15%. AL, PC, KLM: Conception and design, drafting the article, final approval of the article and responsible for the integrity of the article = 15%.
Acknowledgements
Sara Vogrin and Emily Karahalios‐Biostatisticians at Western Health Centre for Health Research and Education, Sunshine hospital, St Albans, Australia. Mary Sulliva, nurse coordinator; Naomi Gandler‐Dietitian and Donna Cooper, data assistant at St. Vincent's Hospital, Melbourne, Australia.
̂ [Correction added on 14 June 2017, after first online publication: Affiliation 4 has been corrected to “Surgery, St. Vincent's Hospital, University of Melbourne, Melbourne Victoria, Australia,”]
References
- 1. March L, Smith EU, Hoy DG et al (2014) Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol 28, 353–66. [DOI] [PubMed] [Google Scholar]
- 2. Cross M, Smith E, Hoy D et al (2014) The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 73, 1323–30. [DOI] [PubMed] [Google Scholar]
- 3. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC (2005) The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology 44, 1531–7. [DOI] [PubMed] [Google Scholar]
- 4. Lybrand S (2003) Health related quality of life and the burden of disease in Australian rheumatology practice [Masters thesis]. University of Queensland, Brisbane. [Google Scholar]
- 5. Fernandes L, Hagen KB, Bijlsma JW et al (2013) EULAR recommendations for the non‐pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 72, 1125–35. [DOI] [PubMed] [Google Scholar]
- 6. Hochberg MC, Altman RD, April KT et al (2012) American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 64, 465–74. [DOI] [PubMed] [Google Scholar]
- 7. Jevsevar DS (2013) Treatment of osteoarthritis of the knee: evidence‐based guideline. J Am Acad Orthop Surg 21, 571–6. [DOI] [PubMed] [Google Scholar]
- 8. McAlindon TE, Bannuru RR, Sullivan M et al (2014) OARSI guidelines for the non‐surgical management of knee osteoarthritis. Osteoarthritis Cartilage 22, 363–88. [DOI] [PubMed] [Google Scholar]
- 9. Bachmeier CJM, March LM, Cross MJ et al (2001) A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis Cartilage 9 (2), 137–46. [DOI] [PubMed] [Google Scholar]
- 10. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster J‐Y (2004) Health‐related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint surg. Am. 86, 963–74. [DOI] [PubMed] [Google Scholar]
- 11. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD (2010) Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res 468 (1), 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schafer T, Pritzkuleit R, Jeszenszky C et al (2013) Trends and geographical variation of primary hip and knee joint replacement in Germany. Osteoarthritis Cartilage 21, 279–88. [DOI] [PubMed] [Google Scholar]
- 13. Ackerman IN, Dieppe PA, March LM et al (2009) Variation in age and physical status prior to total knee and hip replacement surgery: a comparison of centers in Australia and Europe. Arthritis Rheum 61, 166–73. [DOI] [PubMed] [Google Scholar]
- 14. Dixon T, Urquhart DM, Berry P et al (2011) Variation in rates of hip and knee joint replacement in Australia based on socio‐economic status, geographical locality, birthplace and indigenous status. ANZ J Surg 81 (1–2), 26–31. [DOI] [PubMed] [Google Scholar]
- 15. Dixon T, Shaw ME, Dieppe PA (2006) Analysis of regional variation in hip and knee joint replacement rates in England using Hospital Episodes Statistics. Public Health 120 (1), 83–90. [DOI] [PubMed] [Google Scholar]
- 16. Dieppe P, Basler H, Chard J et al (1999) Knee replacement surgery for osteoarthritis: effectiveness, practice variations, indications and possible determinants of utilization. Rheumatology 38 (1), 73–83. [DOI] [PubMed] [Google Scholar]
- 17. Dowsey MM, Choong PF (2013). Predictors of pain and function following total joint replacement. INTECH Open Access Publisher, Rijeka. [Google Scholar]
- 18. March L, Amatya B, Osborne RH, Brand C (2010) Developing a minimum standard of care for treating people with osteoarthritis of the hip and knee. Best Pract Res Clin Rheumatol 24 (1), 121–45. [DOI] [PubMed] [Google Scholar]
- 19. Zhang W, Moskowitz R, Nuki G et al (2008) OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence‐based, expert consensus guidelines. Osteoarthritis Cartilage 16 (2), 137–62. [DOI] [PubMed] [Google Scholar]
- 20. Muraki S, Oka H, Akune T et al (2009) Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population‐based cohorts: The ROAD study. Osteoarthritis Cartilage 17, 1137–43. [DOI] [PubMed] [Google Scholar]
- 21. van Dijk GM, Dekker J, Veenhof C, van den Ende CH (2006) Course of functional status and pain in osteoarthritis of the hip or knee: a systematic review of the literature. Arthritis Care Res 55, 779–85. [DOI] [PubMed] [Google Scholar]
- 22. Dieppe P, Lim K, Lohmander S (2011) Who should have knee joint replacement surgery for osteoarthritis? Int J Rheum Dis 14, 175–80. [DOI] [PubMed] [Google Scholar]
- 23. Dowsey MM, Gunn J, Choong PF (2014) Selecting those to refer for joint replacement: who will likely benefit and who will not? Best Pract Res Clin Rheumatol 28 (1), 157–71. [DOI] [PubMed] [Google Scholar]
- 24. Dowsey MM, Nikpour M, Dieppe P, Choong PF (2016) Associations between pre‐operative radiographic osteoarthritis severity and pain and function after total hip replacement. Clin Rheumatol 35 (1), 183–9. [DOI] [PubMed] [Google Scholar]
- 25. Dowsey MM, Spelman T, Choong PF (2016) Development of a prognostic nomogram for predicting the probability of nonresponse to total knee arthroplasty 1 year after surgery. J Arthroplasty 31 (8), 1654–60. [DOI] [PubMed] [Google Scholar]
- 26. Brand C, Hunter D, Hinman R, March L, Osborne R, Bennell K (2011) Improving care for people with osteoarthritis of the hip and knee: how has national policy for osteoarthritis been translated into service models in Australia? Int J Rheum Dis 14, 181–90. [DOI] [PubMed] [Google Scholar]
- 27. Network AM (2012). Osteoarthritis chronic care program model of care. Agency for Clinical Innovation, Sydney. [Google Scholar]
- 28. Brown C, Gordon B, Bucknill A (2011) The Impact of the Osteoarthritis Hip and Knee Service (OAHKS) in Melbourne health: a review from 2006–2009. Orthopaedic Proc 93‐B (Suppl. II), 211. [Google Scholar]
- 29. Teoh LS, Eyles JP, Makovey J, Williams M, Kwoh CK, Hunter DJ (2016) Observational study of the impact of an individualized multidisciplinary chronic care program for hip and knee osteoarthritis treatment on willingness for surgery. Int J Rheum Dis 2016 Dec 12. doi: 10.1111/1756‐185x.12950. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30. Agency for Clinical Innovation (2015). Osteoarthritis Chronic Care Program evaluation. NSW Health, Sydney, NSW. [Google Scholar]
- 31. Le Marshall K, Yee B, Dieppe P et al (2013) Differences between patients with hip and knee osteoarthritis. Osteoarthritis Cartilage 21, S139. [Google Scholar]
- 32. Gossec L, Paternotte S, Bingham CO 3rd et al (2011) OARSI/OMERACT initiative to define states of severity and indication for joint replacement in hip and knee osteoarthritis. An OMERACT 10 Special Interest Group. J Rheumatol 38, 1765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jordan K, Arden N, Doherty M et al (2003) EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 62, 1145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang W, Doherty M, Arden N et al (2005) EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 64, 669–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osborne R, Bucknill A, De Steiger R, Brand C, Graves S (2012) Validation of the Multi‐Attribute Prioritisation Tool (MAPT) for hip and knee osteoarthritis against WOMAC, Oxford Hip/Knee Score. J Bone Joint Surg Br 94 (Suppl. XXIII), 37.22219245 [Google Scholar]
- 36. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD (1998) Knee injury and osteoarthritis outcome score (KOOS)—development of a self‐administered outcome measure. J Orthop Sports Phys Ther 28 (2), 88–96. [DOI] [PubMed] [Google Scholar]
- 37. Roos EM, Toksvig‐Larsen S (2003) Knee injury and osteoarthritis outcome score (KOOS)–validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes 1 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Collins NJ, Prinsen CAC, Christensen R, Bartels EM, Terwee CB, Roos EM (2016) Knee injury and osteoarthritis outcome score (KOOS): systematic review and meta‐analysis of measurement properties. Osteoarthritis Cartilage 24, 1317–29. [DOI] [PubMed] [Google Scholar]
- 39. Nilsdotter AK, Lohmander LS, Klässbo M, Roos EM (2003) Hip disability and osteoarthritis outcome score (HOOS)–validity and responsiveness in total hip replacement. BMC Musculoskelet Disord 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Altman R, Asch E, Bloch D et al (1986) Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum 29, 1039–49. [DOI] [PubMed] [Google Scholar]
- 41. Altman R, Alarcón G, Appelrouth D et al (1991) The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum 34, 505–14. [DOI] [PubMed] [Google Scholar]
- 42. Kellgren J, Lawrence J (1957) Radiological assessment of osteo‐arthrosis. Ann Rheum Dis 16, 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vincent KR, Conrad BP, Fregly BJ, Vincent HK (2012) The pathophysiology of osteoarthritis: a mechanical perspective on the knee joint. PM R 4 (5 Suppl.), S3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Amin S, Luepongsak N, McGibbon CA, LaValley MP, Krebs DE, Felson DT (2004) Knee adduction moment and development of chronic knee pain in elders. Arthritis Care Res 51, 371–6. [DOI] [PubMed] [Google Scholar]
- 45. Messier SP, Mihalko SL, Legault C et al (2013) Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 310, 1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cammarata ML, Schnitzer TJ, Dhaher YY (2011) Does knee osteoarthritis differentially modulate proprioceptive acuity in the frontal and sagittal planes of the knee? Arthritis Rheum 63, 2681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Culliford DJ, Maskell J, Kiran A et al (2012) The lifetime risk of total hip and knee arthroplasty: results from the UK general practice research database. Osteoarthritis Cartilage 20, 519–24. [DOI] [PubMed] [Google Scholar]
- 48. Eitzen I, Fernandes L, Kallerud H, Nordsletten L, Knarr B, Risberg MA (2015) Gait characteristics, symptoms, and function in persons with hip osteoarthritis: a longitudinal study with 6 to 7 years of follow‐up. J Orthop Sports Phys Ther 45, 539–49. [DOI] [PubMed] [Google Scholar]
- 49. Miller FG, Kaptchuk TJ (2008) The power of context: reconceptualizing the placebo effect. J R Soc Med 101, 222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Doherty M, Dieppe P (2009) The “placebo” response in osteoarthritis and its implications for clinical practice. Osteoarthritis Cartilage 17, 1255–62. [DOI] [PubMed] [Google Scholar]


