Abstract
Elaboration of tumor necrosis factor (TNF) is a very early event in development of ischemia/reperfusion injury pathophysiology. Therefore, TNF may be a prominent mediator of endothelial cell and vascular wall dysfunction in sickle cell anemia, a hypothesis we addressed using NY1DD, S+SAntilles, and SS‐BERK sickle transgenic mice. Transfusion experiments revealed participation of abnormally activated blood monocytes exerting an endothelial activating effect, dependent upon Egr‐1 in both vessel wall and blood cells, and upon NFκB(p50) in a blood cell only. Involvement of TNF was identified by beneficial impact from TNF blockers, etanercept and infliximab, with less benefit from an IL‐1 blocker, anakinra. In therapeutic studies, etanercept ameliorated multiple disturbances of the murine sickle condition: monocyte activation, blood biomarkers of inflammation, low platelet count and Hb, vascular stasis triggered by hypoxia/reoxygenation (but not if triggered by hemin infusion), tissue production of neuro‐inflammatory mediators, endothelial activation (monitored by tissue factor and VCAM‐1 expression), histopathologic liver injury, and three surrogate markers of pulmonary hypertension (perivascular inflammatory aggregates, arteriolar muscularization, and right ventricular mean systolic pressure). In aggregate, these studies identify a prominent—and possibly dominant—role for an abnormal monocyte‐TNF‐endothelial activation axis in the sickle context. Its presence, plus the many benefits of etanercept observed here, argue that pilot testing of TNF blockade should be considered for human sickle cell anemia, a challenging but achievable translational research goal.
1. INTRODUCTION
A chronic and robust systemic inflammatory state is a striking feature and pathogenic factor in sickle cell anemia (SCA).1 Hence, identification of the core vector(s) underlying inflammation's evolution and perpetuation should identify useful therapeutic targets. As general underlying processes, attention has focused upon vascular occlusion as the initiator of ischemia/reperfusion injury (I/R) pathophysiology2 and upon hemolysis as a source of toxic heme.3, 4 Beyond this, however, the role of specific mediators as antecedent agents remains opaque in its intricacy. Indeed, available data on SCA do not even enable parsing potential mediators into those acting proximately versus more distally.
The literature on SCA, however, does document abnormal activation of blood monocytes and their ability to activate and/or damage vascular endothelial cells in vitro.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 This suggests monocyte prominence in clinical disease genesis, if only because monocyte/macrophages are dominant generators of pro‐inflammatory cytokines in the broad context of inflammation's generative role in vascular disease generally.18 The present studies implicate a disease causing vector extending from peripheral blood monocytes (PBM) to the vascular endothelium, with the bridging mediator being tumor necrosis factor (TNF, aka TNFα).
Our focus upon TNF stems from its roles as a “sentinel cytokine,” largely post‐transcriptionally regulated, and as an acute‐phase initiator of oxidant species and NFκB‐driven (and other) responses that have defensive, beneficial roles.19 Yet, its obverse, maladaptive potential can be realized when TNF is produced in excess and/or absent appropriate resolution. Then, its pleiotropic effects can induce multi‐faceted inflammatory pathology. Thus, it is the striking clinical benefit of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases19, 20 that prompts our interest in this approach to the stubborn chronicity of the SCA inflammatory state. Yet, the perplexing complexity of TNF biology makes efficacy of TNF‐blocking agents impossible to predict with assurance.
This deserves exploration because many well‐known TNF effects are directly relevant to pathobiology of clinical sickle disease. To illustrate, we simply focus upon the vascular endothelium, the blood/tissue interface of enormous importance in multiple biological processes. Most globally harmful, TNF causes degradation of the glycocalyx,21 thus jeopardizing its critical roles that include: mediation of shear‐dependent functions (e.g., NO production); anchorage of surface enzymes; and repelling potentially adherent blood cells. Separately, TNF jeopardizes NO bioavailability by activating both endothelial arginase (starving eNOS of its required substrate, arginine22) and endothelial NADPH oxidase (depleting tetrahydrobiopterin to provoke superoxide generation by eNOS23). Experimentally, TNF induces endothelial adhesion molecule expression to promote RBC adhesion24 and vasoocclusion.25 TNF exerts many additional adverse effects upon and beyond endothelial cells.
At the level of clinical disease, TNF plays a prominent causative role in organ diseases of general medicine, and these may be instructive regarding their counterparts in SCA. Examples include TNF's role in: pulmonary hypertension;26 asthma;27 sleep apnea;28 left ventricular dysfunction;29 cognitive, neuropsychiatric and neurologic impairments;30 and pain syndromes.31, 32 For most of these organ manifestations within general medicine, TNF blockade using etanercept has yielded clinical improvement.
Therefore, TNF is a therapeutic target that should be considered in SCA. The studies reported here examined effects of the TNF blocker, etanercept, utilizing three sickle transgenic mouse models that exhibit a systemic inflammatory state mimicking that of human sickle disease.33, 34 The resulting data create a framework within which this intervention can be envisioned in the sickle disease context.
Note that, due to complexity and variety of experiments, interpretation of individual experiment sets is included in Results section, so that Discussion can address the broader issues. (The data reported here were presented, in preliminary form, at meetings of the American Society of Hematology, 2007–2013).
2. MATERIALS/METHODS
Some Methods are presented in greater detail in Supporting Information Methods.
2.1. Drugs
Etanercept, a chimeric fusion of human IgG1 Fc domain and the 75 kDa extracellular portion of human TNFR2,35 acts as a “decoy” by binding TNF. It is known to block TNF in murine experimental inflammatory disease.36 It also binds the lymphotoxin family, less understood mediators that use the same receptors and mimic TNF itself. Our etanercept dosing (3–10 mg/kg) falls in the lower end of the range used during its preclinical development. We estimate that our highest dose would provide ∼103‐fold molar excess over murine blood TNF level.37 By comparison, clinical trials of etanercept for human rheumatoid arthritis used dosing that could have achieved a ∼105‐fold excess over human TNF level.
Infliximab is a chimeric, mouse/human hybrid monoclonal specific for TNF. Dosing here (10 mg/kg) would achieve the same fold‐excess as for our highest‐dose etanercept. The typical human dose is 3 mg/kg, given IV, every few weeks.
Anakinra, a recombinant form of the human IL‐1 receptor antagonist, blocks signaling by IL‐1α and IL‐1β. We examined it because of prior use of an (undefined) IL‐1 blocker by Kaul et al.38 Dosing here was 10 mg/kg, with unknown ratio to IL‐1 receptor. Anakinra is known to impede experimental murine inflammation.39
Scrambled Control Peptide, obtained via custom synthesis, contains the 12 amino acids of the terminal end of the TNFR2 in scrambled order. For simplicity and to economize on animal consumption, this was always given at 0.1 mg/kg per dose, on same schedule as the other agents.
2.2. Mice
Mice were housed and bred in our institution's specific‐pathogen‐free facility, with routine surveillance testing, to avoid confounding infectious disease. Studies were done with the approval of our Institutional Animal Care and Use Committee. We used NY1DD, S+SAntilles and SS‐BERK sickle transgenic mice exhibiting, respectively, mild to moderate to severe phenotypes.34 For NY1DD and S+SAntilles mice we used C57BL/6 controls. For mixed background BERK mice, our breeding strategy ensured background homogenization between SS‐BERK and their AA‐BERK controls.
2.2.1. Cross‐breeding
We separately crossbred knockout states for NFκB(p50)14 and Egr‐140 into NY1DD mice, using the same strategy we previously described.14 Additionally, we crossbred the NFκB(p50) knockout into SAntilles mice. Then NFκB(p50)‐/‐ NY1DD and NFκB(p50)‐/‐ SAntilles were crossbred to ultimately obtain NFκB(p50)‐/‐ S+SAntilles mice. Such knockout transfers always included >10 backcrosses against wild‐type C57BL/6.
2.2.2. Marrow transplantation
As described,14 we previously conducted reciprocal marrow transplantations between wild‐type and NFκB(p50)−/− NY1DD to obtain NY1DD mice having NFκB(p50)−/− in blood cells but not endothelium/tissue, or conversely having NFκB(p50)−/− in endothelium/tissue but not blood cells. Using the same strategy, we here created NY1DD mice that were Egr‐1‐/‐ in blood cells but not endothelium/tissue, or vice versa.
2.3. Study protocols
We tested the impact of drugs, transcription factor knockouts, or transfusion of PBMC using animals at 3.5–4 months of age, unless otherwise indicated. Experiments used pooled animals from multiple litters, with pups from any one litter divided into both test and control groups, using both males and females. Drug treatment studies always included parallel controls receiving scrambled peptide (on same schedule as the test drugs). Drugs were given by intra‐peritoneal (ip) injection for short‐term experiments and by sub‐cutaneous (sq) injection for long‐term experiments.
2.3.1. Ischemia/reperfusion (I/R) model
Some NY1DD mice were subjected to hypoxia/reoxygenation (H/R) stress: 3 hr at normobaric 8% O2, followed by reoxygenation (return to room air, typically overnight).34 This rapidly triggers actual ischemia/reperfusion (I/R) in sickle –but not normal– mice.2, 14, 34 Some animals were pretreated with etanercept or infliximab or anakinra, at 10 mg/kg.
2.3.2. Transfusion of PBMC (peripheral blood mononuclear cells)
Using cardiac blood from pooled donors to isolate PBMC, we transfused 4 × 106 PBMC into naive recipient mice. Some PBMC preparations were monocyte depleted.14 Some PBMC donor mice were post‐H/R; some donors or recipient mice were pretreated with etanercept at 10 mg/kg.
2.3.3. Long‐term therapeutic studies
We treated S+SAntilles with etanercept (3 mg/kg, sq, once weekly, for 13 weeks), while BERK mice received higher dosing (3 mg/kg for 3 weeks or 6 mg/kg for 6 weeks, sq, twice weekly).
2.4. Endpoints examined
2.4.1. Blood counts and inflammatory markers
We performed CBC on cardiac blood using an analyzer standardized for murine blood. We used ELISA kits to assess plasma biomarker levels, and FACS to detect monocyte activation.
2.4.2. Endothelial cell activation
We monitored expression of Tissue Factor (TF; expressed as the percent of pulmonary veins positive) and vascular cell adhesion molecule 1 (VCAM‐1; expressed as percent of 50 ± 10 μm microvessels having >50% circumferential positivity).34
2.4.3. Vascular stasis
Using S+SAntilles mice with implanted dorsal skinfold chambers, we determined the percentage of previously‐flowing microvessels that developed vascular stasis after provocation with either H/R or infusion of hemin.3 Some were pretreated with etanercept.
2.4.4. Dermal Cytokines/neuropeptides
We tested cytokine/neuropeptide levels in conditioned medium from skin biopsies (obtained from long‐term etanercept‐treated SS‐BERK mice) that were incubated ex vivo for 24 hours, as described.41
2.4.5. Peri‐vascular inflammatory aggregates
We used antibodies to CD11b and von Willebrand Factor (vWF) to identify CD11b+ cells that were perivascular. We discovered this abnormality only retrospectively, so retrievable data are from frozen lung sections that were not prepared with prospective intent to quantify lesion prevalence.
2.4.6. Pulmonary arteriolar muscularization
The same caveat applies here as well. We used antibodies to murine smooth muscle actin (SMA) and vWF to score 50 ± 10 μm microvessels as non‐muscular, partially muscular, or fully muscular. A single value for each mouse was derived from at least 50 vessels from three separated sections.
2.4.7. Measurement of RVSP (right ventricular mean systolic pressure)
Using isoflurane anesthesia, standardized mechanical ventilation, and a para‐sternal lateral thoracotomy,42 we placed a right ventricular pressure‐transducing catheter to capture waveform data that enabled calculation of RVSP. We measured multiple parameters to assess RV mass.
2.5. Statistics
We made data comparisons using t testing, paired or unpaired, ANOVA as indicated.
3. RESULTS
We studied transgenic sickle mouse models having phenotypes ranging from mild to severe: NY1DD, S+SAntilles and SS‐BERK.34 We identified endothelial activation by monitoring endothelial expression level of tissue factor (TF) and VCAM‐1. Studies utilized TNF blocker etanercept (E), with a control peptide (CP) always examined in parallel; the latter uniformly failed to exert any effect compared to no treatment. Therefore, to simplify graphics, we focus upon only the most important comparisons. An interpretation of individual experiment sets is included within Results, so Discussion can address the broader issues.
4. IDENTIFICATION OF A MONOCYTE‐TNF‐ENDOTHELIAL ACTIVATION AXIS
4.1. Activated monocytes cause endothelial activation in sickle mice (Figure 1)
Figure 1.
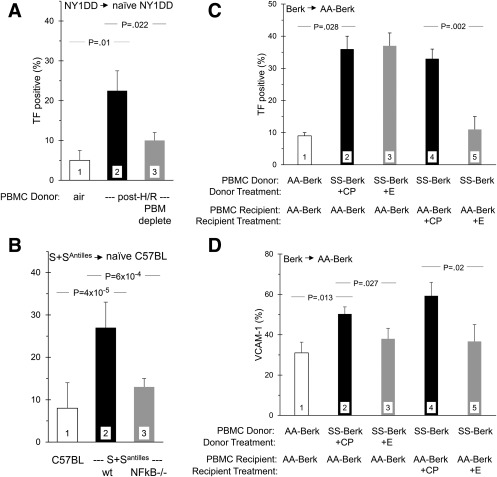
Transfusion experiments identify the role of PBM in endothelial cell activation. Peripheral blood mononuclear cells (PBMC) from donor mice were transfused into recipient mice, and pulmonary endothelial activation was monitored via expression of TF and/or VCAM‐1. Some mice were pretreated either with etanercept (+E) or control peptide (+CP). Since the CP had no impact compared to wholly unmanipulated control animals, that is not shown. Panel A. Transfusion of NY1DD PBMC (from unstressed donors) into naive NY1DD recipients did not increase recipient endothelial TF (bar 1). PBMC from post‐H/R NY1DD donors did do so (bar 2), an effect ameliorated by specific depletion of PBM from the PBMC population (bar 3). For Panel A, n = 3,3,3. Previously reported,14 this single experiment is reproduced in Panel A to establish context. Panel B. Transfusion of C57BL6 PBMC into C57BL6 recipients did not activate recipient TF (bar 1), but transfusion of S+SAntilles PBMC did do so (bar 2). This impact of S+SAntilles PBMC was eliminated if the donor mice had concurrent NFB(p50)‐/‐ (bar 3) For Panel B, n = 5,6,6. Panel C. AA‐BERK PBMC transfused into AA‐BERK recipients did not activate TF expression (bar 1), but SS‐BERK PBMC did do so (bar 2). Pretreatment of the PBMC donors with either control peptide (bar 2) or etanercept (bar 3) had no effect. However, pretreatment of the PBMC recipients with etanercept greatly blunted the TF activating effect (bar 5). For Panel C, n = 3,3,3,3,3. Panel D. For the same mice shown in Panel C, etanercept pretreatment of either PBMC donors (bar 3) or PBMC recipients (bar 5) reduced recipient VCAM‐1 expression. For Panel D, n = 3,3,3,3,3
Using all three sickle mouse models, we demonstrated the importance of peripheral blood monocytes (PBM) via transfusion experiments in which peripheral blood mononuclear cells (PBMC) were infused into appropriate control recipient mice.
To illustrate context, our relevant previously published experiment14 is illustrated here as Figure 1A. When infused into naive (i.e., unstressed) NY1DD recipients, the PBMC harvested from control NY1DD did not activate endothelial TF (bar 1); but infusion of PBMC from H/R‐stressed NY1DD donors did do so (bar 2). This activating effect was ameliorated if that donor PBMC population was selectively depleted of PBM (bar 3). Thus, PBM activation accounts for the endothelial activation state that is triggered when NY1DD mice are experimentally stressed with H/R exposure to induce I/R.2, 14, 34 Therefore, we here sought to corroborate this using sickle mice that spontaneously exhibit an inflammatory state, i.e., not requiring challenge by a stressor.
4.1.1. S+SAntilles mice
Infusion of PBMC from S+SAntilles donors (Figure 1B, bar 2), but not infusion of PBMC from C57BL6 control donors (Figure 1B, bar 1), activated TF in C57BL6 recipient mice. This activating effect was lost if the S+SAntilles donors concurrently were NFκB(p50)‐/‐ (Figure 1B, bar 3). This is supportive because we previously had demonstrated, using NY1DD mice, that NFκB(p50)‐/‐ eliminates ability of PBMC to activate endothelium.14
4.1.2. SS‐BERK mice
Using BERK mice, we also identified the activating role of PBMC on endothelial TF (Figure 1C), as well as endothelial VCAM‐1 (Figure 1D). In AA‐BERK recipients, activation of both endothelial antigens resulted from infusion of PBMC from SS‐BERK donors (bars 2) but not from AA‐BERK control donors (bars 1).
4.2. Endothelial activation is TNF‐dependent in sickle mice
We identified involvement of TNF first using the H/R stressed NY1DD mice that developed increased expression of both TF and VCAM‐1 (Figure 2A,B, bars 2), compared to baseline expression in unstressed control NY1DD (bars 1). This activation was unaffected by pretreatment with control peptide (CP, bars 3) or heat‐inactivated etanercept (bars 4). However, pretreatment with intact etanercept clearly exerted dose‐dependent inhibition (bars 5 and 6), also seen using the TNF‐specific inhibitor, infliximab (bars 8). By comparison, the IL‐1 receptor inhibitor, anakinra, was a less potent inhibitor of TF than was etanercept; but for VCAM‐1 inhibition, anakinra exhibited potency equivalent to etanercept (bars 7).
Figure 2.
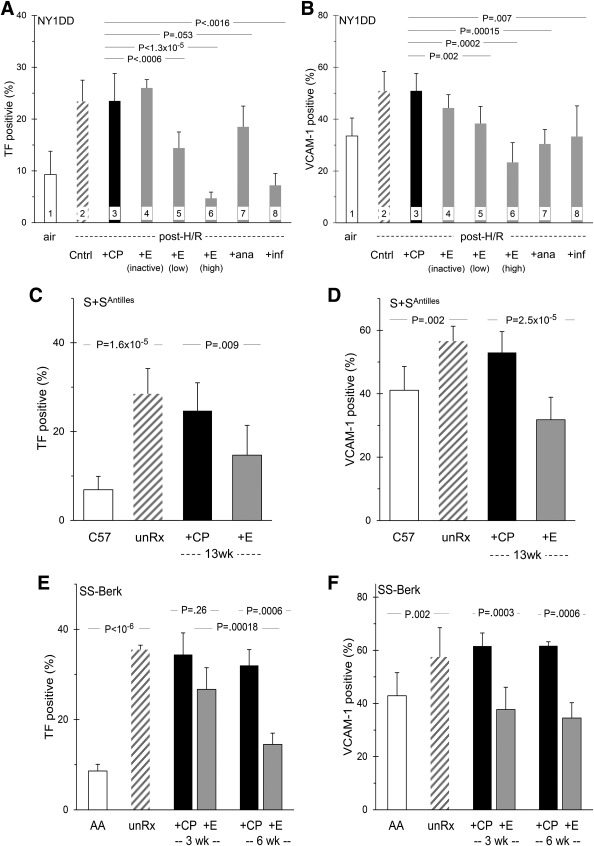
TNF blockade ameliorates endothelial activation in sickle mice. Panel A. The low TF expression of unstressed NY1DD mice (bar 1) is increased by H/R exposure (bar 2). Pretreatment with control peptide (bar 3) or with heat inactivated etanercept (bar 4) had no effect, but etanercept exerted dose‐dependent inhibition (bar 5 and 6). IL‐1 blocker anakinra had very little effect (bar 7), but TNF‐specific blocker infliximab strongly inhibited TF (bar 8). For Panel A, n = 8,8,8,4,10,3,8,5. Panel B shows inhibition of VCAM‐1 expression in those same NY1DD mice. For Panel B, n = 10,10,8,3,8,3,5,4. Panels C and D. S+SAntilles mice had elevated TF and VCAM‐1 (bars 2), compared to their C57BL controls (bars 1). After long‐term treatment (13 weeks), this was inhibited by etanercept (bars 4) but not control peptide (bars 3). For Panel C, n = 7,4,6,11. For Panel D, n = 7,4,5,6. Panels E and F. SS‐BERK mice had elevated TF and VCAM‐1 (bars 2), compared to their AA‐BERK controls controls (bars 1). Etanercept treatment for 3 weeks (bars 4) or 6 weeks (bars 6) inhibited TF and VCAM‐1 expression; control peptide did not (bars 3 and 5). For Panels E and F, n = 6,4,5,6,3,4
4.3. Relationship between PBM and TNF in endothelial activation
Because the biology of TNF is so complex (see Discussion), we examined this apparent PBM‐TNF link in more detail, using PBMC transfusions and the BERK model. AA‐BERK recipient mice that received PBMC from AA‐BERK donors exhibited the lower baseline expression of TF (Figure 1C) and VCAM‐1 (Figure 1D) (bars 1). But recipients that received PBMC from SS‐BERK donors developed activation with higher expression of both TF and VCAM‐1 (bars 2). These starting data enable interpretation of the following experiment.
4.3.1. TF
When we pretreated the SS‐BERK donors of with etanercept, their PBMC still induced endothelial TF expression in the AA‐BERK recipients (Figure 1C, bar 3). However, when we pretreated the AA‐BERK recipients with etanercept, the subsequently transfused SS‐BERK PBMC were unable to activate endothelial TF in those recipients (bar 5). Thus, etanercept's blockade of endothelial TF expression apparently requires the drug to be present when the active PBMC are present. In turn, this implies that etanercept acts either by binding sTNF in plasma or by engaging with tmTNF on endothelial cells themselves.
4.3.2. VCAM‐1
When we examined the same mice for endothelial VCAM‐1 expression, a different result emerged. The elevation of recipient VCAM‐1 caused by transfusion of SS‐BERK PBMC (Figure 1D, bar 2) was ameliorated by etanercept pretreatment of either the PBMC donor (bar 3) or the PBMC recipient (bar 5). Thus, etanercept's blockade of endothelial VCAM‐1 expression presumably results from the drug modifying the PBMC themselves. One possibility is that etanercept could engage tmTNF on the PBM, with a consequent down‐regulating effect on their ability to produce TNF, an effect that persisted when the PBM were then transfused.
4.4. Divergence of TF versus VCAM‐1 activating mechanisms
Thus, results described so far include two suggestions that activation of endothelial TF and VCAM‐1 expression in the sickle I/R context may involve somewhat differing mechanisms. First, the results of treating mice with the TNF blocker etanercept vs. the IL‐1 blocker anakinra suggest that endothelial TF and VCAM‐1 may have non‐identical underlying mechanisms (Figure 2A,B). Second, the data probing the PBM/etanercept relationship suggested that etanercept may be targeting different features of TNF biology in its inhibition of TF vs VCAM‐1 (Figure 1C,D). For this reason, we here describe ancillary experiments that bear on mechanistic divergence of etanercept action and its potential implications for therapeutics.
We focused upon NFκB and Egr‐1 because these are dominant regulators of TF expression.
4.4.1. Egr‐1
Using the post‐H/R NY1DD model, we observed that Egr‐1‐/‐ eliminated the increased endothelial TF expression induced by sickle I/R. This was true both if Egr‐1‐/‐ was in endothelium/tissue but not blood cells, and if Egr‐1‐/‐ was in blood cells but no endothelium/tissue (Supporting Information Figure S1). These findings are consistent, respectively, with Egr‐1′s known prominence as a regulator of TF expression43 and with its suspected role in monocyte TNF expression.44
4.4.2. NFκB(p50)
We previously reported that the impact of PBM on endothelial TF expression actually requires a NFκB(p50) dependent gene within blood cells.14 It is relevant that NFκB(p50) is believed to participate indirectly in PBM production of TNF.45 Interestingly, however, we have now observed that NFκB(p50)‐/‐ in sickle mice actually causes increased expression of endothelial VCAM‐1 (Supporting Information Figure S1). This can be explained simply by the NFκB p50/p65 heterodimer's less robust promotion of gene expression, compared to that of the NFκB p65/p65 homodimer.46 Hence, a knockout of p50 effectively removes a braking effect.
4.4.3. NFκB inhibitors
These data suggest it may be advisable to avoid NFκB inhibitors that are truly specific for the p50 component. Fortunately, most are not p50 specific. Nonetheless, we here examined the VCAM‐1 expression impact of the NFκB inhibitors we previously identified as effective inhibitors of endothelial TF in sickle mice.14, 34, 47 We find several of those agents to be effective also in ameliorating the increased VCAM‐1 in post‐H/R NY1DD mice: lovastatin, andrographolide, curcumin, sulfasalazine and histone deacetylase inhibitors (Supporting Information Table S1).
This VCAM‐1 expression scoring was enabled by our discovery, during the course of these studies of an unsuspected, regional heterogeneity in the dynamism of VCAM‐1′s activation in sickle mice. VCAM‐1 responses, both increases and inhibitions, were detectable only by focusing on vessels 50 ± 10 μm diameter.
5. THERAPEUTIC BENEFIT FROM TNF BLOCKADE
To assess etanercept in a therapeutic context, we conducted long‐term studies giving etanercept vs. control peptide to S+SAntilles or SS‐BERK mice. Doses, schedules and length of treatment are described in Methods. Etanercept exerted a beneficial impact upon nearly all of the endpoints evaluated as being relevant to human sickle disease.
5.1. Inflammatory biomarkers (Table 1)
Table 1.
| Long‐term Treatment Agent | ||||
|---|---|---|---|---|
| Parameters | CP | E | P. | |
| Blood counts | ||||
| Hb | (g/dl) | 9.1 + 1.3 (10) | 11.2 ± 1.1 (9) | .032 |
| Reticulocytes | (%) | 4.1 ± 1.1 (5) | 3.5 ± 2.5 (4) | .674 |
| WBC | (×103/μL) | 9.0 ± 6.8 (9) | 7.3 ± 5.3 (9) | .564 |
| Platelets | (×103/μL) | 834 ± 125 (6) | 1249 ± 46 (4) | .0006 |
| Biomarkers | ||||
| sVCAM‐1 | (ng/mL) | 1053 ± 84 (6) | 827 ± 55 (5) | .027 |
| SAP | (μg/mL) | 555 ± 101 (5) | 367 ± 115 (5) | .025 |
| TNF | (pg/mL) | 14.7 ± 6.1 (4) | 3.5 ± 0.16 (5) | .0053 |
| MCP‐1 | (pg/mL) | 60.9 ± 29.0 (4) | 23.2 ± 13.9 (5) | .0359 |
| Neopterin | (pg/mL) | 650 ± 24 (6) | 600 ± 165 (5) | ns |
| Monocyte Activation | ||||
| TNF + | (%) | 13.2 ± 1.0 (12) | 4.6 ±3.3 (12) | .001 |
| CD11b + | (MFI) | 257 ± 13 (12) | 229 ± 21 (12) | ns |
| IL‐6 + | (MFI) | 20.1 ± 3.6 (12) | 25.0 ± 14.8 (12) | ns |
Data shown as mean ± SD (N), for S+SAntilles mice treated 13 weeks with control peptide (CP) vs the TNF blocker etancercept (E; 3 mg/kg, once weekly). Abbreviations: Hb, hemoglobin; MCP, monocyte chemotactic protein; MFI, mean fluorescence intensity; RVSP, right ventricular mean systolic pressure; SAP, serum amyloid P; TF, tissue factor; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule; WBC, white blood count.
In general, blood biomarkers of inflammation responded favorably to etanercept, with significant improvements in: soluble VCAM‐1 (sVCAM‐1), a marker of endothelial activation; SAP (serum amyloid P), the murine equivalent of human CRP; TNF itself; and MCP‐1 (monocyte chemotactic protein 1) elaborated, e.g., by endothelial cells in response to TNF. Serum neopterin, a product of monocyte activation, was unhelpful. Blood monocyte activation status (by FACS) revealed a response to etanercept in the proportion of monocytes labeling positively for TNF; but proportions of CD11b+ or IL‐6+ did not respond.
Long‐term treatment of S+SAntilles mice with etanercept also improved blood Hb and platelet count. An abnormally low platelet count is a feature of murine sickle models (Supporting Information Table S2), as it is in some humans with sickle cell anemia.
5.2. Pulmonary endothelial activation state
Etanercept was an effective inhibitor of abnormal pulmonary endothelial cell expression of TF and VCAM‐1 in all three sickle mouse models models (Figure 2): the acute I/R triggered in NY1DD by H/R exposure (panels A and B); and the spontaneous, chronic I/R state of S+SAntilles (panels C and D) and SS‐BERK (panels E and F). Notably, the SS‐BERK mice revealed that the suppression of TF expression increased further with longer duration of etanercept treatment.
5.3. Neuro‐inflammatory mediators and pain
In vitro incubation of skin biopsies from SS‐BERK mice that had been treated long‐term with control peptide or etanercept enabled quantitative measurement of neuro‐inflammatory mediator elaboration from actual tissue. As previously reported, such data actually correlate with pain behaviors exhibited by sickle mice.41 Biopsies from the etanercept‐treated mice exhibited decreased elaboration of IL‐6, MCP‐1, substance P and CGRP (calcitonin gene‐related peptide) (Supporting Information Figure S2). Although not achieving significance due to small number of animals available, levels of IL‐1β, IL‐1α, IFNγ, and MIP‐1α suggested a downwards trend.
We know that the biologies of acute and chronic pain both involve inflammation having complicated, bidirectional effects between systemic and neuro‐inflammatory substances.31, 32 Pain behaviors could not be tested here, as the number of available animals was far too small. Yet, the present data suggest that therapy with etanercept might benefit pain.
5.4. Histopathology
In contrast to an absence of pathology in C57BL6 control mice, livers of S+SAntilles mice displayed acute or chronic coagulative necrosis, indicating ischemia or prior infarction. This was ameliorated both by long‐term treatment with etanercept and, revealingly, by presence of the NFκB(p50)‐/‐ state (Supporting Information Table S2).
5.5. Vascular occlusion
To assess etanercept impact upon acute vascular flow deficiency, we studied S+SAntilles mice bearing dermal windows (to enable microvascular flow visualization). We pretreated them with etanercept or control peptide and then challenged them with an insult known to trigger vascular stasis, either H/R2, 14, 34 or infusion of hemin.3 The stasis triggered by H/R was greatly diminished by etanercept (Figure 3A), but that triggered by hemin infusion was not blunted significantly (Figure 3B). This is consistent with our current understanding of these two stasis induction mechanisms. H/R exposure triggers I/R14, 34 which involves TNF elaboration in its very earliest stages. In contrast, hemin directly perturbs endothelium, causing stasis via TNF‐independent mechanisms in addition to TNF elaboration.3
Figure 3.
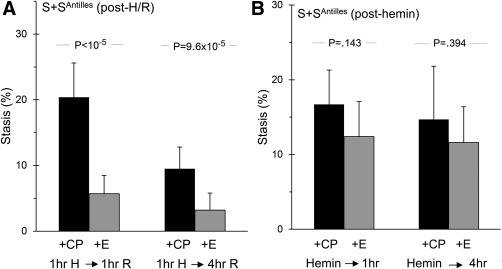
TNF blockade eliminates H/R‐triggered vascular stasis. S+SAntilles mice were pretreated either with control peptide (CP) or etanercept (E) and then provoked with H/R exposure or with infusion of hemin; stasis of previously‐flowing vessels was scored after 1 and 4 hours. Panel A. Pretreatment with etanercept eliminated H/R‐triggered stasis, its normal level in absence of H/R stress being <10%. Number of animals: 11,10,11,10. Panel B. Etanercept failed to significantly blunt stasis triggered by hemin infusion. Number of animals: 6,6,6,6
5.6. Pulmonary arterial disease
We examined three surrogate markers for presence of pulmonary hypertension.
5.6.1. Peri‐vascular inflammatory aggregates
Staining for CD11b+ evinced the presence of perivascular inflammatory cell aggregates (examples shown in Supporting Information Figure S3). Since the available material allowed only semi‐quantitation, we applied three different measures (Table 2). Each parameter revealed increased prevalence of these inflammatory aggregates in S+SAntilles compared to C57Bl6 control, and each was notably diminished in response to etanercept. Because such perivascular inflammatory aggregates are part of the histopathology of human pulmonary hypertension,48 this result prompted the following additional studies.
Table 2.
Prevalence of peri‐vascular inflammatory aggregates (CD11b+ cells)a
| C57BL6 | S+Sa ntilles | |||
|---|---|---|---|---|
| Semi‐quantification method . | control | unRx | CP | E . |
| mice with large aggregates (n) | 0/5 | 4/6 | 4/6 | 0/11 |
| vessels with smaller aggregate (n)b | 9 ± 8 | 47 ± 9 | 45 ± 6 | 22 ± 18 |
| cells per vessel (n)c | 1.5 ± 0.3 | 3.6 ± 0.4 | 2.9 ± 0.2 | 2.2 ± 0.5 |
S+SAntilles mice were untreated (unRx) or treated either with control peptide (CP) or etanercept (E) for 13 weeks. Data for small aggregates and cells/vessel are expressed as mean ± SD. Definitions: large and small aggregates were >20 cells or 3–20 cells, respectively, around any given vessel. Above 20, they were too numerous to be discretely identified or counted.
Untreated S+SAntilles vs. C57, P =.00034. CP vs. E treated S+SAntilles mice, P = .0038.
Untreated S+SAntilles vs. C57, P = 2.4 × 10−5. CP vs. E treated S+SAntilles, P = .042.
5.6.2. Pulmonary arteriolar muscularization
Staining for smooth muscle actin (SMA) (examples shown in Supporting Information Figure S3) suggested a trend towards increasing muscularization of ∼50 μm pulmonary arterial vessels as animals matured from age 1 to 4 months, seen for both C57BL6 and S+SAntilles mice (Figure 4A). However, at both ages, muscularization was greater for sickle than for normal mice. Notably, after 3 months of etanercept therapy (starting at age 1 month) the muscularization in S+SAntilles mice was significantly reduced. Indeed, it appears the etanercept may have both prevented further muscularization and somewhat reduced existing muscularization.
Figure 4.
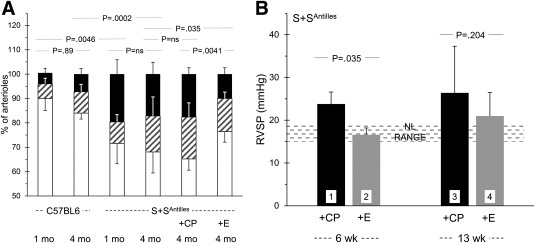
TNF blockade benefits surrogate measures of pulmonary hypertension. Panel A. Pulmonary arteriolar muscularization in S+SAntilles mice is plotted, each bar showing percent of arterioles with full (black), partial (hatched), or no (white) muscularization, defined by staining for SMC actin. [Examples of SMC actin staining images are provided in Supporting Information Figure S3]. Mouse age is shown in months. The P values compare total muscularization (i.e., partial plus full). Some mice received etanercept (E) or control peptide (CP), staring at 1 month of age. These revealed probable time‐dependence of muscularization degree, with more developing in S+SAntilles mice, and a favorable and significant response to etanercept. For Panel A, n = 4,5,5,5,5,5. Panel B. Etanercept given for 6 weeks lowered right ventricular (mean) systolic pressure to the normal range (bar 2). By the age achieved upon 13 weeks of treatment, pressures had risen, but etanercept appears still to have been effective, although this was not provable given the increasing variability that develops as the mice age. For Panel B, n = 3,5,4,6
5.6.3. Elevated right ventricular pressure
As a surrogate measure for possible elevation of pulmonary mean arterial pressure (i.e., pulmonary hypertension), we found that right ventricular (mean) systolic pressure (RVSP) was elevated in S+SAntilles mice compared to C57Bl6 controls. Treatment with etanercept for 6 weeks (started at weaning) eliminated RVSP elevation in sickle mice (Figure 4B). After 13 weeks of therapy, however, the differential between groups was preserved, but the statistical significance was lost. This loss may simply be because, as inspection indicates (Figure 4B), inter‐individual variability increased markedly as age advanced (as often happens in clinical complications of human SCA). The small number of animals precluded resolution of this.
For both treatment durations, the accompanying necropsy measures of right heart weights (RV/RV + LV; RV/tibia length; RV/body weight) did not reveal a significant change in response to etanercept in this study.
6. DISCUSSION
The present studies identify a monocyte‐TNF‐endothelial activation axis promotive of pleiotropic disturbances and damage. Results were consistent for the sickle mice of two different genetic backgrounds, and they were similar for both experimentally triggered I/R (NY1DD mice) and mice having chronic spontaneous I/R (S+SAntilles and SS‐BERK mice). We identified a substantial ameliorating effect of the TNF blocker, etanercept, upon nearly all endpoints evaluated, a diverse group of clinical features shared by sickle mice and humans. Thus, interruption of this monocyte‐TNF‐endothelial vector offers an interesting therapeutic targeting option.
6.1. Interpreting results of TNF blockade
The complexity of TNF biology renders interpretation of experiments challenging.19, 20 Signaling derives from both soluble TNF (sTNF) and cell‐bound trans‐membrane TNF (tmTNF). The sTNF activates via both receptors, TNFR1 and TNFR2; mTNF activates only via latter. One cell's tmTNF can receive outside‐in activating signals if it engages another cell's TNFR. Conversely, a TNF blocking antibody may induce positive or negative signaling within a cell upon engaging its tmTNF.49 Finally, plasma contains released TNFRs that can bind TNF. Despite this bewildering intricacy, its net impact and TNF‐dependence are experimentally demonstrable. In the present studies, the impact of etanercept was uniformly beneficial.
6.2. Evidence for a TNF role in sickle cell anemia
The SCA inflammatory state guarantees participation of TNF, the question being with what primacy. sTNF levels often are elevated in SCA,50, 51 but this itself may not be very useful information, as has been discovered in the classical TNF‐dependent disease, rheumatoid arthritis (RA). Nonetheless, the very spectrum of clinical complications in SCA is consistent with TNF being a participating inflammatory instigator (per Introduction).
Actually, RA provides an instructive paradigmatic context. Since humans having the TNF(‐308) promoter GG polymorphism are predisposed to develop inflammatory RA,52 it is interesting that SCA children having that G allele are predisposed to develop large vessel stroke53 that is caused by an inflammatory vasculopathy in the Circle of Willis.1 Indeed, we found that endothelial cells from sickle children with Circle of Willis disease exhibited an exaggerated NFκB activation response to stimulation with TNF/IL‐1.54
Thus, current concepts about TNF and inflammation generally, and disease consequent to the sickle mutation specifically, are consistent with the conclusions we draw from the present studies.
6.3. Is TNF actually the most proximate mediator?
This really is the wrong question. Inflammatory signaling, rather than a strictly hierarchical cascade, is a vast and intricate network. To illustrate, Kaul et al. preliminarily reported that blockade of IL‐1β ameliorated endothelial activation and inflammation in a sickle mouse model (probing only VCAM‐1).38 This is perfectly consistent with our present observations. Moreover, TNF and IL‐1β exert overlapping effects, and each induces production of the other. Thus, our results reveal only that the participation of TNF is substantial enough that its inhibition is unambiguously effective and beneficial.
Our results do beg the question: How are sickle monocytes activated in the first place? This is not yet answerable, as many mediators within the sickle milieu are capable of doing so; it seems likely that multiple agents are activating. Nonetheless, we find three TLR4 ligands to be highly likely actors in SCA: free heme from hemolysis, HMGB1 (high mobility group box 1) released during I/R, and heparan sulfate released from endothelial glycocalyx. Each of these ligands can be expected to exert activating effects via TLR4 on monocytes, leading to TNF release; and TNF actually enhances activity of this TLR4 pathway.55 This would seem to predict the very cyclicity and apparent perpetuity of inflammation that is evident in SCA.
6.4. Why TNF blockade should work in sickle disease
The remarkably uniform efficacy of etanercept seen here is, we suspect, not because it is exerting “anti‐inflammatory” effects in the traditional sense. Rather, it is impeding the actual triggers that comprise the very inception of I/R pathobiology.2 If so, TNF blockade would inhibit –perhaps even halt—the very driver of the perpetual inflammatory process responsible for the vascular dysfunction of SCA. If this I/R model of SCA pathophysiology2 is valid, TNF can be expected to be ameliorative.
6.5. What next?
The unavoidable uncertainty inherent in the complexity of TNF biology also complicates therapeutic predictability. Beyond data such as ours, only advancing to pilot study in humans can answer whether or not etanercept may exert benefit for SCA. This, however, may require our field to create solutions to several barriers, as we expect efficacy of etanercept might be exerted over longer rather than shorter time‐scales. Additionally, nature and mechanism of benefit could be different for acute intervention vs. chronic prevention.
Finally, we are not arguing that etanercept itself would be an ideal therapeutic, because it is expensive and must be administered subcutaneously, although it could be deployed immediately in health systems that can deal with such issues. As alternatives, ongoing development of small molecule TNF blockers may eventually offer very wide applicability and accessibility. Meanwhile, it would be helpful for us to glean insights about pathophysiology that would derive from a focused pilot test of etanercept in humans with SCA. Attempting that would present several significant challenges for which solutions have been proposed elsewhere. Indeed, that might identify additional opportunities for productive therapeutic targeting.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information.
ACKNOWLEDGMENTS
We thank Jim Kiley, Fuad Abdullah, Sethu Nair, and Chunsheng Chen for technical assistance. We are grateful for editorial expertise provided by Tracy Daye‐Groves and Michael Franklin. This work was supported by the National Institutes of Health: HL55552 to RPH; HL114567 to GV; and HL103773 to KG.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conceived, designed, supervised and guided study: AS, AS, KG, GV, RPH. Conducted experiments: AS, AS, JB, LV, LM, KAN, GOS. Provided critical models, reagents and expertise: RJK, RP, NM, RPH. Wrote or edited manuscript: AS, AS, NM, GOS, JB, RPH.
Solovey A, Somani A, Belcher JD, et al. A monocyte‐TNF‐endothelial activation axis in sickle transgenic mice: Therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119–1130. https://doi.org/10.1002/ajh.24856
Funding information National Institutes of Health, Grant/Award Number: HL55552 to RPH; HL114567 to GV; and HL103773 to KG
Anna Solovey and Arif Somani contributed equally to this work.
REFERENCES
- 1. Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11(2):129–151. [PubMed] [Google Scholar]
- 2. Hebbel RP. Ischemia‐reperfusion injury in sickle cell anemia: relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain. Hematol Oncol Clin North Am. 2014;28(2):181–198. [DOI] [PubMed] [Google Scholar]
- 3. Belcher JD, Chen C, Nguyen J, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso‐occlusion in murine sickle cell disease. Blood. 2014;123(3):377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Setty BN, Betal SG, Zhang J, Stuart MJ. Heme induces endothelial tissue factor expression: potential role in hemostatic activation in patients with hemolytic anemia. J Thromb Haemost. 2008;6(12):2202–2209. [DOI] [PubMed] [Google Scholar]
- 5. Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso‐occlusion. Blood. 2000;96(7):2451–2459. [PubMed] [Google Scholar]
- 6. Wun T, Cordoba M, Rangaswami A, Cheung AW, Paglieroni T. Activated monocytes and platelet‐monocyte aggregates in patients with sickle cell disease. Clin Lab Haematol. 2002;24(2):81–88. [DOI] [PubMed] [Google Scholar]
- 7. Selvaraj SK, Giri RK, Perelman N, Johnson C, Malik P, Kalra VK. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood. 2003;102(4):1515–1524. [DOI] [PubMed] [Google Scholar]
- 8. Perelman N, Selvaraj SK, Batra S, et al. Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood. 2003;102(4):1506–1514. [DOI] [PubMed] [Google Scholar]
- 9. Sloma I, Zilber MT, Charron D, Girot R, Tamouza R, Gelin C. Upregulation and atypical expression of the CD1 molecules on monocytes in sickle cell disease. Hum Immunol. 2004;65(11):1370–1376. [DOI] [PubMed] [Google Scholar]
- 10. Zennadi R, Chien A, Xu K, Batchvarova M, Telen MJ. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood. 2008;112(8):3474–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marcal LE, Dias‐da‐Motta PM, Rehder J, et al. Up‐regulation of NADPH oxidase components and increased production of interferon‐gamma by leukocytes from sickle cell disease patients. Am J Hematol. 2008;83(1):41–45. [DOI] [PubMed] [Google Scholar]
- 12. Brittain JE, Knoll CM, Ataga KI, Orringer EP, Parise LV. Fibronectin bridges monocytes and reticulocytes via integrin alpha4beta1. Br J Haematol. 2008;141(6):872–881. [DOI] [PubMed] [Google Scholar]
- 13. Chaar V, Picot J, Renaud O, et al. Aggregation of mononuclear and red blood cells through an {alpha}4{beta}1‐Lu/basal cell adhesion molecule interaction in sickle cell disease. Haematologica. 2010;95(11):1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kollander R, Solovey A, Milbauer LC, Abdulla F, Kelm RJ, Jr , Hebbel RP. Nuclear factor‐kappa B (NFkappaB) component p50 in blood mononuclear cells regulates endothelial tissue factor expression in sickle transgenic mice: implications for the coagulopathy of sickle cell disease. Transl Res. 2010;155(4):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Safaya S, Steinberg MH, Klings ES. Monocytes from sickle cell disease patients induce differential pulmonary endothelial gene expression via activation of NF‐kappaB signaling pathway. Mol Immunol. 2012;50(1–2):117–123. [DOI] [PubMed] [Google Scholar]
- 16. Setty BN, Key NS, Rao AK, et al. Tissue factor‐positive monocytes in children with sickle cell disease: correlation with biomarkers of haemolysis. Br J Haematol. 2012;157(3):370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ragab SM, Soliman MA. Tissue factor‐positive monocytes expression in children with sickle cell disease: clinical implication and relation to inflammatory and Coagulation Markers. Blood Coagul Fibrinolysis. 2016;27(8):862–869. [DOI] [PubMed] [Google Scholar]
- 18. Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78(6):539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117(2):244–279. [DOI] [PubMed] [Google Scholar]
- 20. Van Hauwermeiren F, Vandenbroucke RE, Libert C. Treatment of TNF mediated diseases by selective inhibition of soluble TNF or TNFR1. Cytokine Growth Factor Rev. 2011;22(5–6):311–319. [DOI] [PubMed] [Google Scholar]
- 21. Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18(8):1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao X, Xu X, Belmadani S, et al. TNF‐alpha contributes to endothelial dysfunction by upregulating arginase in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2007;27(6):1269–1275. [DOI] [PubMed] [Google Scholar]
- 23. Crabtree MJ, Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide. 2011;25(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swerlick RA, Eckman JR, Kumar A, Jeitler M, Wick TM. Alpha 4 beta 1‐integrin expression on sickle reticulocytes: vascular cell adhesion molecule‐1‐dependent binding to endothelium. Blood. 1993;82(6):1891–1899. [PubMed] [Google Scholar]
- 25. Kaul DK, Fabry ME, Nagel RL. Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: pathophysiological implications. Proc Natl Acad Sci U S A. 1989;86(9):3356–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujita M, Mason RJ, Cool C, Shannon JM, Hara N, Fagan KA. Pulmonary hypertension in TNF‐alpha‐overexpressing mice is associated with decreased VEGF gene expression. J Appl Physiol ( Physiol). 2002;93(6):2162–2170. [DOI] [PubMed] [Google Scholar]
- 27. Berry M, Brightling C, Pavord I, Wardlaw A. TNF‐alpha in asthma. Curr Opin Pharmacol. 2007;7(3):279–282. [DOI] [PubMed] [Google Scholar]
- 28. Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor‐alpha antagonist. J Clin Endocrinol Metab. 2004;89(9):4409–4413. [DOI] [PubMed] [Google Scholar]
- 29. Bozkurt B, Kribbs SB, Clubb FJ, Jr. , et al. Pathophysiologically relevant concentrations of tumor necrosis factor‐alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97(14):1382–1391. [DOI] [PubMed] [Google Scholar]
- 30. Tobinick E, Kim NM, Reyzin G, Rodriguez‐Romanacce H, DePuy V. Selective TNF inhibition for chronic stroke and traumatic brain injury: an observational study involving 629 consecutive patients treated with perispinal etanercept. CNS Drugs. 2012;26(12):1051–1070. [DOI] [PubMed] [Google Scholar]
- 31. Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6(7):521–532. [DOI] [PubMed] [Google Scholar]
- 32. Hess A, Axmann R, Rech J, et al. Blockade of TNF‐a rapidly inhibits pain responses in the central nervous system. Proceedings of the National Academy of Sciences. 2011;108(9):3731–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Belcher JD, Bryant CJ, Nguyen J, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003;101(10):3953–3959. [DOI] [PubMed] [Google Scholar]
- 34. Solovey A, Kollander R, Shet A, et al. Endothelial cell expression of tissue factor in sickle mice is augmented by hypoxia/reoxygenation and inhibited by lovastatin. Blood. 2004;104(3):840–846. [DOI] [PubMed] [Google Scholar]
- 35. Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor‐IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991;174(6):1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mazzon E, Esposito E, Di Paola R, et al. Effect of tumour necrosis factor‐alpha receptor 1 genetic deletion on carrageenan‐induced acute inflammation: a comparison with etanercept. Clin Exp Immunol. 2008;153(1):136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luong BT, Chong BS, Lowder DM. Treatment options for rheumatoid arthritis: celecoxib, leflunomide, etanercept, and infliximab. Ann Pharmacother. 2000;34(6):743–760. [DOI] [PubMed] [Google Scholar]
- 38. Kaul DK, Thangaswamy S, Suzuka SM, Fabry ME, Alan A. Wanderer and Hermann Gram Anti‐interleukin‐1B antibody‐based therapy ameliorates endothelial activation and inflammation in sickle mice. Blood 2001;118:848. [abstract]
- 39. Toldo S, Schatz AM, Mezzaroma E, et al. Recombinant human interleukin‐1 receptor antagonist provides cardioprotection during myocardial ischemia reperfusion in the mouse. Cardiovasc Drugs Ther. 2012;26(3):273–276. [DOI] [PubMed] [Google Scholar]
- 40. Pawlinski R, Pedersen B, Kehrle B, et al. Regulation of tissue factor and inflammatory mediators by Egr‐1 in a mouse endotoxemia model. Blood. 2003;101(10):3940–3947. [DOI] [PubMed] [Google Scholar]
- 41. Vincent L, Vang D, Nguyen J, et al. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122(11):1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cruz JA, Bauer EM, Rodriguez AI, et al. Chronic hypoxia induces right heart failure in caveolin‐1‐/‐ mice. Am J Physiol Heart Circ Physiol. 2012;302(12):H2518–H2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mackman N. Regulation of the tissue factor gene. FASEB J. 1995;9(10):883–889. [DOI] [PubMed] [Google Scholar]
- 44. Pritchard MT, Nagy LE. Ethanol‐induced liver injury: potential roles for egr‐1. Alcohol Clin Exp Res. 2005;29(11 Suppl):146S–150S. [DOI] [PubMed] [Google Scholar]
- 45. Joyce DA, Gimblett G, Steer JH. Targets of glucocorticoid action on TNF‐alpha release by macrophages. Inflamm Res. 2001;50(7):337–340. [DOI] [PubMed] [Google Scholar]
- 46. Parry GC, Mackman N. A set of inducible genes expressed by activated human monocytic and endothelial cells contain kappa B‐like sites that specifically bind c‐Rel‐p65 heterodimers. J Biol Chem. 1994;269(33):20823–20825. [PubMed] [Google Scholar]
- 47. Hebbel RP, Vercellotti GM, Pace BS, et al. The HDAC inhibitors trichostatin A and suberoylanilide hydroxamic acid exhibit multiple modalities of benefit for the vascular pathobiology of sickle transgenic mice. Blood. 2010;115(12):2483–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Robinson JC, Graham BB, Rouault TC, Tuder RM. The crossroads of iron with hypoxia and cellular metabolism. Implications in the pathobiology of pulmonary hypertension. Am J Respir Cell Mol Biol. 2014;51(6):721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rossol M, Meusch U, Pierer M, et al. Interaction between transmembrane TNF and TNFR1/2 mediates the activation of monocytes by contact with T cells. J Immunol. 2007;179(6):4239–4248. [DOI] [PubMed] [Google Scholar]
- 50. Tavakkoli F, Nahavandi M, Wyche MQ, Perlin E. Plasma levels of TNF‐alpha in sickle cell patients receiving hydroxyurea. Hematology. 2004;9(1):61–64. [DOI] [PubMed] [Google Scholar]
- 51. Sarray S, Saleh LR, Lisa Saldanha F, Al‐Habboubi HH, Mahdi N, Almawi WY. Serum IL‐6, IL‐10, and TNFalpha levels in pediatric sickle cell disease patients during vasoocclusive crisis and steady state condition. Cytokine. 2015;72(1):43–47. [DOI] [PubMed] [Google Scholar]
- 52. Zeng Z, Duan Z, Zhang T, et al. Association between tumor necrosis factor‐alpha (TNF‐alpha) promoter −308 G/A and response to TNF‐alpha blockers in rheumatoid arthritis: a meta‐analysis. Mod Rheumatol. 2013;23(3):489–495. [DOI] [PubMed] [Google Scholar]
- 53. Hoppe C, Klitz W, D'harlingue K, et al. Confirmation of an association between the TNF(‐308) promoter polymorphism and stroke risk in children with sickle cell anemia. Stroke. 2007;38(8):2241–2246. [DOI] [PubMed] [Google Scholar]
- 54. Chang Milbauer L, Wei P, Enenstein J, et al. Genetic endothelial systems biology of sickle stroke risk. Blood. 2008;111(7):3872–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang WS, Han NJ, Kim JJ, Lee MJ, Park SK. TNF‐α activates high‐mobility group Box 1 ‐ toll‐like receptor 4 signaling pathway in human aortic endothelial cells. Cell Physiol Biochem. 2016;38(6):2139–2151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information.


