ABSTRACT
Background
Idiopathic REM sleep behavior disorder is a prodromal stage of Parkinson's disease and dementia with Lewy bodies. Hyposmia, reduced dopamine transporter binding, and expression of the brain metabolic PD‐related pattern were each associated with increased risk of conversion to PD. The objective of this study was to study the relationship between the PD‐related pattern, dopamine transporter binding, and olfaction in idiopathic REM sleep behavior disorder.
Methods
In this cross‐sectional study, 21 idiopathic REM sleep behavior disorder subjects underwent 18F‐fluorodeoxyglucose PET, dopamine transporter imaging, and olfactory testing. For reference, we included 18F‐fluorodeoxyglucose PET data of 19 controls, 20 PD patients, and 22 patients with dementia with Lewy bodies. PD‐related pattern expression z‐scores were computed from all PET scans.
Results
PD‐related pattern expression was higher in idiopathic REM sleep behavior disorder subjects compared with controls (P = 0.048), but lower compared with PD (P = 0.001) and dementia with Lewy bodies (P < 0.0001). PD‐related pattern expression was higher in idiopathic REM sleep behavior disorder subjects with hyposmia and in subjects with an abnormal dopamine transporter scan (P < 0.05, uncorrected).
Conclusion
PD‐related pattern expression, dopamine transporter binding, and olfaction may provide complementary information for predicting phenoconversion. © 2017 The Authors. Movement Disorders published by Wiley Periodicals, Inc. on behalf of International Parkinson and Movement Disorder Society.
Keywords: idiopathic REM sleep behavior disorder, Parkinson's disease‐related pattern, 18F‐FDG‐PET, dopamine transporter 123I‐FP‐CIT SPECT, olfaction
Longitudinal studies have shown that >80% of individuals with idiopathic REM sleep behavior disorder (RBD) developed Parkinson's disease (PD) or dementia with Lewy bodies (DLB) on long‐term follow‐up.1, 2, 3, 4, 5 RBD subjects represent a suitable group to study the prodromal stage of these disorders and may be crucial for disease‐modification trials. However, such trials require biomarkers that can reliably identify at‐risk individuals and predict clinical manifestation of PD/DLB.
Neurodegenerative disorders are characterized by disease‐specific patterns of altered brain glucose metabolism on 18F‐fluorodeoxyglucose positron emission tomography (18F‐FDG‐PET) brain imaging. Such patterns can be extracted from 18F‐FDG‐PET data with scaled subprofile model and principal component analysis (SSM PCA6). With SSM PCA, a PD‐related pattern (PDRP) has been identified in multiple cohorts.7, 8, 9, 10 The degree to which the PDRP is present in a new 18F‐FDG‐PET scan can be quantified, resulting in a subject score. PDRP subject scores increase with disease progression and decrease with effective therapy.10, 11
To date, 2 groups have reported that RBD subjects have higher PDRP subject scores compared with controls.12, 13 In a longitudinal study of 17 RBD subjects, baseline PDRP expression was associated with a high risk of developing PD or DLB within 5 years.12
Other markers have also been considered. Loss of striatal dopamine transporter (DAT) binding on single photon emission computed tomography (DAT‐SPECT) indicates imminent phenoconversion.14, 15 In addition, RBD subjects with baseline hyposmia have a high risk of developing PD/DLB within 5 years of follow‐up.16, 17
The PDRP has potential as a disease biomarker in prodromal subjects, but further validation by an independent research group is essential. Moreover, direct comparisons between PDRP expression, DAT binding, and olfaction in the same RBD subjects have never been made. We therefore studied these 3 markers in 21 RBD patients.
Methods
Twenty‐one subjects with RBD (polysomnographically confirmed18) were evaluated with 18F‐FDG‐PET, DAT‐SPECT, and olfactory testing. Per inclusion criteria, RBD subjects did not have parkinsonism19 or DLB20 at the time of the study. Participants with a history of psychotropic medication use before the onset of RBD were excluded.21
Nineteen age‐matched healthy controls were studied with 18F‐FDG‐PET and olfactory testing. Controls did not have RBD (score < 5 on the RBD screening questionnaire22) and furthermore had no first‐degree family members with a neurodegenerative disease.
RBD subjects and controls were investigated with the Unified Parkinson's Disease Rating Scale (UPDRS, version 200323) and the Montreal Cognitive Assessment (MoCA).24 Olfactory function was assessed with Sniffin' Sticks.15, 16, 25 Total olfaction scores (TDI) were obtained by summing the threshold (T), discrimination (D), and identification (I) subscores. Five olfactory stages were defined as follows: anosmia, TDI ≤ 15; severe hyposmia, 15 < TDI ≤ 20); moderate hyposmia, 20 < TDI ≤ 25; mild hyposmia, 25 < TDI ≤ 30; and normosmia, TDI > 30. In a previous study, it was determined that a baseline TDI score < 18 was associated with increased risk of phenoconversion to PD/DLB within 5 years of follow‐up.16 We therefore divided RBD patients into 2 groups: patients with TDI scores < 18 and patients with TDI scores ≥ 18.
For reference, we studied the 18F‐FDG‐PET scans of retrospectively‐included patients with clinical diagnoses of “probable PD” (n = 20, nondemented, aged 67.5 ± 8.6 years; 16 men; median disease duration, 2 years; interquartile range, 1‐7 years) and “probable DLB” (n = 22, aged 73.7 ± 7 years; 17 men; median disease duration, 3 years; interquartile range, 1‐4 years) according to consensus criteria.19, 20
Exclusion criteria for all subjects included a history of (other) neurological diseases, diabetes mellitus, stroke, significant head trauma, or other relevant comorbidities. The study was approved by local institutional review boards. Voluntary written informed consent was obtained from each subject after verbal and written explanation of the study, in accordance with the Declaration of Helsinki.
All subjects underwent static 18F‐FDG‐PET imaging on a Siemens Biograph mCT‐64 PET/CT camera (Siemens, Munich, Germany) at the University Medical Center Groningen, the Netherlands. Images were reconstructed with OSEM3D, including point‐spread function and time‐of‐flight modeling, and smoothed with a Gaussian 8‐mm full‐width at half‐maximum filter. Central nervous system depressants were discontinued in all subjects for at least 24 hours before each scan. In RBD patients, all RBD‐related medications (eg, melatonin or clonazepam) were discontinued for at least 48 hours prescan. In PD and DLB patients, dopamimetics were not withheld.
All images were spatially normalized onto an 18F‐FDG‐PET template in Montreal Neurological Institute brain space26 using SPM12 software (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK) implemented in Matlab (version 2012b; MathWorks, Natick, Massachusetts). Expression of the previously identified PDRP8 was calculated in the new 18F‐FDG‐PET data as described previously.27 All PDRP subject scores were z‐transformed to the controls (n = 19), such that the average PDRP z‐score in controls was 0, with a standard deviation of 1.
In future clinical trials of RBD, diagnostic tool specificity will be more important than sensitivity (ie, RBD subjects who will not phenoconvert should be excluded). We therefore reanalyzed the PDRP identification cohort8 and selected a cutoff z‐score that gave 100% specificity. At PDRP z = 1.8, there was no misclassification of controls in the identification cohort (data not shown). This threshold was applied to the PDRP z scores in the current study (ie, a z score ≥ 1.8 was considered indicative of PD).
RBD subjects underwent DAT imaging with ¹²³I‐2β‐carbomethoxy‐3β‐(4‐iodophenyl)‐N‐(3‐fluoropropyl)nortropane single photon emission computed tomography. DAT binding in striatal regions was quantified with the Brain Registration & Analysis Software Suite (BRASS; HERMES Medical, Sweden). Non‐specific‐binding ratios were calculated in the caudate nucleus and putamen bilaterally, using the occipital cortex for reference (ie, nonspecific binding). DAT‐binding ratios that were 2 or more standard deviations lower than age‐matched expected control values were considered abnormal (see Supplementary Material). The lowest putamen DAT‐binding ratio of each subject was used for further analyses. The median interval between acquisition of the 123I‐FP‐CIT‐SPECT and 18F‐FDG‐PET was 2.7 months (interquartile range, 1.3‐4.6 months; total range, 12 days to 9.6 months). Loss of striatal DAT binding in the putamen was considered abnormal for age in 9 of 21 RBD subjects.
Statistical Analysis
The normality of distribution of each variable was assessed with the Shapiro‐Wilk test, Q‐Q plots, and box plots. PDRP z‐scores and DAT‐binding ratios were parametric. PDRP z‐scores were compared across controls, RBD, PD, and DLB with a 1‐way analysis of variance (ANOVA) with post hoc Bonferroni corrections.
PDRP z‐scores were compared between RBD subjects with normal and abnormal DAT scans with an independent t test. PDRP z‐scores and DAT‐binding ratios were also compared between the 2 olfaction categories (TDI score < 18 or ≥ 18) with an independent t test. These analyses were not corrected for multiple comparisons.
In the 21 RBD subjects, correlations between PDRP z‐scores and DAT‐binding ratios were tested for significance with a Pearson correlation coefficient. TDI, MoCA, and UPDRS‐III scores were nonparametric. Correlations between these variables and the imaging metrics (PDRP z‐scores and DAT binding) were assessed with a Spearman's rank correlation coefficient. Correlations were considered significant at P < 0.05 (uncorrected). All analyses were performed using SPSS software version 23 (SPSS Inc., Chicago, IL).
Results
UPDRS‐III scores were significantly higher in RBD subjects compared with controls. MoCA and olfaction scores were significantly lower in RBD patients (P < 0.01; Supplementary Table).
PDRP subject scores were not significantly different between men (n = 9) and women (n = 10) in the control group (P = 0.75, independent t test). Stepwise increases in PDRP z scores were observed across groups (ANOVA F 81 = 59.06, P < 0.0001; Fig. 1). In 12 of 21 RBD subjects (57%), the PDRP z score surpassed the threshold (z ≥ 1.8; Table 1).
Figure 1.
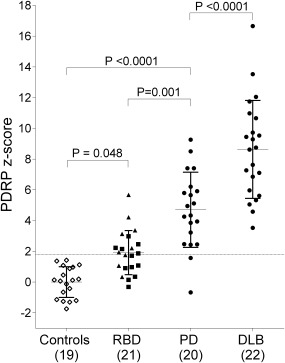
PDRP z scores across groups. PDRP expression was calculated in all groups and z‐transformed to the healthy controls. PDRP expression z scores were compared across groups with a 1‐way analysis of variance. Post hoc comparisons were Bonferroni‐corrected. The dashed line (z = 1.8) indicates the cutoff for PDRP expression. Triangles indicate RBD subjects with abnormal DAT scans. Squares indicate subjects with normal DAT scans.
Table 1.
Clinical and imaging characteristics of the 21 RBD subjects
| PDRP z score category | DAT scan category | RBD subject | PDRP z score | Lowest putamen DAT‐binding ratio | Total olfaction score (TDI)b | Sex | Age | RBD duration (years) | Age at onset RBD | MoCA | UPDRS‐III |
|---|---|---|---|---|---|---|---|---|---|---|---|
| <1.8 | Normal | 1 | 1.7 | 2.0c | 33.8 | Male | 57.4 | 5.0 | 52.4 | 30.0 | 4.0 |
| 2 | 1.0 | 2.3 | 33.5 | Female | 58.9 | 7.0 | 51.9 | 27.0 | 0.0 | ||
| 3 | ‐0.3 | 2.5 | 33.5 | Female | 68.3 | 6.0 | 62.3 | 23.0 | 2.0 | ||
| 4 | 1.1 | 2.5 | 29.5 | Male | 54.0 | 6.0 | 48.0 | 26.0 | 4.0 | ||
| 5 | 0.9 | 2.4 | 28.0 | Male | 56.4 | 6.0 | 50.4 | 27.0 | 1.0 | ||
| 6 | 0.2 | 2.2 | 19.5 | Male | 67.1 | 25.0 | 42.1 | 28.0 | 0.0 | ||
| 7 | 0.4 | 2.9 | 0.0 | Male | 56.0 | 9.0 | 47.0 | 25.0 | 1.0 | ||
| Abnormal | 8 | 0.3 | 1.2 | 19.0 | Male | 65.9 | 12.0 | 53.9 | 26.0 | 2.0 | |
| 9 | 1.1 | 1.0 | 13.0 | Male | 66.4 | 6.0 | 60.4 | 27.0 | 3.0 | ||
| ≥1.8 | Normal | 10 | 2.2 | 2.5 | 29.0 | Male | 57.8 | 5.0 | 52.8 | 28.0 | 1.0 |
| 11a | 2.2 | 2.3 | 23.5 | Male | 62.6 | 14.0 | 48.6 | 24.0 | 5.0 | ||
| 12 | 3.0 | 2.5 | 20.5 | Male | 57.5 | 2.5 | 55.0 | 27.0 | 6.0 | ||
| 13 | 1.9 | 2.3 | 16.5 | Male | 64.5 | 2.0 | 62.5 | 26.0 | 2.0 | ||
| 14a | 2.5 | 2.0c | 15.5 | Female | 70.1 | 3.0 | 67.1 | 28.0 | 4.0 | ||
| Abnormal | 15 | 2.2 | 1.7 | 27.5 | Male | 64.0 | 14.0 | 50.0 | 28.0 | 4.0 | |
| 16 | 1.8 | 1.6 | 25.8 | Male | 66.9 | 3.0 | 63.9 | 27.0 | 2.0 | ||
| 17 | 3.4 | 0.9 | 17.0 | Male | 61.5 | 4.0 | 57.5 | 27.0 | 0.0 | ||
| 18 | 3.1 | 1.7 | 13.0 | Male | 65.4 | 6.0 | 59.4 | 27.0 | 6.0 | ||
| 19 | 4.2 | 2.0c | 2.0 | Male | 49.9 | 4.0 | 45.9 | 24.0 | 1.0 | ||
| 20 | 5.7 | 1.2 | 0.0 | Male | 63.2 | 4.0 | 59.2 | 28.0 | 1.0 | ||
| 21 | 1.9 | 1.8 | 0.0 | Male | 66.6 | 2.0 | 64.6 | 22.0 | 5.0 |
In these 2 RBD subjects, 18F‐FDG‐PET was performed respectively 3.4 and 1.5 months before DAT‐SPECT.
bOlfaction was measured with the Sniffin' Sticks test; total TDI scores are reported in this table (see main text). A TDI > 30 indicates normal olfactory function; a TDI ≤ 20 indicates severe hyposmia. A TDI score < 18 was previously associated with an increased risk of phenoconversion to PD/DLB.16
cSubjects 1, 14, and 19 all have putamen DAT‐binding ratios of 2.0. Subjects 1 and 14 are still in the “normal DAT” category, and subject 19 is in the “abnormal DAT” category. This is because DAT‐binding ratios were considered abnormal if they were 2 standard deviations below the value expected for age. For subjects 1 and 14, the ratio of 2.0 is still normal for age (57 and 70 years old, respectively); however, for subject 19, this ratio is abnormal for age (50 years old). We note that subject 1 has a borderline‐normal DAT‐binding ratio and PDRP z‐score (z = 1.7).
RBD, idiopathic REM sleep behavior disorder; PDRP, Parkinson's disease‐related pattern; DAT, dopamine transporter; MoCA, Montreal Cognitive Assessment; UPDRS‐III, part 3 of the Unified Parkinson's Disease Rating Scale (2003 version).
In Table 1, PDRP z‐scores, putamen DAT‐binding ratios, and TDI scores are shown for each RBD patient. This permits identification of several RBD subgroups. Subjects 1‐3 have normal values for all 3 markers. Subjects 17‐21 have abnormal values for all 3 markers: suprathreshold PDRP z scores, putamen DAT‐binding too low for age, and TDI scores < 18. Subjects 15 and 16 have suprathreshold PDRP z‐scores and abnormal DAT scans, but TDI scores ≥ 18. Of the 9 subjects with abnormal DAT scans, 7 had suprathreshold PDRP z‐scores (subjects 15‐21). Interestingly, of the 12 subjects with normal DAT scans, 5 (42%) had suprathreshold PDRP z‐scores (subjects 10‐14).
On average, subjects with abnormal DAT scans (n = 9) had higher PDRP z‐scores compared with subjects with normal DAT scans (P = 0.044, uncorrected). Subjects with olfaction scores < 18 (n = 9) had higher PDRP z‐scores compared with subjects with olfaction scores ≥ 18 (P = 0.032, uncorrected). Putamen DAT‐binding ratios were not significantly different between the 2 olfaction groups (P = 0.117). PDRP z‐scores, DAT binding, and olfaction were not significantly correlated, but trends were observed (n = 21; Supplementary Fig. 1).
Discussion
Our findings underscore the value of the PDRP as a potential disease biomarker in idiopathic RBD. In line with 2 previous studies, RBD subjects significantly expressed the PDRP.12, 13 Although on average, PDRP z‐scores were lower in RBD subjects compared with PD/DLB, more than half of the RBD subjects already had a PDRP z‐score in the range of PD patients.
This study is the first to directly compare PDRP expression, striatal DAT binding, and olfaction in RBD. Although a trend was observed, PDRP and striatal DAT binding were not significantly correlated. Previous studies in PD have shown that PDRP expression shows only modest correlation to DAT binding.10, 28, 29 This may indicate a partly nondopaminergic genesis of the PDRP. Remarkably, 5 of 12 RBD patients with normal striatal DAT binding had suprathreshold PDRP z‐scores. In 2 of these cases, 18F‐FDG‐PET was performed before DAT‐SPECT. It has been shown that some DLB patients may initially have unremarkable DAT scans.30 It is possible that RBD subjects with significant PDRP expression but normal DAT binding will eventually develop DLB. Longitudinal imaging studies of RBD subjects are needed to further investigate the relationship between PDRP expression and loss of DAT binding in relation to the final clinical diagnosis.
That there was no direct significant correlation between PDRP z‐scores, DAT binding, and olfaction could indicate that the 3 markers provide complementary information. For example, 2 cases had suprathreshold PDRP z scores and abnormal DAT scans, but TDI scores ≥ 18. These subjects would have been considered at low risk of phenoconversion if the olfaction scores alone had been considered.16 We also identified 3 subjects with normal values for all 3 markers. These individuals may have a low risk of converting to PD/DLB. In contrast, 5 subjects had suprathreshold PDRP z scores, putamen DAT binding too low for age, and TDI scores < 18; these subjects may be considered to have a particularly high risk of conversion within the next 5 years.
The data presented in this report are cross‐sectional. A longitudinal study of our RBD cohort is ongoing. Follow‐up data will be essential to elucidate if DAT‐SPECT‐negative DLB cases, and perhaps subjects who later developed multiple system atrophy, contributed to the aforementioned findings. We expect that the PDRP will be especially informative, because in contrast to olfaction,31 the PDRP is a progression marker.11 Moreover, PDRP expression is useful in the differential diagnosis of parkinsonian disorders,32 whereas DAT imaging is not.33
Author Roles
1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B. Review and Critique.
S.K.M.: 1ABC, 2ABC, 3AB
D.V.: 1BC, 3B
R.J.R.: 2ABC, 3B
E.S.W.: 1ABC, 3B
G.M.: 1ABC, 3B
C.D.: 1ABC, 3B
K.R.: 1ABC, 3B
S.O.: 1ABC, 3B
A.P.: 1ABC, 3B
F.E.R.: 1C, 3B
T. van L.: 1C, 3B
L.H.: 1ABC
L.K.T.: 1A, 3B
H.H.: 1ABC, 3B
M.L.: 1ABC, 3B
K.K.: 1ABC, 3B
S.M.A.: 2ABC 3B
J.B.: 2ABC, 3B
K.L.L.: 1ABC, 2ABC, 3B
W.H.O.: 1ABC, 2ABC, 3B
FINANCIAL DISCLOSURE OF ALL AUTHORS
This study was funded by the Dutch Stichting Parkinson Fonds and the German Parkinson Fonds Deutschland.
S.K.M.: Stichting Parkinson Fonds (grant); D.V.: none; R.J.R.: none; E.S.W.: Parkinson Fonds Deutschland (grant); G.M.: none; C.D.: none; K.R.: German Federal Ministry of Education and Research (BMBF 01GQ1402; grant); S.O.: the Netherlands Organization for Scientific Research (grant 016.116.371), UCB Pharma, Boehringer Ingelheim, and Novartis (other); A.P.: UCB Pharma (other); F.E.R.: none; T.v.L.: Brittania (advisory boards and honoraria), neuroderm (advisory board), Medtronic, AbbVie (honoraria), Stichting Parkinson Fonds, Mosadex Pharma, EGRET European Fund, Weston Brain Institute, BCN Institute at University of Groningen (grants); L.H.: none; L.K.T.: none; H.H.: none; M.L.: Michael J. Fox Foundation for Parkinson's Research (grant).; K.K: none; S.A.M.: none; J.B.: GE Healthcare (grant); K.L.L.: Stichting Parkinson Fonds (grant); W.H.O.: BiogenIdec, Medigene, Merck Darmstadt, Roche (stock ownership in medically related fields), Adamas, BristolMyerSquibb, GE Health, Mundipharma, Novartis, Roche, Teva, UCB (advisory boards), AbbVie, Desitin, Lundbeck, Mundipharma, Novartis, UCB (honoraria), German Ministry of Education and Research, German Research Foundation, Michael J. Fox Foundation, Internationaal Parkinson Fonds, Novartis Pharma Germany (grant).
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's website.
Supplementary Information
Acknowledgments
We thank R.V. Kogan for proofreading the manuscript. W.H. Oertel, MD, PhD, is Hertie‐Senior‐Research Professor, supported by the Charitable Hertie Foundation, Frankfurt/Main, Germany.
Relevant conflicts of interest/financial disclosures: Jan Booij is a consultant at GE Healthcare, and received grant support from GE Healthcare.
Funding agencies: This study was funded by the Dutch Stichting ParkinsonFonds and the German ParkinsonFonds Deutschland.
References
- 1. Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte‐Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009;72:1296‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain 2012;135:1860‐1870. [DOI] [PubMed] [Google Scholar]
- 3. Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post‐mortem pathology in idiopathic rapid‐eye‐movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 2013;12:443‐453. [DOI] [PubMed] [Google Scholar]
- 4. Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16‐year update on a previously reported series. Sleep Med 2013;14:744‐748. [DOI] [PubMed] [Google Scholar]
- 5. Iranzo A, Fernandez‐Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One 2014;9:e89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci 2009;32:548‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson's disease: test‐retest reproducibility. J Cereb Blood Flow Metab 2007;27:597‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teune LK, Renken RJ, de Jong BM, et al. Parkinson's disease‐related perfusion and glucose metabolic brain patterns identified with PCASL‐MRI and FDG‐PET imaging. Neuroimage Clin 2014;5:240‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meles SK, Teune LK, de Jong BM, Dierckx RA, Leenders KL. Metabolic imaging in Parkinson disease. J Nucl Med 2017;58:23‐28. [DOI] [PubMed] [Google Scholar]
- 10. Niethammer M, Eidelberg D. Metabolic brain networks in translational neurology: Concepts and Applications. Ann Neurol 2012;72(5):635‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson's disease. Brain 2007;130:1834‐1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holtbernd F, Gagnon JF, Postuma RB, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology 2014;82:620‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu P, Yu H, Peng S, et al. Consistent abnormalities in metabolic network activity in idiopathic rapid eye movement sleep behaviour disorder. Brain 2014;137:3122‐3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iranzo A, Lomena F, Stockner H, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid‐eye‐movement sleep behaviour disorder: a prospective study [corrected. Lancet Neurol 2010;9:1070‐1077. [DOI] [PubMed] [Google Scholar]
- 15. Stiasny‐Kolster K, Doerr Y, Moller JC, et al. Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha‐synucleinopathy demonstrated by dopamine transporter FP‐CIT‐SPECT. Brain 2005;128:126‐137. [DOI] [PubMed] [Google Scholar]
- 16. Mahlknecht P, Iranzo A, Hogl B, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology 2015;84:654‐658. [DOI] [PubMed] [Google Scholar]
- 17. Ponsen MM, Stoffers D, Booij J, van Eck‐Smit BL, Wolters EC, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol 2004;56:173‐181. [DOI] [PubMed] [Google Scholar]
- 18. Schenck CH, Montplaisir JY, Frauscher B, et al. Rapid eye movement sleep behavior disorder: devising controlled active treatment studies for symptomatic and neuroprotective therapy‐‐a consensus statement from the International Rapid Eye Movement Sleep Behavior Disorder Study Group. Sleep Med 2013;14:795‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863‐1872. [DOI] [PubMed] [Google Scholar]
- 21. Frauscher B, Jennum P, Ju YE, et al. Comorbidity and medication in REM sleep behavior disorder: a multicenter case‐control study. Neurology 2014;82:1076‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stiasny‐Kolster K, Mayer G, Schafer S, Moller JC, Heinzel‐Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire‐‐a new diagnostic instrument. Mov Disord 2007;22:2386‐2393. [DOI] [PubMed] [Google Scholar]
- 23. Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease . The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738‐750. [DOI] [PubMed] [Google Scholar]
- 24. Gagnon JF, Postuma RB, Joncas S, Desjardins C, Latreille V. The Montreal Cognitive Assessment: a screening tool for mild cognitive impairment in REM sleep behavior disorder. Mov Disord 2010;25:936‐940. [DOI] [PubMed] [Google Scholar]
- 25. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 1997;22:39‐52. [DOI] [PubMed] [Google Scholar]
- 26. Della Rosa PA, Cerami C, Gallivanone F, et al. A standardized [18F]‐FDG‐PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics 2014;12:575‐593. [DOI] [PubMed] [Google Scholar]
- 27. Spetsieris PG, Eidelberg D. Scaled subprofile modeling of resting state imaging data in Parkinson's disease: methodological issues. Neuroimage 2011;54:2899‐2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holtbernd F, Ma Y, Peng S, et al. Dopaminergic correlates of metabolic network activity in Parkinson's disease. Hum Brain Mapp 2015;36:3575‐3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang CC, Poston KL, Dhawan V, Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson's disease. J Neurosci 2010;30:1049‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Zande JJ, Booij J, Scheltens P, Raijmakers PG, Lemstra AW. (123)]FP‐CIT SPECT scans initially rated as normal became abnormal over time in patients with probable dementia with Lewy bodies. Eur J Nucl Med Mol Imaging 2016;43:1060‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iranzo A, Serradell M, Vilaseca I, et al. Longitudinal assessment of olfactory function in idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord 2013;19:600‐604. [DOI] [PubMed] [Google Scholar]
- 32. Tripathi M, Tang CC, Feigin A, et al. Automated differential diagnosis of early parkinsonism using metabolic brain networks: a validation study. J Nucl Med 2016;57(1):60‐68. [DOI] [PubMed] [Google Scholar]
- 33. Stoffers D, Booij J, Bosscher L, Winogrodzka A, Wolters EC, Berendse HW. Early‐stage [123I]beta‐CIT SPECT and long‐term clinical follow‐up in patients with an initial diagnosis of Parkinson's disease. Eur J Nucl Med Mol Imaging 2005;32:689‐695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's website.
Supplementary Information


