Abstract
The term avian osteopetrosis is used to describe alterations to the skeletal elements of several species of domestic bird, most typically the chicken, Gallus gallus domesticus (L. 1758). Such lesions are routinely identified in animal bones from archaeological sites due to their distinctive appearance, which is characterised by proliferative diaphyseal thickening. These lesions are relatively uncomplicated for specialists to differentially diagnose and are caused by a range of avian leucosis viruses in a series of subgroups. Only some avian leucosis viruses cause the development of such characteristic lesions in osteological tissue. Viraemia is necessary for the formation of skeletal pathology, and avian osteopetrosis lesions affect skeletal elements at different rates. Lesion expression differs by the age and sex of the infected individual, and environmental conditions have an impact on the prevalence of avian leucosis viruses in poultry flocks. These factors have implications for the ways in which diagnosed instances of avian osteopetrosis in archaeological assemblages are interpreted. By integrating veterinary research with archaeological evidence for the presence of avian leucosis viruses across Western Europe, this paper discusses the nature of these pathogens, outlines criteria for differential diagnosis, and offers a fresh perspective on the human‐aided movement of animal disease in the past through investigation of the incidence and geographic distribution of avian osteopetrosis lesions from the first century BC to the post‐medieval period. © 2017 The Authors International Journal of Osteoarchaeology Published by John Wiley & Sons Ltd.
Keywords: animal husbandry, avian leucosis virus, avian osteopetrosis, palaeopathology, Western Europe
Dedication
This work owes an intellectual debt to the pioneering research of Don Brothwell, whose seminal paper on avian osteopetrosis in archaeological assemblages from England highlighted the potential interpretive value of these lesions (Brothwell, 2002). Professor Brothwell passed away during the preparation of this paper, which is dedicated to his memory.
Introduction
Viral diseases of domestic animals impacted past human communities in often severe ways. Rinderpest (cattle plague, RPV) and swine fevers (CSFV and ASFV) have devastated herds in the last centuries. Beyond the threat to livelihoods, the former is likely the evolutionary source of the measles virus in humans (MeV) (Furuse et al., 2010), and zoonotic viruses are a serious human health threat (Murray et al., 2016). Archaeological evidence for viral infections in livestock is rare, and neither rinderpest nor swine fevers can be detected in skeletal remains. Although poultry are ‘household livestock’ and therefore not ideal models for viral transmission between cattle or caprines and humans, avian leucosis viruses (ALVs) (archaeologically visible as avian osteopetrosis) present opportunities to differentially diagnose and interpret macroscopic lesions resulting from infection by a known group of viruses, and contextualise viral disease in domestic animals in the past.
Non‐human palaeopathology specialists routinely analyse disarticulated, incomplete, and fragmentary skeletal remains, and the nature of these assemblages can prevent the advancement of interpretations beyond simple description and broad nosological classification. Pathologies which can be differentially diagnosed from a single element or partial specimen are of particular utility in interpreting relationships between human and non‐human animals in the past. Avian osteopetrosis, caused by ALV infection, is characterised by a distinctive lesion affecting osseous tissue (Figure 1) and has been included in animal palaeopathology manuals since the first major volume on the subject was published (Baker & Brothwell, 1980, 61–62). These lesions and their aetiology continue to be discussed in major works on palaeopathology (Bartosiewicz & Gál, 2013; Thomas, 2012). In addition to Brothwell's article (Brothwell, 2002), avian osteopetrosis lesions have been identified in a wide range of European archaeozoological investigations over the last few decades (Allison, 2010; Baker, 1998; Fabiš, 1997; Fothergill & Best, In press; Gál, 2008; Gál & Kunst, 2014; Gordon, 2016; Lentacker et al., 2004; Luff & Brothwell, 1993; Morel, 1991; Peters, 1998; Prummel, 1987; van Wijngaarden‐Bakker & Krauwer, 1979; von den Driesch & Pöllath, 2000).
Figure 1.

Osteopetrotic chicken ulna excavated from Causeway Lane, Leicester, shown with a comparative element. [Colour figure can be viewed at wileyonlinelibrary.com]
In noting the presence of avian osteopetrosis in an assemblage, the appearance of affected elements is customarily described and occasionally photographed, but discussion is rare and either limited to concepts such as stocking density or the spread of disease (Gál, 2008; Thomas, 2012) or supplied as an explanation for the disposal of an individual (Groot, 2008: 108). Furthermore, statements regarding the species vulnerable to infection by ALVs, the impact of the infection on soft tissue, which age groups and sexes of birds are susceptible, and the order in which elements across the skeleton are affected are presented with few references to the veterinary research (e.g. Albarella et al., 2009; Fabiš, 1997).
This paper sheds new light on avian osteopetrosis and discusses the viruses which cause these characteristic lesions. It will examine: issues of problematic nomenclature and terminology; differential diagnosis of archaeological material; variation in lesion progression across sexes and the skeleton; links between environment, husbandry, and transmission; the physical and ethological effects of ALV infection; the impact upon flock productivity; and the archaeological evidence for the temporal and spatial dimensions of the disease.
Avian leucosis viruses
Avian leucosis viruses (ALVs), first established as a cause for tumour diseases in the early twentieth century (Ellermann & Bang, 1908; Rous, 1910), are members of the Alpharetrovirus genus of the family Retroviridae. Avian leucosis virus infections are still routinely observed in modern broiler and egg‐laying flocks of chickens (Gao et al., 2010) and have a negative effect on productivity (Payne & Venugopal, 2000). Despite their pervasiveness and the economic importance of controlling avian leucoses, the resulting skeletal lesions have been discussed using variable terminology which has been debated since they were first medically described. ‘Sporadic diffuse osteoperiostitis’, a condition bearing a resemblance to avian osteopetrosis with regard to obliteration of the medullary (endosteal) cavity in avian long bones, was described by Pugh (1927), then referred to as ‘osteopetrosis gallinarum’ when identified by Jungherr and Landauer a decade later (Jungherr & Landauer, 1938). When it became evident that lesions affecting avians were aetiologically distinct from those affecting mammals and that characteristics such as the obliteration of the endosteal cavity developed secondarily, scholars began to suggest alternative terminologies. These were consistent with perceptions of viral infections at the time, with ‘transmissible multiple juvenile hyperostotic sclerosing osteopathia’ put forward by Thiersch (1948), ‘avian osteogenic osteoblastoma’ by Boyde et al. (1978), and ‘leukosarcomatous osteodysplasia’ most recently (Uzunova et al., 2014). None has yet been fully embraced in non‐human palaeopathology, and ‘avian osteopetrosis’ continues to be used to describe bony lesions of the avian skeleton from ALV infection; it is favoured by most archaeological specialists, with some exceptions (e.g. Fabiš, 1997). The archaeological application of this term has resulted in a view of the disease as a simple viral infection, with little consideration for the relevance of environment, husbandry practices, or the impact of infection on productivity. In fact, such lesions reflect infection by a possible range of viruses in a series of subgroups (A‐J, of which some are endogenous and J is recently emergent) rather than a simple single agent (Payne, 1992; Vogt, 1977). Their prevalence in modern populations is firmly linked to the environment in which flocks are raised (Uzunova et al., 2014), and husbandry methods are therefore a key component of pathogenesis.
Avian leucosis viruses vary in their ability to induce avian osteopetrosis and the rapidity of skeletal lesion development; persistent viraemia (the presence of the virus in the bloodstream) is essential for lesion formation but does not guarantee it (Robinson & Miles, 1985). In an experiment inducing avian osteopetrosis in chickens using leukaemic blood from mammalian sources, Thiersch noted that the time required for skeletal lesions to develop was reduced by repeated transmission of the virus in question (Thiersch, 1948: 101, 108). Male birds have been shown to be more susceptible to the development of avian osteopetrosis (Holmes, 1961; Robinson & Miles, 1985; Robinson et al., 1992). Table 1 shows the range of domestic and commensal species known to be vulnerable to ALV infection, namely members of Galloanserae and Columbea (Vogt, 1977 373–376; Payne, 1992; Weiss, 2006). Passerines (parakeets) were experimentally investigated but found to be resistant (Vogt, 1977, 314).
Table 1.
Species affected by avian leucosis viruses and the viral subgroups to which they are susceptible, adapted from Table 5 in Vogt, 1977:375
| Species affected | Subgroups affected by | Reference |
|---|---|---|
| Chicken (Gallus gallus) | A‐E | Payne, 1992; many others |
| Guinea fowl (Numida meleagris) | A‐E | Payne, 1992; Uzunova et al., 2014 |
| Turkey (Meleagris gallopavo) | Not B | Holmes, 1963; Weiss, 2006 |
| Common quail (Coturnix coturnix) | E | Weiss, 2006 |
| Japanese quail (Coturnix japonica) | Not B | Moscovici and MacIntyre, 1966 |
| King quail (Excalfactoria chinensis) | F, G | Vogt, 1977 |
| Bobwhite quail (Colinus sp.) | D | Payne, 1992 |
| Pigeon (Columba livia) | D | Sarma et al., 1969; Payne, 1992 |
| Chukar (Alectoris chukar) | A | Payne, 1992 |
| Duck (Anas platyrhnchos) | C | Payne, 1992; Pruková et al., 2007 |
| Goose (Anser anser) | C | Payne, 1992 |
| Pheasant (Chrysolophus sp., Lophura sp., Phasianus sp., Syrmaticus sp.) | F, G, otherwise by species; not B | Payne, 1992; Weiss, 2006 |
Aside from experimental techniques, ALVs are transmitted by three methods: horizontal (direct or indirect contact); vertical (congenital from female to offspring); and genetic (viral genome transmission from parent) (Pruková et al., 2007). Quantities of ALVs are shed by both oral and cloacal routes, with the highest viral concentration in faeces; exposed skin is the portal of entry most conducive to infection (Weyl & Dougherty, 1977). Experimental transmission research demonstrated that ALVs can survive desiccation for 105 days (Simpson & Sanger, 1968: 272). A study of infected chickens found that whilst three in four viraemic hens transmitted ALV to their chicks, one in 12 non‐viraemic hens also did so (Rubin et al., 1961). Because viraemia is necessary for skeletal pathology, this suggests that hens without easily detectable lesions could continue to perpetuate transmission. Cross‐species transmission of ALVs does occur and turkeys, quails, pheasants, and pigeons have been investigated in this regard (Holmes, 1963; Sarma et al., 1969; Weiss, 2006).
The vulnerability of any given individual to infection is determined by a host of factors, including nutritional and immune state, climate, genetic predisposition, age and sex, environment, and sanitation (Inhorn & Brown, 1997; Roberts & Manchester, 2007: 167).
Description and differential diagnosis
Avian osteopetrosis lesions appear as hypermineralised osseous lumps projecting from the diaphyses of affected elements (Robinson et al., 1992), which become thickened and expand periosteally and endosteally as total bone mass increases (Biltz et al., 1965). Studies indicate that viral strains which cause a more rapid osteological lesion progression result in the formation of lesions with a rougher texture and appearance (Robinson & Miles, 1985; Simpson & Sanger, 1968). Complete obliteration of the endosteal cavity can occur (Figure 2), and increased radio‐opacity is universally evident (O'Connor & O'Connor, 2005: Figures 13 and 14). Lesions are consistently bilateral, and articular surfaces are unaffected (Simpson & Sanger, 1968; Thiersch, 1948).
Figure 2.

Chicken ulna from Causeway Lane in Leicester showing near‐complete occlusion of the endosteal cavity. [Colour figure can be viewed at wileyonlinelibrary.com]
Short‐term investigations (typically of less than a year in duration) of lesion development report that long bones are affected (Biltz et al., 1965; Boyde et al., 1978; Jungherr & Landauer, 1938; Simpson & Sanger, 1968; Thiersch, 1948), and that the tarsometatarsus (Simpson & Sanger, 1968), or the humerus, femur, or tibiotarsus are first to show lesions (Boyde et al., 1978; Franklin & Martin, 1980). However, Holmes's longitudinal study demonstrated that all elements of the skeleton showed radiographically identifiable changes, but that they were affected at different rates (Holmes, 1961). Furthermore, the humerus, radius, ulna, femur, and tibiotarsus showed signs of skeletal pathology first and concurrently (Holmes, 1961: 370), which may partly explain why these elements are more frequently identified archaeologically (Figure 7). One possible instance of archaeological avian osteopetrosis has been recorded in a cranium from Mogador, Morocco (Becker et al., 2013: 68). This element is one of the last to show skeletal lesions, and if this individual was suffering from ALV infection, they would have survived for approximately six months based on the rate of progression documented by Holmes (1961: 368). It may be that individuals were culled or disposed of soon after showing signs of illness. Although many elements affected later in the disease progression (e.g. the axial skeleton, metacarpals, pedal and wing phalanges) are difficult to identify to taxon and vulnerable to taphonomic and recovery biases, avian osteopetrosis would increase their potential survivability (Bartosiewicz, 2008).
Avian osteopetrosis lesions have a characteristic appearance which should not be confused with rickets or osteomalacia (as bowing of the elements does not occur), angular deformities (which can affect articulations and are not typically characterised by extensive new bone formation), or chondrodystrophies (which do not affect diaphyseal width). Trauma with extensive callus formation can be more difficult to eliminate by macroscopic inspection. Both avian osteopetrosis and callus formation involve endosteal and periosteal new bone proliferation and can be accompanied by element foreshortening. However, angular deviation from the natural axis of the element is not typical of osteopetrotic elements, and it does not affect articulations. Additionally, a fracture line is not present in avian osteopetrosis lesions but is diagnostic of some forms of trauma and can be detected using radiography (O'Connor & O'Connor, 2005: Figure 9). Despite these distinctions, some authors have excluded avian osteopetrosis from differential diagnosis based upon the assumption that ‘circumferential deposition of bone around the entire diaphysis’ is the defining characteristic of the condition (Cubo et al., 2015: 9). Although this can occur, previous radiographic studies have demonstrated that avian osteopetrosis lesions are not consistently circumferential, especially in early stages (Holmes, 1961; Thiersch, 1948). Radiography is therefore key in attempting differential diagnosis of avian osteopetrosis.
Ethological and external physical changes have also been documented in birds infected with ALVs, as set out in Table 2.
Table 2.
Ethological and externally observable physical changes in chickens which result from avian leucosis virus infection
| Behaviour | External physical changes | Reference |
|---|---|---|
| Lethargy (30% of infected birds) | Uzunova et al., 2014: 188 | |
| Anxiety/depression (20% of infected birds) | Uzunova et al., 2014: 189 | |
| Cannibalism | Watts & Smith, 1980: 504 | |
| Lameness or leg deformity sufficient to encumber movement | Uzunova et al., 2014: 188–189; Robinson & Miles, 1985: 131 | |
| Failure to develop sex characteristics, e.g. ‘infantile testicles’ in males | Holmes, 1961: 24 | |
| Anaemia, ‘pallid yellow cast to legs and combs’ | Holmes, 1961: 24; Paterson & Smith, 1978: 891 | |
| Stunting | Holmes, 1961: 24–25; Hirota et al., 1980: 929; Banes & Smith, 1977: 876–884 | |
| Area of lesions hotter than surrounding tissue/body temperature | Simpson & Sanger, 1968: 274, 277 | |
| Limb paralysis | Robinson et al., 1992: 870 | |
| Emaciation or low body weight | Uzunova et al., 2014: 188–189; Hirota et al., 1980: 932; Smith & Morgan, 1982: 492 | |
| Reduced growth rate (26% of control rate) | Banes & Smith, 1977: 876–884 | |
| Diarrhoea | Pruková et al., 2007; 15 | |
| Delayed sexual maturation in females | Payne, 1992: 338 (citing Gavora et al., 1980) | |
| Reduction in laying | Payne, 1992: 338 (citing Gavora et al., 1980) | |
| Increased eggshell fragility | Payne, 1992: 338 (citing Gavora et al., 1980) | |
| Decreased fertility and hatchability of eggs | Payne, 1992: 338 (citing Gavora et al., 1980) | |
| Decreased egg size | Gavora et al., 1991 |
Many of these changes would have been apparent to observers of diseased flocks in the past, and people may have chosen to selectively dispose of infected individuals later recovered as associated bone groups (deposits of animal bone, sometimes articulated skeletons or portions thereof, hereafter referred to as ABGs following Morris, 2010) (Groot, 2008). These viruses would have had other meaningful impacts: productivity of affected flocks would have decreased, both with regard to egg‐laying and meat yield, making the disease a likely concern for those engaged in avian husbandry.
Disease narrative
An extensive search of published and grey literature reports concerning archaeological chicken remains was conducted for this study: major journals, excavation volumes, non‐human palaeopathology literature, and other resources such as the Archaeological Data Service (ADS, hosted by the University of York). Additionally, further specimens were identified by the author and responses from analysts contacted in person or via the ZOOARCH e‐mail list over a period of 18 months (Table 3). Although there can be some confidence that the vast majority of known incidences have been collected in this search, there remains considerable potential for avian osteopetrosis lesions to remain unidentified in assemblages, particularly in regions where detailed recording of either pathologies or avian remains may not have been routine. Furthermore, chicken elements are small in size, and the identification of pathology in assemblages from unsieved sites could have been affected by this. Lesions identified by the author were recorded using a modified version of Vann & Thomas (2006), photographed, and subjected to digital radiography using a Xograph DRagon mobile x‐ray unit (52 KvP; 1.6 mAs; 0.025 s) for differential diagnosis. Metrical sex was not assessed due to the documented impact of avian leucosis infection on skeletal element length (Holmes, 1961: 24–25; Hirota et al., 1980: 929; Banes & Smith, 1977: 876–884), and no spurs or medullary bone were observed by the author.
Table 3.
Archaeological sites with reported finds of avian osteopetrosis, including suspected cases. The elements listed in bold font have been differentially diagnosed
| Site name | Element(s) | Date (AD) | Reference |
|---|---|---|---|
| Sanctuary of Jupiter Heliopolitanus, Carnuntum‐Muhlacker, Austria | Not noted/accessible | 100–410 | Gál & Kunst, 2014 |
| Tienen, Belgium | Tarsometatarsus | 250–300 | Lentacker et al., 2004 |
| Amiens (Rue Lavalard), France | Tibiotarsus | 150–250 | Meniel, pers. comm., |
| Aunedonnacum, France | Femur | 14–37 | Lignereux et al., 1997 |
| Bondorf (Villa Rustica), Germany | Not noted/accessible | 100–300 | Kokabi et al., 1994 |
| Künzing, Bavaria | Coracoid | 200–250 | von den Driesch & Pöllath, 2000 |
| Buda, Hungary | ABG (sternum, humeri, ulnae, radius, femur and tibiotarsi) | 1247–1686 | Gál, 2008 |
| Intercisa, Hungary | Tibiotarsus | 81–500 | Gál, 2008 |
| Apollonia‐Arsur, Israel | Femur | 1265–1265 | Pines, pers. comm. |
| Naples, Italy | Not noted/accessible | −400–1500 | Albarella, pers. comm. |
| Mogador, Morocco | Cranium | 1–400 | Becker et al., 2013 |
| Dordrecht, Netherlands | Tibiotarsus | 1400–1900 | van Wijngaarden‐Bakker & Krauwer, 1979 |
| Tiel‐Passewaaij, Netherlands | ABG (humerus, ulna, tibiotarsus, tarsometatarsus) | 60–270 | Groot, 2008 |
| Velsen, Netherlands | Not noted/accessible | 15–30 | Prummel, 1987 |
| Vicus Vitudurum (Oberwinterthur), Switzerland | Not noted/accessible | 1–400 | Morel, 1991 |
| Troia, Turkey | Two tibiotarsi | 1–500 | Fabiš, 1997 |
| 1 Poultry, London, UK | Tibiotarsus | 95–125 | MOLA database; Morris, pers. comm. |
| 2‐12 Gresham Street, London EC2, UK | Tibiotarsus | 43–410 | MOLA database; Pipe, pers. comm. |
| 28 Park Street, London SE1, UK | Tibiotarsus | 43–1750 | MOLA database; Pipe, pers. comm. |
| 35 Basinghall Street, London EC2, UK | Tibiotarsus | 43–1750 | MOLA database; Pipe, pers. comm. |
| Ashton Roman Town, UK | Ulna | 43–410 | Mahoney, pers. comm. |
| Blossoms Inn, 30 Gresham Street, London EC2, UK | Five elements, not further specified | 200–400 | MOLA database; Pipe, pers. comm. |
| Causeway Lane, Leicester, UK | Ulna | 43–410 | Connor & Buckley, 1999 |
| Chester (Nicholas St. Mews), UK | Femur | 1600–1800 | Gordon, 2016 |
| Cirencester, UK | Tibiotarsus | 43–410 | Strid, pers. comm. |
| Colchester, UK | 29 elements: 11 tibiotarsi, 10 humeri, four ulnae, two tarsometatarsi, one femur, one fibula | 40–400 | Luff & Brothwell, 1993 |
| Docklands Light Railway, UK | Humerus | 43–1750 | MOLA database; Morris, pers. comm. |
| Dudley Castle, UK | Tibiotarsus | 1533–1647 | Thomas, 2005 |
| Fishbourne Roman Palace, UK | Two tibiotarsi and a coracoid | 43–410 | Fothergill and Best, In Prep. |
| Little Lane, Leicester, UK | Tibiotarsus | 100–500 | Gidney, 1989 |
| London Bridge station, Jubilee Line extension, London SE1, UK | Tibiotarsus | 140–250 | MOLA database; Pipe, pers. comm. |
| Norwich (Barbican Well), UK | One element, not identified | 1400–1600 | Albarella et al., 2009 |
| Old Grapes Lane Site A, Carlisle, UK | Two tibiotarsi | 43–100 | Allison, 2010 |
| Plantation House, 23 Fenchurch Street, London EC3, UK | Tibiotarsus | 1220–1550 | MOLA database; Pipe, pers. comm. |
| Princesshay, Exeter, UK | Radius | 43–410 | Fothergill and Best, In Prep. |
| Rochester Riverside, Kent, UK | Femur | 150–400 | Rielly, pers. comm. |
| Roman Southwark, Union Street, UK | Tibiotarsus | 43–410 | MOLA database; Pipe, pers. comm. |
| Scole‐Dickleburgh, UK | Two humeri | 43–410 | Baker, 1998 |
| St Mary Axe, London EC3, UK | Tarsometatarsus | 120–160 | MOLA database; Morris, pers. comm. |
| Uley, UK | Tibiotarsus | 43–410 | Fothergill and Best, In Prep. |
The geographic distribution of avian osteopetrosis lesions spans the Mediterranean and large swathes of Europe, with concentrations of sites near the Danube and the Rhine, in the Netherlands, and in the south of England (Figures 3 and 4). Excavation policies and faunal bone retention, analysis, and publication practices across this substantial area vary considerably and are likely to have affected this distribution. However, some spatial patterns are evident, particularly when temporal dimensions are also taken into account. The earliest instances of avian osteopetrosis (Figure 3, indicated in white) come from sites connected with the Roman military. Two have been identified in early first century AD (Tiberian) assemblages excavated from the fort at Aulnay in central France and the fort and naval base at Velsen in the Netherlands (Lignereux et al., 1997; Prummel, 1987). Additionally, two possible instances of avian osteopetrosis from the first century AD were identified in the Old Grapes Lane assemblage from Roman Carlisle, UK (Allison, 2010).
Figure 3.

Map of Roman‐period archaeological sites with avian osteopetrosis lesions reported. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.
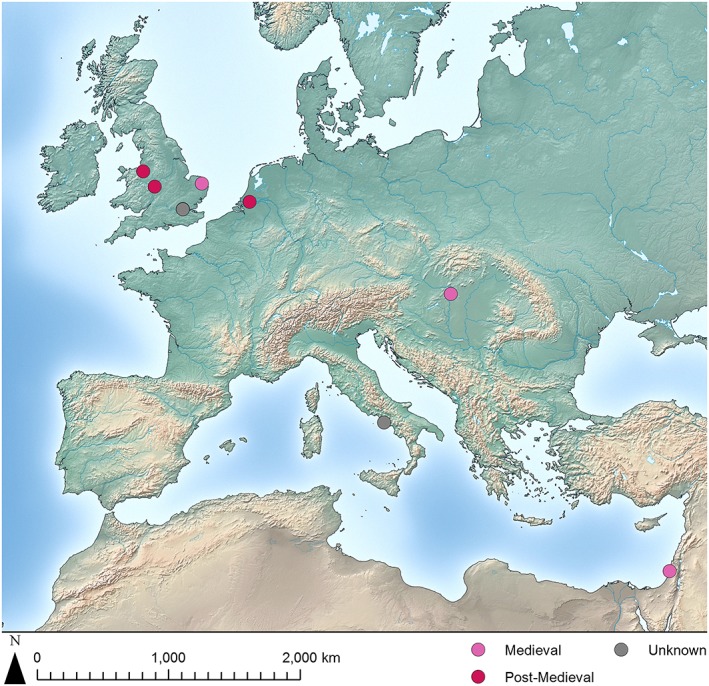
Map of Medieval and Post‐Medieval archaeological sites with avian osteopetrosis lesions reported. [Colour figure can be viewed at wileyonlinelibrary.com]
The majority of sites with lesions described as avian osteopetrosis date to the first half of the first millennium AD. Most of these contexts have broad ranges, but none are definitively dated later than ad 250, and all are located within the boundaries of the Roman Empire at that time.
No lesions were identified in assemblages dating from the sixth to the twelfth centuries, but five sites have evidence of avian osteopetrosis from the Medieval and Post‐Medieval periods in areas near the Roman‐period distribution (Figure 4). Avian osteopetrosis lesions have been reported in modern Bulgarian flocks (Uzunova et al., 2014), and the lack of sites from later Post‐Medieval contexts is probably related to the small, fragmentary nature of these assemblages and the lower perceived value of archaeological material from that time period (Thomas, 2009).
Another visualisation of this data is provided in Figure 5, which shows the temporal spread of the date midpoints of assemblages with avian osteopetrosis using a linear distribution based on Willet's Gaussian method (Willet, 2014), smoothed using a 125‐year rolling average.
Figure 5.
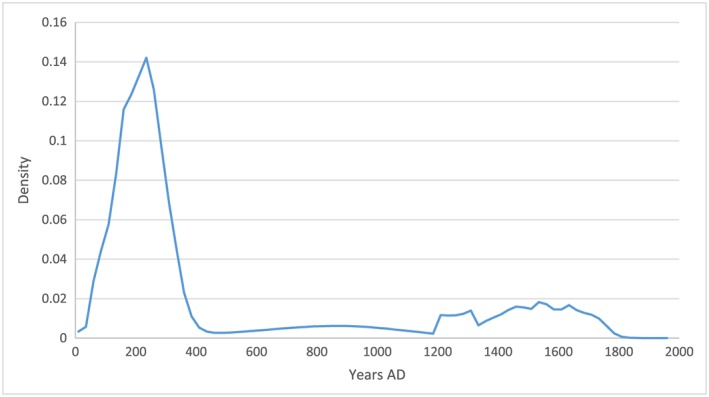
Linear distribution of the date midpoints of assemblages with avian osteopetrosis. [Colour figure can be viewed at wileyonlinelibrary.com]
This distribution illustrates a trend for higher numbers of skeletal lesions in the first few centuries AD, which in turn suggests that flocks with viraemic individuals and higher rates of infection were more prevalent in the Roman period, coincident with the greatest extent of the Empire. A re‐emergence of the disease at densely‐populated urban sites is evident after the thirteenth century. Other factors are likely to have influenced this pattern, including fewer reported assemblages from the early medieval period and a drop in the presence and proportion of chickens in early medieval assemblages. For comparison, Figure 6 shows the distribution of identifiable chicken elements (NISP, weighted by the temporal range of the assemblage) over the same two millennia.
Figure 6.

Linear distribution of chicken NISP weighted by date range of the assemblage. [Colour figure can be viewed at wileyonlinelibrary.com]
Based on data from Europe and North Africa collated from the Cultural and Scientific Perceptions of Human–Chicken Interactions project (2016) and other sources, the chicken NISP is highest at late Roman sites, which do not have correspondingly high lesion counts, and although the number of chicken elements increases just after ad 1000, avian osteopetrosis lesions are not identified with proportionate frequency.
Of the 74 archaeological chicken elements with avian osteopetrosis, none has been directly dated, and many have not been differentially diagnosed or described in detail (Table 3). Even so, the clustering of lesions in the first few centuries AD suggests that the disease spread rapidly across Europe, and that the movement of people and goods in relation to imperial Roman activity, especially given the presence of diagnosed lesions at sites along the limes, may have been a factor (Gál, 2008: 45 notes the spread of the disease in the Roman period). This has more recent historical parallels with the outbreaks of rinderpest in the eighteenth century, which took place in the context of increased movement of human and non‐human animals due to war, as well as reliance on cattle and cattle trading (Roeder et al., 2013).
Archaeological avian osteopetrosis lesions are not evenly distributed across the skeleton, and the most frequently affected element is the tibiotarsus, followed by the humerus. In addition to their high survivability, comparatively large size and the ease with which they can be identified, both of these elements are amongst the first to show skeletal lesions resulting from infection by ALVs (Holmes, 1961: 370). Figure 7 compares the archaeological elements affected by avian osteopetrosis lesions to the progression of lesions through the chicken skeleton (Holmes, 1961).
Figure 7.
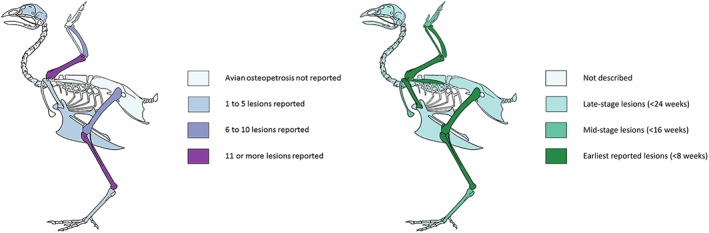
Skeletal diagram of archaeological elements with avian osteopetrosis lesions (left) and lesion progression according to Holmes (1961, right). [Colour figure can be viewed at wileyonlinelibrary.com]
The elements initially affected during the progression of avian osteopetrosis are also those in which the condition is most frequently identified, which might suggest that the illness did not often progress beyond the initial skeletal stage and was terminated either by the effects of the virus or the actions of those responsible for the flocks. However, these elements are also large and survive both taphonomic and excavation processes reasonably well (Ericson, 1987); furthermore, they are often less complicated to identify to taxon than axial elements, phalanges, and ribs.
Discussion
Without advanced genetic investigations and the application of direct dating methods to archaeological lesions, it is not yet possible to reconstruct the precise spatial and temporal origins of ALVs and therefore avian osteopetrosis, particularly as avian bone can be quite mobile with respect to archaeological stratigraphy (Lebrasseur et al., In press). None of the earliest examples reported in this paper should be treated as a ‘patient zero’; indeed, the first cases of the disease may be much earlier. Instead, at some point in the early first century AD, ALVs took hold and spread widely within a few decades, undoubtedly the result of human action. Although archaeological perspectives on the movement of animal pathogens are rare, such activity has economic and social implications.
The appearance of avian osteopetrosis lesions in less suitable ecological contexts for keeping chickens may inform upon the spatial distribution. No lesions have been reported from Spain, Portugal, and most of North Africa (with the exception of Mogador on the west coast of Morocco), which are much warmer climes. Whilst the influence of under‐reporting cannot be ruled out for reasons discussed previously, the entirety of the Mediterranean offered a total of three assemblages with avian osteopetrosis present, and other factors may also be at work. Based on new ecological niche modelling using nine environmental variables (Pitt et al., 2016), roughly half of the locations from which avian osteopetrosis lesions have been excavated are not suitable for chickens. The variables used included temperature seasonality, maximum and minimum temperatures, precipitation seasonality, maximum and minimum precipitation, maximum and minimum vegetation cover, and the availability of grit (Pitt et al., 2016: 4). Chickens are descended from a jungle‐dwelling species, and most of Europe is not environmentally ideal, particularly with regard to low temperatures. It may be that the very measures which ensured flock survival and even marginal productivity (e.g. sheltering chickens or keeping them indoors) exacerbated the spread of ALVs by increasing chances of exposure. Conversely, the management of poultry species in areas which appear unaffected could have played a role in preventing transmission. Additionally, archaeological sampling, analysis, and publication policies with regard to faunal material vary widely and continue to evolve. It is likely that a combination of these factors has contributed to the geographic lacunae without archaeological evidence for avian osteopetrosis.
The frequency of sites related to the Roman military in northern and central Europe cannot solely be explained by ecological context and excavation strategies. The term ‘Roman military’ is used here in the broadest possible sense, to encompass the many distinct and diverse communities associated with it, rather than only soldiers (e.g. James, 2006). Movement of people and goods between Roman forts and their civilian settlements was routine and necessary. Although chickens are rarely mentioned in written sources, ALVs could have been transported in chickens or eggs as part of supply shipments (Erdkamp, 2002; Stallibrass & Thomas, 2008), or by soldiers and their families moving household or personal items (Allison, 2013: 20). Given that such viruses can withstand desiccation for more than three months (Simpson & Sanger, 1968: 272), and that the highest concentration of virus is shed in faecal material, even the surfaces of a cart, ship, or shoe could have played a role in spreading ALVs across the Roman Empire, especially considering the high value of chicken dung as fertiliser.
The osteopetrotic ABGs recovered from Tiel‐Passewaaij (Groot, 2008), Buda (Gál, 2008), and Colchester (Luff & Brothwell, 1993) may be the remains of individuals disposed of due to their diseased state. However, because non‐viraemic hens can pass ALVs to their offspring, even selective slaughter of birds with obvious symptoms would not have prevented perpetuation of the viruses responsible for avian osteopetrosis.
Chickens were introduced to Europe and North Africa in the first millennium BC, potentially via multiple east to west routes (Becker et al., 2013; West & Zhou, 1988), and their remains are found archaeologically in contexts from across the study area by the second century BC. A programme of chicken element radiocarbon dating is currently being undertaken by Julia Best and colleagues, and a forthcoming publication will clarify the chronology of the introduction and early husbandry of the species. Although chickens were present in some Mediterranean regions for centuries before reaching northern Europe, the overall population appears to have boomed across the broad area considered here only in the first millennium AD.
Although poultry husbandry was practiced in diverse ways across the spatial and temporal extent considered here, Roman military sites warrant attention in this respect due to their strategic transport links and the presence of early avian osteopetrosis (although it is likely that the developed urban networks of the first centuries AD also played a role). The pullari, keepers of the chickens used in augury (often as part of military expeditions), are referred to by Cicero and Livy (Cic. Fam. 10.12.3, Cic. Div. 2.34; Liv. 8.30, Liv. 10.40, Liv. 9.14), and the Vindolanda tablets refer to chickens (and eggs) in the context of what appears to be a shopping list (Tab. Vindol. II 302), but their husbandry is not described. However, the management of other domestic animals is mentioned, and poultry keeping may have followed a similar practical arrangement. References are made to Alio, a veterinary doctor who would have been concerned primarily with equines (Tab. Vindol. II 181), those directly responsible for other domestic species such as cattle herders, and specific individuals named Lucco and Candidus, who were in charge of pigs (Tab. Vindol. II 180, 183). Prummel concluded that the chickens at Velsen were raised inside the camp, perhaps for cockfighting (Prummel, 1987: 186), and this may have been the case for other military sites. Also, given the importance of chickens at sites such as Uley and their dominance in assemblages from Mithraea (Lentacker et al., 2004; Levitan, 1993; von den Driesch & Pöllath, 2000), it is also possible that the movement of birds destined for sacred roles increased the spread of ALVs. If the Roman military was a catalyst for technological change (as argued by Davies (2002) and others), and animal husbandry practices altered as a result, some changes may have fostered the emergence and transmission of viral disease. Given that the distribution of lesions is weighted towards the north‐west, we should keep in mind that military communities in the Roman world developed their own distinctive identities and practices. These trends may be the result of an intersection of different groups, environments, cultural practices, and diets that encouraged higher populations of chickens or the implementation of specific agricultural and husbandry practices that facilitated the spread of avian leucoses.
It is also probable that the drop in lesion frequency between the sixth and twelfth centuries was caused by multiple factors. A widespread decrease in the number and proportions of chickens in archaeological assemblages occurs across much of Europe, and this is likely to be linked to a lower number of reported assemblages from this period. The Medieval Warm Period also took place from c. ad 950 to 1250, temporarily altering the climate. Another possibility, especially as the later sites would have provided ideal conditions, is that this trend represents the cyclical re‐emergence of the viruses responsible, a process mired in complexity (Morens et al., 2004). If this is the case, epidemiologic transition theory suggests that the sociocultural processes responsible for the pattern are diverse and challenging to frame (Barrett et al., 1998). The subsequent rise in lesion frequency between the thirteenth and sixteenth centuries is of significant interest, but caution must be exercised as this trend is dependent upon a handful of specimens and is unlikely to have statistical validity. We can be confident, however, that husbandry practices had changed, although it is possible that the rediscovery of Roman agricultural writers and application of their methods during the Renaissance may have had an impact.
Conclusions
This paper has considered the nature of ALVs, the bony lesions arising from infection, provisional differential diagnosis of lesions, and the role of human involvement, especially transportation of pathogens and application of husbandry methods, in the occurrence of avian osteopetrosis. Avian leucosis viruses were spread across Europe as a result of human action, likely connected in some way to the activities of the Roman military. The presence and progression of the infection are evident in affected individuals, some of whom may have been culled or otherwise disposed of due to their symptoms or behaviour. Apart from the usual biases affecting archaeological practice, ecological factors, cultural perceptions, and management techniques are likely to be partly responsible for the spatial and temporal distribution of avian osteopetrosis. The potential interpretive utility of diverse strands of evidence in non‐human palaeopathological investigation has also been demonstrated.
In addition to noting the impact of residuality, taphonomy, and archaeological practicalities (e.g. excavation, retention, analysis, and publication policies) on such findings, other factors should also be taken into account when constructing interpretations of past human‐animal relationships. Disease progression is often qualified (e.g. describing arthropathy as ‘advanced’), but other characteristics such as sex and individual or group ethology are intrinsically linked to processes of pathogenesis and transmission, and should be incorporated into analysis and interpretation of pathology whenever possible. The ways in which environmental influences relate to the presence, frequency, transmission, and movement of pathogens should also be considered. Finally, whilst pinpointing the first archaeological evidence for the emergence of ALVs is an endeavour of interest, future investigation of transmissible livestock diseases in the past should critically examine their impacts on past flocks, herds, and the lives of the peoples who kept them in the appropriate biological and cultural contexts.
Acknowledgements
I am grateful to many colleagues for observations of avian osteopetrosis lesions and kindly granting access to specimens or images, including Umberto Albarella, Polydora Baker, Charlotte Coles, Rebecca Gordon, Laura Hadland (and the Leicester Museums), Meghann Mahoney, Patrice Meniel, Jim Morris, Terry O'Connor, Joris Peters, Miriam Pines, Alan Pipe (and Museum of London Archaeology), Kevin Rielly, Lena Strid (and Oxford Archaeology), Richard Thomas, and Jean‐Hervé Yvinec. Thanks also to Mark Maltby, Terry O'Connor, and Richard Thomas for very useful comments on an early draft of this work. This research was supported by the Arts and Humanities Research Council‐funded Cultural and Scientific Perspectives of Human‐Chicken Interactions project (AH/L006979/1).
Fothergill, B. T. (2017) Human‐Aided Movement of Viral Disease and the Archaeology of Avian Osteopetrosis. Int. J. Osteoarchaeol., 27: 853–866. doi: 10.1002/oa.2599.
References
- Albarella U, Beech M, Curl J, Locker A, Moreno‐García M, Mulville J. 2009. Norwich castle: excavations and historical surveys 1987–98. Part III: a zooarchaeological study. East Anglian Archaeology Occasional Papers 22(21). [Google Scholar]
- Allison EP. 2010. The bird bones In The Southern Lanes, Carlisle. Publication of existing unpublished fascicules, Fasicule 1, Oxford Archaeology North, English Heritage; pp. 161–162. [Google Scholar]
- Allison PM. 2013. People and Spaces in Roman Military Bases. Cambridge University Press: Cambridge. [Google Scholar]
- Baker JR, Brothwell DR. 1980. Animal Diseases in Archaeology. Academic Press: London. [Google Scholar]
- Baker P. 1998. The vertebrate remains from Scole‐Dickleburgh, excavated in 1993 (Norfolk and Suffolk), A140 and A143 Road Improvement Project. Report 29/1998.
- Banes AJ, Smith RE. 1977. Biological characterization of avian osteopetrosis. Infection and Immunity 16(3): 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett R, Kuzawa CW, McDade T, Armelagos GJ. 1998. Emerging and re‐emerging infectious diseases: the third epidemiologic transition. Annual Review of Anthropology: 247–271. [Google Scholar]
- Bartosiewicz L. 2008. Taphonomy and palaeopathology in archaeozoology. Geobios 41(1): 69–77. [Google Scholar]
- Bartosiewicz L, Gál E. 2013. Shuffling Nags, Lame Ducks: The Archaeology of Animal Disease. Oxbow: Oxford. [Google Scholar]
- Becker C, von den Driesch A, Küchelmann HC. 2013. Mogador, eine Handelsstation am westlichen Rand der phönizischen und römischen Welt – die Tierreste Current Discoveries from Outside and Within. Field Explorations and Critical Comments from the Lab. Documenta Archaeobiologiae 10, Grupe G, McGlynn G. (eds.). Marie Leidorf: Rahden; 11–159. ISBN:978‐3‐89646‐625‐9. [Google Scholar]
- Biltz RM, Pellegrino ED, Rogers P. 1965. Avian osteopetrotic bone. The Journal of Bone and Joint Surgery 47(7): 1365–1377. [PubMed] [Google Scholar]
- Boyde A, Banes AJ, Dillaman RM, Mechanic GL. 1978. A morphological study of an avian bone disorder caused by myetosis‐associated virus. Metabolic Bone Disease & Related Research 1(3): 235–242. [Google Scholar]
- Brothwell DR. 2002. Ancient avian osteopetrosis: the current state of knowledge. Acta Zoologica Cracoviensia 45: 315–318. [Google Scholar]
- Cicero . 1901. –1902. Epistulae ad familiares [Translation by LC Purser].
- Cicero . 1923. De divinatione [Translation by WA Falconer].
- Connor A, Buckley R. 1999. Roman and Medieval Occupation in Causeway Lane, Leicester. University of Leicester Archaeological Services: Leicester. [Google Scholar]
- Cubo J, Woodward H, Wolff E, Horner JR. 2015. First reported cases of biomechanically adaptive bone modeling in non‐avian dinosaurs. PloS One 10(7): e0131131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cultural and Scientific Perceptions of Human‐Chicken Interactions Database . 2016. http://www.nottingham.ac.uk/~aczzoo/chickencoop/search/ [Accessed 19/10/2016]
- Davies JL. 2002. Soldiers, peasants, industry and towns. The Roman army in Britain. A Welsh perspective The Roman Army and the Economy, Erdkamp P. (ed.). J.C. Gieben: Amsterdam; 169–197. [Google Scholar]
- Ellermann V, Bang O. 1908. Experimentelle Leukamie bei Huhnern. Zentralblatt fuer Bakteriologie, Parasitenkunde und Infektionskrankheiten 46: 595–609. [Google Scholar]
- Erdkamp P. 2002. The Roman Army and the Economy. J.C. Gieben: Amsterdam. [Google Scholar]
- Ericson PG. 1987. Interpretations of archaeological bird remains: a taphonomic approach. Journal of Archaeological Science 14(1): 65–75. [Google Scholar]
- Fabiš M. 1997. A case of an infectious disease among domestic fowl of Troia IX Studia Troica Vol. 7 Verlag Philipp von Zabern: Mainz am Rhein; 539–548. [Google Scholar]
- Fothergill BT, Best J. In press. Hens, health, and husbandry: new approaches to past poultry‐keeping in England. Open Quaternary. [Google Scholar]
- Franklin RM, Martin MT. 1980. In ovo tumorigenesis induced by avian osteopetrosis virus. Virology 105(1): 245–249. [DOI] [PubMed] [Google Scholar]
- Furuse Y, Suzuki A, Oshitani H. 2010. Origin of measles virus: divergence from rinderpest virus between the 11th and 12th centuries. Virology Journal 7(52): 7–52. https://doi.org/10.1186/1743‐422X‐. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gál E, Kunst GK. 2014. Offered to Gods, eaten by people: bird bones from the sanctuary of Jupiter Heliopolitanus in Carnuntum–Mühläcker (Austria). International Journal of Osteoarchaeology 24(3): 336–346. [Google Scholar]
- Gál E. 2008. Bone evidence of pathological lesions in domestic hen (Gallus domesticus Linnaeus, 1758). Veterinarija ir Zootechnika 41(63): 42–48. [Google Scholar]
- Gao YL, Qin LT, Pan W, Wang YQ, Qi XL, Gao HL, Wang XM. 2010. Avian leukosis virus subgroup J in layer chickens, China. Emerging Infectious Disease 16: 1637–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavora JS, Kuhnlein U, Crittenden LB, Spencer JL, Sabour MP. 1991. Endogenous viral genes: association with reduced egg production rate and egg size in White Leghorns. Poultry Science 70(3): 618–623. [DOI] [PubMed] [Google Scholar]
- Gavora JS, Spencer JL, Gowe RS, Harris DL. 1980. Lymphoid leukosis virus infection: effects on production and mortality and consequences in selection for high egg production. Poultry Science 59: 2165–2178. [DOI] [PubMed] [Google Scholar]
- Gidney L. 1989. Leicester, the shires 1988 excavations: further identifications of small mammal and bird bones Ancient Monuments Laboratory Report 92/93. Historic Building and Monuments Commission for England: London. [Google Scholar]
- Gordon RL. 2016. Feeding the city: zooarchaeological perspectives on urban provisioning and consumption behaviours in post‐medieval England (AD1500–AD1900) (Doctoral dissertation, School of Archaeology and Ancient History, University of Leicester; ). [Google Scholar]
- Groot M. 2008. Animals in Ritual and Economy in a Roman Frontier Community: Excavations in Tiel‐Passewaaij. Amsterdam University Press: Amsterdam. [Google Scholar]
- Hirota Y, Martin MT, Viljanen M, Toivanen P, Franklin RM. 1980. Immunopathology of chickens infected in vivo and at hatching with the avian osteopetrosis virus MAV. 2–0. European Journal of Immunology 10(12): 929–936. [DOI] [PubMed] [Google Scholar]
- Holmes JR. 1961. Postmortem findings in avian osteopetrosis. The Journal of Comparative Pathology and Therapeutics 71: 20–27. [DOI] [PubMed] [Google Scholar]
- Holmes JR. 1963. Experimental osteopetrosis in the turkey. The Journal of Comparative Pathology and Therapeutics 73: 136IN10–145IN11. [DOI] [PubMed] [Google Scholar]
- Inhorn MC, Brown PJ. 1997. The anthropology of infectious disease The Anthropology of Infectious Disease: International Health Perspectives, Brown PJ, Inhorn MC. (eds.). Routledge: London and New York; 31–67. [Google Scholar]
- James S. 2006. Engendering change in our understanding of the structure of Roman military communities. Archaeological Dialogues 13(1): 31–36. [Google Scholar]
- Jungherr EL, Landauer W. 1938. Studies on fowl paralysis III. A condition resembling osteopetrosis (marble bone) in the common fowl. Storrs Agricultural Experimental Station Bulletin; 222. [Google Scholar]
- Kokabi M, Amberger G, Wahl J. 1994. Die Knochenfunde aus der Villa rustica von Bondorf Die Villa rustica von Bondorf. Forschungen und Berichte zur Vor‐ und Frühgeschichte in Baden‐Württemberg 51, Gaubatz‐Sattler A. (ed.). Theiss: Stuttgart; 285–335. [Google Scholar]
- Lebrasseur O, Best J, Colonese A, Miller H, Peters J, O'Connor T. In press. Chicken bones and stratigraphy: cautionary tales. Journal of Archaeological Science. [Google Scholar]
- Lentacker A, Ervynck A, Van Neer W. 2004. The symbolic meaning of the cock. The animal remains from the Mithraeum at Tienen (Belgium) Roman Mithraism: The Evidence of the Small Finds. Instituut voor het Archeologisch Patrimonium, Museum Het Toreke: Bruxelles; 57–80. [Google Scholar]
- Levitan B. 1993. Vertebrate remains The Uley Shrines: Excavation of a Ritual Complex on West Hill, Uley: Gloucestershire, 1977–9, Woodward A, Leach P. (eds.). Vol. 17, English Heritage Archaeological Report English Heritage: London; 257–302. [Google Scholar]
- Lignereux Y, Peters J, Tassaux F, Tronche P. 1997. Viandes, volailles et fruits de mer a la table des légions Romaines d'Aunedonnacum, 20–30 après Jésus‐Christ (Aulnay‐de‐Saintonge, Charente‐Maritime). Revue de Médecine Vétérinaire 148(5): 399–412. [Google Scholar]
- Livy . The history of Rome [Translation by Rev. Canon Roberts, 1912].
- Luff RM, Brothwell DR. 1993. Animal bones from excavations in Colchester, 1971–85 Colchester Archaeological Report 12. Colchester: Colchester Archaeological Trust. [Google Scholar]
- Morel P. 1991. Untersuchungen des osteologischen Fundgutes aus dem Vicus Vitudurum‐Oberwinterthur. Beiträge zum römischen Oberwinterthur‐Vitudurum 5 . Berichte der Zürcher Denkmalpflege, Archäologische Monographien 10: 79–176. [Google Scholar]
- Morens DM, Folkers GK, Fauci AS. 2004. The challenge of emerging and re‐emerging infectious diseases. Nature 430(6996): 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. 2010. Associated bone groups; beyond the Iron Age Integrating Social and Environmental Archaeologies; Reconsidering Deposition, Morris J, Mark M. (eds.), International Series, 2077 British Archaeological Reports: Oxford; 12–23. ISBN:9781407306384. [Google Scholar]
- Moscovici C, MacIntyre EH. 1966. Effect of avian myeloblastosis virus in the Japanese quail. Journal of Bacteriology 92(4): 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KA, Allen T, Loh E, Machalaba C, Daszak P. 2016. Emerging viral zoonoses from wildlife associated with animal‐based food systems: risks and opportunities Food Safety Risks from Wildlife. Springer International Publishing; 31–57. [Google Scholar]
- O'Connor T, O'Connor S. 2005. Digitising and image‐processing radiographs to enhance interpretation in avian palaeopathology Documenta Archaeobiologiae 3: Feathers, Grit and Symbolism. Birds and Humans in the Ancient Old and New Worlds, Grupe G, Peters J. (eds.). Verlag Marie Leidorf GmbH: Rahden; 69–82. [Google Scholar]
- Paterson RW, Smith RE. 1978. Characterization of anemia induced by avian osteopetrosis virus. Infection and Immunity 22(3): 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne LN, Venugopal K. 2000. Neoplastic diseases: Marek's disease, avian leukosis and reticuloendotheliosis. Revue scientifique et technique (International Office of Epizootics). 19(2): 544–564. [DOI] [PubMed] [Google Scholar]
- Payne LN. 1992. Chapter 6: Biology of avian retroviruses The Retroviridae, Levy JA. (ed.). Vol. 1 Plenum Press: New York; 299–404. [Google Scholar]
- Peters J. 1998. Römische Tierhaltung und Tierzucht. Eine Synthese aus archäozoologischer Untersuchung und schriftlichbildlicher Überlieferung. Passauer Universitätsschriften zur Archäologie 5 Westf: Rahden. [Google Scholar]
- Pitt J, Gillingham PK, Maltby M, Stewart JR. 2016. New perspectives on the ecology of early domestic fowl: an interdisciplinary approach. Journal of Archaeological Science 74: 1–10. [Google Scholar]
- Pruková D, Vernerová Z, Pilcík T, Stepanets V, Indrová M, Geryk J, Plachý J, Hejnar J, Svoboda J. 2007. Differences in pathogenicity among strains of the same or different avian leukosis virus subgroups. Avian Pathology 36(1): 15–27. [DOI] [PubMed] [Google Scholar]
- Prummel W. 1987. Poultry and fowling at the roman castellum Velsen I Palaeohistoria Vol. 29 Balkema: Rotterdam; 183–201. [Google Scholar]
- Pugh LP. 1927. Sporadic diffuse osteoperiostitis in fowls. Veterinary Review 7: 189–190. [Google Scholar]
- Roberts CA, Manchester K. 2007. The Archaeology of Disease. Cornell University Press: Ithaca. [Google Scholar]
- Robinson HL, Foster RG, Blais BP, Reinsch SS, Newstein M, Shank PR. 1992. 5′ Avian leukosis virus sequences and osteopetrotic potential. Virology 190(2): 866–871. [DOI] [PubMed] [Google Scholar]
- Robinson HL, Miles BD. 1985. Avian leukosis virus‐induced osteopetrosis is associated with the persistent synthesis of viral DNA. Virology 141(1): 130–143. [DOI] [PubMed] [Google Scholar]
- Roeder P, Mariner J, Kock R. 2013. Rinderpest: the veterinary perspective on eradication. Philosophical Transactions of the Royal Society B 368(1623) 20120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rous P. 1910. A transmissible avian neoplasm. (Sarcoma of the common fowl). Journal of Experimental Medicine 12: 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H, Cornelius A, Fanshier L. 1961. The pattern of congenital transmission of an avian leukosis virus. Proceedings of the National Academy of Sciences 47(7): 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma PS, Log TS, Huebner RJ, Turner HC. 1969. Studies of avian leukosis group‐specific complement‐fixing serum antibodies in pigeons. Virology 37(3): 480–483. [DOI] [PubMed] [Google Scholar]
- Simpson CF, Sanger VL. 1968. A review of avian osteopetrosis: comparisons with other bone diseases. Clinical Orthopaedics and Related Research 58: 271–282. [PubMed] [Google Scholar]
- Smith RE, Morgan JH. 1982. Identification of plaque isolates of an avian retrovirus causing rapid and slow onset osteopetrosis. Virology 119(2): 488–499. [DOI] [PubMed] [Google Scholar]
- Stallibrass S, Thomas R. 2008. Feeding the Roman Army: The Archaeology of Production and Supply in NW Europe. Oxbow Books: Oxford. [Google Scholar]
- Thiersch JB. 1948. Contribution to the radiology and pathology of transmissible avian osteopetrosis—lymphomatosis. Radiology 51(1): 100–110. [DOI] [PubMed] [Google Scholar]
- Thomas R. 2005. Animals, economy and status: integrating zooarchaeological and historical data in the study of Dudley Castle, West Midlands (c. 1100–1750). BAR Archaeopress: Oxford. [Google Scholar]
- Thomas R. 2009. Bones of contention: why later post‐medieval faunal assemblages in Britain matter Crossing Paths or Sharing Tracks: Future Directions in the Archaeological Study of Post‐1550 Britain and Ireland, Horning A, Palmer M. (eds.). Boydell and Brewer Ltd.: Woodbridge; 133–148. [Google Scholar]
- Thomas R. 2012. Nonhuman palaeopathology The Global History of Paleopathology: Pioneers and Prospects, Roberts C. (ed.). Oxford University Press on Demand: Oxford; 652–666. [Google Scholar]
- Uzunova K, Stamatova‐Yovcheva K, Dimova V, Yovchev D, Halil M. 2014. Anatomical and ethological changes in poultry affected by osteopetrosis. Scientific Papers on Animal Science and Biotechnologies 47(1): 188–191. [Google Scholar]
- van Wijngaarden‐Bakker LH, Krauwer M. 1979. Animal palaeopathology. Some examples from the Netherlands. Helinium Wetteren 19(2): 37–53. [Google Scholar]
- Vann S, Thomas R. 2006. Humans, other animals and disease: a comparative approach towards the development of a standardised recording protocol for animal palaeopathology. Internet Archaeology 20 https://doi.org/10.11141/ia.20.5. [Google Scholar]
- Vindolanda Tablets Online . Vindolanda inventory no. 88.944. http://vindolanda.csad.ox.ac.uk/TVII‐181 [Accessed 09/11/2016a]
- Vindolanda Tablets Online . Vindolanda inventory no. 89.978. http://vindolanda.csad.ox.ac.uk/TVII‐183 [Accessed 09/11/2016b]
- Vindolanda Tablets Online . Vindolanda inventory no. 88.839.f http://vindolanda.csad.ox.ac.uk/TVII‐302 [Accessed 09/11/2016c]
- Vindolanda Tablets Online . Vindolanda inventory no. 88.943. http://vindolanda.csad.ox.ac.uk/TVII‐180 [Accessed 09/11/2016d]
- Vogt PK. 1977. Chapter 8: Genetics of RNA tumor viruses Comprehensive Virology, Vol. 9: Regulation and Genetics, Genetics of Animal Viruses, Fraenkel‐Conrat H, Wagner RR. (eds.). Plenum Press: New York and London; 341–455. [Google Scholar]
- von den Driesch A, Pöllath N. 2000. Tierknochen aus den Mithrastempel von Künzing Lkr. Deggendorf. Vorträge des Niederbayerischen Archäologentages Vol. 18; 145–162. [Google Scholar]
- Watts SL, Smith RE. 1980. Pathology of chickens infected with avian nephoblastoma virus MAV‐2 (N). Infection and Immunity 27(2): 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RA. 2006. The discovery of endogenous retroviruses. Retrovirology 3(67). https://doi.org/10.1186/1742‐4690‐3‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West B, Zhou BX. 1988. Did chickens go North? New evidence for domestication. Journal of Archaeological Science 15(5): 515e533. [Google Scholar]
- Weyl KG, Dougherty RM. 1977. Contact transmission of avian leukosis virus. Journal of the National Cancer Institute 58(4): 1019–1025. [DOI] [PubMed] [Google Scholar]
- Willet R. 2014. Experiments with diachronic data distribution methods applied to Eastern Sigillata A in the eastern Mediterranean. Herom 3(1): 39–69. [Google Scholar]


