The history of metformin‐associated lactic acidosis (MALA) is rooted in the earlier experience with phenformin, another biguanide treatment for type 2 diabetes that was removed from the market owing to a clear causal association with cases of lactic acidosis (LA). Although metformin is less problematic in this regard, metformin can and does induce LA, and under the right circumstances it continues to present a potential risk to patients. Recent liberalization of the prescribing guidelines in the USA and European Union specifically regarding use in more advanced stages of chronic kidney disease (CKD) have led to renewed interest in the topic of MALA, and this issue of Diabetes Obesity Metabolism presents two timely articles on the topic, by Lalau et al.1 and Connelly et al.2
Lalau et al.1 outline a new way of thinking regarding the extremely rare, but often serious and potentially fatal, condition of MALA. They present a thoughtful analysis of existing MALA data and propose a more precise framework and nomenclature for categorizing LA with regard to whether metformin is clearly implicated causally, or is more of an “innocent bystander”. Specifically, the authors propose a framework, primarily based on the presence of any other underlying condition that can cause LA, as well as a metformin measurement, within the proposed “umbrella” category of “lactic acidosis in metformin therapy” (LAMT) as follows. (1) Metformin‐induced LA (MILA): cases where (i) no known additional conditions that could lead to LA are present and (ii) a high plasma metformin concentration value is available or there is evidence suggestive of metformin accumulation based on the patient's metformin dose, renal function, and time since last dose. (2) Metformin‐unrelated LA (MULA): cases with good evidence of an intercurrent acute illness or condition that can lead to LA, together with low actual or assumed metformin concentration(s). (3) MALA: cases with insufficient information to rule out metformin involvement.
While a more precise categorization paradigm may provide useful guidance to clinicians when dealing with suspected cases of MALA, the authors suggest that metformin does not play a contributing role in cases in which any other condition could be attributed to the LA event, and conclude that MULA “is probably by far the most common.” The authors do not provide new data to justify such a conclusion, and this conclusion does not incorporate the additive and perhaps synergistic effect metformin concentration has on inducing LA when a secondary event occurs. Contrary to the authors’ assessment, MALA represents the majority of cases as there is often insufficient information in real‐world clinical settings to definitively exclude metformin as a contributing cause of LA.
The underlying assumption made by Lalau et al. is that a single low measurement of plasma metformin and the presence of any other hypoxic condition is sufficient to rule out metformin relatedness. We do not believe that metformin's involvement in the development of LA can be so easily excluded for two reasons. Firstly, metformin increases the risk of LA. Because of its ability to disrupt lactate metabolism, metformin accumulation can cause LA on its own in extreme cases (eg, renal failure, overdose), and moderate accumulation can increase the likelihood that a secondary hypoxic event would lead to LA even though the severity of such an event may not have been sufficient to lead to LA in the absence of metformin accumulation. Thus, metformin use presents a risk of metformin plasma accumulation, which lowers the threshold for any relevant other intercurrent event to induce LA. As such, a secondary condition/event is often present in cases of MALA, but that is an insufficient reason to exclude metformin accumulation as a contributing factor. The relationship between degree of metformin accumulation and severity of a secondary hypoxic condition as it relates to LA risk is complex, as exemplified in Figure 1.
Figure 1.
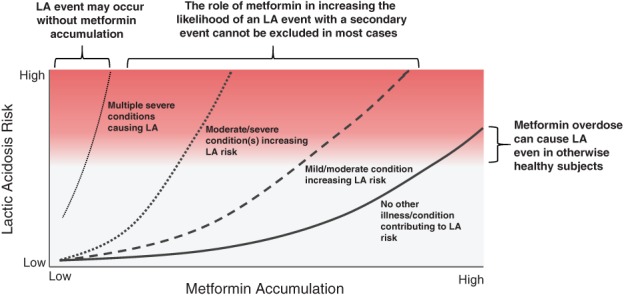
Representation of lactic acidosis risk as a function of metformin accumulation and other precipitating factors
Secondly, the temporal relationship between metformin accumulation and effects on oxidative metabolism and ensuing acidosis risk are not completely understood. A recent publication by Neal et al.3 showed that exposure to high concentrations of metformin can have prolonged effects on oxidative metabolism, even after a lengthy washout. Thus, even if metformin concentrations are low at the time of assessment (possibly many hours after LA ensued), a contributing role of metformin cannot be excluded. This is consistent with reports of MALA that are refractory to the commonly employed treatment haemodialysis.4, 5
Thus, for reasons stated we believe that, in the vast majority of cases, we do not have the ability to exclude the known ability of metformin to increase LA risk. Our concern is that, while attempting to add more precision to the terms used to define LA in the presence of metformin use, a binary classification system that would discount the contribution of metformin based on a cross‐sectional assessment of the patient would be inappropriate from a pharmacovigilance perspective as it will probably lead to underreporting of metformin's association with LA. We refer the reader to a recent report by Boucaud‐Maitre et al.6 not discussed by Lalau et al, which provides a rich data set regarding MALA incidence and the relationship between metformin plasma concentration, lactate concentration, pH and renal function. The conclusions drawn in that study point to a strong relationship among circulating concentrations of metformin, LA and mortality. MALA is indeed an imprecise term that speaks to the often multivariate nature of LA events in patients treated with metformin.
Also in this issue, Connelly et al.2 present a case–control study of the GoDARTs database to assess the association between biochemically confirmed LA, metformin use, and acute kidney injury. Despite metformin‐treated patients being younger and having better kidney function, they exhibited increased mean lactate concentrations, and were at significantly higher risk of an LA event than non‐metformin‐treated patients with type 2 diabetes, having an incidence of 45.7 per 100 000 patient‐years compared with 11.8 per 100 000 patient‐years in the non‐metformin treated patients. The relationship between metformin use, lactate concentration and LA was strongest in cases of acute kidney injury. This was to be expected, given the increased metformin accumulation that results from decreased renal function and suggests an interaction between metformin and LA risk. Consistent with this relationship, Connelly et al. found a statistically significant interaction between metformin use and AKI status on lactate levels. Perhaps most relevant to the discussion regarding diagnosis of LA in patients using metformin was the observation that only 11% of the biochemically confirmed cases of LA identified by Connelly et al. were International Classification of Disease‐coded as acidosis events. Despite the limitations of a study of this nature, highlighted by the authors themselves, the results suggest an underreporting of LA in these patients. This report, together with the recent publication by Boucaud‐Maitre et al.6 reinforces the contributing role of metformin in the aetiology of LA.
Recent publications7, 8 have advocated broadening the use of metformin in patients with more advanced CKD, including patients on dialysis and/or awaiting renal transplant. Treatment options for these patients are limited, and metformin has generally not been widely used for the reasons stated earlier. Although these publications acknowledge the need for controlled clinical trial data, which is currently lacking, they propose that patients with advanced renal disease could be treated with lower doses of metformin to improve glycaemic control without excessive plasma accumulation. Dose reduction in renally impaired patients is a common strategy for renally cleared drugs that act systemically, and while such a strategy would probably result in acceptable exposure in the context of safety, the relationship between metformin plasma exposure and efficacy is not that simple. There is increasing evidence that a substantial contribution to the glycaemic effect of metformin is mediated through the intestine rather than primarily through systemic exposure.9, 10, 11, 12, 13 Given that the bioavailability of metformin increases with decreasing dose,14 it is unlikely that the low doses of metformin required to maintain acceptable plasma concentrations would deliver sufficient metformin to the intestine to leverage the gut‐based mechanisms of action.15
In summary, classification of LA in metformin‐treated patients should acknowledge the complexity of the contributing factors and weigh the potential roles of both metformin accumulation and underlying hypoxic diseases/conditions based on all the information available. This is particularly important in patients at inherently higher risk of MALA, such as the elderly and those with advanced CKD.
ORCID
Thomas A. Bicsak http://orcid.org/0000-0001-8629-9733
REFERENCES
- 1. Lalau J‐D, Kajbaf F, Protti A, Christensen MM, Broe ME, Wiernsperger N. Metformin‐associated lactic acidosis (MALA): moving towards a new paradigm. Diabetes Obes Metab. 2017. https://doi.org/10.1111/dom.12974. [DOI] [PubMed] [Google Scholar]
- 2. Connelly PJ, Lonergan M, Soto‐Pedre E, Donnelly L, Zhou K, Pearson ER. Acute kidney injury, plasma lactate concentrations and lactic acidosis in metformin users: a GoDarts study. Diabetes Obes Metab. 2017. https://doi.org/10.1111/dom.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neal A, Rountree AM, Philips CW, et al. Quantification of low‐level drug effects using real‐time, in vitro measurement of oxygen consumption rate. Toxicol Sci. 2015;148(2):594‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozeki T, Kawato R, Watanabe M, et al. A fatal case of metformin‐associated lactic acidosis. Intern Med. 2016;55(7):775‐778. [DOI] [PubMed] [Google Scholar]
- 5. Zibar L, Zibar K. Hemodialysis‐refractory metformin‐associated lactate acidosis with hypoglycemia, hypothermia, and bradycardia in a diabetic patient with belated diagnosis and chronic kidney disease. Int J Clin Pharmacol Ther. 2017;55(4):348‐351. [DOI] [PubMed] [Google Scholar]
- 6. Boucaud‐Maitre D, Ropers J, Porokhov B, et al. Lactic acidosis: relationship between metformin levels, lactate concentration and mortality. Diabet Med. 2016;33(11):1536‐1543. [DOI] [PubMed] [Google Scholar]
- 7. Chowdhury TA, Srirathan D, Abraham G, et al. Could metformin be used in patients with diabetes and advanced chronic kidney disease? Diabetes Obes Metab. 2017;19(2):156‐161. [DOI] [PubMed] [Google Scholar]
- 8. Kumar SS, Graham GG, Smith FC, et al. Could metformin be used in patients with advanced chronic kidney disease? Diabetes Obes Metab. 2017;19(2):302‐303. [DOI] [PubMed] [Google Scholar]
- 9. Napolitano A, Miller S, Nicholls AW, et al. Novel gut‐based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS ONE. 2014;9(7):e100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duca FA, Cote CD, Rasmussen BA, et al. Metformin activates a duodenal Ampk‐dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21(5):506‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buse JB, DeFronzo RA, Rosenstock J, et al. The primary glucose‐lowering effect of metformin resides in the gut, not the circulation. results from short‐term pharmacokinetic and 12‐week dose‐ranging studies. Diabetes Care. 2016;39(2):198‐205. [DOI] [PubMed] [Google Scholar]
- 13. McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59(3):426‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81‐98. [DOI] [PubMed] [Google Scholar]
- 15. Jones GC, Sainsbury CAR. Comment on ‘A justification for less restrictive guidelines on the use of metformin in stable chronic renal failure’. Diabet Med. 2015;32(2):287. [DOI] [PubMed] [Google Scholar]


