Abstract
Cystic fibrosis (CF) is an autosomal recessive disease that is potentially treatable by gene therapy. Since the identification of the gene encoding CF transmembrane conductance regulator, a number of preclinical and clinical trials have been conducted using the first generation of adeno-associated virus, AAV2. All these studies showed that AAV gene therapy for CF is safe, but clinical benefit was not clearly demonstrated. Thus, a new generation of AAV vectors based on other serotypes is needed to move the field forward. This study tested two AAV serotypes (AAV1 and AAV5) using a dual-luciferase reporter system with firefly and Renilla luciferase genes packaged into AAV1 or AAV5, respectively. Two male and two female Rhesus macaques were each instilled in their lungs with both serotypes using a Penn-Century microsprayer. Both AAV1 and AAV5 vector genomes were detected in all the lung samples when measured at the time of necropsy, 45 days after instillation. However, the vector genome number for AAV1 was at least 10-fold higher than for AAV5. Likewise, luciferase activity was also detected in the same samples at 45 days. AAV1-derived activity was not statistically greater than that derived from AAV5. These data suggest that gene transfer is greater for AAV1 than for AAV5 in macaque lungs. Serum neutralizing antibodies were increased dramatically against both serotypes but were less abundant with AAV1 than with AAV5. No adverse events were noted, again indicating that AAV gene therapy is safe. These results suggest that with more lung-tropic serotypes such as AAV1, new clinical studies of gene therapy using AAV are warranted.
Keywords: : cystic fibrosis, lung, rhesus, AAV1, AAV5
Introduction
Cystic fibrosis (CF) is an autosomal recessive disorder common within the Caucasian population.1,2 CF is caused by mutations in the gene encoding the CF transmembrane conductance regulator (CFTR), which functions as a chloride channel.3 The chloride ions that flow function out of airway cells via CFTR functions in the production of thin, freely flowing mucus.4 Mutations in the CFTR gene disrupt the function and trafficking of CFTR.3 As a result, the cells that line the passageways of the lungs, pancreas, and other organs produce mucus that is unusually thick and sticky.5 Absence of functioning CFTR also leads to altered bicarbonate transport, resulting in a more acidic pH of mucosal fluids. The latter adversely diminishes the ability of the airway to defend itself against bacterial challenges.6 Thus, individuals with CF are more susceptible to pulmonary infections that lead to bronchiectasis and diminution of lung function,7 one of the primary causes of their reduced life-span when compared to individuals without CF (https://www.cff.org/Research/Researcher-Resources/Patient-Registry). Thus, treatments that improve lung function and prevent bacterial lung infection and inflammation would increase the average age of survival of CFTR patients.8,9
Gene therapy
Shortly after the CF gene was identified, intense efforts were undertaken as part of a race to develop an effective gene therapy,10 and several clinical trials were conducted with this purpose in mind. These clinical trials utilized full-length CFTR packaged into adenovirus vectors, adeno-associated virus (AAV) vectors, or liposomes and delivered to the airways. AAV11 vectors emerged from these studies as the most promising viral vector with regard to safety profile.
AAV
AAV is a single-stranded DNA parvovirus commonly found in humans and primates. AAV cannot propagate on its own, since it is naturally defective for replication, requiring co-infection with a helper adenovirus or herpesvirus for replication [19]. The AAV genome consists of inverted terminal repeats (ITR) and the rep and cap proteins. The ITRs play a key role in integration, replication (ori), excision, and packaging, and the rep genes are involved in replication. Recombinant AAV (rAAV) manufactured for gene therapy12 contains only the ITRs, the desired coding sequence of a gene such as CFTR, encapsulated into an AAV particle. Since the rAAV does not contain the rep genes, rAAV cannot replicate, even in the presence of a naturally occurring helper virus. Thus, for human therapy, removal of the genes involved in AAV replication is a safety feature that helps prevent inappropriate spread of rAAV following clinical application.
CF preclinical and clinical trials using AAV
A number of preclinical and clinical studies have been conducted utilizing an AAV2 serotype into which was packed either a reporter gene or CFTR.13–19 All of the clinical trials utilized a recombinant virus, tgAAV2-CFTR, within which the full-length coding sequence of CFTR was packaged.20 In tgAAV2, CFTR expression was driven by the natural promoter contained within the ITR.21 In all the animal and human studies involving AAV in airways, this recombinant virus was found to be safe, an extremely important prerequisite for its development for clinical therapy.22–27 In the preclinical animal studies, rAAV2 infection and transduction were both observed.28 However, the results of the clinical trials were more varied. Viral gene transfer was often detected, in terms of the persistent presence of the viral genome in the airways for long periods.13,14,16–18,29,30 However, demonstration of transduction, in the form of vector-derived mRNA expression, proved to be difficult to detect (see Guggino and Cebotaru31 for a review). When AAV2 gene transfer was tested in humans,14 it was also found to be safe, but it did not show a sustained clinical effect.13 There were two major reasons for this lack of clinical efficacy: the weak promoter in the vector construct did not express enough protein to be effective,32 and the AAV2 was not very efficient in transducing lung cells.
With the discovery of many more serotypes of AAV showing a more pronounced tropism for the airways when compared to AAV2,33 the prospect of boosting gene transfer using these new serotypes33 has recently become feasible. In order to begin to determine which AAV is the most efficient in transduction, this study focused on AAV1 and AAV5. Both AAVs utilize the same receptor: a2-3 and a2-6 N-linked sialic acid (see Huang et al.34 for a review). Several studies have shown that AAV5 transduces the airways of Rhesus macaque (see Guggino and Cebotaru31 for a review). Similarly, AAV1 has been shown to transduce polarized primary human airway cells in culture with a preference toward transducing the cell via the apical plasma membrane in contrast to other AAVs, such as AAV6, which have a preference for transducing the cells via the basolateral cell membrane.35 These data indicate that AAV1 may be the ideal vector for airway cell transduction. To test this further, a dual-reporter assay was developed based upon firefly and Renilla luciferases36 cloned into the AAV1 and AAV5 vectors, respectively. The two enzymes differ in their substrate and cofactor requirements and can be easily distinguished in assays. First, a non-lethal study was conducted utilizing chimpanzees, chosen because they are the closest primate to man.36 However, given the recent restrictions regarding the use of chimpanzees in non-lethal studies, the usefulness of substituting Rhesus macaques as a standard model for testing the tropism of virus for airway delivery has now been assessed.
Experimental Design
Viral particles
The Renilla (RL) luciferase-containing virus was packaged utilizing the proviral plasmid pAAV2-CBA-HA-Rl-plus, and the firefly (FL) luciferase-containing virus utilized pAAV2-CBA-HA-FL for packaging. Both plasmids were manufactured so that they were of identical length and contained the cytomegalovirus enhancer, β-actin promoter segment from pTR2CB-264 CFTR.36 Recombinant AAV virus particles were produced by a standard co-transfection protocol, as described previously.36 The firefly luciferase gene was packaged into AAV1 capsids and is referred to as AAV1-CB-FL, and the Renilla luciferase gene was packaged into AAV5 capsids and is referred to as AAV5-CB-RL.36 The vector combination was formulated on the day of dosing by combining the two vectors, as provided by the Vector Core at the University of Massachusetts, to achieve equal concentrations of the genome copies (gc) of the two vectors in the final mixtures. Specifically, 13.5 mL of the AAV5-CB-RL vector (1.2 × 1013 gc/mL) was combined with 16.2 mL of the AAV1-CB-FL vector (1.0 × 1013 gc/mL) to provide a mixture with 0.54 × 1013 gc/mL of each vector.
Rhesus macaques
Healthy male and female Rhesus monkeys (Macaca mulatta; two male and two female) between 2 and 3 years of age (based on dentition) were purchased from Global Research Supply, LLC (Reno, NV). The animal identifiers were: RQ8425 (F), RQ8571 (M), RQ8676 (F), and RQ8872 (M). The males weighed between 3.7 and 4.1 kg, and the females between 3.4 and 4.3 kg at the time of vector dosing. The macaques were housed in individual stainless-steel cages according to the requirements of the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. They were fed Harlan Tekland Certified Monkey Diet (Madison, WI). The macaques were fed an appropriate number of biscuits twice daily. Three times per week, each macaque received enrichment in the form of food supplements (i.e., fruit/vegetable). Tap water was provided ad libitum. Prior to randomization to the dosage groups, each animal underwent a complete physical examination by a staff veterinarian. The examination included evaluations of behavioral and clinical observations, body weight and rectal temperature, respiratory and heart rate, a complete blood count (CBC) and serum chemistry screen, as well as fecal ova and parasite counts (O&P). Only macaques considered asymptomatic and clinically healthy were assigned to the study. Animals were euthanized at 45 days post vector instillation.
This study was conducted in the Lovelace Respiratory Research Institute (LRRI) animal facilities, which are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. This study complied with all applicable sections of the Final Rules of the Animal Welfare Act regulations (9 CFR Parts 1, 2, and 3) and the Guide for the Care and Use of Laboratory Animals (NRC 1996). The study was approved by the Institutional Animal Care and Use Committee of both the LRRI and the Johns Hopkins University.
Those portions of the study not conducted at the LRRI were: evaluation of vector in-use stability (University of Massachusetts), quantitation of luciferase activity in lung samples (University of Iowa), quantitation of AAV vector in blood and tissues (Powell Gene Therapy Center), and quantitation of antibodies recognizing the AAV core protein (University of Pennsylvania, Immunology Core).
Technetium-99m delivery using an Areoneb/Idehaler combination
The Aeroneb Solo nebulizer, in combination with the Idehaler holding chamber, was selected for delivery of the colloidal technetium-99m (99mTc). This system was selected on the basis of published data37,38 that reference high delivery efficiency and a particle size of 4–5 μm mass median aerodynamic diameter. The nebulizer and controller were used as recommended by the manufacturer for clinical applications, except that for the NHP exposures, it was fitted directly with a face mask, and during exhalation, the nebulizer output was retained in the holding chamber, minimizing the potential waste of aerosol.
99mTc was conjugated with sulfur colloid particles for aerosol delivery according to the kit instructions (Pharmalucence, Inc., Billerica, MA). The suspension was directly transferred to each nebulizer for aerosolization. A portion of the radiolabeled colloid was placed in the Aeroneb nebulizer, and the system was activated. The output from the Idehaler was passed to a facemask for inhalation by the animal. In a second attempt, the Idehaler was modified to reduce its dead volume.
A mechanical breathing system (Harvard pump) was used to simulate the inspiration/expiration of the macaque. The Harvard pump was connected at the mouthpiece of the Idehaler. The ventilatory settings were as follows: 50 mL/breath, 18 breaths/min, and a 40% inspiratory cycle. These settings were selected based on previous ventilatory data obtained from free-breathing nonhuman primates under anesthesia.
During the exposures, the macaques were sedated with a combination of ketamine (3–10 mg/kg administered intramuscularly [i.m.]) and dexmedetomidine (0.018–0.050 mg/kg i.m.) and then transferred to the exposure room in a primate transfer box. Each macaque was placed in a prone position on a slanted board (head upwards) and exposed by face mask inhalation to the 99mTc aerosol. After the exposure, the macaques were immediately transferred to the gamma camera for image acquisition. Planar images were collected for quantification of regional deposition in regions that included, but were not limited to, the lungs, oral cavity, and trachea/esophagus. After imaging was completed for each animal, atipamezole (0.18–0.50 mg/kg) was administered intravenously to reverse the anesthetic effects of the dexmedetomidine, and the macaques were returned to their home cages.
AAV administration
AAV vectors were administered by trans-oral insertion of a Penn-Century rigid microsprayer into an endotracheal tube inserted into the trachea of the anesthetized macaque. The spray was initiated using the designated 1 mL polystyrene syringes: 4 × 1 mL were administered to each macaque. Each animal received 2.16 × 1014 vg of each vector. An attempt was made to deliver each spray during an inhalation cycle. Following administration of the vectors to the macaques, additional test article was aerosolized and collected. This collected material, as well as aliquots of the combined, non-aerosolized test article, and the individual vector solutions, were stored at 2–8°C for 4 days, until they could be shipped by overnight delivery to Dr. Guangping Gao, University of Massachusetts, for evaluation of stability. The effect of passing the vector mixture through the microsprayer was assessed by comparing the expression of the two types of luciferase in 293 cells, compared to the mixture without having been sprayed.
Blood sampling
Clinical pathology
Blood samples were collected (1) between 14 and 28 days prior to 99mTc inhalation exposure (during physical examination), (2) within 30 days prior to AAV administration, and (3) on the day of necropsy. Endpoints for analyses included hematology, coagulation, clinical chemistry, and anti-AAV1/AAV5 antibody assessment. A sufficient blood volume was collected via the femoral vein while the animals were anesthetized. Blood totals did not exceed 7.5% of the total circulating blood volume of each animal for each draw.
Vector concentration in blood
For quantitation of vector copies in blood, additional blood samples (∼0.5 mL each) were collected into ethylenediaminetetraacetate (EDTA) tubes prior to the administration of the AAV1/AAV5 vector and at 1, 3, and 8 h after AAV1/AAV5 inhalation exposure, 2 days after AAV1/AAV5 administration, and at necropsy. Samples were flash-frozen and submitted to Dr. Thomas Conlon, University of Florida, for determination of vector genomes (see below).
Hematology and clinical chemistry
Hematology and clinical chemistry evaluations were performed on blood samples collected as described above. For hematology analyses, about 0.3–0.5 mL of whole blood was placed into tubes containing EDTA as an anticoagulant. The blood was stored at room temperature prior to analysis. Samples were analyzed on an ADVIA™ 120 Hematology System (Siemens Medical Solutions Diagnostics, Tarrytown, NY). Any unused blood was discarded.
Neutralizing antibodies
AAV-1 firefly-LacZ or AAV5 Renilla-LacZ was incubated with serial dilutions of heat-inactivated serum samples in Dulbecco's modified Eagle's medium (DMEM) for 1 h at 37°C. Subsequently, the serum–vector mixture was added to 96-well plates with seeded Huh7 cells at 1 × 105 cells/well. After 1 h, the cells in each well were supplemented with 100 μL of 20% fetal bovine serum–DMEM and incubated for 18–22 h at 37°C and 5% CO2. The neutralizing antibody titer was reported as the highest serum dilution that inhibited AAV1 or AAV5 LacZ transduction by 50%, when compared to its own AAV1 or AAV5 vector-positive/serum-negative control.
Real-time polymerase chain reaction
Real-time polymerase chain reactions (PCRs) were run in triplicate, with the third replicate containing an approximate 10:1 ratio of plasmid spike-in to 0.1 μg gDNA loaded. The spike-in for negative samples was evaluated for inhibition and assay confirmation. A spike-in ≥40 copies/μg of gDNA confirmed the assay. For samples being assayed by real-time PCR, any value ≥100 copies/μg of gDNA was considered a positive signal. Numerical values of copy numbers normalized to 1 μg of gDNA are reported for tissue and blood gDNA samples that were positive. Because of the dual-vector nature of this study, first there was a need to determine the specificity of each primer and probe set designed to target the RL and FL DNA sequences. For firefly luciferase, the forward primer was ATCCGGTGGCGGTGG, and the reverse was CGCCGGGCCTTTCTTT. For Renilla, the forward primer was AATTTGCAGCATATCTTGAACCATT, and the reverse was GGATTTCACGAGGCCATGAT. The probe was 6FAM-TCGGTCACCGACGCCAAAAACA-TAMRA.
To do this, the 1 × 108 of the FL and RL standards was cycled with the primers and probe designed to detect the opposing sequence. The lack of cycling, “undetermined” in the two runs, demonstrates the specificity to the intended target and no cross-reactivity with the alternate construct.
Dual-luciferase assays
Lung samples were lysed in 200 μL of Passive Lysis Buffer (Promega, Madison, WI). FL and RL activities in the cell lysates were measured with the Promega Dual-Luciferase Reporter Assay System in a 20/20 luminometer (Turner BioSystem, Sunnyvale, CA) according to the manufacturer's instruction. An empirical correction (determined in zz) of 1 Fl = 14 RL units was applied to equalize the intensity of expression between the two reporters. This was determined by independently infecting primary human airway cells with either AAV1-CB-FL or AAV1-CB-RL and comparing the sensitivity of the luciferase activity measurement derived from the FL or RL assay (see fig. 2 of Flotte et al.36).
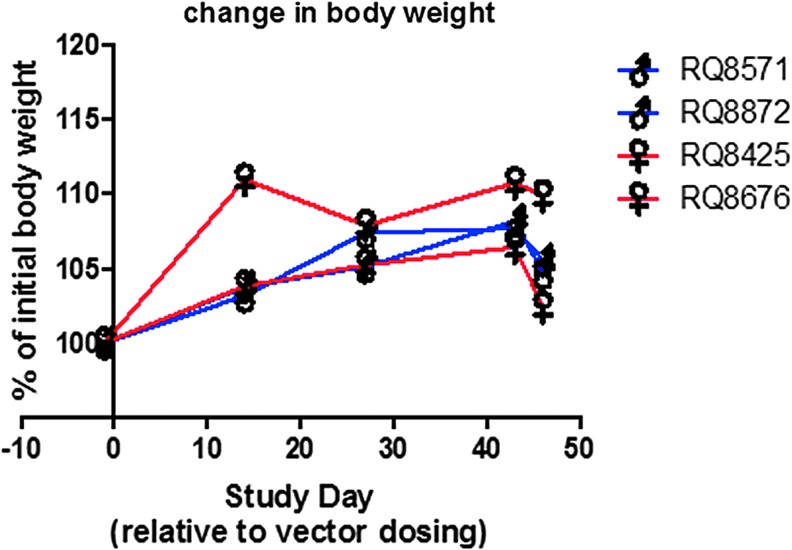
Figure 2.
Body weight. Animals were weighed as an assessment of health. Note that all animals gained weight, except on the last day, when there was a short-term food restriction prior to necropsy. Data from all four animals are included along with their sex. Two male and two female monkeys were used.
Results
Aerosol versus brochosprayer delivery
The objective was to determine the feasibility and efficiency of aerosol delivery to nonhuman primates using the Aeroneb Solo nebulizer/Aeroneb ProX Controller in combination with the Idehaler chamber. The results of this characterization allowed us to determine whether the system would be suitable for conducting a subsequent AAV1/AAV5 vector aerosol deposition study in the Rhesus macaques, as described below.
Radiolabeled test aerosols of sulfur colloid labeled with 99mTc that could be measured with a gamma camera imaging and analysis system were used to define the delivery efficiency and distribution of the aerosols in the macaques. The efficiency of delivery from the nebulizer to the lungs was determined, together with the percentage of the inhaled dose that was delivered to the lungs and a qualitative assessment of central airway versus peripheral lung deposition.
The efficiency of aerosol delivery to the macaque lungs averaged ∼1.2%. The remaining aerosol was retained primarily in the Idehaler holding chamber, and approximately 10% was found in the face/throat, face mask, and exhaust filter (Fig. 1A and Table 1). The data were further refined to determine that ∼27% of what was deposited in the animals was located in the lungs. Based on the results of this experiment, it was hypothesized that the delivery of aerosol to the macaques was low because the volume of the Idehaler chamber (400–500 mL) is much greater than the tidal volume of the macaques (30–50 mL). Therefore, the animals were unable to pull sufficient aerosol from the chamber into their airways. Further, since their nares were not blocked to prevent nasal inhalation of aerosol, it is possible that nasal deposition of the 5 μm aerosol may have contributed to the low thoracic/pulmonary deposition. Therefore, the Idehaler chamber was modified to reduce the volume, and methods were identified to provide oral-only aerosol inhalation in a subsequent limited study. To test this setup, one macaque was exposed to the Tc aerosol to determine whether modification of the Idehaler chamber to a smaller volume (∼33% less) and oral-only inhalation (achieved by blocking the nares) would improve the delivery of the aerosol to the lungs. The results of this analysis are shown in Table 1, and the image of the deposition pattern from these exposures is shown in Fig. 1B. The results showed that these modifications provided no improvement in the delivery efficiency of the system. However, there may have been a slight qualitative increase in central deposition. Given that the deposition was unacceptably low using the Idehaler, the administration of the AAV vectors was accomplished via trans-oral insertion of a Penn-Century microsprayer into an endotracheal tube inserted into the trachea of the anesthetized macaques, as described in the Methods section.
Figure 1.
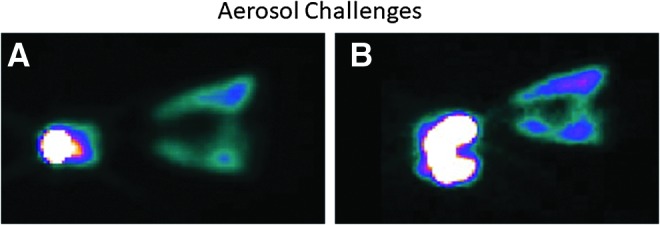
Aerosol challenges. Total lung deposition of technetium-99m (99mTc) measured by gamma scintigraphy. (A) The Idehaler was used according to the manufacturer's specifications. (B) The Idehaler chamber was reduced to a smaller volume (approximately 33% less). Images were acquired with the animal in a prone position and represent the buccal cavity on the left and lung regions on the right. Note that most of the deposition was in the buccal cavity.
Table 1.
Ratio of deposition
| NHP ID | Lung | Face/throat | Face mask | Exhaust filter | Idehaler chamber |
|---|---|---|---|---|---|
| RQ8425a | 0.03 | 0.06 | 0.21 | 0.69 | 99.0 |
| RQ8676 | 2.15 | 2.82 | 3.96 | 8.36 | 82.7 |
| RQ8872 | 0.78 | 2.73 | 3.07 | 8.80 | 84.6 |
| RQ8571 | 0.66 | 4.03 | 4.68 | 9.29 | 81.3 |
| Average | 1.20 | 3.19 | 3.90 | 8.81 | 82.89 |
| SD | 0.83 | 0.73 | 0.81 | 0.47 | 1.65 |
Gamma images were acquired with the animal in a prone position. Immediately following each image, the nebulizer components (face mask, exhaust filter, and Idehaler chamber) were imaged. Images were acquired with a 128 × 128 matrix, zoom factor of 1, and as static planar images. Images were analyzed with the ICON software. Radioactivity was determined for each region of interest (ROI) including the lungs, oral/nasal cavity, face mask, exhaust filter, and Idehaler holding chamber. Counts in each ROI were converted to radioactivity through a daily curve, prepared on the dose calibrator. The percentage in each ROI was determined based on the amount consumed by the nebulizer.
Note that most of the technetium remained in the chamber of the inhaler.
NHP, nonhuman primate; SD, standard deviation.
Body weights
All animals gained weight during the study, as expected. The percentage change as a function of time is shown in Fig. 2. The small decrease on the last day is presumed to be due to food having been withheld prior to euthanasia to minimize the possibility of aspiration of any gastric contents during the procedure.
Clinical observations
The only significant clinical observation concerning these animals was a footpad laceration in RQ8425. The laceration was evaluated and determined to be superficial, and it was treated with chlorhexidine and skin glue. The laceration healed normally and was not a test article–related finding.
The complete results for the hematology and clinical chemistry analyses are provided in Tables 2–4. The coagulation parameters were not collected for the pre-vector dosing blood draw because of an error in the type of blood collection tubes used, and the blood from one male and one female collected the day prior to necropsy clotted, so that coagulation parameters could not be reported. There were no unusual observations in the chemistry profiles. However, one of the females (RQ8676) demonstrated slightly elevated neutrophils in the pre-study and pre-dosing blood samples and a substantial further increase at the terminal collection. In addition, the total eosinophils for the other female (RQ8425) more than tripled between the pre-dosing and terminal blood collections. Because similar increases were not observed in the two male macaques, it is unlikely this was a test article–related effect, but because these changes in the two animals occurred between the pre-dosing and terminal time points, that possibility cannot be excluded. In summary, there were no clinical observations attributable to vector administration
Table 2.
Summary of hematology results
| Sex | ID | WBC 103/mL | RBC 106/mL | HGB g/dL | HCT % | MCV fL | MCH pg | MCHC g/dL | PLT 103/mL | NEUT %WBC | LYM %WBC | MONO %WBC | EOS %WBC | BASO %WBC | LUC %WBC | Abs. NEUT 103/mL | Abs. LYM 103/mL | Abs. MONO 103/mL | Abs. EOS 103/mL | Abs. BASO 103/mL | Abs. LUC 103/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | |||||||||||||||||||||
| Male | RQ8571 | 13.22 | 5.85 | 14.6 | 44.3 | 75.6 | 25.0 | 33.1 | 435 | 39.9 | 55.9 | 1.7 | 0.3 | 0.7 | 1.5 | 5.28 | 7.39 | 0.23 | 0.04 | 0.09 | 0.19 |
| RQ8872 | 5.56 | 5.87 | 14.8 | 46.2 | 78.7 | 25.3 | 32.1 | 310 | 44.5 | 52.3 | 1.5 | 0.1 | 0.7 | 0.9 | 2.47 | 2.91 | 0.08 | 0.01 | 0.04 | 0.05 | |
| Female | RQ8425 | 9.40 | 5.87 | 14.1 | 42.6 | 72.6 | 23.9 | 33.0 | 291 | 28.1 | 64.7 | 4.1 | 1.0 | 0.9 | 1.3 | 2.64 | 6.08 | 0.39 | 0.09 | 0.08 | 0.12 |
| RQ8676 | 8.88 | 5.56 | 14.1 | 42.3 | 76.0 | 25.4 | 33.5 | 257 | 67.3 | 29.7 | 1.9 | 0.4 | 0.4 | 0.4 | 5.98 | 2.63 | 0.17 | 0.03 | 0.03 | 0.04 | |
| Pre | |||||||||||||||||||||
| Male | RQ8571 | 14.00 | 5.54 | 13.9 | 42.6 | 77.0 | 25.0 | 32.5 | 410 | 20.8 | 74.2 | 1.9 | 1.1 | 0.5 | 1.5 | 2.91 | 10.38 | 0.27 | 0.15 | 0.07 | 0.21 |
| RQ8872 | 4.47 | 5.59 | 14.3 | 46.2 | 82.6 | 25.6 | 31.0 | 267 | 24.2 | 72.4 | 2.1 | 0.2 | 0.2 | 1.0 | 1.08 | 3.23 | 0.09 | 0.01 | 0.01 | 0.04 | |
| Female | RQ8425 | 11.28 | 5.69 | 13.8 | 43.5 | 76.3 | 24.2 | 31.7 | 332 | 14.2 | 78.5 | 4.0 | 0.9 | 0.6 | 1.8 | 1.60 | 8.85 | 0.45 | 0.11 | 0.07 | 0.20 |
| RQ8676 | 8.28 | 5.36 | 13.6 | 42.0 | 78.3 | 25.4 | 32.5 | 280 | 61.2 | 34.6 | 2.4 | 0.9 | 0.3 | 0.6 | 5.07 | 2.87 | 0.20 | 0.07 | 0.02 | 0.05 | |
| Post | |||||||||||||||||||||
| Male | RQ8571 | 12.51 | 5.34 | 13.6 | 41.3 | 77.3 | 25.4 | 32.8 | 385 | 48.6 | 45.4 | 3.8 | 0.6 | 0.3 | 1.3 | 6.07 | 5.67 | 0.48 | 0.08 | 0.04 | 0.16 |
| RQ8872 | 5.39 | 6.11 | 15.9 | 50.5 | 82.6 | 26.1 | 31.6 | 243 | 34.0 | 63.2 | 1.3 | 0.1 | 0.3 | 1.1 | 1.84 | 3.40 | 0.07 | 0 | 0.02 | 0.06 | |
| Female | RQ8425 | 10.03 | 5.82 | 14.3 | 44.0 | 75.6 | 24.5 | 32.5 | 261 | 16.7 | 72.5 | 4.9 | 3.9 | 0.4 | 1.5 | 1.68 | 7.28 | 0.49 | 0.39 | 0.04 | 0.16 |
| RQ8676 | 15.39 | 5.35 | 13.8 | 42.8 | 80.0 | 25.9 | 32.4 | 280 | 82.8 | 14.4 | 1.9 | 0.2 | 0.3 | 0.4 | 12.75 | 2.21 | 0.30 | 0.03 | 0.04 | 0.06 | |
Blood samples were collected within 30 days prior to adeno-associated virus administration and on the day of necropsy.
Abs. BASO, Absolute Basophil count; Abs. EOS, absolute eosinophil count; Abs. LUC, absolute large unstained cell count; Abs. LYM, absolute lymphocyte count; Abs. MONO, absolute monocyte count; Abs. NEUT, absolute neutrophil count; BASO, basophils; EOS, eosinophils; HGB, hemoglobin; HCT, hematocrit; ID (animal), (animal) identification number; LUC, large unstained cells; LYM, lymphocytes; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MONO, monocytes; NEUT, neutrophils; PLT, platelet count; RBC, red blood cell Count.
Table 3.
Summary of hematology results
| Sex | ID | NA-S mM | K-S mM | CL-S mM | GLU mg/dL | BUN mg/dL | CRE-S mg/dL | PHOS mg/dL | BILIT mg/dL | ALP IU/L | ALT IU/L | AST IU/L | TP g/dL | ALB g/dL | CA mg/dL | CK IU/L | CHOL mg/dL | GLOBN g/dL | AG ratio | BC ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | ||||||||||||||||||||
| Male | RQ8571 | 147 | 3.7 | 109 | 56 | 23 | 0.6 | 4.6 | 0.2 | 446 | 38 | 46 | 7.3 | 4.7 | 9.9 | 575 | 166 | 2.6 | 1.8 | 38 |
| RQ8872 | 152 | 4.5 | 110 | 115 | 18 | 0.8 | 4.3 | 0.2 | 560 | 23 | 35 | 7.3 | 4.9 | 10.8 | 702 | 155 | 2.4 | 2 | 23 | |
| Female | RQ8425 | 146 | 3.7 | 108 | 81 | 24 | 0.7 | 4.8 | 0.1 | 379 | 20 | 26 | 6.7 | 4.5 | 10.3 | 430 | 139 | 2.2 | 2 | 34 |
| RQ8676 | 144 | 3.2 | 107 | 66 | 19 | 0.6 | 3.6 | 0.2 | 260 | 26 | 26 | 6.9 | 4.6 | 10.4 | 556 | 147 | 2.3 | 2 | 32 | |
| Pretreatment | ||||||||||||||||||||
| Male | RQ8571 | 146 | 3.5 | 107 | 61 | 23 | 0.5 | 5.1 | 0.2 | 561 | 25 | 48 | 7.1 | 4.4 | 9.4 | 1078 | 132 | 2.7 | 1.6 | 46 |
| RQ8872 | 149 | 3.9 | 104 | 82 | 19 | 0.9 | 4.6 | 0.3 | 571 | 25 | 36 | 7.4 | 5.1 | 10 | 490 | 171 | 2.3 | 2.2 | 21 | |
| Female | RQ8425 | 144 | 3.4 | 104 | 77 | 23 | 0.7 | 5.9 | 0.2 | 398 | 29 | 35 | 6.9 | 4.5 | 9.5 | 801 | 135 | 2.4 | 1.9 | 33 |
| RQ8676 | 145 | 3.6 | 107 | 43 | 24 | 0.6 | 4.5 | 0.2 | 265 | 44 | 31 | 7.1 | 4.5 | 9.6 | 764 | 126 | 2.6 | 1.7 | 40 | |
| Post treatment | ||||||||||||||||||||
| Male | RQ8571 | 149 | 3.8 | 113 | 63 | 20 | 0.5 | 4.7 | 0.3 | 632 | 24 | 37 | 7.3 | 4.5 | 9.4 | 234 | 126 | 2.8 | 1.6 | 40 |
| RQ8872 | 146 | 3.1 | 104 | 111 | 17 | 0.8 | 5.1 | 0.3 | 441 | 24 | 32 | 7.7 | 5.2 | 9.9 | 425 | 157 | 2.5 | 2.1 | 21 | |
| Female | RQ8425 | 144 | 3.4 | 104 | 58 | 23 | 0.7 | 5.9 | 0.2 | 369 | 30 | 46 | 7 | 4.5 | 9.2 | 465 | 147 | 2.5 | 1.8 | 33 |
| RQ8676 | 143 | 3.1 | 106 | 51 | 21 | 0.5 | 4.9 | 0.2 | 296 | 31 | 33 | 7.3 | 4.5 | 9.8 | 394 | 147 | 2.8 | 1.6 | 42 | |
No adverse clinical changes were noted between the pre and post dosing periods.
AAV1-CB-FL, AAV1- firefly luciferase; AAV5-RL, AAV5- renilla luciferase; AB, Albumin/Globulin; ALB, Albumin; ALP, serum alkaline phosphatase; ALT, serum alanine transaminase (aminotransferase); AST, serum aspartate transaminase (aminotransferase); BILIT, bilirubin (total); BUN/CRE, blood urea nitrogen/creatinine; BUN, blood urea nitrogen; CA, serum calcium; CBC, complete blood count; cDNA, copy deoxyribonucleic acid; CHOL, serum cholesterol (total); CK, serum creatine kinase; CL-S, serum chloride (serum); CRE-S, serum creatinine (serum); DPD, days post-dosing; EDTA, ethylene diamine tetra acetate; F, female; gc, genome copies; GLOBN, serum globulin; GLP, good laboratory practices; GLU, serum glucose; ID (animal), (animal) identification number; K-S, serum potassium; M, male; NA-S, serum sodium; O&P, fecal ova and parasite; PBS, phosphate buffered Saline; PHOS, serum phosphorous; PT, prothrombin time; PTT, partial thromboplastin time; SD, standard deviation; TP, serum protein (total).
Table 4.
Summary of coagulation results
| Sex | Animal ID | Pro-thrombin time (s) | APTT (s) |
|---|---|---|---|
| Pretreatment | |||
| Male | RQ8571 | 19 | 30.8 |
| RQ8872 | 18 | 37.6 | |
| Female | RQ8425 | 17.4 | 37.8 |
| RQ8676 | 17.9 | 33.2 | |
| Post treatment | |||
| Male | RQ8571 | UTR | UTR |
| RQ8872 | 16.6 | 31.4 | |
| Female | RQ8425 | UTR | UTR |
| RQ8676 | 17.2 | 27.9 | |
ID (animal), (animal) identification number.
AAV vector genomes
The stability of the AAV1 and AAV5 vectors after spraying through the microsprayer was assessed by passing the viral vectors through the device and measuring luciferase activity of non-sprayed versus sprayed vector. Figure 3 shows that there was some decrement of activity post spraying.
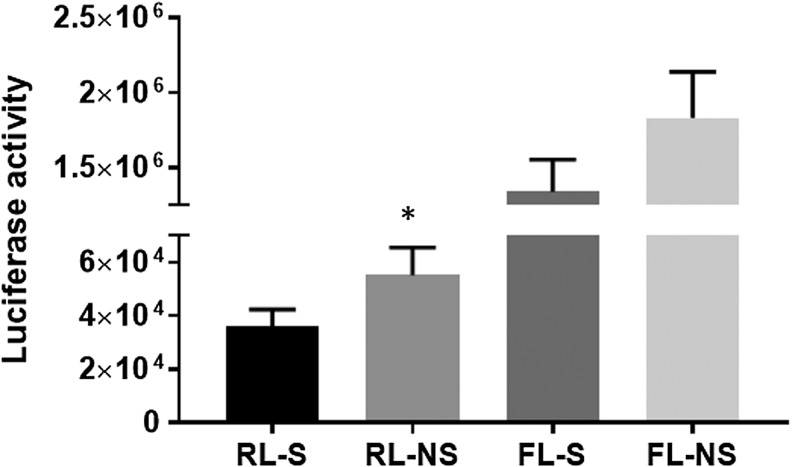
Figure 3.
Vector stability. To assess vector stability, remaining sample fluid from each individual animal dosing was sprayed into 15 mL sterile polypropylene tubes and stored frozen in cryovials. Luciferase activity was measured. There was approximately 35% less luciferase activity from renilla viral vector (RL) and 27% less activity from the firefly viral vector (although the data were not significantly different) when sprayed (s) versus not-sprayed (ns) were compared. *p < 0.05.
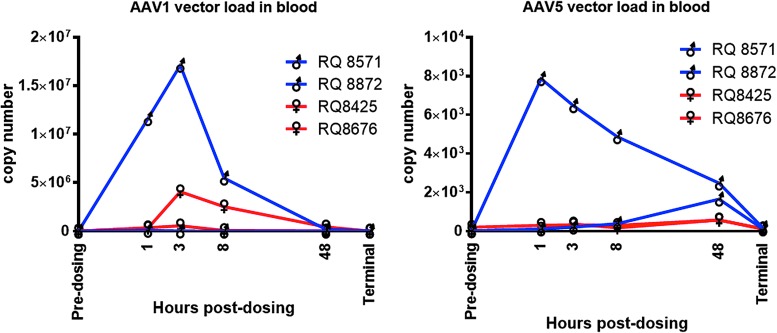
AAV1 and AAV5 vector loads were measured in the blood before dosing, and at 1, 3, 8, and 48 h after dosing (Fig. 4). All blood samples were negative for RL and FL vector genomes prior to dosing. There was a much greater detection of AAV1 than of AAV5 genomes in the blood. Interestingly, the levels of both vectors were considerably higher for male RQ8872 and considerably lower for male RQ8571 than for either of the females. AAV5 appeared to be cleared from the blood more slowly than was AAV1, with substantial amounts of AAV5 remaining at the 48 h post-dosing time.
Figure 4.
Viremia. To assess the passage of the virus from the lungs to the bloodstream, vector copy numbers were measured in blood up to 48 h post infection. Note the difference in scale between the two graphs. Primers were designed to detect LacZ DNA sequences from RL or FL. See the Methods section.
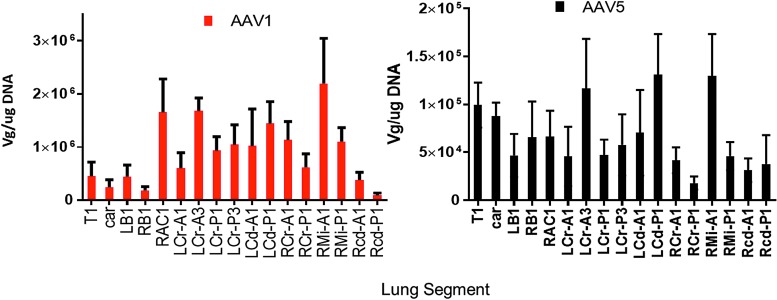
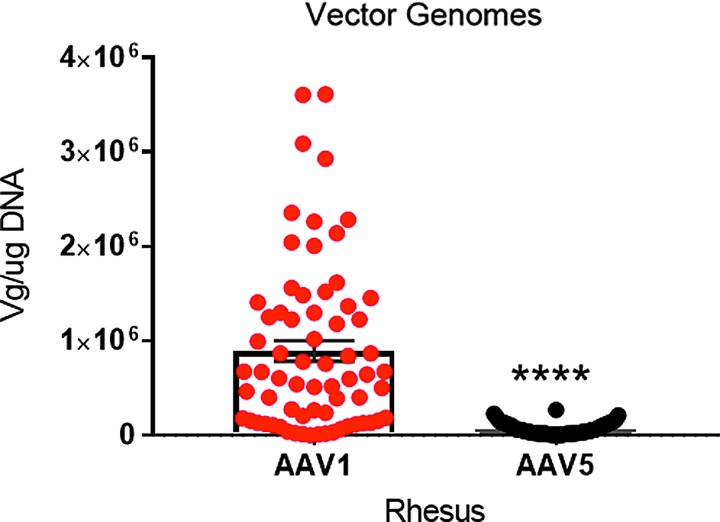
Samples were taken from 17 different lung regions, as shown in Fig. 5. Figure 6A and B shows the vector genomes measured in each lung region at necropsy. rAAV1-CB-FL and rAAV5-CB-RL vector DNA was detectable at ≥100 copies of single-stranded DNA/μg of genomic DNA in all lungs tissues for all four vector-treated test subjects. Note that the vector was widely distributed throughout the lung by the microsprayer, demonstrating conclusively that the droplet size was sufficient to support widespread distribution. Figure 7 shows the PCR data for 17 lung samples from the four monkeys. The data clearly demonstrate a dramatic difference between the number of vector genomes detected between AAV1 and AAV5: AAV1 was approximately 10 times more prevalent than AAV5.
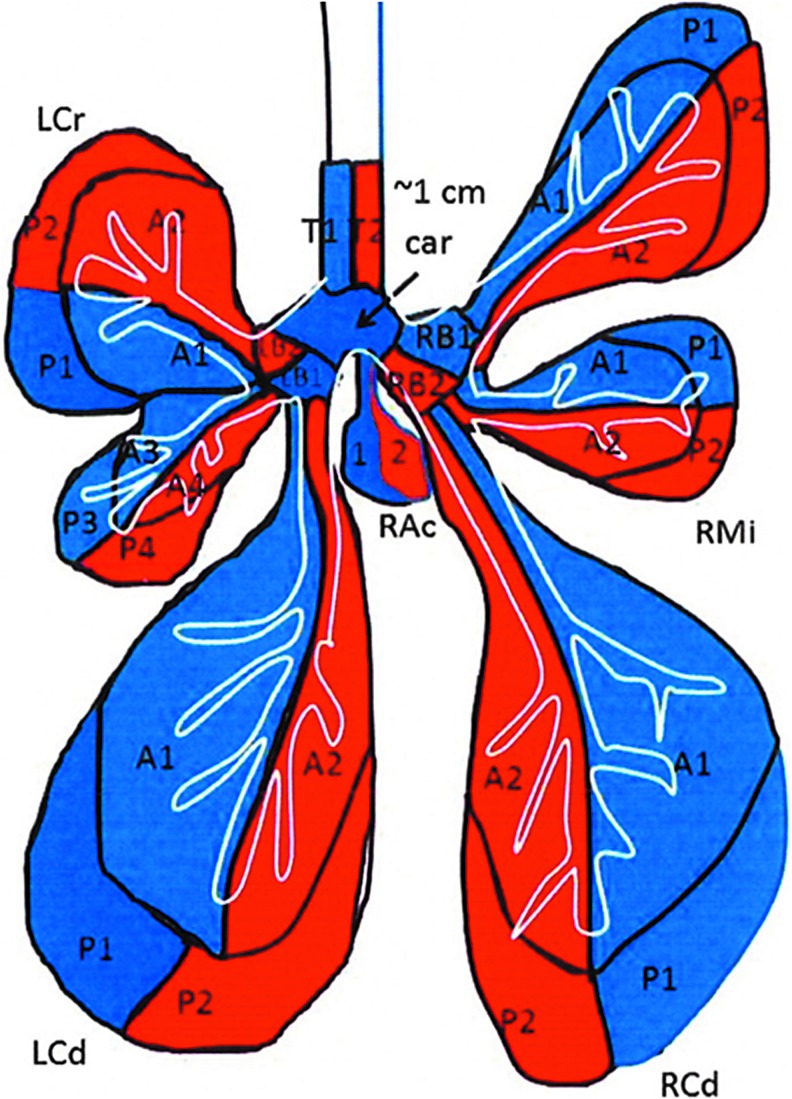
Figure 5.
Location of samples taken from the lung: A, airway; B, bronchus; Car, carina; Cd, caudal; Cr, cranial; P, parenchymal; T, trachea; R, right; L, left.
Figure 6.
Distribution of AAV1 versus AAV5. Vector genomes/μg of lung DNA were measured in the 17 lung segments shown in Fig. 5. Note that although there were some differences from segment to segment, it is clear that the vector genomes from AAV1 were a ten-fold higher than those measured from AAV5. Both AAV1 and AAV5 were distributed throughout the lung following microspraying. Data are averages ± standard error of the mean (SEM); n = 4 for each column. Measurements from samples taken at necropsy. Note the difference scale between the two graphs. Primers were designed to detect LacZ DNA sequences from RL or FL. See the Methods section.
Figure 7.
Distribution of AAV1 versus AAV5. The average values among all the samples are given. Note the statistically significant difference between the two viruses, with AAV1 being much higher than AAV5. Data are averages ± SEM; n = 68 samples from four monkeys. Paired t-test; ****p < 0.001. Measurements from samples taken at necropsy. Primers were designed to detect LacZ DNA sequences from RL or FL. See the Methods section.
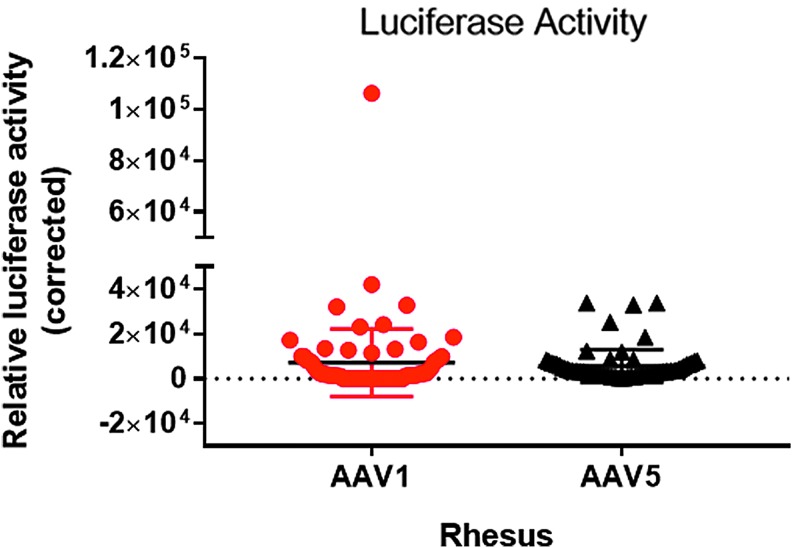
AAV vector transduction
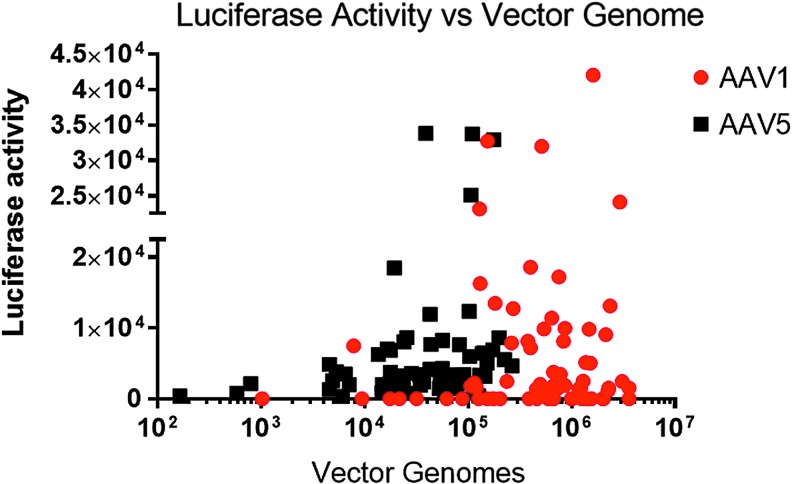
To assess functional expression from the transgenes, luciferase activity was measured in the various lung sections and normalized to the protein content of the sections (Fig. 8). Values for AAV5-RL were corrected for the 14-fold lower signal per expressed protein, and all values were normalized to the protein content of the sample. Despite the small number of macaques, the data show slightly better transduction with AAV1 than with AAV5. Plotted in Fig. 9 is a comparison of relative luciferase activity versus vector DNA measured in each of the lung sections. Note that there was no clear correlation between the presence of vector genomes and luciferase activity, such that the greater the number of vector genomes did not necessarily result in a greater the luciferase activity. In many of the samples, particularly in the assay for AAV1 using the firefly luciferase, the samples had high levels of vector particles but no detectable luciferase activity.
Figure 8.
Luciferase activity. The average values measured for all samples are given. Note that the activity derived from AAV1 transduction was not significantly different from that of AAV5. Data are averages ± SEM; n = 68 samples from four monkeys. Paired t-test; NS, not significant; p = 0.45. Measurements from samples taken at necropsy.
Figure 9.
Luciferase activity versus vector genomes. Note that as the number of vector genomes increased, there was no concomitant increase in luciferase activity. Data are taken from Figs. 7 and 8. Linear regression analysis was performed. R2 = 0.0065 for AAV1; R2 = 0.0637 for AAV5.
Neutralizing antibodies
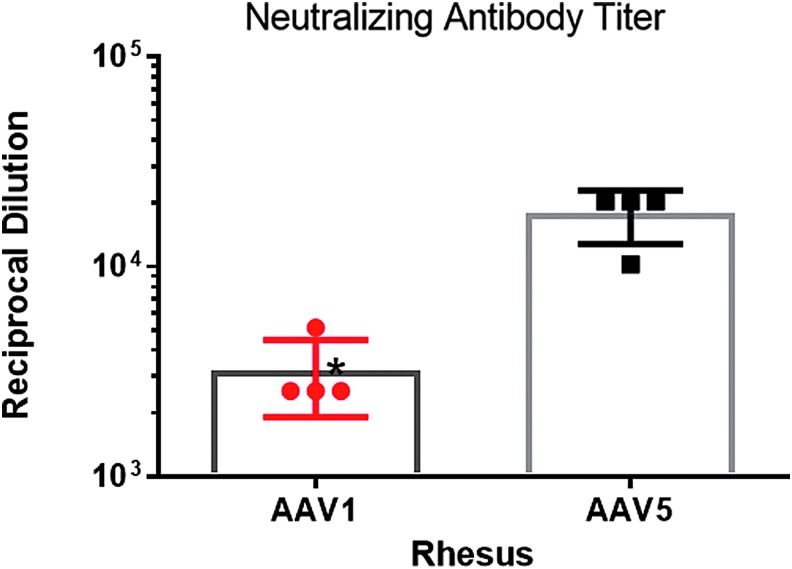
The results of an evaluation of anti-AAV antibodies are shown in Fig. 10. All animals used in the study had low neutralizing antibodies (<5 reciprocal dilution). The levels increased dramatically in all animals between the pre-study time points and the time of necropsy on study day 45. Considerably higher titers were observed for AAV5 than for AAV1.
Figure 10.
Neutralizing antibody. The neutralizing antibody titer increased significantly following vector instillation for both viruses. However, the magnitude of the increase in AAV1 neutralizing antibodies was considerably lower than that for AAV5, despite the presence of more AAV1 particles in the airways. Data are averages ± SEM; n = 4 samples from four monkeys. Paired t-test; *p < 0.05, p = 0.0193. Measurements from samples taken at necropsy. y-Axis is to the power of 10.
Discussion
AAV2 was the first generation of AAV vectors used in gene therapy for CF, but a number of limitations compromised the outcomes of the clinical studies using this vector. For example, the AAV2 heparin sulfate proteoglycan receptor is more abundant in the basolateral cell membrane than in the apical cell membrane.39 The AAV2 virus that does manage to enter the cell from the apical membrane does so via a different mechanism compared to the basolateral membrane. When AAV2 is endocytosed across the apical membrane, the viral particles are ubiquitinated and ultimately degraded in proteasomes and therefore do not reach the nucleus, where transduction normally occurs.40 This limitation, among others, led to the hunt for new and more efficient viral vectors (both natural and synthetic) at transducing airways.
For example, Steines et al.41 combined a number of AAV capsid libraries, including shuffled AAV1-9 capsid genes, an error-prone AAV2 library, and another in which the AAV capsid loops were varied. They infected the airways of non-CF pigs, isolated the airway epithelia, infected with wild-type adenovirus 5 to induce the replication cycle, and harvested the viral particles. After three rounds of infection, they isolated a synthetic virus referred to as AAV2H22. This synthetic virus was identical to AAV2, except for five amino acid changes in the capsid proteins, but it was approximately 240 times more infectious than AAV2 and was better at transducing the large and small airway epithelia of pigs.41
This study took a different approach in testing known AAV serotypes such as AAV1 and AAV5, conducting a non-lethal study in chimpanzees, the closest genetic relative to Homo sapiens. The same dual-reporter assay based upon firefly and Renilla luciferase36 was used as was applied in the current study. The results of the analysis performed on bronchial brushings were similar to those shown here, namely a rather large increase in luciferase activity in the animals treated with the AAV1 construct when compared to AAV5. Similar results were obtained with primary human airway cells grown in tissue culture. In these cells, AAV1 was approximately 100-fold more effective in transduction than was AAV5.
The results reported here for Rhesus macaques showed that AAV1 vector genomes were almost 10-fold more prevalent than AAV5 at necropsy. However, the abundance of AAV1 genomes did not translate into significantly more luciferase activity compared to AAV5. One possibility is that the PCR techniques for detecting AAV1 genomes at necropsy could have detected AAV1-derived DNA, which was present on both the outside and the inside of the cells of the airways, although it is unlikely that AAV1 viral particles would persist intact on the surface of the airways for 45 days post instillation. Another explanation is that although genomes for AAV1 were present in the cells, they may not have been in a form or location, which would have allowed them to be transcribed and ultimately translated into luciferase activity. For example, the AAV1 particles could have been slower to convert from single- to double-stranded DNA as needed prior to transcription.42 It is also possible that the particles did get into the cell but did not reach the nucleus (see Sanlioglu et al.43 for a review). It is important to note that chimpanzees exposed to AAV1 did indeed take up the viral particles, and significant luciferase activity was detected.36 The difference in AAV1 transduction between chimpanzees and Rhesus macaques may reflect differences between new and old world monkeys. Given that chimpanzees are closer to man, the chimpanzee results will be more applicable to human clinical trials.
AAV1 is an interesting serotype that is considered to be a simian virus, isolated from nonhuman primates. It has been reported that approximately 61% of Rhesus macaques are positive for AAV1 neutralizing antibodies. Indeed, in choosing seronegative animals for this study, it was found that several of the animals tested positive for AAV1 prior to vector instillation; in this colony, approximately a third were positive. Therefore, it is important to pretest the animals before entering them in any preclinical studies using AAV1. The worldwide prevalence of neutralizing antibodies against AAV1 measured in humans is estimated at approximately 30% of the population,44 similar to what was obtained in this study in the Rhesus macaques. On the other hand, only 10–20% of adults are seropositive for AAV5.45
In view of the new rules restricting the use of chimpanzees in research, it became necessary to verify that similar results could be obtained in Rhesus macaques. Given that in previous clinical studies, tgAAV2 CFTR was supplied via an inhaler, the ability of an inhaler to deliver the virus to the macaques was tested. Unlike humans who use inhalers while awake, the animals were anesthetized during the inhalation exposure. Therefore, it is not surprising that the monkeys did not participate in the inhalation process, and thus the efficiency of delivery was very low. With these results in hand, the study switched to a microsprayer to spray the virus, via positive pressure, into the airways. As has been shown, this technique provides efficient transduction of the lungs, with viral particle genomes present at necropsy (at 45 days). The presence of viral particles and the successful transduction, as assessed by the presence of luciferase activity, demonstrated that one application of virus was not only effective at gene transfer, but also led to sustained transduction for >1 month. If these findings are transferable to a CFTR-based treatment, they bode well for the possibility of a long-term therapeutic effect in humans.
As we had previously shown in chimpanzees, the gene transfer and transduction by AAV1 surpassed that of AAV5 in the Rhesus macaques, pointing to AAV1 as the more effective clinical product.36 However, it was previously shown that AAV5 produced better gene transfer and transduction than AAV2 did.26 Thus, although the results of the current study would suggest that AAV1 is the product of choice, AAV5 still transduces airway cells, and it may still be useful if patients are unable to receive AAV1 because of pre-existing antibodies.
It is commonly understood that neutralizing antibodies are a barrier to repeated dosing of AAV vectors (reviewed by Mingozzi and High46). This study has shown that all of the macaques had neutralizing antibody titers to both AAV1 and AAV5 at necropsy. As was observed for the chimpanzees,36 AAV1 stimulated far fewer neutralizing antibodies than AAV5 did. The fact that the antibodies are found in the serum and AAV is administered to the lungs suggests that the lungs may be one of the organs in which repeat dosing is feasible. The prospect of repeated delivery for gene therapy to the lungs needs to be tested in several animal models using AAV-CFTR and/or GFP. In all the studies reported thus far, the presence of neutralizing antibodies in the serum (defined as inhibition of wild-type AAV2 replication) increased after the first dose, but seroconversion (defined as a more than fourfold rise in titer) was not observed in any of the animals until at least two or more doses were applied. Interestingly, no neutralizing antibodies, or many fewer antibodies, were detected in the bronchial lavage fluid (see Guggino and Cebotaru31 for a review). Given this experience and the observations from the current study, as well as the earlier chimpanzee study36 in which AAV1 neutralizing titers were low to begin with, repeated dosing of AAV1 virus in the airways may be feasible. One common conclusion from both the chimpanzee and macaque studies is that single-dose delivery of AAV1 or AAV5 is generally safe. It should be mentioned that AAV gene therapy still had a number of challenges to overcome (recently reviewed in Guggino and Cebotaru31). However, given this body of promising data, it appears that further development of AAV with the goal of resumption of gene therapy clinical trials is certainly warranted.
Acknowledgments
The authors would like to acknowledge the Gene Therapy Resource Program of the National Heart, Lung, and Blood Institute, National Institutes of Health, for providing the toxicology testing services for this study. The work was funded by National Institutes of Health grant numbers P01 HL051811 to W.B.G. and R01 HL122267 to L.C. and W.B.G., and by a CF grant # GUGGIN14P0 to L.C. and W.B.G.
Author Disclosure
L.C. has licensed cells to Vertex. W.B.G. is a consultant for Vertex. No competing financial interests exist for the remaining authors.
References
- 1.Sosnay PR, Castellani C, Corey M, et al. Evaluation of the disease liability of CFTR variants. Methods Mol Biol 2011;742:355–372 [DOI] [PubMed] [Google Scholar]
- 2.Sosnay PR, Siklosi KR, Van GF, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet 2013;45:1160–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuller CM, Benos DJ. CFTR. Am J Physiol Cell Physiol 1992;263:C267–C286 [DOI] [PubMed] [Google Scholar]
- 4.Guggino WB. Cystic fibrosis and the salt controversy. Cell 1999;96:607–610 [DOI] [PubMed] [Google Scholar]
- 5.Cohen-Cymberknoh M, Shoseyov D, Kerem E. Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med 2011;183:1463–1471 [DOI] [PubMed] [Google Scholar]
- 6.Pezzulo AA, Tang XX, Hoegger MJ, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012;487:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bombieri C, Claustres M, De BK, et al. Recommendations for the classification of diseases as CFTR-related disorders. J Cyst Fibros 2011;10:S86–102 [DOI] [PubMed] [Google Scholar]
- 8.Clancy JP, Rowe SM, Accurso FJ, et al. Results of a Phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 2012;67:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle MP, Bell SC, Konstan MW, et al. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a Phase 2 randomised controlled trial. Lancet Respir Med 2014;2:527–538 [DOI] [PubMed] [Google Scholar]
- 10.Flotte TR. Gene therapy for cystic fibrosis. Curr Opin Mol Ther 1999;1:510–516 [PubMed] [Google Scholar]
- 11.Flotte TR. Recent developments in recombinant AAV-mediated gene therapy for lung diseases. Curr Gene Ther 2005;5:361–366 [DOI] [PubMed] [Google Scholar]
- 12.Clement N, Knop DR, Byrne BJ. Large-scale adeno-associated viral vector production using a herpesvirus-based system enables manufacturing for clinical studies. Hum Gene Ther 2009;20:796–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss RB, Milla C, Colombo J, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled Phase 2B trial. Hum Gene Ther 2007;18:726–732 [DOI] [PubMed] [Google Scholar]
- 14.Moss RB, Rodman D, Spencer LT, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 2004;125:509–521 [DOI] [PubMed] [Google Scholar]
- 15.Silver JN, Elder M, Conlon T, et al. Recombinant adeno-associated virus-mediated gene transfer for the potential therapy of adenosine deaminase-deficient severe combined immune deficiency. Hum Gene Ther 2011;22:935–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner JA, Messner AH, Moran ML, et al. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 1999;109:266–274 [DOI] [PubMed] [Google Scholar]
- 17.Wagner JA, Moran ML, Messner AH, et al. A Phase I/II study of tgAAV-CF for the treatment of chronic sinusitis in patients with cystic fibrosis. Hum Gene Ther 1998;9:889–909 [DOI] [PubMed] [Google Scholar]
- 18.Wagner JA, Nepomuceno IB, Messner AH, et al. A Phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther 2002;13:1349–1359 [DOI] [PubMed] [Google Scholar]
- 19.Wagner JA, Reynolds T, Moran ML, et al. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet 1998;351:1702–1703 [DOI] [PubMed] [Google Scholar]
- 20.Conrad CK, Allen SS, Afione SA, et al. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Ther 1996;3:658–668 [PubMed] [Google Scholar]
- 21.Sirninger J, Muller C, Braag S, et al. Functional characterization of a recombinant adeno-associated virus 5-pseudotyped cystic fibrosis transmembrane conductance regulator vector. Hum Gene Ther 2004;15:832–841 [DOI] [PubMed] [Google Scholar]
- 22.Beck SE, Jones LA, Chesnut K, et al. Repeated delivery of adeno-associated virus vectors to the rabbit airway. J Virol 1999;73:9446–9455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck SE, Laube BL, Barberena CI, et al. Deposition and expression of aerosolized rAAV vectors in the lungs of Rhesus macaques. Mol Ther 2002;6:546–554 [DOI] [PubMed] [Google Scholar]
- 24.Boyle MP, Enke RA, Adams RJ, et al. In utero AAV-mediated gene transfer to rabbit pulmonary epithelium. Mol Ther 2001;4:115–121 [DOI] [PubMed] [Google Scholar]
- 25.Fischer AC, Beck SE, Smith CI, et al. Successful transgene expression with serial doses of aerosolized rAAV2 vectors in rhesus macaques. Mol Ther 2003;8:918–926 [DOI] [PubMed] [Google Scholar]
- 26.Fischer AC, Smith CI, Cebotaru L, et al. Expression of a truncated cystic fibrosis transmembrane conductance regulator with an AAV5-pseudotyped vector in primates. Mol Ther 2007;15:756–763 [DOI] [PubMed] [Google Scholar]
- 27.Afione SA, Conrad CK, Kearns WG, et al. In vivo model of adeno-associated virus vector persistence and rescue. J Virol 1996;70:3235–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flotte TR, Laube BL. Gene therapy in cystic fibrosis. Chest 2001;120:124S–131S [DOI] [PubMed] [Google Scholar]
- 29.Aitken ML, Moss RB, Waltz DA, et al. A Phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum Gene Ther 2001;12:1907–1916 [DOI] [PubMed] [Google Scholar]
- 30.Moss RB, Rodman D, Spencer T, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: A multicenter, double-blind, placebo-controlled trial. Chest 2004;125:509–521 [DOI] [PubMed] [Google Scholar]
- 31.Guggino WB, Cebotaru L. Adeno-associated virus (AAV) gene therapy for cystic fibrosis: current barriers and recent developments. Expert Opin Biol Ther 2017. July 6 [Epub ahead of print]. DOI: 10.1080/14712598.2017.1347630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flotte TR, Afione SA, Solow R, et al. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem 1993;268:3781–3790 [PubMed] [Google Scholar]
- 33.Gao G, Vandenberghe LH, Alvira MR, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol 2004;78:6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang L-Y, Halder S, Agbandje-McKenna M. Parvovirus glycan interactions. Curr Opin Virol 2014;7:108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Z, Lei-Butters DC, Keiser NW, et al. Distinct transduction difference between adeno-associated virus type 1 and type 6 vectors in human polarized airway epithelia. Gene Ther 2013;20:328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flotte TR, Fischer AC, Goetzmann J, et al. Dual reporter comparative indexing of rAAV pseudotyped vectors in chimpanzee airway. Mol Ther 2010;18:594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coates A, Fink J, Chantrel G, et al. In vivo justification of a physiological insiratory: expiratory ratio to predict deposition of a novel valved spacer for liquid aerosol. Am J Respir Crit Care Med 2006;3:A84 [Google Scholar]
- 38.Fink JB. New technology offers new opportunities: continuous bronchodilator therapy during mechanical ventilation. Respir Ther 2007;2:29–32 [Google Scholar]
- 39.Duan D, Yue Y, Yan Z, et al. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum Gene Ther 1998;9:2761–2776 [DOI] [PubMed] [Google Scholar]
- 40.Duan D, Yue Y, Yan Z, et al. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest 2000;105:1573–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steines B, Dickey DD, Bergen J, et al. CFTR gene transfer with AAV improves early cystic fibrosis pig phenotypes. JCI Insight 2016;1:e88728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afione SA, Wang J, Walsh S, et al. Delayed expression of adeno-associated virus vector DNA. Intervirology 1999;42:213–220 [DOI] [PubMed] [Google Scholar]
- 43.Sanlioglu S, Monick MM, Luleci G, et al. Rate limiting steps of AAV transduction and implications for human gene therapy. Curr Gene Ther 2001;1:137–147 [DOI] [PubMed] [Google Scholar]
- 44.Calcedo R, Vandenberghe LH, Gao G, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halbert CL, Miller AD, Mcnamara S, et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum Gene Ther 2006;17:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 2013;122:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]