Abstract
Introduction: The high prevalence of adolescent cannabis use, the association between this use and later psychiatric disease, and increased access to high-potency cannabis highlight the need for a better understanding of the long-term effects of adolescent cannabis use on cognitive and behavioral outcomes. Furthermore, increasing Δ9-tetrahydrocannabinol (THC) in high-potency cannabis is accompanied by a decrease in cannabidiol (CBD), thus an understanding of the interactions between CBD and THC in the neurodevelopmental effects of THC is also important. The current study examined the immediate and long-term behavioral consequences of THC, CBD, and their combination in a mouse model of adolescent cannabis use.
Materials and Methods: Male CD1 mice received daily injections of THC (3 mg/kg), CBD (3 mg/kg), CBD+THC (3 mg/kg each), vehicle, or remained undisturbed in their home cage (no handling/injections), either during adolescence (postnatal day [PND] 28–48) or during early adulthood (PND 69–89). Animals were then evaluated with a battery of behavioral tests 1 day after drug treatment, and again after 42 drug-free days. The tests included the following: open field (day 1), novel object recognition (NOR; day 2), marble burying (day 3), elevated plus maze (EPM; day 4), and Nestlet shredding (day 5).
Results: Chronic administration of THC during adolescence led to immediate and long-term impairments in object recognition/working memory, as measured by the NOR task. In contrast, adult administration of THC caused immediate, but not long term, impairment of object/working memory. Adolescent chronic exposure to THC increased repetitive and compulsive-like behaviors, as measured by the Nestlet shredding task. Chronic administration of THC, either during adolescence or during adulthood, led to a delayed increase in anxiety as measured by the EPM. All THC-induced behavioral abnormalities were prevented by the coadministration of CBD+THC, whereas CBD alone did not influence behavioral outcomes.
Conclusion: These data suggest that chronic exposure to THC during adolescence leads to some of the behavioral abnormalities common in schizophrenia. Interestingly, CBD appeared to antagonize all THC-induced behavioral abnormalities. These findings support the hypothesis that adolescent THC use can impart long-term behavioral deficits; however, cotreatment with CBD prevents these deficits.
Keywords: : cannabis, neurodevelopment, object recognition, schizophrenia, working memory
Introduction
Cannabis contains more than 60 cannabinoids.1–3 Of these, tetrahydrocannabinol (THC) is the major psychoactive and cannabidiol (CBD) is the main nonpsychoactive component of cannabis. Through genetic interactions, breeding for high-THC content (to create high-potency cannabis) results in very low-CBD levels. The market demand for high-potency cannabis has seen the average THC content of U.S. cannabis increasing from ∼4% to 12% in the past 20 years. This has been accompanied by a corresponding decrease in CBD content, increasing the average THC/CBD ratio ∼20-fold over the same period.4
Cannabis is the most widely used illicit drug among adolescents in the United States.5 In 2016, 12.8% of 8th graders have used cannabis in their lifetime, and by 12th grade 44.5% have tried cannabis.5 Furthermore, daily use increases from 0.7% among 8th graders to 6% among 12th graders.5 The high prevalence of frequent adolescent cannabis use, and the increased presence of high-THC/low-CBD cannabis, emphasizes a need for research examining the long-lasting effects of chronic adolescent exposure to major cannabis constituents on neurobehavioral outcomes.
During human adolescence (age ∼10–25), neuroanatomical and functional changes lead to increased neural and cognitive efficiency.6–8 As the prefrontal cortex (PFC) matures during adolescence, it undergoes significant synaptic remodeling and changes in myelination.9–12 The PFC is responsible for higher order cognitive function such as executive function,13–15 and disruption of maturation PFC can be expected to have long-lasting adverse consequences.
Because of the prominent role of endocannabinoids in neurodevelopment,16 cannabis exposure during adolescence might perturb PFC neurodevelopment and cause long-term cognitive, emotional, and behavioral impairments. Supporting this hypothesis, many studies indicate a positive correlation between adolescent cannabis use and aspects of schizophrenia (age of first psychotic episode, severity).17–22 Factors such as onset of use, frequency of use, and the THC/CBD ratio in cannabis consumed have been shown to modulate this risk.17,23,24 For instance, after controlling for confounding variables (e.g., IQ, psychiatric illness in family members), a large population-based study found a dose-dependent relationship of adolescent cannabis use and the development of schizophrenia.19 In addition, the risk of developing a psychotic disorder was shown to increase threefold among users of high-potency cannabis compared with subjects who never used cannabis.25 In this study, daily use of high-potency cannabis conferred an even higher (>fivefold) risk for developing schizophrenia.25
A causal relationship between adolescent cannabis use and an increased risk for schizophrenia is unlikely to be proven given ethical constraints. Thus, research utilizing animal models, rigorously controlled experimental design, and examination of specific cannabis components can identify potential mechanisms linking frequent adolescent cannabis use and an increased risk for schizophrenia. Furthermore, examining the variables involved in producing schizophrenia-associated phenotypes—such as impairment of working and object recognition memory, anxiety, and repetitive and compulsive behaviors—might provide valuable insight into schizophrenia's etiology. Working26–29 and object recognition memory30–32 deficits are frequent in schizophrenia. Furthermore, repetitive and compulsive behaviors33,34 as well as anxiety35,36 are common in schizophrenia.
This study examined the immediate and long-term effects of chronic cannabinoid exposure on object recognition/working memory, repetitive and compulsive behaviors, and anxiety in adolescent and adult mice. We found that adolescent THC impaired selected behavioral domains and that this impairment was prevented by concurrent CBD treatment.
Materials and Methods
Subjects
Male CD1 mice (n=221) were bred and housed three to four per cage under a standard reversed light cycle housing facility (light–dark cycle of 12 h: lights on at 7 p.m.). Food and water were available ad libitum. All experimental procedures were approved by Indiana University's Bloomington Institutional Animal Care and Use Committee.
Drugs
Injection solutions were made by dissolving THC, CBD, or CBD+THC in 100% ethanol to a concentration of 6 mg/mL for each drug. In a second tube, Kolliphor® EL (synonym: Cremophor® EL; Sigma-Aldrich) was mixed with sterile 0.9% saline. The dissolved cannabinoid was then mixed with the Cremophor/NaCl solution to a final ratio of 1:1:18 (cannabinoid/ethanol:Cremophor:saline). The final concentration of each component in the injection solutions was 0.3 mg/mL cannabinoid, 5% ethanol, 5% Cremophor, and 0.81% NaCl. Mice were injected intraperitoneally (i.p.) with 3 mg/kg of the indicated drug (in a volume of 10 mL/kg). Controls received a vehicle consisting of 5% ethanol, 5% Cremophor, and 0.81% NaCl (also at a volume of 10 mL/kg).
Injection and behavioral testing schedule
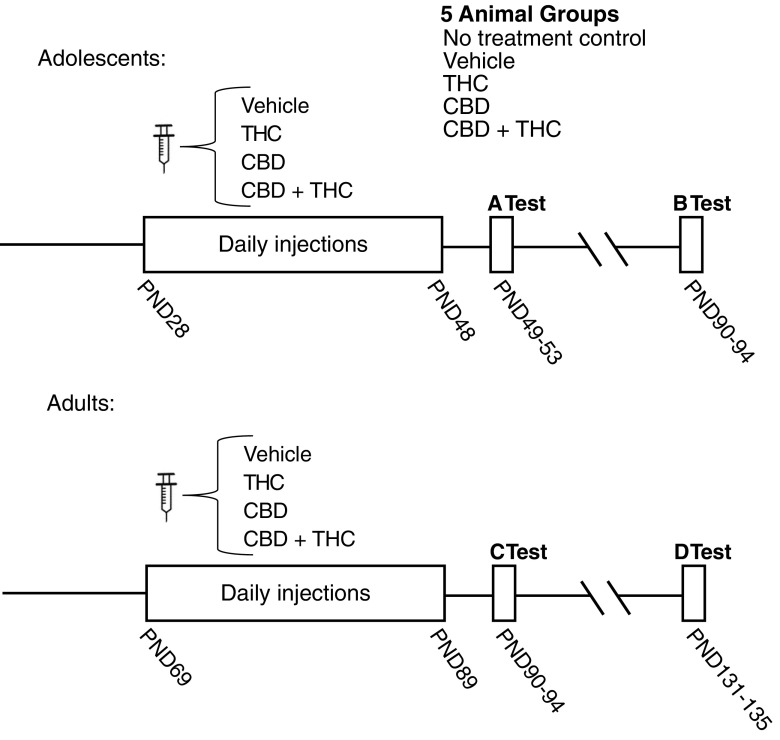
Mice were randomly assigned to one of five groups: no treatment control (remained in home cages during 3 weeks of treatment, subjected only to routine cage changes); vehicle control; THC (3 mg/kg); CBD (3 mg/kg); or CBD+THC (3 mg/kg each). Animals underwent daily i.p. injections either during adolescence (postnatal day [PND] 28–48) or during adulthood (PND 69–89; Fig. 1). Animals were behaviorally tested starting 24 h after the last injection, and again 6 weeks later. The behavioral battery included open field (day 1), novel object recognition (NOR; day 2), marble burying (day 3), elevated plus maze (EPM; day 4), and Nestlet shredding (day 5).
FIG. 1.
Injection and behavioral testing schedule. (Top graph) Adolescent-treated animals: no treatment control (n=11), vehicle (n=9), THC (n=10), CBD (n=11), CBD+THC (n=11). Behavioral testing during (A) early adulthood and (B) after a 42-day drug-free delay. (Bottom graph) Adult-treated animals: no treatment control (n=9), vehicle (n=10), THC (n=12), CBD (n=8), CBD+THC (n=12). Behavioral testing during (C) early adulthood and (D) after a 42-day drug-free delay. Panels A–D in Figures 2–7 will use this same convention where panel A represents adolescently treated animals tested immediately after 3 weeks of treatment, etc. CBD, cannabidiol; THC, tetrahydrocannabinol
Procedures
Animals were habituated to the testing room for 30 min before testing. Testing was conducted within a 100 lux illuminated room, during the dark phase of the light–dark cycle. Conditions were counterbalanced across the testing sessions. All apparatuses were wiped down with a diluted 2% chlorhexidine solution (AgriLabs, St. Joseph, MO) between trials. An observer blind to group allocations manually scored video-recorded trials, except for the open field assay (OFA), which utilized an automated scoring system (The Fusion software program; Omnitech Electronics, Inc.; Columbus, OH).
Novel object recognition
The NOR task evaluates working and object recognition memory and takes advantage of a mouse's inherent tendency to explore novel more than familiar objects.37 During the NOR's familiarization phase, mice were exposed to two equivalent objects within a Plexiglass chamber (42L×42W×32H cm; AccuScan Instruments, Columbus, OH) for 5 min. (Untreated mice spent an equal amount of time exploring the two objects used in the NOR study; Supplementary Fig. S1.) The same pairs of objects were used for all testing. After a 105-min intersession interval, mice were returned to the chamber; however, one of the previously shown “familiar” objects was removed and replaced with a novel object. Familiar and novel object positions were counterbalanced across testing sessions. Testing lasted 3 min and was recorded using a webcam (Logitech Pro 9000 PC Internet Camera Webcam, 2.0-Megapixel Video Resolution and Carl Zeiss Lens Optics; Romanel-sur-Morges, Switzerland).
Exploration was defined by the mouse's nose pointed toward and within a 2-cm radius of an object. To assess performance in this task, a discrimination index (DI) was calculated. The DI quantified the difference between time spent exploring the novel and familiar object during testing as a fraction of total exploratory time, DI=(TN − TF)/(TN + TF), where novel object exploration time is TN and familiar object exploration time is TF. The DI can range between +1 and −1, where a positive score indicates more time exploring the novel object, a negative score indicates more time exploring the familiar object, and zero indicates null preference.37 Important to the interpretation of these studies, chronic THC administration to mice leads to impaired object recognition, which takes several days to resolve.38
Nestlet shredding
The Nestlet shredding task is a measure of compulsive and repetitive-like behavior in mice, which takes advantage of a mouse's inherent tendency to shred material for nest building.39 In this task, mice were individually placed in a clean mouse cage with ∼2 cm (height) of woodchip bedding material and one 2×2 in. packed cotton Nestlet (Ancare Corp; Bellmore, NY) laid on top of the bedding. Testing lasted 75 min. The remaining Nestlet was collected, dried overnight, and weighed, and the percentage of the Nestlet shredded was calculated.
Marble burying
The marble burying task is a measure of repetitive-like behavior in rodents, and takes advantage of a mouse's inherent tendency to bury objects,39,40 although marble burying can also be affected by changes in aversion to novelty, novelty-induced anxiety, excessive locomotion, etc. Mice were individually placed into a clean mouse cage containing 5 cm (height) of corncob bedding, with 20 evenly placed blue marbles (5×4) laid on top. Testing was for 8 min. Marbles that were at least three-fourth covered by bedding were considered to be buried.
Elevated plus maze
The EPM measures anxiety-like behavior in rodents.41 Mice were individually placed in the central-open area within the EPM. Testing was for 5 min. Scoring was based on (1) time spent in the open versus closed arms and (2) entries into the open versus closed arms. As an index of locomotor activity, the total number of entries into all four EPM arms was also counted. Behavior was recorded using the same webcam as above.
Open field
The OFA was used to assess locomotion and thigmotaxis, the latter as an alternative measure of anxiety.42 Mice were placed into a Plexiglass chamber (42L×42W×32H cm) and allowed to acclimate to the chamber for ∼3 min. Activity was then measured for three, consecutive 10-min bins. The Fusion software program collected data from sensors located along the bottom periphery of the chamber. Fusion distinguishes the interior (21×21 cm within the center of the chamber) versus peripheral zones (10.5 cm from edge to center) of the chamber, which is used for scoring. Two variables were analyzed in this study: duration (whole body) and distance traveled (whole body) within each zone.
Determination of THC plasma levels
Lipid extractions of plasma obtained by exsanguination 24 h after the last cannabinoid injection were performed in a separate group of adolescent male CD1 mice receiving 21 days of THC or THC+CBD, as previously described.43 Deuterium-labeled anandamide (d8AEA; Cayman Chemical, Ann Arbor, MI) was used as an internal standard to determine extraction efficiency. Samples were analyzed using an Applied Biosystems API 3000 triple quadrupole mass spectrometer with electrospray ionization (Foster City, CA). THC levels were determined by running each sample using a multiple reactions monitoring method to detect the 315.2 parent ion mass and the 123.2 fragment. Analysis of the HPLC/MS/MS data was performed using Analyst software (Applied Biosystems) as previously described.43
Statistical analysis
Analyses were conducted using Microsoft Excel 2016 (Redmond, WA) and GraphPad Prism 7 (La Jolla, CA), with the level of significance set at 0.05. Significance was assessed by one-way analysis of variance (ANOVA), or by Student's t-test if two samples were compared. If a significant difference in ANOVA was detected, Tukey's post hoc analyses were conducted.
Results
Adolescent THC leads to long-term impairment in NOR
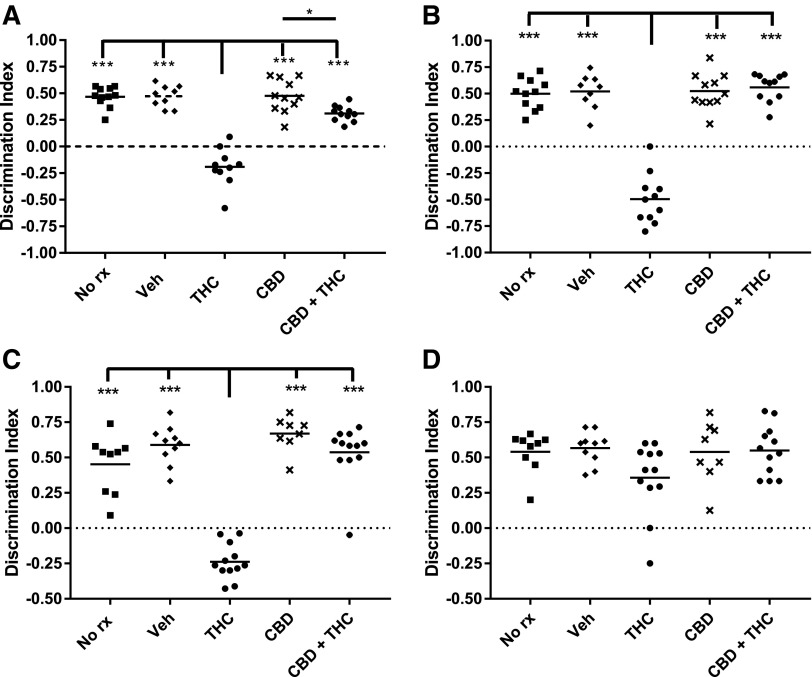
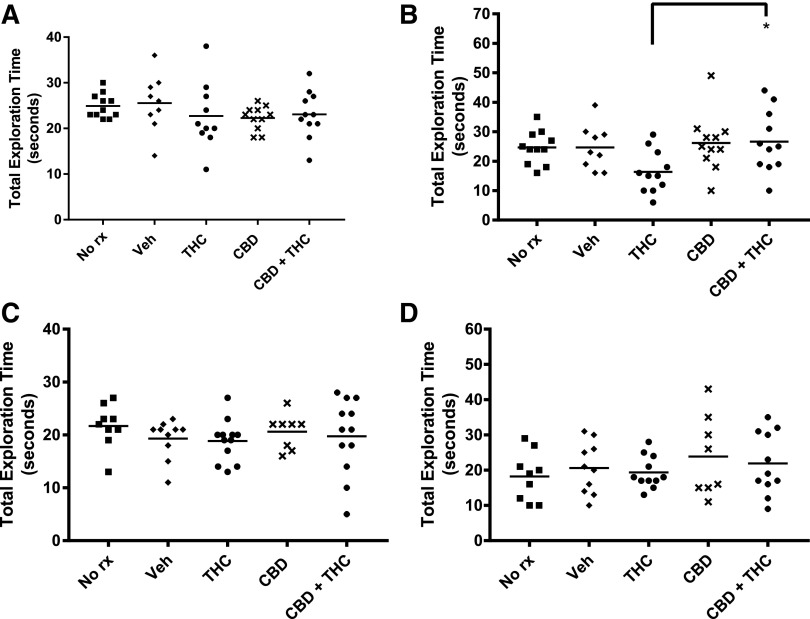
Three weeks of THC treatment significantly decreased the DI in both adolescent and adult mice relative to all other treatment groups (Fig. 2). However, although the DI remained decreased in adolescent-treated mice 6 weeks after the final THC dose, the DI had recovered to control levels in the adult-treated mice (Fig. 2). Thus, 3 weeks of THC treatment similarly impaired NOR in adolescent and adult mice, but although the impairment was transient in the adult-treated mice, it was persistent in mice treated during adolescence. If a treatment markedly decreased exploratory time, then the DI may also be decreased. However, Figure 3 shows no major differences in total exploration times among treatment groups, or testing times.
FIG. 2.
Adolescent THC impairs NOR performance. Object recognition was scored using a discrimination index (see Materials and Methods section). Adolescent treated and (A) immediately tested, (B) delay tested. Adult treated and (C) immediately tested, (D) delay tested. ***p<0.001 and *p<0.05. NOR, novel object recognition.
FIG. 3.
Similar exploration times in the NOR test among all treatment groups. Exploration time was the time the mouse spent engaged with either object (see Materials and Methods section for details). Adolescent treated and (A) immediately tested, (B) delay tested. Adult treated and (C) immediately tested, (D) delay tested. *p<0.05.
CBD has been observed to attenuate various undesirable effects of THC,44–47 and the risk of developing schizophrenia among heavy cannabis users appears to increase as CBD content decreases.23–25,48 Therefore, we examined whether concurrently administered CBD would prevent either the immediate or long-term consequences of THC administration on NOR. Interestingly, chronic administration of CBD alone did not affect NOR performance (Fig. 2), whereas coadministration of CBD+THC completely abolished the deleterious consequences of THC on DI among adolescent- and adult-treated mice (Fig. 2).
Nestlet shredding
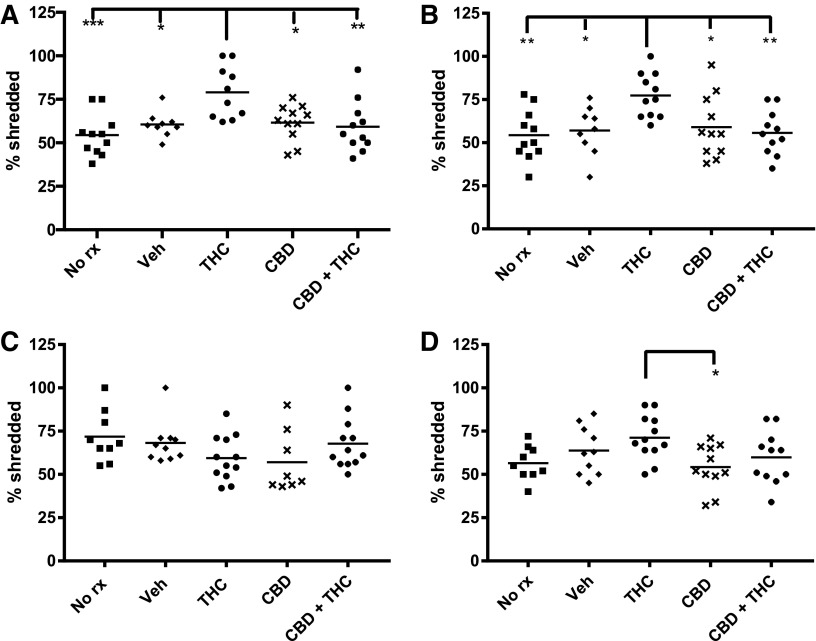
Figure 4 shows that adolescent THC treatment increases Nestlet shredding at both time periods investigated. In contrast, adult THC treatment did not increase Nestlet shredding when compared with vehicle controls. As with impaired NOR, concurrent treatment with CBD prevented both the immediate and long-lasting THC-induced increases in Nestlet shredding (Fig. 4).
FIG. 4.
Adolescent THC increases Nestlet shredding. The amount of Nestlet shred was determined by measuring unshred Nestlet 75 min after placing the Nestlet in with the mouse. Adolescent treated and (A) immediately tested, (B) delay tested. Adult treated and (C) immediately tested, (D) delay tested. ***p<0.001, **p<0.01, *p<0.05.
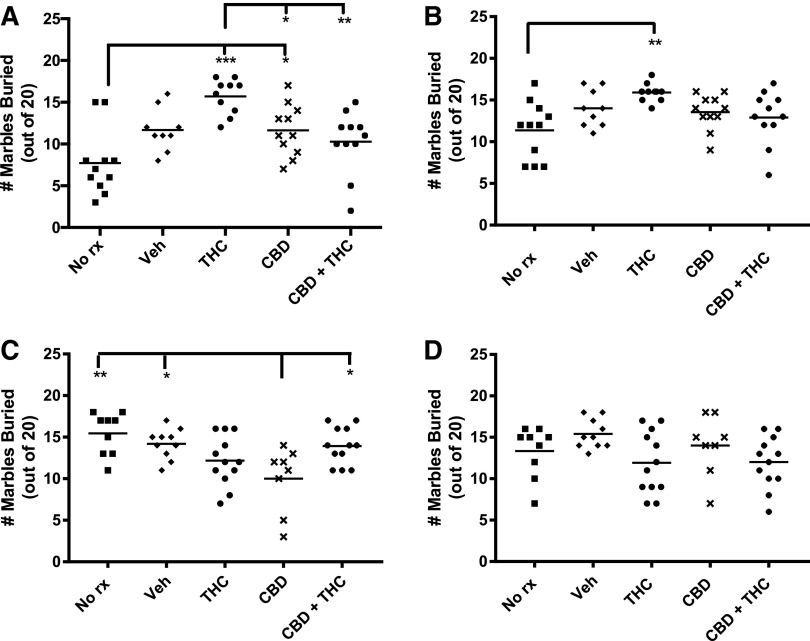
Marble burying
Figure 5 shows marble burying was enhanced immediately after 3 weeks of THC treatment of adolescent mice. Although not reaching our threshold for statistical significance, there was a strong trend for a difference in the adolescent THC-treated mice versus vehicle (p=0.06). Six weeks later, there were no apparent differences between any of the groups receiving injections. The pattern was quite different in the adult-treated mice. Chronic CBD treatment resulted in fewer marbles buried relative to the two control groups and the combined CBD+THC group when tested immediately after the end of injections (Fig. 5). However, when adults were tested 6 weeks later, no significant differences were apparent between the various treatment groups.
FIG. 5.
Adolescent THC transiently enhances marble burying. Buried marbles were those that were at least three-fourth covered with bedding. Adolescent treated and (A) immediately tested, (B) delay tested. Adult treated and (C) immediately tested, (D) delay tested. ***p<0.001, **p<0.01, *p<0.05.
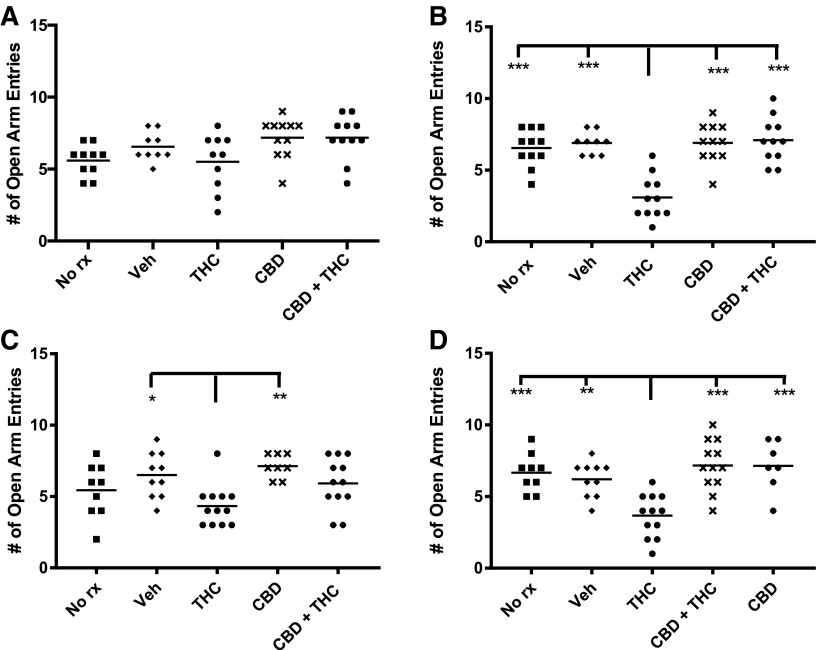
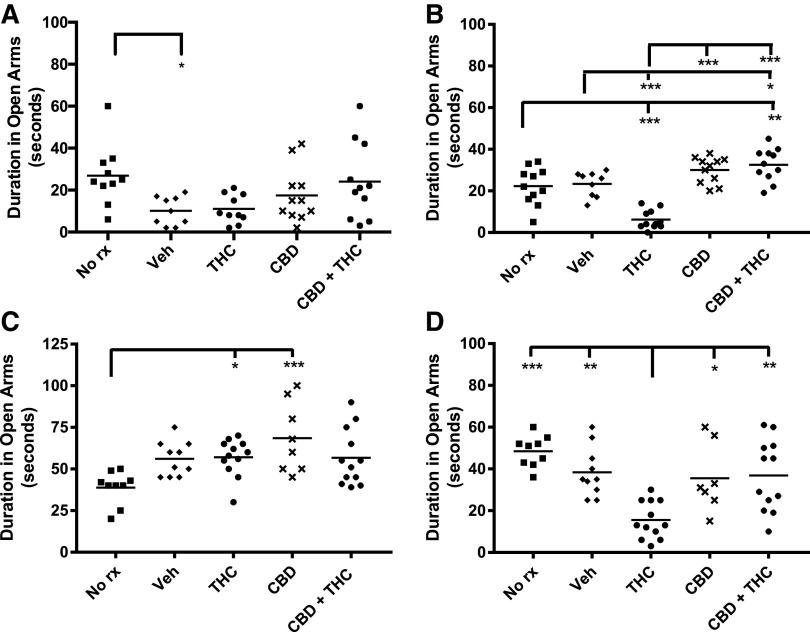
Elevated plus maze
Both adolescent- and adult-treated mice exhibited decreased EPM open arm entries when tested 6 weeks after THC treatment, suggesting the late emergence of anxiety (Fig. 6). This was accompanied by a decrease in open arm time (Fig. 7). The THC-induced decrease in open arm entries and duration was prevented by cotreatment with CBD (Figs. 6 and 7). Adult THC-treated mice also showed a decrease in open arm entries immediately after chronic treatment. There were no consistent differences in total arm entries between the various treatment groups (data not shown), suggesting that the various treatments did not have major effects on locomotor activity in the EPM.
FIG. 6.
Adult THC consistently decreases EPM open arm entries and adolescent THC decreases open arm entries after a delay. A movement was considered an entry into the maze arm if head and shoulders entered the arm. Adolescent treated and (A) immediately tested, (B) delay tested. Adult treated and (C) immediately tested, (D) delay tested. ***p<0.001, **p<0.01, *p<0.05. EPM, elevated plus maze.
FIG. 7.
Adolescent and adult THC decrease EPM open arm duration after a THC-free delay. Mice were considered to be within an arm until their entire body had exited the arm. Adolescent treated and (A) immediately tested, (B) delay tested. Adult treated and (C) immediately tested, (D) delay tested. ***p<0.001, **p<0.01, *p<0.05.
Open field
There were no significant differences in total distance traveled or thigmotaxis within the OFA arena between any of the treatment groups (data not shown).
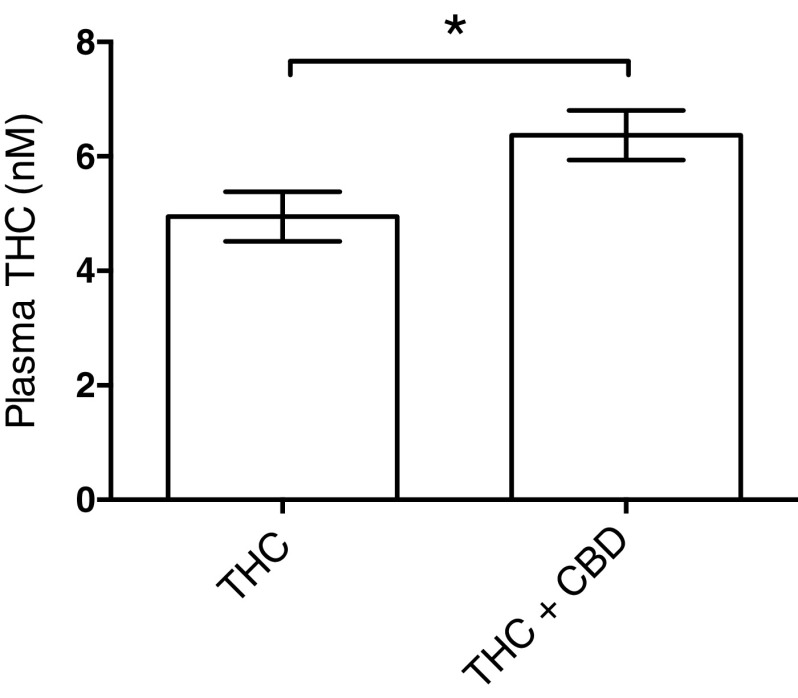
Plasma THC levels
One possible mechanism by which CBD could attenuate THC effect is if CBD increased THC metabolism and lowered THC plasma levels. This seems unlikely as most studies find that concurrent CBD+THC does not increase THC metabolism,49 nonetheless, to test this possibility, THC plasma levels were measured after 3 weeks of THC or THC+CBD. As shown in Figure 8, concurrent CBD administration modestly, but significantly, increased plasma THC levels (p=0.027). Thus the attenuation of THC's effects across multiple behavioral tests is not due to increased THC metabolism and is likely due to a pharmacodynamic interaction.
FIG. 8.
Combining THC with CBD mildly increases THC plasma levels. THC levels were determined 24 h after 21 days of daily THC injections. Plasma was collected and THC levels were analyzed as described in Materials and Methods section. Concurrent CBD treatment increased plasma THC levels by ∼30%. * p<0.027.
Discussion
This study found that THC administration to adolescent male mice for 3 weeks led to long-lasting deficits in NOR performance and repetitive behaviors, whereas a similar treatment of adults results in only transient abnormalities. Furthermore, chronic THC administration to both adolescent and adult mice induced anxiety behaviors after a delay. Very interestingly, coadministration of CBD prevented all of the THC-induced impairments observed in this study, whereas chronic CBD alone was inactive in the assays used here.
THC-induced cognitive impairment
While the current study used mice, previous studies using rats found chronic cannabinoid (THC, CP 55940, or WIN55,212-2) administration during adolescence, but not during adulthood, causes object recognition impairment after a drug-free delay.50–52 Similarly to our findings with mice and THC, chronic WIN55,212-2 administration during adolescence leads to immediate object recognition deficits in rats.53 Therefore, this study provides additional support that THC exposure during adolescence can lead to enduring cognitive impairment that is absent if THC is given during adulthood.
THC-induced behavioral impairment
Although this study is the first to specifically examine the effects of chronic cannabinoid administration on Nestlet shredding in mice, other studies have found both acute54 and chronic55 THC disrupted nest building. Although shredding material is the first step in nest building, increases in shredding behavior detected by the Nestlet shredding task are considered a measure of repetitive and compulsive-like behavior56 and as a model of obsessive-compulsive disorder.39 This study is the first to find that chronic adolescent THC administration leads to a long-lasting increase in this repetitive behavior.
Our findings also suggest that THC administration during adolescence transiently increases repetitive behaviors, as assessed by the marble-burying task. This difference was clear when THC mice were compared with adolescent mice treated with CBD or CBD+THC. In addition, although just missing our significance threshold, chronic adolescent THC treatment likely leads to an immediate increase in marble burying compared with vehicle. No biologically significant differences in marble burying were found among adolescent-treated mice tested 6 weeks after the last injection. This is the first study to examine the effects of chronic THC on marble burying. Several earlier studies have found that a single dose of synthetic cannabinoid, CBD, and/or acutely increasing endocannabinoid levels, suppressed marble burying.57–59 The decrease in CBD-treated adult mice (Fig. 5C) is consistent with these earlier findings.
THC-induced anxiety
This study is the first to examine the persistent effects of chronic cannabinoid administration on EPM behaviors in adolescent or adult mice. We found that chronic THC administration to either adult or adolescent mice produced a delayed anxiogenic effect, as measured through EPM. An earlier study60 using adult mice that examined the effects of THC or CBD on anxiety behaviors found that a chronic dose of THC (10 mg/kg), higher than that used in this study, increased anxiety when mice were tested immediately after dosing (vs. 4 days later as in this study). A previous study examining the effects of chronic, incrementing (2.5 mg/kg PND 35–37; 5 mg/kg PND 38–41; 10 mg/kg PND 42–45) THC administration in adolescent rats of both sexes during the early light cycle found no differences in anxiety-like behavior in the EPM, when measured after a drug-free delay (PND 75).61 The difference between the cited studies and this study may be due to species, escalating dose, or delay in testing, etc.
In contrast to the EPM results, no evidence of anxiety was apparent in any of the open field measures, for any treatment, age, or post-test interval. Thus, these results are similar to the previously cited study in rats.61 Owing to the inclusion of a ∼3 min acclimation period, it was not possible to determine whether the various treatments affected acclimation to a novel environment.
CBD protects against THC-induced deficits
Perhaps the most significant finding of our study is that coadministration of CBD+THC protected against all of the cognitive and behavioral impairments induced by THC. Interestingly, this was observed even though coadministration of CBD with THC actually increased plasma THC levels (Fig. 8). The mechanism of this protection is unclear, but does not appear to be pharmacokinetic. Numerous targets have been proposed for CBD.62,63 A particularly interesting potential mechanism that could explain the current results is CBD's negative allosteric modulation of CB1 cannabinoid receptors.64 The finding that coadministration of CBD+THC blocks the deleterious effects of THC may help explain the higher psychosis/schizophrenia risk among users of high-potency THC (and low CBD) cannabis.23,25,48,65 We chose to administer equal amounts of CBD and THC for these experiments, as this approximates the ratio of the acid forms of THC and CBD present in Cannabis sativa strains expressing both CBD and THC syntheses.66 However, it will be quite interesting to determine the minimal amount of CBD that is protective.
Conclusions
Chronic THC during adolescence in male mice produced a selective spectrum of persistent behavioral consequences. Based on the rat literature, it is likely that treatment of female mice will also lead to persistent behavioral abnormalities.67–70 Other than late-emerging anxiety, these abnormalities were absent in similarly treated adult male mice. These findings reinforce the notion that adolescence is a vulnerable developmental period, and that sustained exposure to THC during this time may have long-lasting and detrimental consequences on cognition and behavior. The finding that coadministration of equal amounts of CBD with THC prevented the emergence of all behavioral deficits supports the epidemiological finding that higher CBD content in cannabis reduces risk for psychotic disorders.48 Furthermore, encouraging the cultivation and consumption of “balanced” CBD cannabis (i.e., roughly equal proportions of CBD and THC), over lower CBD cannabis, may be a reasonable strategy for harm reduction.71
Supplementary Material
Abbreviations Used
- CBD
cannabidiol
- DI
discrimination index
- EPM
elevated plus maze
- NOR
novel object recognition
- OFA
open field assay
- PFC
prefrontal cortex
- THC
tetrahydrocannabinol
Acknowledgments
We thank Ben Cornett for his careful mouse husbandry for this study. This study was funded by the NIH: DA021696 and DA039463.
Author Disclosure Statement
The authors declare no competing financial interests exist.
References
- 1.Pertwee RG. Handbook of cannabis. Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- 2.Ahmed SA, Ross SA, Slade D, et al. Minor oxygenated cannabinoids from high potency cannabis sativa l. Phytochemistry. 2015;117:194–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A. Phytochemistry of Cannabis sativa L. In: Kinghorn A, Falk H, Gibbons S, Kobayashi J. (eds.) Phytocannabinoids. Progress in the Chemistry of Organic Natural Products, vol 103. Springer: Cham, Switzerland, 2017 [DOI] [PubMed] [Google Scholar]
- 4.ElSohly MA, Mehmedic Z, et al. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biol Psychiatry. 2016;79:613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NIDA. Monitoring the future study: Marijuana. 2016. https://www.drugabuse.gov/drugs-abuse/marijuana (accessed May12, 2017)
- 6.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47:296–312 [DOI] [PubMed] [Google Scholar]
- 7.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74 [DOI] [PubMed] [Google Scholar]
- 8.Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drzewiecki CM, Willing J, Juraska JM. Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: a role for pubertal onset. Synapse. 2016;70:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnea-Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854 [DOI] [PubMed] [Google Scholar]
- 11.Sowell ER, Thompson PM, Tessner KD, et al. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asato M, Terwilliger R, Woo J, et al. White matter development in adolescence: a DTI study. Cereb Cortex. 2010;20:2122–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray N. Prefrontal cortex: dopamine double rules. Nat Rev Neurosci. 2015;16:68–68 [DOI] [PubMed] [Google Scholar]
- 14.Moriguchi Y, Hiraki K. Prefrontal cortex and executive function in young children: a review of NIRS studies. Front Hum Neurosci. 2013;7:86–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev. 2014;42:180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maccarrone M, Guzman M, Mackie K, et al. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci. 2014;15:786–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arseneault L, Cannon M, Poulton R, et al. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol. 2010;160:511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zammit S, Allebeck P, Andreasson S, et al. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325:119–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-o-methyltransferase gene: longitudinal evidence of a gene x environment interaction. Biol Psychiatry. 2005;57:1117–1127 [DOI] [PubMed] [Google Scholar]
- 21.Manrique-Garcia E, Zammit S, Dalman C, et al. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol Med. 2012;42:1321–1328 [DOI] [PubMed] [Google Scholar]
- 22.Henquet C, Murray R, Linszen D, et al. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612 [DOI] [PubMed] [Google Scholar]
- 23.Di Forti M, Morgan C, Dazzan P, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195:488–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192:306–307 [DOI] [PubMed] [Google Scholar]
- 25.Di Forti M, Marconi A, Carra E, et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry. 2015;2:233–238 [DOI] [PubMed] [Google Scholar]
- 26.Carter CS, Perlstein W, Ganguli R, et al. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287 [DOI] [PubMed] [Google Scholar]
- 27.Gold JM, Carpenter C, Randolph C, et al. Auditory working memory and Wisconsin card sorting test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165 [DOI] [PubMed] [Google Scholar]
- 28.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357 [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:59–9. [DOI] [PubMed] [Google Scholar]
- 30.Crespo‐Facorro B, Wiser AK, Andreasen NC, et al. Neural basis of novel and well‐learned recognition memory in schizophrenia: a positron emission tomography study. Hum Brain Map. 2001;12:219–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doniger GM, Foxe JJ, Murray MM, et al. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020 [DOI] [PubMed] [Google Scholar]
- 32.Heckers S, Curran T, Goff D, et al. Abnormalities in the thalamus and prefrontal cortex during episodic object recognition in schizophrenia. Biol Psychiatry. 2000;48:651–657 [DOI] [PubMed] [Google Scholar]
- 33.Berman I, Merson A, Viegner B, et al. Obsessions and compulsions as a distinct cluster of symptoms in schizophrenia: a neuropsychological study. J Nerv Ment Dis. 1998;186:150–156 [DOI] [PubMed] [Google Scholar]
- 34.Poyurovsky M, Fuchs C, Weizman A. Obsessive-compulsive disorder in patients with first-episode schizophrenia. Am J Psychiatry. 1999;156:1998–2000 [DOI] [PubMed] [Google Scholar]
- 35.McReynolds P. Anxiety, perception and schizophrenia. In: Jackson D. (ed.). The etiology of schizophrenia. Basic Books: New York, 1960, pp. 248–292. [Google Scholar]
- 36.Seedat S, Fritelli V, Oosthuizen P, et al. Measuring anxiety in patients with schizophrenia. J Nerv Ment Dis. 2007;195:320–324 [DOI] [PubMed] [Google Scholar]
- 37.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Proc. 2012;13:93–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puighermanal E, Busquets-Garcia A, Gomis-Gonzalez M, et al. Dissociation of the pharmacological effects of THC by mTOR blockade. Neuropsychopharmacology. 2013;38:1334–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witkin JM. Animal models of obsessive‐compulsive disorder. Curr Protoc Neurosci. 2008;45:9..30:9.30.1–9.30.9. [DOI] [PubMed] [Google Scholar]
- 40.Thomas A, Burant A, Bui N, et al. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204:361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30 [DOI] [PubMed] [Google Scholar]
- 42.Seibenhener ML, Wooten MC. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015;(96):e5243–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leishman E, Mackie K, Luquet S, et al. Lipidomics profile of a NAPE-PLD KO mouse provides evidence of a broader role of this enzyme in lipid metabolism in the brain. Biochim Biophys Acta. 2016;1861:491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein C, Karanges E, Spiro A, et al. Cannabidiol potentiates δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology. 2011;218:443–457 [DOI] [PubMed] [Google Scholar]
- 45.Morgan CJ, Schafer G, Freeman TP, et al. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study. Br J Psychiatry. 2010;197:285–290 [DOI] [PubMed] [Google Scholar]
- 46.Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19–27 [DOI] [PubMed] [Google Scholar]
- 48.Schubart CD, Sommer IE, van Gastel WA, et al. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr Res. 2011;130:216–221 [DOI] [PubMed] [Google Scholar]
- 49.Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4:1770–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quinn HR, Matsumoto I, Callaghan PD, et al. Adolescent rats find repeated δ9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–1126 [DOI] [PubMed] [Google Scholar]
- 51.O'shea M, Singh ME, McGregor IS, et al. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18:502–508 [DOI] [PubMed] [Google Scholar]
- 52.Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769 [DOI] [PubMed] [Google Scholar]
- 53.Abush H, Akirav I. Short-and long-term cognitive effects of chronic cannabinoids administration in late-adolescence rats. PLoS One. 2012;7:e3173–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moschovakis A, Liakopoulos D, Armaganidis A, et al. Cannabis interferes with nest-building behavior in mice. Psychopharmacology. 1978;58:181–183 [DOI] [PubMed] [Google Scholar]
- 55.Frischknecht HR, Sieber B, Waser PG. Effects of multiple, chronic and early hashish exposure on mating behavior, nest-building and gestation in mice. Comp Biochem Physiol C. 1982;72:363–368 [DOI] [PubMed] [Google Scholar]
- 56.Angoa-Pérez M, Kane MJ, Briggs DI, et al. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J Vis Exp. 2013;(82):e5097–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinsey SG, O'Neal ST, Long JZ, et al. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav. 2011;98:21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deiana S, Watanabe A, Yamasaki Y, et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and cbd action on obsessive–compulsive behaviour. Psychopharmacology. 2012;219:859–873 [DOI] [PubMed] [Google Scholar]
- 59.Nardo M, Casarotto PC, Gomes FV, et al. Cannabidiol reverses the mcpp-induced increase in marble-burying behavior. Fundam Clin Pharmacol. 2014;28:544–550 [DOI] [PubMed] [Google Scholar]
- 60.Long LE, Chesworth R, Huang XF, et al. A behavioural comparison of acute and chronic delta9-tetrahydrocannabinol and cannabidiol in c57bl/6jarc mice. Int J Neuropsychopharmacol. 2010;13:861–876 [DOI] [PubMed] [Google Scholar]
- 61.Rubino T, Realini N, Guidali C, et al. Chronic δ9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771 [DOI] [PubMed] [Google Scholar]
- 62.Pisanti S, Malfitano AM, Ciaglia E, et al. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–150 [DOI] [PubMed] [Google Scholar]
- 63.Campos AC, Fogaca MV, Sonego AB, et al. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016;112:119–127 [DOI] [PubMed] [Google Scholar]
- 64.Laprairie R, Bagher A, Kelly M, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid cb1 receptor. Br J Pharmacol. 2015;172:4790–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Forti M, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2013;40:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiblen GD, Wenger JP, Craft KJ, et al. Gene duplication and divergence affecting drug content in cannabis sativa. New Phytol. 2015;208:1241–1250 [DOI] [PubMed] [Google Scholar]
- 67.Llorente-Berzal A, Puighermanal E, Burokas A, et al. Sex-dependent psychoneuroendocrine effects of THC and MDMA in an animal model of adolescent drug consumption. PLoS One. 2013;8:e7838–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubino T, Prini P, Piscitelli F, et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis. 2015;73:60–69 [DOI] [PubMed] [Google Scholar]
- 69.Rubino T, Vigano D, Realini N, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771 [DOI] [PubMed] [Google Scholar]
- 70.Zamberletti E, Gabaglio M, Prini P, et al. Cortical neuroinflammation contributes to long-term cognitive dysfunctions following adolescent delta-9-tetrahydrocannabinol treatment in female rats. Eur Neuropsychopharmacol. 2015;25:2404–2415 [DOI] [PubMed] [Google Scholar]
- 71.Fischer B, Russell C, Sabioni P, et al. Lower-risk cannabis use guidelines: a comprehensive update of evidence and recommendations. Am J Public Health. 2017;107:e1–e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
References
Cite this article as: Murphy M, Mills S, Winstone J, Leishman E, Wager-Miller J, Bradshaw H, Mackie K (2017) Chronic adolescent Δ9-tetrahydrocannabinol treatment of male mice leads to long-term cognitive and behavioral dysfunction, which are prevented by concurrent cannabidiol treatment, Cannabis and Cannabinoid Research 2:1, 235–246, DOI: 10.1089/can.2017.0034.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.