Abstract
Background
Follicle-stimulating hormone (FSH) is a gonadotropin synthesized and secreted by the pituitary gland. FSH stimulates follicle development and maturation in females. It also plays an important role in spermatogenesis in males, including humans and mice. However, the effects of FSH on male pigs are largely unknown. In this study, we generated transgenic pigs to investigate the effects of FSHα/β overexpression on reproductive traits in boars.
Results
After five transgenic F0 founders were crossed with wide-type pigs, 193 F1 animals were obtained. Of these, 96 were confirmed as transgenic. FSHα and FSHβ mRNAs were detected only in pituitary tissue. Transgenic boars exhibited significantly higher levels of FSHα and FSHβ mRNA, serum FSH, and serum testosterone, compared to full-sib non-transgenic boars. Significant increases in testis weight, vas deferens diameter, seminiferous tubule diameter, and the number of Leydig cells were observed, suggesting that the exogenous FSHα/β affects reproductive traits. Finally, transgenic and non-transgenic boars had similar growth performance and biochemical profiles.
Conclusions
Pituitary-specific overexpression of FSHα/β genes is likely to impact reproductive traits positively, as indicated by enhancements in serum testosterone level, testis weight, the development of vas deferens, seminiferous tubules, and Leydig cells in transgenic boars. A high level of serum FSH induces secretion of serum testosterone, possibly by boosting the number of Leydig cells, which presumably increases the libido and the frequency of sexual activity in transgenic boars. Our study provides a preliminary foundation for the genetic improvement of reproductive traits in male pigs.
Keywords: Boar, FSHα/β, Reproductive traits, Transgene
Background
Follicle-stimulating hormone (FSH) is a gonadotropin and glycoprotein polypeptide hormone with a mass of 35.5 kDa [1]. As a member of the glycoprotein hormone superfamily, it consists of two subunits (α and β) that combine non-covalently to form an active heterodimer, as is also the case for luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and human chorionic gonadotropin (hCG) [1]. The synthesis and secretion of FSHα and FSHβ is regulated by gonadotropin-releasing hormone (GnRH). FSHβ is also regulated by inhibin, leptin, and activins derived from brain, pituitary, placenta, and other tissues [2–4]. In females, FSH plays a key role in antral follicle development and stimulates preovulatory follicular growth in cooperation with LH [5, 6]. In males, FSH is required for the mitotic division of germ cells, and together with testosterone, is involved in spermatocyte maturation and spermatogenesis [7].
Transgenic mouse models incorporating human FSHα and FSHβ genes have been used to study the effect of FSH on reproductive function [8]. In transgenic mice carrying a 10 kb human FSHβ construct, the inserted gene is highly and specifically expressed in pituitary tissue and the mice exhibit normal fertility [9, 10]. FSH-null (knockout) male mice are fertile and sire normal-sized litters, although they show reductions in epididymal sperm number, sperm motility, and testicle size, while female knockouts are infertile [5]. FSHβ has been verified to be an important gene controlling litter size in Chinese Erhualian pigs, one of the most prolific pig breeds in the world [11]. In transgenic mice exhibiting pituitary-specific overexpression of the Chinese Erhualian FSH gene, ovulation rate and litter size increase markedly [12].
F0 transgenic pigs, in which FSHα/β expression is pituitary-specific, were generated previously [13]. In this study, we obtained 193 F1 transgenic animals derived from five F0 founders crossed to wild-type Large White pigs. Integration of the exogenous FSHα/β genes and their expression were confirmed. Since genetic improvements are more efficiently transferred by males than by females in pig breeding, we focused on the effects of FSHα/β on reproductive traits in boars. As is typical in reproductive trait studies, multiple traits were assessed, including semen volume, sperm quality parameters, sperm per ejaculate, epididymis weight, reproductive tract weight, and seminiferous tubule diameters (Animal QTL database) [14]. Hormone assays and histological analyses were performed to investigate the effects of exogenous FSH expression on the reproductive traits of male offspring. In addition, the health status of transgenic pigs was evaluated based on growth and various biochemical criteria. The results are directly relevant to strategies for improving the fecundity of multiparous mammals.
Methods
Generation of transgenic pigs
BAC DNA used for the production of transgenic animals in this study was described previously [12]. BAC clones for FSHα (BAC412H8) and FSHβ (BAC183O11) were isolated from a BAC library constructed using genomic DNA from a male Erhualian pig [15]. The LoxP-neo-LoxP cassette was introduced into two BAC constructs (FSHα and FSHβ) by homologous recombination (Fig. 1). BAC DNAs were linearized with NotI and co-transfected into fetal fibroblast cells. Positive cells were used as donors to produce transgenic founder pigs following standard procedures [16]. Transgenic F0 pigs were mated with non-transgenic Large White pigs to produce F1 pigs.
Fig. 1.
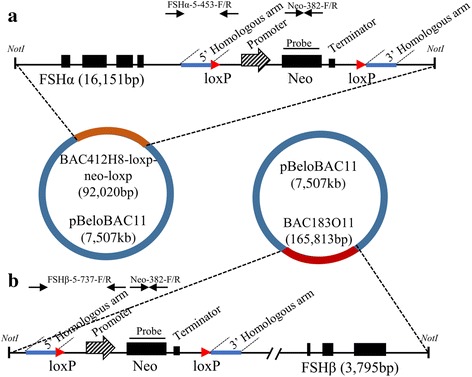
Schematic view of FSHα and FSHβ expression vectors. The vectors include the complete DNA sequences of the FSHα and FSHβ genes, along with the Neo gene and its promoter and terminator. Solid boxes represent exons. The red arrows represent LoxP, and the homologous arms are represented in blue. PCR primers (FSHα-5-453-F/R, FSHβ-5-737-F/R and Neo-382-F/R) are represented by black arrows. Southern blot probes are indicated by the label “probe”. NotI was the restriction enzyme cutting site
Identification of transgenic pigs and detection of gene expression
Transgenic pigs were identified by PCR and Southern blot using genomic DNA extracted from ear tissue. Three pairs of primers, FSHα-5-453-F/R (453 bp product), FSHβ-5-737-F/R (737 bp product) [13], and Neo-382-F/R (382 bp product), were used to amplify FSHα, FSHβ, and Neo, respectively. PCR products were digested with AvaII and PstI prior to gel electrophoresis. The primers Neo-382-F/R (forward 5′-GTTGTCACTGAAGCGGGAAG-3′ and reverse 5′-CACAGTCGATGAATCCAGAAAA-3′) were used to generate a digoxigenin (DIG)-labeled probe for the Southern blot assay (Roche Diagnostics, Mannheim, Germany). All primers were synthesized by the Sangon Company (Shanghai, China).
Three F1 transgenic (Tg) boars and three non-transgenic (NTg) full-sib boars were slaughtered at approximately 300 d of age. Tissue samples from hypothalamus, pituitary, testis, epididymis, vas deferens, seminal vesicle, prostate, Cowper’s gland, heart, liver, spleen, lung, kidney, and pancreas were collected, rapidly frozen in liquid nitrogen, and stored at −80 °C. Tissue-specific expression of the FSHα and FSHβ transgenes was determined by reverse transcription PCR (RT-PCR) and quantified by real-time PCR [17]. Total RNA was extracted using an animal total RNA extraction kit according to the manufacturer’s instructions (Tiangen, Beijing, China). cDNA synthesis was performed with 1 μg total RNA following the protocol accompanying the FastQuant RT Kit (Tiangen, Beijing, China). GADPH expression was used for normalization. The specific primers used for quantifying expression were: FSH-α (forward: 5′-GGGTGCCCCAATCTATCAGTG-3′, reverse: 5′-GTGGCATTCGGTGTGGTTCTC-3′), FSH-β (forward: 5′-CACCCCAAGATGAAGTCGCTG-3′, reverse: 5′-GCCAGGTACTTTCACGGTCTCG-3′), and GADPH (forward: 5′-GTTTGTGATGGGCGTGAAC-3′, reverse: 5′-ATGGACCTGGGTCATGAGT-3′).
Phenotype measurements
Body weight
Body weight of 20 F1 pigs (10 Tg and 10 NTg half-sib individuals) was recorded at the ages of 1 d (birth weight), 10 d and 21 d (weaning weight), 60 d, 90 d, and 150 d.
Serum biochemistry
Serum was separated from blood samples obtained from F1 pigs (5 Tg and 5 of NTg half-sib individuals) at 300 d, 307 d, and 315 d. The following compounds were measured: glucose (GLU), urea (UREA), creatinine (CREA), blood urea nitrogen/creatinine (BUN/CREA), phosphorus (PHOS), calcium (CA), total protein (TP), albumin (ALB), globulin (GLB), alanine aminotransferase (ALT), alkaline phosphatase (ALKP), γ-glutamyl transpeptidase (GGT), cholesterol (CHOL), triglyceride (TRIG), amylase (AMYL), lipase (LIPA), and creatine kinase (CK). All assays were conducted at Beijing Tianzewanwu Veterinary Hospital, China.
Hormone assays
Serum from three pairs of randomly chosen Tg and NTg full-sib boars was collected 3 times within one week at ~300 d of age. Levels of FSH, LH, testosterone, and estradiol (E2) were measured in triplicate using a standard radioimmunoassay. Assays were conducted at the Beijing North Institute of Biological Technology, China.
Assessment of sperm quality
Semen collection and quality assessments were performed as described [18]. Briefly, semen was collected from five pairs of Tg and NTg half-sib boars at an approximate age of 300 d. Three successive collections were performed at 7-day intervals. Semen volume was measured using graduated semen collection jars. Sperm concentration and motility were analyzed using the Sperm Quality Analyzer (Beijing, China). Total sperm number per ejaculate was calculated using the formula: sperm concentration × semen volume. The fraction of sperm exhibiting teratospermia, intact acrosomes, and normal mitochondrial function was assessed using methods described previously [19]. Seminal plasma quality was assessed by measuring levels of zinc, fructose, neutral α-glucosidase (NAG), and acid phosphatase (ACP), using a ChemWell BRED Analyzer (Guangdong, China) at the Beijing North Institute of Biological Technology.
Histological analysis
After slaughter, testes and epididymis were isolated and weighed. Testes tissue and vas deferens was fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned. Tissue sections were stained with hematoxylin-eosin (H&E) and observed with a light microscope (Nikon, Japan). The diameters of vas deferens and seminiferous tubules were measured in ~30 fields. Leydig cells were counted in ~10 fields for each pig at 200× magnification and the average value was calculated.
Statistical analysis
Student’s t-test was performed using SPSS Statistics (IBM Corporation, USA). All values are presented as mean ± standard error (SEM). P < 0.05 was the threshold for statistical significance.
Results
Transgenic pigs exhibiting pituitary-specific overexpression of the FSHα/β genes were generated using the BAC DNAs (FSHα and FSHβ) shown in Fig. 1. Five F0 transgenic animals (two boars and three sows), in which both BACs were intact, were identified by PCR and Southern blot analysis, as described by Bi [13].
Integration and expression of exogenous FSH
Five founders were crossed with wild-type Large White pigs to obtain 193 F1 progenies, of which nearly half (43 boars and 53 sows) were positive for the exogenous FSHα, FSHβ and Neo genes, as determined by PCR (Fig. 2a). The Neo gene was also detected by Southern blot in all 96 F1 pigs (Fig. 2b). These data confirm that the integrated FSHα, FSHβ and Neo genes were transmitted to both male and female F1 pigs with the expected Mendelian ratio.
Fig. 2.
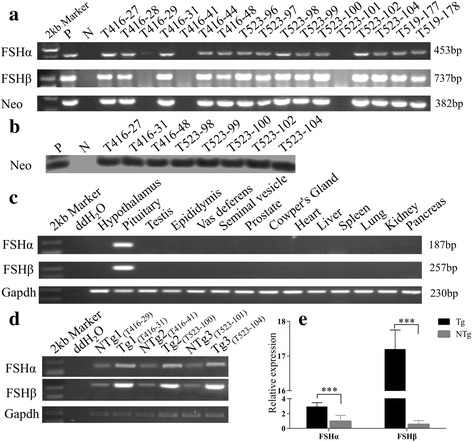
Identification of exogenous FSHα/β insertion and expression analysis. (a) Identification of F1 transgenic pigs by PCR using DNA obtained from ear tissue. P, a single F0 transgenic pig as positive control; N, a single non-transgenic Large White pig as negative control; T416–27, 28, 29, 31, 41, T523–96, 97, 98, 99, 100, 101, 102, 104, and T519–177, 178 are identifiers for F1 transgenic pigs. (b) Southern blot for transgenic pig identification. The Neo gene in transgenic pigs was detected using the probe shown in Fig. 1. DNAs were digested with AvaII and PstI to generate a target fragment of 463 bp. (c) RT-PCR analysis of FSHα and FSHβ from pituitary and 13 other tissues. GADPH was used as a control. (d) RT-PCR analysis of FSHα and FSHβ expression using mRNA from the pituitaries of six transgenic pigs. (e). FSHα and FSHβ mRNA expression levels in the pituitaries of Tg and NTg pigs analyzed using qPCR. Relative expression was calculated relative to β-actin (reference gene). Values are expressed as means ± SEM. ***, P < 0.001
To determine whether the exogenous FSHα and FSHβ genes in the F1 transgenic pigs were expressed in a tissue specific manner, FSH mRNA from pituitary gland and 13 other tissues was subjected to RT-PCR. FSHα and FSHβ expression was observed only in pituitary tissue (Fig. 2c). Because this experiment does not distinguish between contributions made by exogenous and endogenous FSH genes, FSHα and FSHβ expression in the pituitary glands of three pairs of full-sib transgenic and non-transgenic boars was compared by RT-PCR (Fig. 2d), and total FSH mRNA expression was quantified in the same samples using qPCR (Fig. 2e). As expected, mRNA levels of both FSHα and FSHβ were significantly higher in transgenic animals (P < 0.001).
Serum concentrations of FSH, LH, testosterone, and E2
To examine the effects of FSHα/β overexpression on hormone levels, FSH, LH, testosterone and E2 levels were compared in full-sib transgenic and non-transgenic boars at an approximate age of 300 d (Fig. 3). Serum levels of FSH were significantly higher in transgenic animals (2.25 ± 0.18 mIU/mL vs. 1.75 ± 0.20 mIU/mL, P < 0.05, Fig. 3a). Similarly, testosterone levels in transgenic boars were significantly higher than in non-transgenic boars (3.26 ± 0.64 ng/mL vs. 1.67 ± 0.60 ng/mL, P < 0.05, Fig. 3b). Although serum levels of both LH and E2 were higher in transgenic boars, the differences were not significant (LH: 9.16 ± 0.70 mIU/mL vs. 8.19 ± 0.67 mIU/mL, P > 0.05; E2: 29.71 ± 3.46 pg/mL vs. 25.00 ± 3.22 pg/mL, P > 0.05; Fig. 3c-d).
Fig. 3.
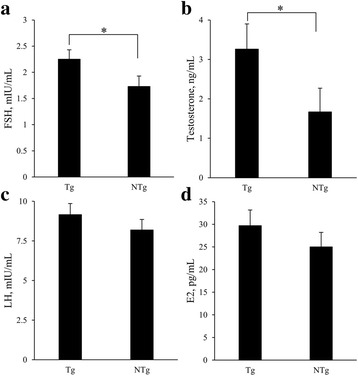
Hormone assays. (a) Serum FSH, (b) testosterone, (c) LH, and (d) E2 levels in F1 pigs. All assays were conducted in triplicate. Bars represent means ± SEM. *, P < 0.05
Effect of FSH overexpression on reproductive traits
Several semen quality indicators and seminal plasma components were compared between transgenic and non-transgenic boars at ~300 d of age. No significant differences were observed in any of the seven semen quality indicators (P > 0.05, Table 1). Transgenic and non-transgenic boars exhibited similar values for all four seminal plasma components (P > 0.05, Table 2).
Table 1.
Semen characteristics in transgenic and non-transgenic boars
| Items | Tg | NTg | P-value |
|---|---|---|---|
| Semen volume per ejaculate, mL | 218.75 ± 28.73 | 237.00 ± 29.54 | 0.079 |
| Sperm concentration, 108/mL | 3.58 ± 0.09 | 3.46 ± 0.08 | 0.619 |
| Total sperm per ejaculate, 108 | 794.74 ± 28.35 | 832.78 ± 25.36 | 0.314 |
| Sperm mobility, % | 77.11 ± 2.63 | 73.57 ± 2.36 | 0.411 |
| Teratospermia, % | 8.23 ± 0.30 | 7.30 ± 0.38 | 0.764 |
| Acrosome intactness, % | 81.68 ± 0.25 | 81.34 ± 0.23 | 0.890 |
| Normal mitochondria function, % | 80.18 ± 1.52 | 82.22 ± 1.36 | 0.930 |
Table 2.
Biochemical indicators for seminal plasma in transgenic and non-transgenic boars
| Items | Tg | NTg | P-value |
|---|---|---|---|
| Seminal plasma zinc, μmol | 0.76 ± 0.16 | 0.54 ± 0.15 | 0.248 |
| Seminal plasma fructose, mIU | 1.01 ± 0.05 | 0.95 ± 0.05 | 0.919 |
| Neutral α-glucosidase, IU | 0.95 ± 0.06 | 0.70 ± 0.05 | 0.324 |
| Acid phosphatase, μmol | 236.90 ± 14.17 | 217.89 ± 12.67 | 0.406 |
We also compared testis and epididymis characteristics between transgenic and non-transgenic boars. As shown in Fig. 4a, the testis weight in transgenic boars was significantly higher (501.6 ± 35.6 g vs. 355.2 ± 32.8 g, P < 0.05). Transgenic boars exhibited higher epididymis weight but the levels were statistically indistinguishable (149.6 ± 10.6 g vs. 138.0 ± 11.0 g, P > 0.05, Fig. 4a). Vas deferens and seminiferous tubule diameters were also compared. Interestingly, both diameters were significantly higher in transgenic boars (vas deferens, 2216.25 ± 173.24 μm vs. 1894.72 ± 270.86 μm, P < 0.001; seminiferous tubules, 117.30 ± 6.65 μm vs. 107.79 ± 6.79 μm, P < 0.001, Fig. 4b-f). Enlargement of the vas deferens occurred mainly in the muscular layer of the wall. Finally, the number of Leydig cells in transgenic boars was significantly higher than in non-transgenic boars (337.6 ± 14.3 vs. 178.9 ± 23.4, P < 0.01, Fig. 4g-i).
Fig. 4.
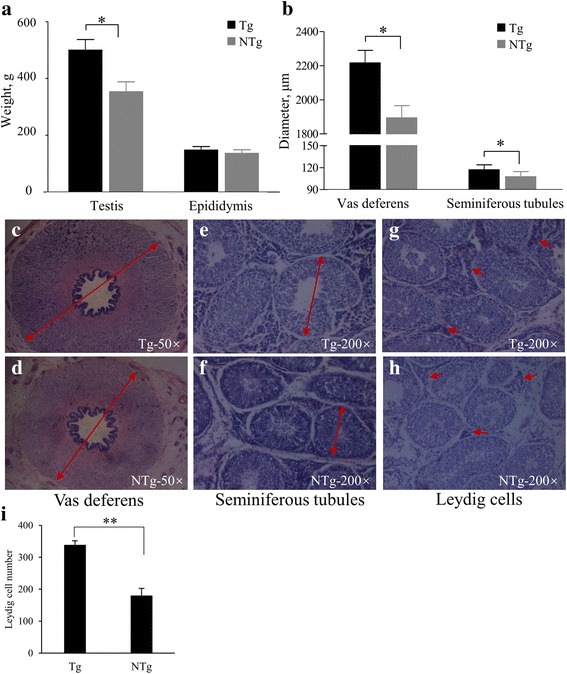
Histological assessment of reproductive tissue from F1 boars. (a) Comparison of testis and epididymis weight in Tg and NTg boars. (b) Vas deferens and seminiferous tubule diameters in Tg and NTg boars. (c-i) Histological sections of testis tissue. (c-d) Vas deferens at 50× magnification. Red arrows span the vas deferens diameter. (e-f) Seminiferous tubules at 200× magnification. Red arrows span tubule diameter. (g-h) Leydig cells at 200× magnification. Red arrows indicate Leydig cells between the seminiferous tubules. (i) The number of Leydig cells in Tg and NTg boars. Data are expressed as means ± SEM. *, P < 0.05. **, P < 0.01
Growth and biochemical analysis
Body weight at six growth stages (from birth to 150 d) was compared between transgenic and non-transgenic boars. There were no significant differences, although transgenic boar body weight was slightly higher from birth to 90 d, while non-transgenic boars exhibited higher body weight at 150 d (Fig. 5). In addition, no significant differences in blood chemistry were observed (Table 3). We conclude that the transgenic boars in this study exhibited no detectable health defects relative to wild-type controls.
Fig. 5.
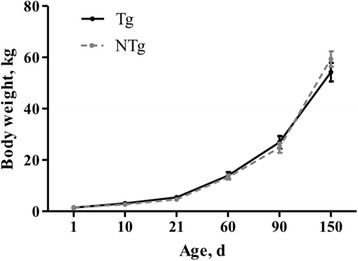
Growth of F1 Tg and NTg boars from birth to 150 d. Data is expressed as means ± SEM
Table 3.
Blood biochemistry in transgenic and non-transgenic boars
| Items | Tg | NTg | P-value |
|---|---|---|---|
| Glucose, mmol/L | 4.58 ± 0.18 | 4.33 ± 0.16 | 0.334 |
| Urea, mmol/L | 6.87 ± 0.24 | 7.19 ± 0.21 | 0.785 |
| Creatinine, μmol/L | 118.42 ± 3.17 | 122.67 ± 2.83 | 0.0629 |
| Blood urea nitrogen/creatinine | 16.50 ± 0.52 | 15.80 ± 0.47 | 0.254 |
| Phosphorus, mmol/L | 2.28 ± 0.14 | 2.10 ± 0.12 | 0.0516 |
| Calcium, mmol/L | 2.29 ± 0.03 | 2.33 ± 0.03 | 0.580 |
| Total protein, g/L | 70.75 ± 0.36 | 70.27 ± 0.32 | 0.491 |
| Albumin, g/L | 32.50 ± 0.17 | 32.27 ± 0.16 | 0.526 |
| Globulin, g/L | 38.25 ± 0.19 | 38.00 ± 0.17 | 0.831 |
| Albumin/ Globulin | 0.84 ± 0.02 | 0.87 ± 0.02 | 0.545 |
| Alanine aminotransferase, IU/L | 59.17 ± 0.62 | 60.00 ± 0.56 | 0.809 |
| Alkaline phosphatase, IU/L | 76.75 ± 4.61 | 82.93 ± 4.12 | 0.366 |
| γ-glutamyl transpeptidase, IU/L | 32.92 ± 1.25 | 34.60 ± 1.22 | 0.802 |
| Cholesterol, mmol/L | 1.89 ± 0.07 | 1.80 ± 0.06 | 0.182 |
| Triglyceride, mmol/L | 0.59 ± 0.06 | 0.50 ± 0.06 | 0.406 |
| Amylase, IU/L | 434.03 ± 30.35 | 474.80 ± 27.14 | 0.617 |
| Lipase, IU/L | 23.50 ± 1.78 | 21.08 ± 1.63 | 0.496 |
| Creatine kinase, IU/L | 606.64 ± 18.65 | 591.08 ± 15.93 | 0.729 |
Discussion
Pig fecundity is one of the most economically important traits in pig production. Because pig reproductive traits have low heritability [20], only a few candidate genes affecting pig reproduction have been identified, such as estrogen receptor 1 (ESR1) and FSHβ [11, 21]. Transgenic mice in which porcine FSH is overexpressed exhibit significantly increased female fertility [12]. In this study, we investigated the effects of porcine FSH on reproductive traits in male transgenic pigs.
In 193 F1 progenies, 96 transgenic pigs were identified. The transmission rate was 49.74%, consistent with ordinary Mendelian inheritance. FSH expression occurred in a pituitary-specific pattern (Fig. 2c), similar to results reported for FSHβ-overexpressing mice [12]. Because the exogenous and endogenous porcine FSHα/β are nearly identical in sequence, we could not distinguish between them using molecular methods. However, when total FSHα/β mRNA and serum FSH were compared in transgenic and non-transgenic pigs, transgenic animals exhibited significantly higher levels. These results suggest that pituitary-specific overexpression of FSH was successfully established in our transgenic pig model. While FSHβ mRNA increased approximately 10-fold in the transgenic animals, and FSHα mRNA increased about 3-fold, we observed only a modest increase in serum FSH levels (Figs. 2e and 3a). This is expected because serum FSH is a heterodimer, consisting of two subunits of FSHα and FSHβ, and FSH levels are probably limited by the lower level of FSHα mRNA expression (Fig. 2d-e) [1].
Male fertility is important in reproductive performance [22], and growing evidence suggests that FSH may be an important factor. In our study, the diameter of vas deferens and seminiferous tubules (Fig. 4b-f) increased with the increasing levels of serum FSH in transgenic boars (Fig. 3a). The enlargement of vas deferens mainly occurred in the muscular layer of the wall. In humans, the vas deferens wall is thinner after vasectomy [23]. We suggest that the thickened muscular layer of the vas deferens might affect sperm transportation and the ejaculation process, but the hypothesis has not yet been tested. Seminiferous tubule diameter correlates positively with semen quality parameters (sperm concentration, sperm motility, and total sperm per ejaculate) in rabbits [24]. In contrast, no improvement in semen quality was identified in transgenic boars in this study. In addition, semen quality in pigs does not change after treatment with FSH, although serum testosterone level increases [25]. Testosterone levels are enhanced in male mice that overexpress FSH [10]. In contrast, FSH and FSH receptor knockout mice have smaller testes and exhibit reduced numbers of germ and Leydig cells [5, 26]. In this study, we also observed that the serum testosterone level (Fig. 3b), testis weight (Fig. 4a) and the number of Leydig cells (Fig. 4g-i) increased in transgenic boars. The main function of Leydig cells is testosterone synthesis and secretion [27], and serum testosterone concentration is strongly related to libido in humans [28], rams [29], rats [30], and mice [31]. Testosterone also enhances libido, frequency of sexual acts, and sleep-related erections in humans [32]. If the underlying biology is similar in pigs, the increased number of Leydig cells in transgenic boars would be expected to increase testosterone levels and thereby enhance libido, increase the frequency of sexual activity, and increase the frequency of semen collection. Because our results indicate that overexpression of FSH increases serum testosterone levels in boars, the effect is likely to be an improvement in the downstream reproductive traits.
Finally, we evaluated whether the exogenous FSHβ gene exerts deleterious effects on the transgenic pigs. Body weight, levels of various biochemical components in blood plasma and semen plasma, and semen quality, were similar in transgenic and non-transgenic animals. This suggests that FSH overexpression has no detectable adverse impact on pig health.
Conclusions
In summary, we successfully produced transgenic pigs in which exogenous FSHα/β genes were integrated and expressed at high levels in a pituitary-specific manner. The high level of serum FSH increases the level of serum testosterone, possibly by increasing the number Leydig cells. Higher levels of testosterone would be expected to enhance the libido and the frequency of sexual activity in transgenic boars. Nevertheless, augmented FSH levels did not improve semen quality, even though testis weight and seminiferous tubules diameter increased. Finally, the expression of exogenous FSHα/β genes resulted in no detectable adverse effects on growth or the overall health of transgenic boars.
Acknowledgements
We thank for Mr. Qiao Xu, Mr. Guoying Hua, Mr. Chunzheng Fu, Mr. Yu Feng, and Mr. Linchao Wang for collecting the samples.
Funding
Funding for this study was provided by National Basic Research Program of China (973 Program, Grant 2014CB138501), the National Transgenic Animal Breeding Grand Project (2014ZX08006–005), and the Program for Changjiang Scholars and Innovation Research Teams in the University (IRT_15R62). The funding sources had no role in the study design.
Availability of data and materials
Data sharing not applicable to this article.
Abbreviations
- ACP
Acid phosphatase
- ALB
Albumin
- ALKP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AMYL
Amylase
- BUN/CREA
Blood urea nitrogen/creatinine
- CA
Calcium
- CHOL
Cholesterol
- CK
Creatine kinas
- CREA
Creatinine
- DIG
Digoxigenin
- E2
Estradiol
- ESR1
Estrogen receptor 1
- FSH
Follicle-stimulating hormone
- FSHα
Follicle-stimulating hormone subunit α
- FSHβ
Follicle-stimulating hormone subunit β
- GGT
γ-glutamyl transpeptidase
- GLB
Globulin
- GLU
Glucose
- GnRH
Gonadotropin-releasing hormone
- hCG
Human chorionic gonadotropin
- LH
Luteinizing hormone
- LIPA
Lipase
- NAG
Neutral α-glucosidase
- PHOS
Phosphorus
- TP
Total protein
- TRIG
Triglyceride
- TSH
Thyroid-stimulating hormone
Authors’ contributions
KW conceived and designed the experiments. WL, YQ, MZ, MZ, YC and QL performed the experiments and collected the samples. WL and KW analyzed the data, wrote the main manuscript, and prepared the figures. All authors reviewed and approved the final manuscript.
Ethics approval
All experiments involving transgenic pig production and sample collection strictly followed protocols approved by the Animal Welfare Committee of China Agricultural University (Approval Number: XK257).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Wenting Li, Email: liwenting@cau.edu.cn.
Yujun Quan, Email: quanyj2013@sina.com.
Mengmeng Zhang, Email: zhangmm@cau.edu.cn.
Kejun Wang, Email: wangkejun.me@163.com.
Muzhen Zhu, Email: 809314640@qq.com.
Ye Chen, Email: 673348465@qq.com.
Qiuyan Li, Email: liqiuyan@cau.edu.cn.
Keliang Wu, Email: liangkwu@cau.edu.cn.
References
- 1.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 2.Matzuk MM, Kumar TR, Shou W, Coerver KA, Lau AL, Behringer RR, et al. Transgenic models to study the roles of inhibins and activins in reproduction, oncogenesis, and development. In: conn PM (ed.) Recent Prog Horm Res, vol. 51; 1996: 123–157. [PubMed]
- 3.Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular-biology of the pituitary gonadotropins. Endocr Rev. 1990;11:177–199. doi: 10.1210/edrv-11-1-177. [DOI] [PubMed] [Google Scholar]
- 4.Kato Y, Imai K, Sakai T, Inoue K. Simultaneous effect of gonadotropin-releasing hormone (GnRH) on the expression of 2 gonadotropin-beta genes by passive-immunization to GnRH. Mol Cell Endocrinol. 1989;62:135–139. doi: 10.1016/0303-7207(89)90122-6. [DOI] [PubMed] [Google Scholar]
- 5.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 6.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 7.Ruwanpura SM, McLachlan RI, Meachem SJ. Hormonal regulation of male germ cell development. J Endocrinol. 2010;205:117–131. doi: 10.1677/JOE-10-0025. [DOI] [PubMed] [Google Scholar]
- 8.Kumar TR. Mouse models for gonadotropins: a 15-year saga. Mol Cell Endocrinol. 2007;260-262:249–254. doi: 10.1016/j.mce.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Kumar TR, Fairchild-Huntress V, Low MJ. Gonadotrope-specific expression of the human follicle-stimulating hormone beta-subunit gene in pituitaries of transgenic mice. Mol Endocrinol. 1992;6:81–90. doi: 10.1210/mend.6.1.1738375. [DOI] [PubMed] [Google Scholar]
- 10.Kumar TR, Low MJ. Gonadal steroid hormone regulation of human and mouse follicle stimulating hormone beta-subunit gene expression in vivo. Mol Endocrinol. 1993;7:898–906. doi: 10.1210/mend.7.7.8413314. [DOI] [PubMed] [Google Scholar]
- 11.Zhao YF, Li N, Xiao L, Cao GS, Chen YZ, Zhang S, et al. FSH beta subunit gene is associated with major gene controlling litter size in commercial pig breeds. Science in China Series C-Life Sciences. 1998;41:664–668. doi: 10.1007/BF02882910. [DOI] [PubMed] [Google Scholar]
- 12.Bi M, Tong J, Chang F, Wang J, Wei H, Dai Y, et al. Pituitary-specific overexpression of porcine follicle-stimulating hormone leads to improvement of female fecundity in BAC transgenic mice. PLoS One. 2012;7:e42335. doi: 10.1371/journal.pone.0042335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bi M. Construction of BAC transgenic mice and pigs harboring porcine follicle-stimulating hormone gene. Beijing: China Agricultural University; 2013. [Google Scholar]
- 14.Hu Z, Park CA, Wu X, Reecy JM. Animal QTLdb: an improved database tool for livestock animal QTL/association data dissemination in the post-genome era. Nucleic Acids Res. 2013;41:D871–D879. doi: 10.1093/nar/gks1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Zhang Y, Liu Z, Guo L, Wang X, Fei J, et al. A five-fold pig bacterial artificial chromosome library: a resource for positional cloning and physical mapping. Prog Nat Sci. 2006;16:889–892. doi: 10.1080/10020070612331343207. [DOI] [Google Scholar]
- 16.Gong G, Dai Y, Fan B, Zhu H, Wang H, Wang L, et al. Production of transgenic blastocyst by nuclear transfer from different types of somatic cells in cattle. Sci China C Life Sci. 2004;47:183–189. doi: 10.1360/03yc0015. [DOI] [PubMed] [Google Scholar]
- 17.Bai Y, Zhang JB, Xue Y, Peng YL, Chen G, Fang MY. Differential expression of CYB5A in Chinese and European pig breeds due to genetic variations in the promoter region. Anim Genet. 2015;46:16–22. doi: 10.1111/age.12257. [DOI] [PubMed] [Google Scholar]
- 18.Hu JH. Study on cryoperservation of boar semen. Yangling: Northwest A&F university; 2006. [Google Scholar]
- 19.Cai W, Su W, Fang Y, Chang Q, Sun S, Zhao Y, et al. Analysis of semen quality, routine blood, biochemical indexes of serum and seminal plasma in transgenic goats. Chinese. J Anim Sci. 2014;50:28–32. [Google Scholar]
- 20.Zhang Y. Animal breeding. Beijing: China Agricultural University Press; 2001. [Google Scholar]
- 21.Rothschild M, Jacobson C, Vaske D, Tuggle C, Wang L, Short T, et al. The estrogen receptor locus is associated with a major gene influencing litter size in pigs. Proc Natl Acad Sci U S A. 1996;93:201–205. doi: 10.1073/pnas.93.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger T. Male Effects on Reproductive Performance. J. Anim. Sci. 1998;(Suppl 3):47–51. doi: 10.2527/1998.76suppl_347x. [DOI] [Google Scholar]
- 23.Schmidt SS, Brueschke EE. Anatomical sizes of the human vas deferens after vasectomy. Fertil Steril. 1976;27:271–274. doi: 10.1016/S0015-0282(16)41716-4. [DOI] [PubMed] [Google Scholar]
- 24.Princewill Ogbuewu I, Charles Okoli I, Uwaezuoke Iloeje M. Semen quality characteristics, reaction time, testis weight and seminiferous tubule diameter of buck rabbits fed neem (Azadirachta Indica a. Juss) leaf meal based diets. International journal of reproductive. Biomedicine. 2009;7:23–28. [Google Scholar]
- 25.Wagner A, Claus R. The effects of postnatal FSH substitution on Sertoli cell number and the sperm production capacity of the adult boar. Anim Reprod Sci. 2009;110:269–282. doi: 10.1016/j.anireprosci.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 26.O'Shaughnessy PJ, Monteiro A, Abel M. Testicular development in mice lacking receptors for follicle stimulating hormone and androgen. PLoS One. 2012;7:e35136. doi: 10.1371/journal.pone.0035136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodgson Y, Hudson B. The pituitary and testis: springer. 1983. Leydig cell function; pp. 107–132. [DOI] [PubMed] [Google Scholar]
- 28.Travison TG, Morley JE, Araujo AB, Donnell AB O, JB MK. The relationship between libido and testosterone levels in aging men. J Clin Endocrinol Metab. 2006;91:2509–2513. doi: 10.1210/jc.2005-2508. [DOI] [PubMed] [Google Scholar]
- 29.Aguirre V, Orihuela A, Vázquez R. Effect of semen collection frequency on seasonal variation in sexual behaviour, testosterone, testicular size and semen characteristics of tropical hair rams (Ovis Aries) Trop Anim Health Prod. 2007;39:271. doi: 10.1007/s11250-007-9010-8. [DOI] [PubMed] [Google Scholar]
- 30.Damassa DA, Smith ER, Tennent B, Davidson JM. The relationship between circulating testosterone levels and male sexual behavior in rats. Horm Behav. 1977;8:275–286. doi: 10.1016/0018-506X(77)90002-2. [DOI] [PubMed] [Google Scholar]
- 31.Luttge WG, Hall NR. Differential effectiveness of testosterone and its metabolites in the induction of male sexual behavior in two strains of albino mice. Horm Behav. 1973;4:31–43. doi: 10.1016/0018-506X(73)90014-7. [DOI] [Google Scholar]
- 32.Shabsigh R. The effects of testosterone on the cavernous tissue and erectile function. World J Urol. 1997;15:21–26. doi: 10.1007/BF01275152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article.


