Abstract
Background
Antibodies against equine influenza virus (EIV) are traditionally quantified by haemagglutination inhibition (HI) or single radial haemolysis (SRH).
Objectives
To evaluate an ELISA for the detection of antibodies against influenza nucleoprotein in the diagnosis and surveillance of equine influenza (EI).
Methods
The ELISA was compared with the SRH and HI tests. Serial serum samples from 203 naturally and 14 experimentally infected horses, from 60 weanlings following primary vaccination with five different vaccines (two whole inactivated vaccines, two ISCOM‐based subunit vaccines and a recombinant canarypox virus vaccine) and from 44 adult horses following annual booster vaccination with six different vaccines were analysed.
Results
Fewer seroconversions were detected in clinical samples by ELISA than by SRH or HI but ELISA was more sensitive than SRH in naïve foals post‐experimental infection. The ELISA did not detect the antibody response to vaccination with the recombinant canarypox virus vaccine confirming the usefulness of the combination of this kit and vaccine to differentiate between naturally infected and vaccinated horses, that is, DIVA. No DIVA capacity was evident with the other vaccines.
Conclusion
The results suggest that this ELISA is a useful supplementary test for the diagnosis of EI although less sensitive than HI or SRH. It is an appropriate test for EI surveillance in a naïve population and may be combined with the recombinant canarypox virus vaccine but not with other commercially available subunit vaccines, in a DIVA strategy.
Keywords: Antibody, DIVA, ELISA, equine, influenza, nucleoprotein
Introduction
Antibodies against equine influenza virus (EIV) are traditionally quantified by haemagglutination inhibition (HI) or single radial haemolysis (SRH).1 Neither test requires costly equipment but both need to be performed and interpreted by trained personnel. HI is frequently the serological test of choice for diagnosis as the results can be obtained within hours. Furthermore, in the absence of EIV isolation from infected horses that have seroconverted, the HI assay may be used with reference viruses to determine in some measure the antigenic characteristics of the infecting EIV isolate. The SRH test is more reproducible between laboratories2, 3 but is more complicated and time consuming than HI and usually only performed in specialist laboratories. A significant increase in antibody titre in paired sera (acute and convalescent samples) can be a useful indicator of recent infection. As many horses have been vaccinated against equine influenza (EI) or have been previously infected, the analysis of single samples is less informative and does not offer a definitive diagnosis. However, testing of single samples by SRH is useful to determine the immune status of a horse as a definite correlation between SRH antibody levels and protective immunity against EI has been established in both experimental challenge studies and in the field.4, 5, 6 The SRH test is frequently used in vaccine efficacy and duration of immunity studies for marketing purposes or submission to the regulatory authorities.7, 8, 9, 10
During the Australian outbreak in 2007, virus spread was limited by restriction of horse movement and strategic vaccination.11 The vaccine used in the eradication programme was Proteq Flu‐Te, which contains recombinant canarypox viruses that express only the HA gene from two EIV strains. One of the reasons for choosing this vaccine was that it was possible to differentiate between infected and vaccinated horses (DIVA) using an ELISA developed primarily for poultry, which detected antibodies against the viral nucleoprotein (NP) of type A influenza viruses.11, 12, 13, 14 The ELISA proved to be extremely useful in the control and eradication of EI in Australia particularly in surveillance of vaccinated horses in the buffer zones surrounding the infected areas.13, 15 Such surveillance was crucial to provide confidence in a declaration of freedom from EI.11, 16
The aim of this study was to evaluate the sensitivity of an ELISA for the detection of EI in infected horses and the monitoring of antibody response to vaccination, and to compare it to existing methodologies.
Materials and methods
Serum samples
Paired serum samples collected from 203 horses during EI outbreaks in 14 yards were tested by ELISA, HI and SRH. Sera collected from 14 weanlings at 1 day prior to experimental infection with an aerosol of 10 ml A/equine/Kildare/89 at 106 EID50/ml, and seven and 14 days post‐infection were tested by ELISA and SRH. Sera collected and tested by SRH for two comparative vaccine studies were tested by ELISA. The vaccines used, the vaccination and sampling regimes and the SRH results for these two studies have been described previously.17, 18 Briefly, in the first study, sixty seronegative Thoroughbred weanlings (circa 6–10 months of age) received their primary vaccination course of three vaccines in line with regulations of the Irish Turf Club. The vaccines included as follows: a whole inactivated vaccine, Duvaxyn IE‐T Plus1 (11 horses), a whole inactivated vaccine, Equilis Resequin2 (11 horses), an ISCOM‐based subunit vaccine, Equip FT3 (14 horses), an ISCOM matrix‐based subunit vaccine, Equilis Prequenza Te2 (13 horses) and a recombinant canarypox virus vaccine, Proteq Flu‐Te4 (11 horses).
Blood samples were collected on the day of first vaccination (V1), 2 weeks post‐V1, on the day of second vaccination (V2), that is, 5 weeks after V1, 2 weeks post‐V2, 13 weeks post‐V2, on the day of third vaccination (V3), that is, 26 weeks after V2, 2 weeks post‐V3, 13 weeks post‐V3 and 26 weeks post‐V3.
In the second comparative vaccine study, 44 National Hunt racehorses (mean age 5·8 years) received their annual booster vaccination with Duvaxyn IE‐T Plus1 (seven horses), Equilis Resequin2 (seven horses), Equip FT3 (nine horses), Proteq Flu‐Te4 (six horses), an inactivated whole virus vaccine, Prevac T Pro2 (eight horses) and an ISCOM ‐based subunit vaccine, Equilis Equenza T2 (seven horses). Blood samples were taken on the day of vaccination and at two, four, 12 and 24 weeks post‐vaccination.
Haemagglutination inhibition
The HI test was performed according to standard procedures1 and as described previously19 except that the viruses used as antigens (HA titre of 1/4 per well) in this study were a representative of the European lineage, A/equine/Kildare/89 (H3N8) and a representative of the American lineage, A/equine/Kildare/92 (H3N8) or A/equine/Meath/07 (H3N8). The HI titre of each serum sample was the highest dilution of serum that caused 50% inhibition of haemagglutination of red blood cells. A seroconversion was defined as an increase of fourfold or greater in the titre between paired samples.
Single radial haemolysis
The SRH test was performed according to standard procedures1 and as described previously.20 The viruses used as antigens for the testing of clinical samples were representatives of the American lineage A/equine/Kildare/92(H3N8) or A/equine/Meath/07 (H3N8). In the experimental infection study, representatives of the European, A/equine/Newmarket/2/93 (H3N8) and of the American lineage, A/equine/Kildare/92(H3N8), were used. The viruses used as antigens for the comparative vaccine studies were representatives of the European lineage, A/equine/Newmarket/2/93 (H3N8) and American lineage, A/equine/Kildare/92(H3N8) and A/equine/South Africa/4/03 (H3N8). A reference serum from the European Directorate for the Quality of Medicines and Healthcare (EDQM) was included on each plate. The results were expressed in mm2 and accepted when the EDQM standard was within 5% of its assigned value. A difference of 25 mm2 or greater between paired samples was considered significant.5
ELISA
The competition ID Screen Influenza A Antibody Competition Multispecies ELISA for the detection of antibodies to the internal nucleocapsid of the Influenza A virus in bird, pig and horse sera was carried out in accordance with the manufacturer's instructions (ID Vet Innovative Diagnostics, Montpellier, France). Briefly, test sera (diluted 1 in 10) were incubated for one hour at 37°C in the antigen‐coated plate. After washing, an anti‐antigen peroxidase conjugate fixed to epitopes that had not reacted with the sera. The results were read and recorded at the O.D. (Optical Density) at 450 nm using Magellan software and the Sunrise Absorbance Reader (Tecan; Männedorf, Switzerland). For each sample, the competition percentage was calculated by dividing the O.D. value of the specimen by the O.D. value of the negative control and multiplying by one hundred. A competition percentage of 45% or less was defined as positive and a conversion from negative to positive or a decrease in competition percentage of 50% or more was considered a seroconversion.
Statistical analysis
To examine symmetry in classification as positive or negative and seroconversion or otherwise, a two‐way classification table was constructed. The symmetry was then measured using the McNemar Test, and associated chi‐square statistic and P value were obtained. In examining the association between SRH and ELISA competition percentage, a simple linear regression model was fitted, and the predicted value associated with a number of SRH cut‐off values was estimated. Analysis was carried out using R Studio running R version 2.13.
Results
On testing paired samples from 203 horses during influenza outbreaks, 90 (44%) seroconverted by SRH, 84 (41%) by HI and 52 (26%) by ELISA (Table S1). By comparison with the HI as a gold standard diagnostic test for EI, the ELISA had a sensitivity and specificity of 69% and 95%, respectively. By comparison with the SRH as a gold standard, the ELISA had a sensitivity and specificity of 67% and 94%, respectively. The mean competition percentage of the 45 horses that seroconverted by SRH but not by ELISA was 9·37%. In comparison, the mean competition percentage of the 52 horses that seroconverted by ELISA was 54·92%. Of the 203 horses studied, 28 were seronegative and 175 were seropositive by SRH on initial sampling. Of the 28 SRH seronegative horses, 24 and 19 were also seronegative by HI and ELISA, respectively. Twenty‐five of the 28 (89%) seroconverted by HI, 22 (79%) by SRH and 21 (75%) by ELISA. Twenty of the 28 horses seroconverted by all three methods, two seroconverted by SRH and HI, one seroconverted by ELISA and HI and two seroconverted by HI alone. Only three did not seroconvert by any of the assays. Sixty‐eight (39%) of the 175 horses that were seropositive by SRH on initial sampling seroconverted by SRH, 59 (34%) by HI and 31 (18%) by ELISA. Twelve of the 31 SRH‐positive horses that seroconverted by ELISA tested negative by this assay on the initial screen.
Examinations of seropositive versus seronegative results with the three assays indicated that there was consistent and close agreement between HI and SRH but borderline disagreement between ELISA and SRH (chi‐square 3·1, P = 0·07) and significant disagreement between ELISA and HI (chi‐square 5·5, P = 0·02). A best‐fit regression line between SRH value and ELISA competition percentage yielded that 25 mm2 was equivalent to 49%, 50 mm2 was equivalent to 44%, 85 mm2 was equivalent to 38% and 150 mm2 was equivalent to 26%. Changing the ELISA cut‐off from 45% to 65% increased the symmetry between the ELISA and other methods, but it also increased the overall misclassification rate. Examination of seroconversions with the three assays indicated that there was a greater disagreement between the ELISA and the SRH (chi‐square 26·3, P < 0·001) and the ELISA and the HI (chi‐square 21·8, P < 0·001). However, SRH and HI showed symmetry in classification (chi‐square 1·6 and P = 0·21).
Fourteen seronegative weanlings experimentally infected with EIV were serologically monitored by SRH and ELISA. The percentage of seroconversions detected by both assays on each day of sampling, and the ELISA results are summarised in Figure 1A,B. All the horses were seronegative by both assays on Day‐1, prior to infection. All horses seroconverted by SRH testing by Day 14, but not by Day 7 post‐infection. The ELISA detected that all horses had seroconverted at 14 days post‐infection but also detected two seroconversions at 7 days post‐infection. Furthermore, several horses exhibited some response by ELISA at Day 7 but insufficient to be defined a seroconversion. Their mean competition percentage was 65 (Figure 1B).
Figure 1.
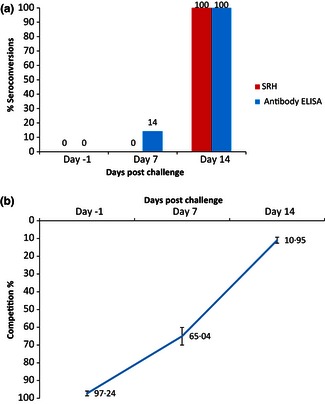
(A) Percentage seroconversions detected by SRH and ELISA post‐experimental infection (n = 14). (B) Mean Competition% detected by ELISA on day‐1, and days 7 and 14 post‐experimental infection (n = 14).
Samples from 60 seronegative weanlings that had participated in a comparative vaccine study were tested by ELISA, and the results compared with those previously obtained by SRH.17 The ELISA did not detect an antibody response in weanlings vaccinated with the recombinant canarypox virus vaccine (Proteq Flu‐Te), which does not contain influenza nucleocapsid protein. The pattern of antibody response post‐vaccination detected by the ELISA was broadly similar to that detected by the SRH test for the whole inactivated virus vaccines and the subunit ISCOM vaccines (Figures 2 and 3). A higher and more persistent ELISA antibody response was observed in horses vaccinated with the whole inactivated vaccine Duvaxyn IE‐T Plus than in horses vaccinated with the other vaccines. For the weanlings vaccinated with the subunit vaccines Equip FT and Equilis Prequenza Te, the pattern of agreement between the two tests was consistent with that observed in the group vaccinated with the whole virus vaccine Equilis Resequin. However, the ELISA response post‐V2 and V3 was more persistent than that observed post‐vaccination with Equilis Resequin.
Figure 2.
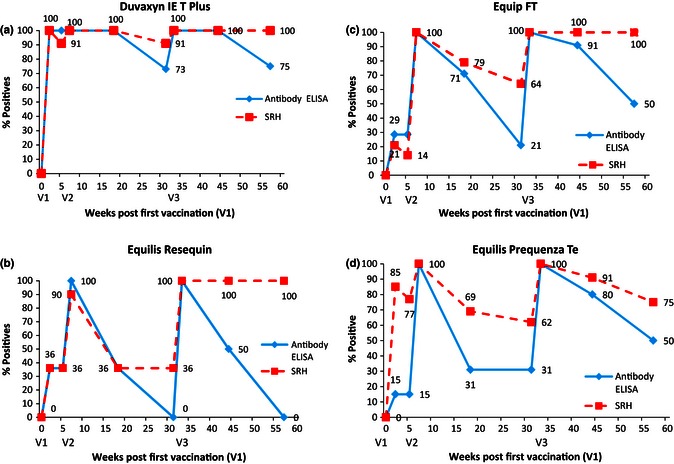
(A) Percentage of EI positive horses detected by ELISA or SRH following vaccination with Duvaxyn IE‐T Plus (B) Percentage of EI positive horses detected by ELISA or SRH following vaccination with Equilis Resequin. (C) Percentage of EI positive horses detected by ELISA or SRH following vaccination with Equip FT. (D) Percentage of EI positive horses detected by ELISA or SRH following vaccination with Equilis Prequenza Te. ELISA = ID Screen Influenza A Antibody Competition ELISA, SRH = Single Radial Haemolysis.
Figure 3.
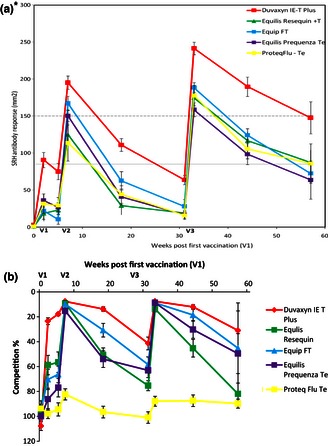
Mean antibody levels and competition percentage post‐EI vaccination as measured by SRH against A/equine/South Africa/4/03 (upper panel) and ELISA (lower panel) (n = 491). *SRH graph has been published17 and is shown here for comparative purposes.
Similar to the SRH results reported previously,17 some horses did not respond initially to vaccination as measured by ELISA. These data are summarised in Table 1. The weanlings vaccinated with Proteq Flu‐Te were excluded from this analysis. Two weeks post‐V1, the SRH and the ELISA failed to detect seroconversions in 19 (39%) and 27 (55%) weanlings, respectively (Table 1). The seroconversion of all weanlings vaccinated with Duvaxyn IE‐T Plus was detected with both assays. The number of weanlings vaccinated with Equilis Resequin and Equip FT that failed to seroconvert post‐V1 was similar with both the SRH and ELISA assay. However, 77% of the weanlings vaccinated with Equilis Prequenza Te failed to seroconvert by ELISA but only 8% failed to seroconvert by SRH. Two weeks post‐V2 (S4), 36% and 9% of horses vaccinated with Duvaxyn IE‐T Plus and Equilis Resequin, respectively, failed to seroconvert by ELISA, but these horses were strongly seropositive by ELISA at the time of vaccination (S3). Their mean competition percentage was 13·28%. Similarly, one horse failed to seroconvert to V3 with Equip FT by ELISA but was strongly positive (16·42%) at the time of vaccination.
Table 1.
Number of weanlings that failed to seroconvert by SRH or ELISA
| (S2) | (S3) | (S4) | (S7) | |||||
|---|---|---|---|---|---|---|---|---|
| Vaccine | SRH (%) | E (%) | SRH (%) | E (%) | SRH (%) | E (%) | SRH (%) | E (%) |
| Duvaxyn IE‐T Plus | 0/11 (0) | 0/11 (0) | 0/11 (0) | 0/11 (0) | 0/11 (0) | 4/11 (36) | 0/11 (0) | 0/11 (0) |
| Equilis Resequin | 7/11 (64) | 7/11 (64) | 6/11 (55) | 6/11 (55) | 1/11 (9) | 1/11 (9) | 0/11 (0) | 0/11 (0) |
| Equip FT | 11/14 (79) | 10/14 (71) | 11/14 (79) | 9/14 (64) | 0/14 (0) | 0/14 (0) | 0/14 (0) | 1/14 (7) |
| Equilis Prequenza Te | 1/13 (8) | 10/13 (77) | 1/13 (8) | 10/13 (77) | 0/13 (0) | 0/13 (0) | 0/13 (0) | 0/13 (0) |
S2 = two weeks post‐V1; S3 = at the time of V2; S4 = two weeks post‐V2; S7 = two weeks post‐V3; SRH = Single Radial Haemolysis, E = ID Screen Influenza A Antibody Competition ELISA.
Samples from 44 horses that had participated in a comparative vaccine study following annual booster vaccination were tested by ELISA (Figure 4). No increase in antibodies was detected by ELISA in the horses vaccinated with Proteq Flu‐Te. The antibody profiles against the nucleocapsid protein of the horses vaccinated with the other vaccines followed a similar pattern to the antibodies against the HA as measured by SRH. They peaked 2 weeks post‐booster vaccination, decreased by 3 months post‐vaccination and declined to near their original levels by 6 months post‐vaccination. All of the horses remained positive by SRH for the duration of the study with the exception of one horse that tested negative by 3 months post‐vaccination.18 This horse also tested negative by ELISA 3 months post‐booster vaccination, as did four other horses. However, the SRH levels for these four horses (82–157 mm2) at that time point were consistent with clinical protection. Three additional horses tested negative by ELISA 6 months post‐booster vaccination when their SRH levels ranged from 60 to 149 mm2. Six months post‐vaccination, five horses had SRH levels <85 mm2, that is, below that required for clinical protection and one horse was seronegative.18 Only one of these horses tested positive by ELISA at this time point.
Figure 4.
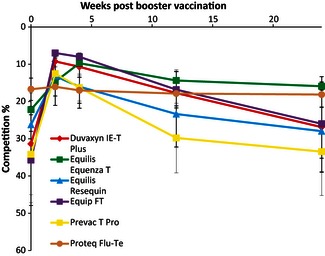
Mean ELISA competition percentage post‐booster vaccination (n = 44).
Excluding the horses vaccinated with Proteq Flu‐Te, 22 of the 38 horses (58%) did not seroconvert by ELISA and 13 (34%) did not seroconvert by SRH. Table 2 summarises the effect of pre‐existing antibody levels on the response to booster vaccination. The ELISA test was similar to the SRH in that pre‐existing antibody levels correlated with response to vaccination. Horses with ELISA competition percentage >15 responded best to vaccination, that is, 15 of 18 seroconverted. Only one of 20 horses with a competition percentage <15 seroconverted. The mean acute ELISA competition percentage of the horses that did not seroconvert post‐booster vaccination was 12·79% compared with a mean competition percentage of 54·56% for the horses that did seroconvert.
Table 2.
Influence of pre‐existing H3N8 antibody levels on booster vaccination response
| Pre‐existing antibody levels at time of vaccination | Number of animals responding by SRH (%) | Number of animals responding by ELISA (%) |
|---|---|---|
| Negligible | 3/3 (100) | 3/3 (100) |
| Low | 6/6 (100) | 5/6 (83) |
| Medium | 13/15 (87) | 7/9 (78) |
| High | 3/14 (21) | 1/20 (5) |
| Total | 25/38 (66) | 16/38 (42) |
For SRH antibody level Negligible ≤ 50 mm2; Low ≥ 50 mm2 < 85 mm2; Medium ≥ 85 mm2 < 150 mm2; High ≥ 150 mm2.
For ELISA competition percentage Negligible ≥ 85%; Low ≥ 50% < 85%; Medium ≥ 15% < 50%; High ≤ 15%.
Discussion
The sensitivity of the ELISA in the serological diagnosis of EI was compared with that of the gold standard assays HI and SRH. Examination of paired serum samples from 203 horses on 14 premises affected by EI indicated that the ELISA detected only 52 seroconversions compared with over 80 by HI or SRH. Many of the horses that seroconverted by the traditional tests but failed to seroconvert by the ELISA were seropositive by ELISA; at the time, the first or acute sample was collected. The results suggest that the ELISA is a less‐sensitive test than HI or SRH for the detection of EI in a country where the virus is endemic, and many of the horses are seropositive by ELISA at the time of exposure. In such circumstances, the ELISA should only be the test of choice in laboratories that do not have the expertise to perform HI or SRH. However, analysis of samples from experimentally infected foals confirmed the findings of Kittelberger et al.,21 and Read et al.,15 that in the naïve animal, an ELISA is extremely sensitive and can detect an antibody response by day seven post‐infection. This sensitivity combined with the fact that the ELISA, unlike the HI or the SRH, can be readily automated and used to rapidly screen large numbers of samples suggest that it is an appropriate test for monitoring the spread of EI in a naïve population. A nucleoprotein ELISA was used to screen approximately 62 000 samples in 6 months after EI was detected in Australia in 2007.15
In this study, serum samples from weanlings that had participated in a comparative vaccine study were tested by ELISA and the results compared with those previously obtained by SRH.17 Only the antibody response of those vaccinated with Proteq Flu‐Te was not detected by ELISA. Thus, this technique could not be used to differentiate infected horses from those vaccinated with the ISCOM‐based subunit vaccines Equip FT and Equilis Prequenza Te or the whole inactivated vaccines Duvaxyn IE‐T Plus and Equilis Resequin. A combination of this kit and any of these four vaccines would not have been suitable for the eradication programme in Australia as there is no DIVA capacity, and it would not be possible to prove freedom from infection. This was to be expected for the whole virus inactivated vaccines. However, some DIVA capacity might have been anticipated with the subunit vaccines which are not expected to contain NP, for example, Equip FT and Equilis Prequenza Te, which contain purified HA and neuraminidase subunits adjuvanted with ISCOM and ISCOM matrix, respectively.22, 23 Similar limitations have been noted with the purity of vaccines against avian influenza and other pathogens when developing DIVA test strategies.24, 25
The pattern of antibody response as measured by the ELISA post‐vaccination was similar to that of the SRH for the four vaccines, and the decline in antibodies after the second vaccination (V2) and prior to third vaccination (V3) was evident. The ELISA determined response to vaccination was less persistent than that measured by the SRH. This lack of persistence was consistent with observations during the outbreak in Australia. Read et al.,15 reported that the ELISA detected antibody in samples from only half of the horses tested 12 months following natural infection. In this study, the ELISA was similar to the SRH in detecting a greater antibody response in horses vaccinated with Duvaxyn IE‐T Plus than other vaccines.17 The ELISA was also similar to SRH in that no poor responders to the first dose (V1) of Duvaxyn IE‐T Plus were identified. The number of weanlings that failed to seroconvert to V1 following vaccination with Equilis Prequenza Te far exceeded those identified by SRH.17 A possible explanation for this finding is that Equilis Prequenza Te is a subunit vaccine that should contain at most, traces of NP. However, the amount of NP contained in any of the vaccines included in this study is unknown. Furthermore, the fact that the incidence of poor responders to V1 with the whole virus vaccine Equilis Resequin was similar to that of V1 with the subunit vaccine Equip FT suggests that a poor response by ELISA is not entirely due to the variable amount of NP from structural component to only traces, in the different types of EI vaccines. On comparison of the two whole virus vaccines, Equilis Resequin has a higher antigenic load than Duvaxyn IE‐T Plus. Thus, it is likely that the higher incidence of poor responders observed with Equilis Resequin is due at least in part to the composition of the adjuvant. As with SRH, almost all of the weanlings that failed to seroconvert to V1 seroconverted to V2 by ELISA. The high incidence of poor responders amongst these Thoroughbred weanlings reported by Gildea et al.,17 was not reported previously in experimental studies with these vaccines. This study using a different serological test corroborates the original findings of Gildea et al.17 Poor responders are a source of concern to horse owners and trainers. They are considered most likely to be the index case in the event of an outbreak in a vaccinated population.26
Samples from a second comparative vaccine study in racehorses following booster vaccination18 were analysed by ELISA. The DIVA capacity of the ELISA combined with Proteq Flu‐Te was again evident as no antibody response was detected post‐vaccination. The ELISA response to all other vaccines used was similar to the SRH response, an initial peak followed by a decline within 3 months. For several horses, the ELISA determined response to booster vaccination was less persistent than that measured by the SRH. The ELISA results in the present study corroborated the SRH data in that a large number (58%) of these horses did not seroconvert post‐vaccination. In many cases, these were not the same horses as those that failed to seroconvert by SRH, but for both assays, the antibody response correlated inversely with the level of antibody at the time of vaccination.
In summary, the results suggest that this ELISA is a useful supplementary test for the diagnosis of EI. It is suited to high throughput surveillance of EI in a naive population. However, the DIVA capacity evident when combined with Proteq Flu‐Te does not extend to the two ISCOM‐based subunit vaccines Equilis Prequenza Te and Equip FT tested in this study.
Supporting information
Table S1. SRH, HI and ELISA results for acute and convalescent serum samples collected from 203 horses during EI outbreaks.
Galvin et al (2013) The evaluation of a nucleoprotein ELISA for the detection of equine influenza antibodies and the differentiation of infected from vaccinated horses (DIVA). Influenza and Other Respiratory Viruses 7(Suppl. 4), 73–80.
Footnotes
Fort Dodge now ELANCO Animal Health.
Intervet now MSD Animal Health.
Pfizer now Zoetis.
Merial.
References
- 1. OIE 2012. Manual of standards for diagnostics tests and vaccines, Equine Influenza. Chapter 2.5.7, 1–14.
- 2. Mumford J. Collaborative study for the establishment of three European Pharmacopoeia Biological Reference Preparations for equine influenza horse antiserum. Pharmeuropa 2000; 1:7–21. [Google Scholar]
- 3. Daly J, Daas A, Behr‐Gross ME. Collaborative study for the establishment of a candidate equine influenza subtype 2 American‐like strain A/EQ/South Africa/4/03 ‐ horse antiserum biological reference preparation. Pharmeuropa Bio 2007; 1:7–14. [PubMed] [Google Scholar]
- 4. Mumford JA, Jessett DM, Rollinson EA, Hannant D, Draper ME. Duration of protective efficacy of equine influenza immunostimulating complex/tetanus vaccines. Vet Rec 1994; 134:158–62. [DOI] [PubMed] [Google Scholar]
- 5. Newton JR, Townsend HG, Wood JL, Sinclair R, Hannant D, Mumford JA. Immunity to equine influenza: relationship of vaccine‐induced antibody in young Thoroughbred racehorses to protection against field infection with influenza A/equine‐2 viruses (H3N8). Equine Vet J 2000; 32:65–74. [DOI] [PubMed] [Google Scholar]
- 6. Mumford JA. Biology, Epidemiology and Vaccinology of Equine Influenza. Quality Control of Equine Influenza Vaccines, Proceedings of an International Symposium organised by the European Directorate for the Quality of Medicines (EDQM), Council of Europe, Budapest 10–11 December 2001; 7–12.
- 7. Daly JM, Sindle T, Tearle J, Barquero N, Newton JR, Corning S. Equine influenza vaccine containing older H3N8 strains offers protection against A/eq/South Africa/4/03 (H3N8) strain in a short‐term vaccine efficacy study. Equine Vet J 2007; 39:446–50. [DOI] [PubMed] [Google Scholar]
- 8. Paillot R, Prowse L, Donald C et al Efficacy of a whole inactivated EI vaccine against a recent EIV outbreak isolate and comparative detection of virus shedding. Vet Immunol Immunopathol 2010; 136:272–83. [DOI] [PubMed] [Google Scholar]
- 9. Paillot R, Prowse L, Montesso F, Huang CM, Barnes H, Escala J. Whole inactivated equine influenza vaccine: efficacy against a representative clade 2 equine influenza virus, IFNgamma synthesis and duration of humoral immunity. Vet Microbiol 2013; 162:396–407. [DOI] [PubMed] [Google Scholar]
- 10. El‐Hage CM, Savage CJ, Minke JM, Ficorilli NP, Watson J, Gilkerson JR. Accelerated vaccination schedule provides protective levels of antibody and complete herd immunity to equine influenza. Equine Vet J 2013; 45:235–9. [DOI] [PubMed] [Google Scholar]
- 11. Garner MG, Cowled B, East IJ, Moloney BJ, Kung N. Evaluating the effectiveness of the response to equine influenza in the Australian outbreak and the potential role of early vaccination. Aust Vet J 2011; 89(Suppl 1):143–5. [DOI] [PubMed] [Google Scholar]
- 12. Selleck PW, Kirkland PD. Avian Influenza. In: Sub‐Committee on Animal Health Laboratory Standards for Animal Health Committee (www.scahls.org.au). Australian and New Zealand Standard Diagnostic Procedures for Animal Diseases.
- 13. Sergeant ES, Kirkland PD, Cowled BD. Field evaluation of an equine influenza ELISA used in New South Wales during the 2007 Australian outbreak response. Prev Vet Med 2009; 92:382–5. [DOI] [PubMed] [Google Scholar]
- 14. Kirkland PD, Delbridge G. Use of a blocking ELISA for antibodies to equine influenza virus as a test to distinguish between naturally infected and vaccinated horses: proof of concept studies. Aust Vet J 2011; 89(Suppl 1):45–6. [DOI] [PubMed] [Google Scholar]
- 15. Read AJ, Arzey KE, Finlaison DS et al A prospective longitudinal study of naturally infected horses to evaluate the performance characteristics of rapid diagnostic tests for equine influenza virus. Vet Microbiol 2012; 156:246–55. [DOI] [PubMed] [Google Scholar]
- 16. Cowled B, Ward MP, Hamilton S, Garner G. The equine influenza epidemic in Australia: spatial and temporal descriptive analyses of a large propagating epidemic. Prev Vet Med 2009; 92:60–70. [DOI] [PubMed] [Google Scholar]
- 17. Gildea S, Arkins S, Walsh C, Cullinane A. A comparison of antibody responses to commercial equine influenza vaccines following primary vaccination of Thoroughbred weanlings‐a randomised blind study. Vaccine 2011; 29:9214–23. [DOI] [PubMed] [Google Scholar]
- 18. Gildea S, Arkins S, Walsh C, Cullinane A. A comparison of antibody responses to commercial equine influenza vaccines following annual booster vaccination of National Hunt horses ‐ a randomised blind study. Vaccine 2011; 29:3917–22. [DOI] [PubMed] [Google Scholar]
- 19. Cullinane A, Weld J, Osborne M, Nelly M, McBride C, Walsh C. Field studies on equine influenza vaccination regimes in thoroughbred foals and yearlings. Vet J 2001; 161:174–85. [DOI] [PubMed] [Google Scholar]
- 20. Gildea S, Arkins S, Cullinane A. A comparative antibody study of the potential susceptibility of Thoroughbred and non‐Thoroughbred horse populations in Ireland to equine influenza virus. Influenza Other Respi Viruses 2010; 4:363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kittelberger R, McFadden AM, Hannah MJ et al Comparative evaluation of four competitive/blocking ELISAs for the detection of influenza A antibodies in horses. Vet Microbiol 2011; 148:377–83. [DOI] [PubMed] [Google Scholar]
- 22. Paillot R, Hannant D, Kydd JH, Daly JM. Vaccination against equine influenza: quid novi? Vaccine 2006; 24:4047–61. [DOI] [PubMed] [Google Scholar]
- 23. Paillot R, Prowse L. ISCOM‐matrix‐based equine influenza (EIV) vaccine stimulates cell‐mediated immunity in the horse. Vet Immunol Immunopathol 2012; 145:516–21. [DOI] [PubMed] [Google Scholar]
- 24. Suarez DL. Overview of avian influenza DIVA test strategies. Biologicals 2005; 33:221–6. [DOI] [PubMed] [Google Scholar]
- 25. Caporale V, Giovannini A, Zepeda C. Surveillance strategies for foot and mouth disease to prove absence of disease and absence of viral circulation. Rev Sci Tech 2012; 31:747–59. [DOI] [PubMed] [Google Scholar]
- 26. Wood JLN. A Review of the history and epidemiology and a description of recent outbreak. [MSc Dissertation]. London: University of London; 1991.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. SRH, HI and ELISA results for acute and convalescent serum samples collected from 203 horses during EI outbreaks.


